Changes in Surface Characteristics of BOPP Foil after Treatment by Ambient Air Plasma Generated by Coplanar and Volume Dielectric Barrier Discharge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
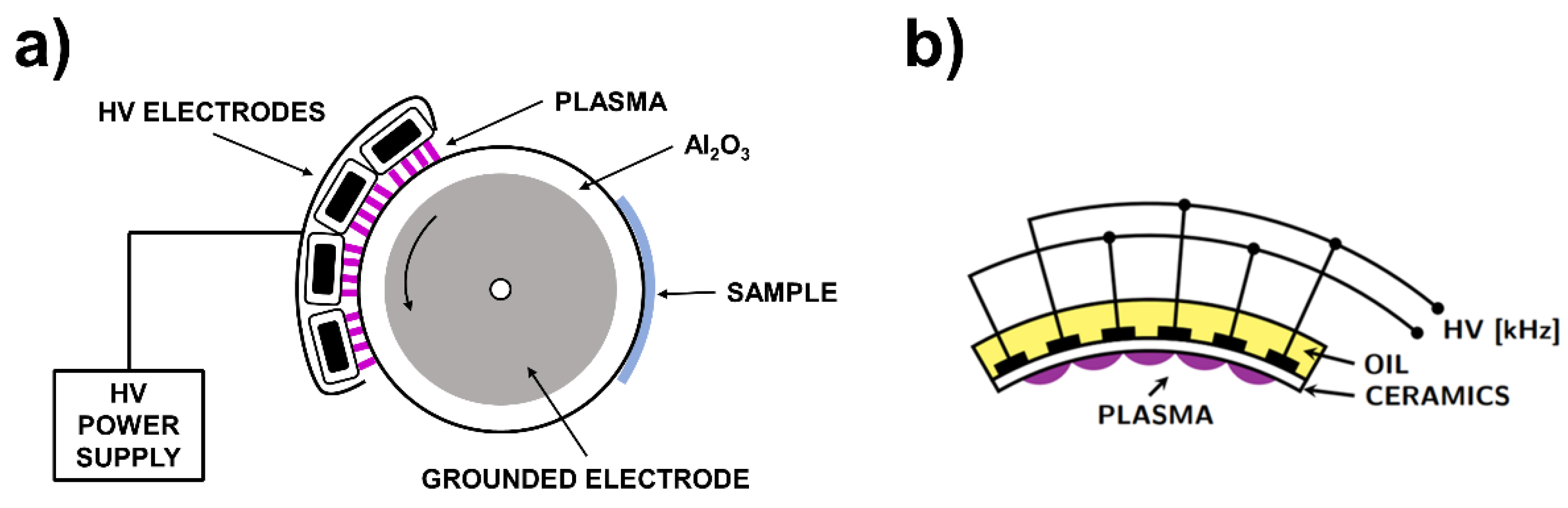
2.2. Plasma Treatment
2.3. Analytical Methods
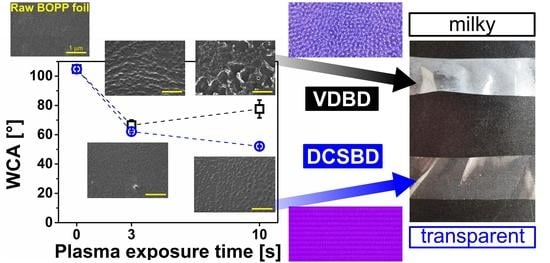
3. Results
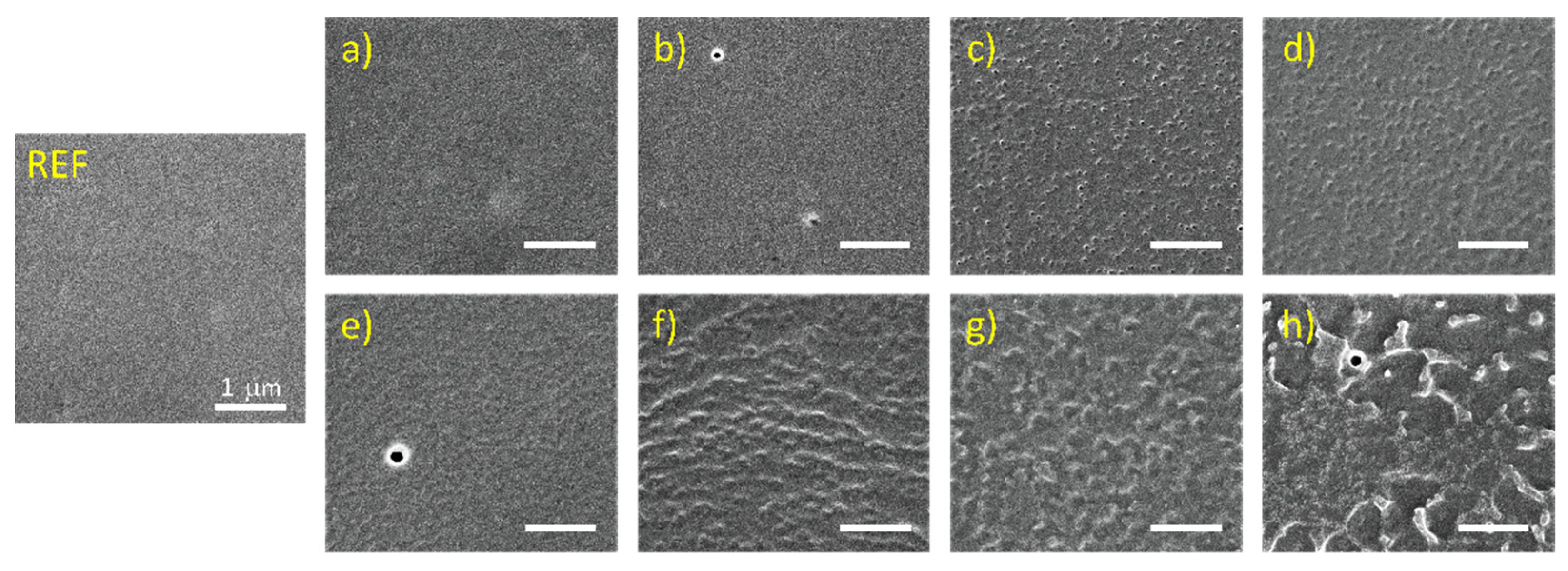
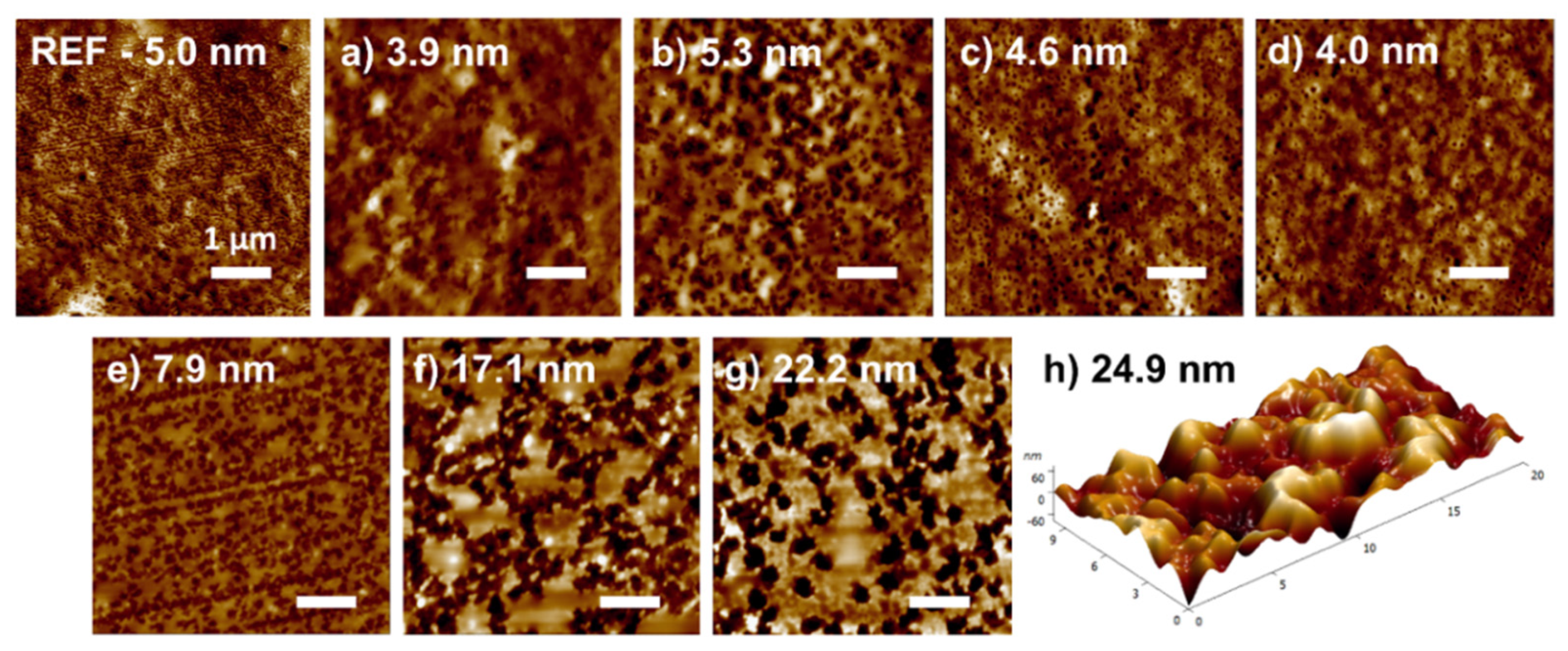
3.1. Surface Morphology
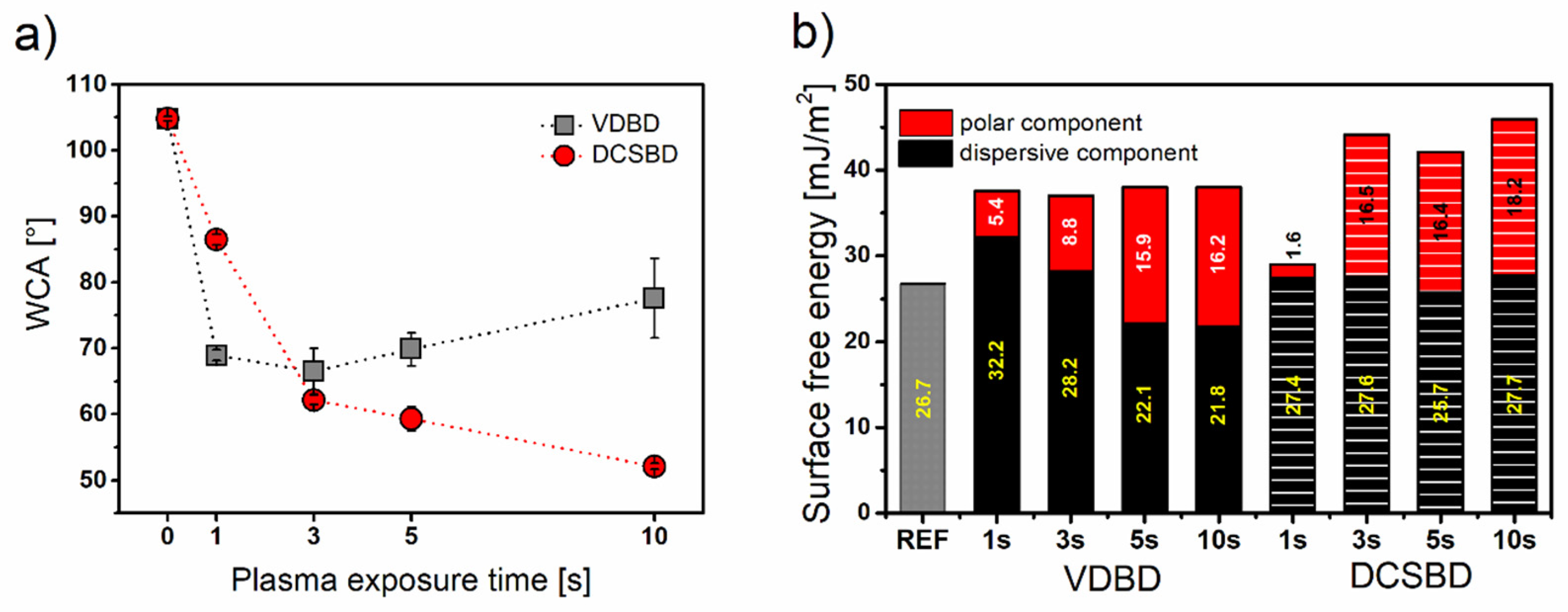
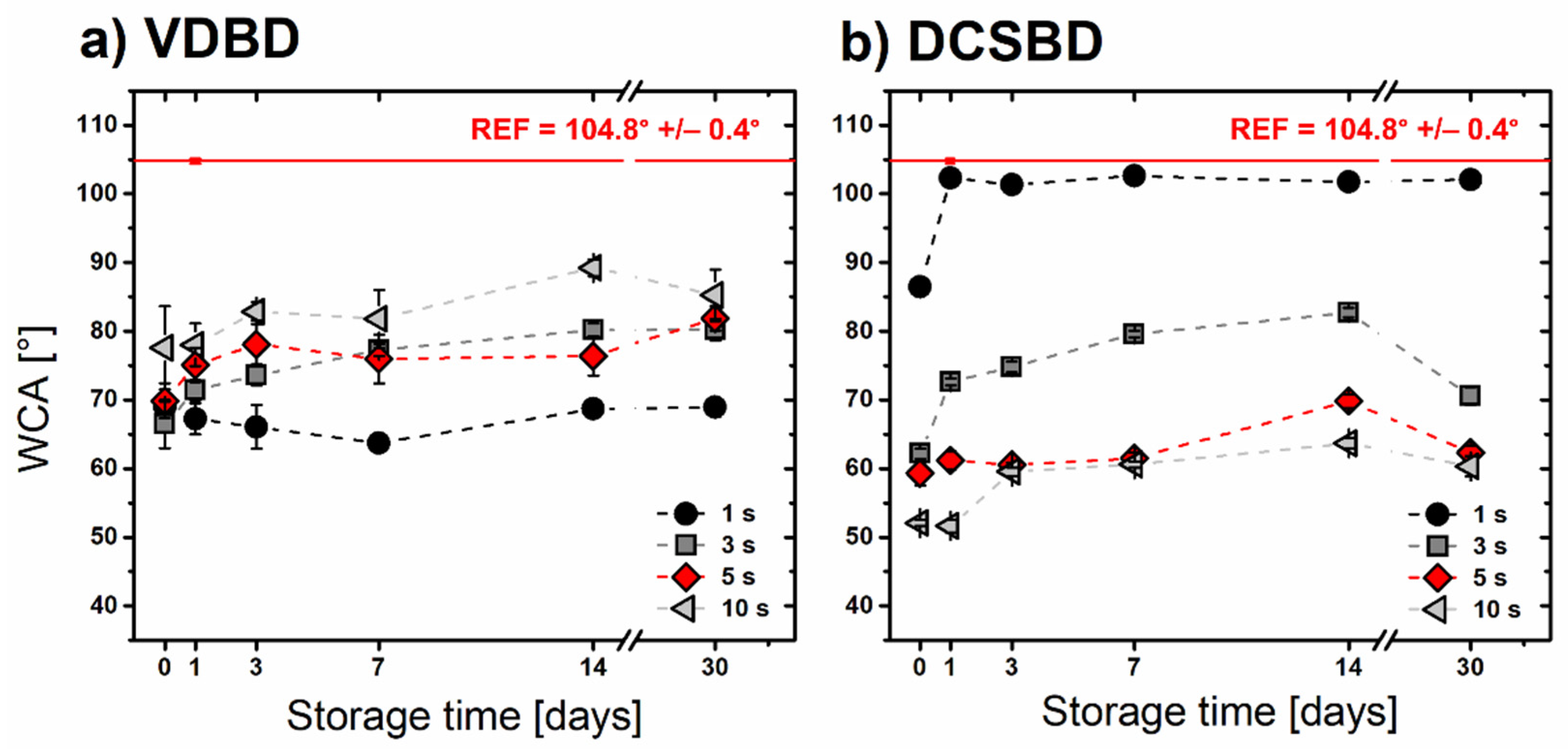
3.2. Wettability and Ageing Study
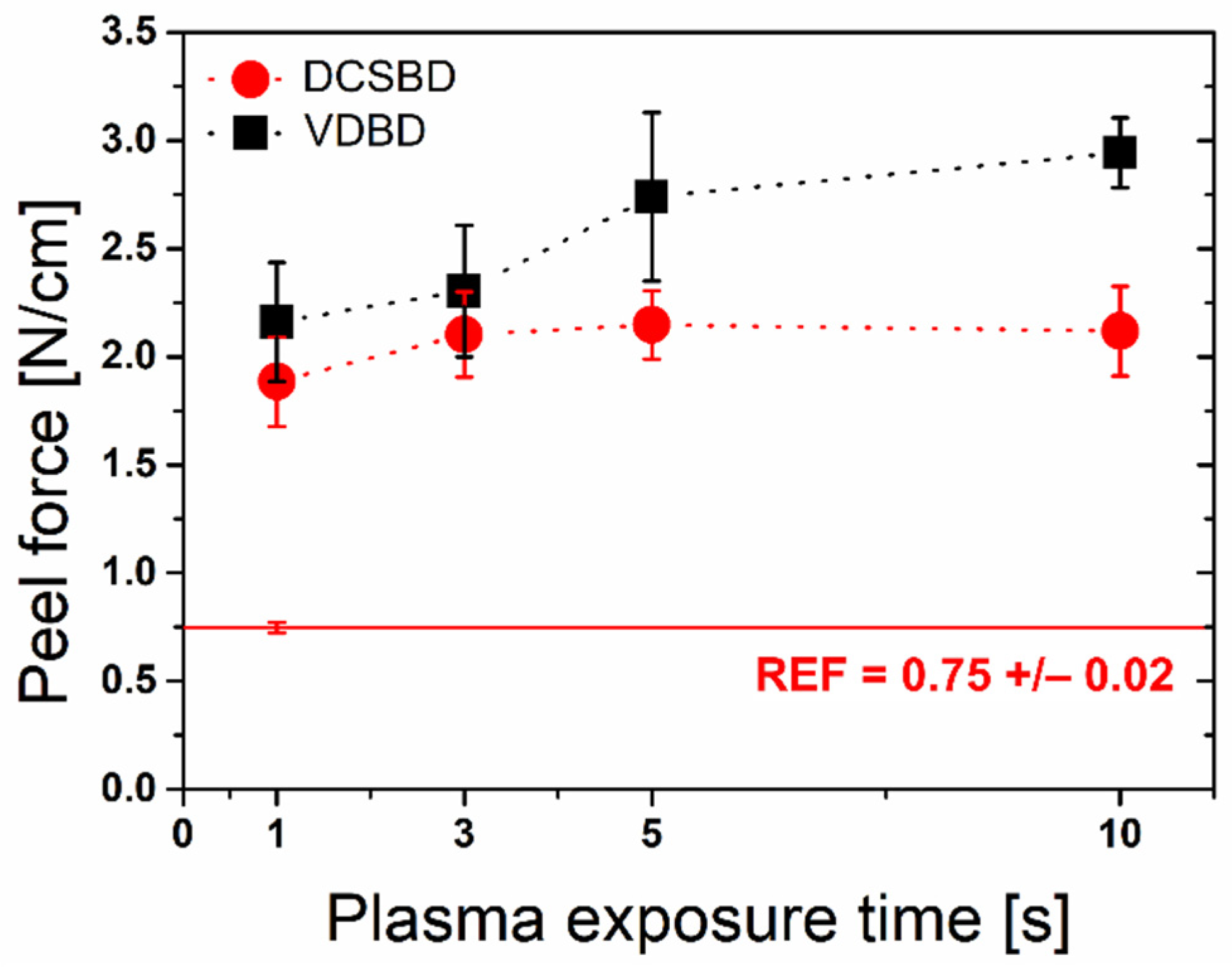
3.3. Peel Force
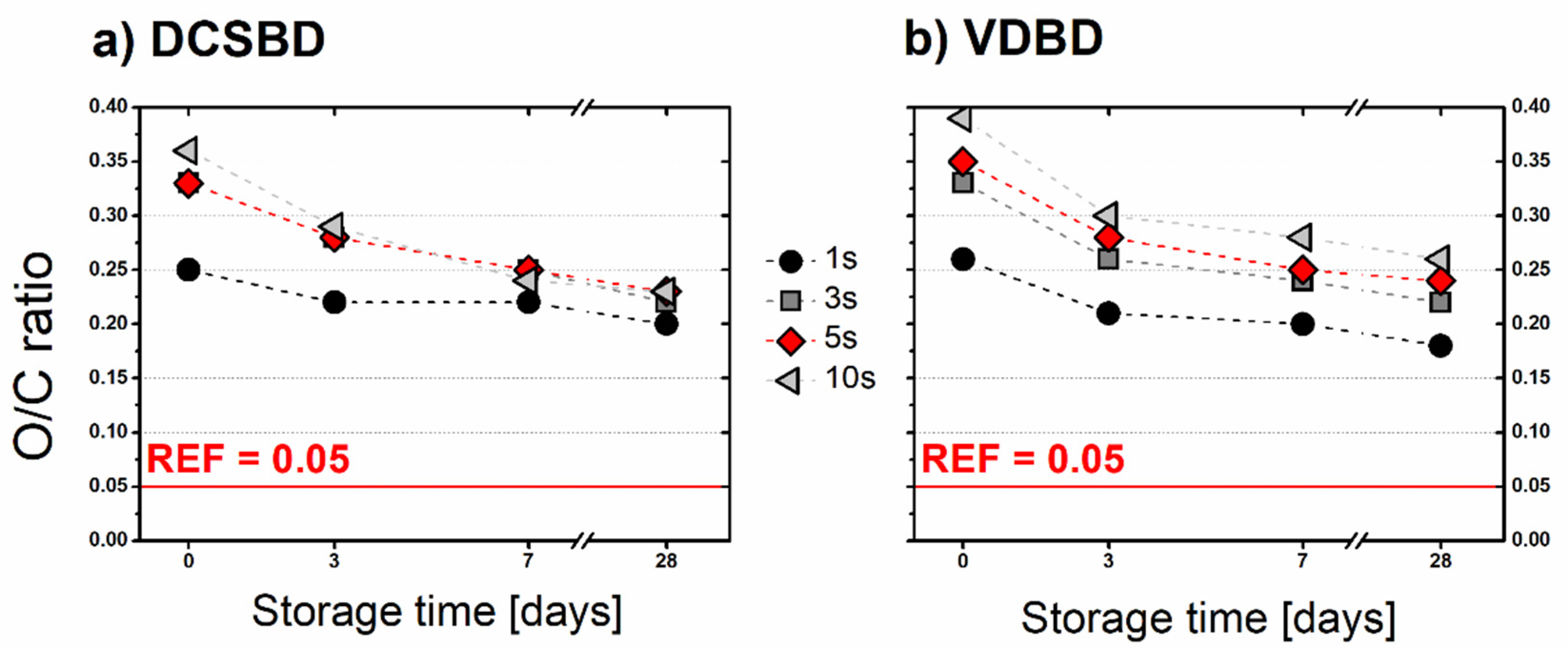
3.4. Surface Chemical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calafut, T. Polypropylene Films. In Plastic Films in Food Packaging; Elsevier: Amsterdam, The Netherlands, 1998; pp. 17–20. [Google Scholar]
- Ščetar, M.; Kurek, M.; Režek Jambrak, A.; Debeaufort, F.; Galić, K. Effect of high power ultrasound on physical–chemical properties of polypropylene films aimed for food packaging: Structure and surface features. Polym. Bull. 2019, 76, 1007–1021. [Google Scholar] [CrossRef]
- Gull, A.; Prasad, K.; Kumar, P. Quality Changes in Functional Pasta During Storage in Two Different Packaging Materials: LDPE and BOPP. J. Food Process. Preserv. 2017, 41, 1–7. [Google Scholar] [CrossRef]
- Huan, T.D.; Boggs, S.; Teyssedre, G.; Laurent, C.; Cakmak, M.; Kumar, S.; Ramprasad, R. Advanced polymeric dielectrics for high energy density applications. Prog. Mater. Sci. 2016, 83, 236–269. [Google Scholar] [CrossRef]
- Guimond, S.; Radu, I.; Czeremuszkin, G.; Carlsson, D.J.; Wertheimer, M.R. Biaxially Oriented Polypropylene (BOPP) Surface Modification by Nitrogen Atmospheric Pressure Glow Discharge (APGD) and by Air Corona. Plasmas Polym. 2002, 7, 71–88. [Google Scholar] [CrossRef]
- Novák, I.; Florián, Š. Investigation of long-term hydrophobic recovery of plasma modified polypropylene. J. Mater. Sci. 2004, 39, 2033–2036. [Google Scholar] [CrossRef]
- Štěpánová, V.; Šrámková, P.; Sihelník, S.; Stupavská, M.; Jurmanová, J.; Kováčik, D. The effect of ambient air plasma generated by coplanar and volume dielectric barrier discharge on the surface characteristics of polyamide foils. Vacuum 2021, 183, 109887. [Google Scholar] [CrossRef]
- Galmiz, O.; Kelar Tučeková, Z.; Kelar, J.; Zemánek, M.; Stupavská, M.; Kováčik, D.; Černák, M. Effect of atmospheric pressure plasma on surface modification of paper. AIP Adv. 2019, 9, 105013. [Google Scholar] [CrossRef]
- Černák, M.; Kováčik, D.; Ráhel’, J.; St’ahel, P.; Zahoranová, A.; Kubincová, J.; Tóth, A.; Černáková, L. Generation of a high-density highly non-equilibrium air plasma for high-speed large-area flat surface processing. Plasma Phys. Control. Fusion 2011, 53, 124031. [Google Scholar] [CrossRef]
- Kormunda, M.; Homola, T.; Matoušek, J.; Kováčik, D.; Černák, M.; Pavlik, J. Surface analysis of poly(ethylene naphthalate) (PEN) films treated at atmospheric pressure using diffuse coplanar surface barrier discharge in air and in nitrogen. Polym. Degrad. Stab. 2012, 97, 547–553. [Google Scholar] [CrossRef]
- Shekargoftar, M.; Kelar, J.; Krumpolec, R.; Jurmanová, J.; Homola, T. A Comparison of the Effects of Ambient Air Plasma Generated by Volume and by Coplanar DBDs on the Surfaces of PP/Al/PET Laminated Foil. IEEE Trans. Plasma Sci. 2018, 46, 3653–3661. [Google Scholar] [CrossRef]
- Hergelová, B.; Zahoranová, A.; Kováčik, D.; Stupavská, M.; Černák, M. Polylactic acid surface activation by atmospheric pressure dielectric barrier discharge plasma. Open Chem. 2015, 13, 564–569. [Google Scholar] [CrossRef] [Green Version]
- Kelar, J.; Shekargoftar, M.; Krumpolec, R.; Homola, T. Activation of polycarbonate (PC) surfaces by atmospheric pressure plasma in ambient air. Polym. Test. 2018, 67, 428–434. [Google Scholar] [CrossRef]
- Homola, T.; Matoušek, J.; Hergelová, B.; Kormunda, M.; Wu, L.Y.L.; Černák, M. Activation of poly(methyl methacrylate) surfaces by atmospheric pressure plasma. Polym. Degrad. Stab. 2012, 97, 886–892. [Google Scholar] [CrossRef]
- Saranko, A.; Szakal, Z.; Kalacska, G.; Samyn, P.; Sukumaran, J.; Klebert, S.; Karoly, Z. Adhesion and sliding tribological properties of polyolefins treated by diffuse coplanar surface barrier discharges. Express Polym. Lett. 2018, 12, 972–985. [Google Scholar] [CrossRef]
- Janík, R.; Kohutiar, M.; Pajtášová, M.; Dubec, A.; Pagáčová, J.; Šulcová, J. The impact of DCSBD plasma discharge on polypropylene. IOP Conf. Ser. Mater. Sci. Eng. 2020, 776, 012090. [Google Scholar] [CrossRef]
- Kohutiar, M.; Janík, R.; Pajtášová, M.; Ondrušová, D.; Labaj, I.; Zvoláneková Mezencevová, V. Study of structural changes in thermoplastics using dynamic mechanical analysis. IOP Conf. Ser. Mater. Sci. Eng. 2020, 776, 012092. [Google Scholar] [CrossRef] [Green Version]
- Oravcová, A.; Hudec, I. The influence of atmospheric pressure plasma treatment on surface properties of polypropylene films. Acta Chim. Slovaca 2010, 3, 57–62. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Wokovich, A.M.; Brown, S.A.; McMaster, F.J.; Doub, W.H.; Cai, B.; Sadrieh, N.; Mei, L.C.; Machado, S.; Shen, M.; Buhse, L.F. Evaluation of substrates for 90° peel adhesion—A collaborative study: I. Medical tapes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 105–113. [Google Scholar] [CrossRef]
- McGuiggan, P.M.; Chiche, A.; Filliben, J.J.; Yarusso, D.J. Peel of an adhesive tape from a temperature-gradient surface. Int. J. Adhes. Adhes. 2008, 28, 185–191. [Google Scholar] [CrossRef]
- Junkar, I.; Cvelbar, U.; Vesel, A.; Hauptman, N.; Mozetič, M. The role of crystallinity on polymer interaction with oxygen plasma. Plasma Process. Polym. 2009, 6, 667–675. [Google Scholar] [CrossRef]
- Strobel, M.; Strobel, J.M.; Jones, V.; Lechuga, H.; Lyons, C.S. Effect on wettability of the topography and oxidation state of biaxially oriented poly(propylene) film. J. Adhes. Sci. Technol. 2019, 33, 1644–1657. [Google Scholar] [CrossRef]
- Chen, W.X.; Yu, J.S.; Chen, G.L.; Qiu, X.P.; Hu, W.; Bai, H.Y.; Shao, J.Z. Development of a novel protocol for the permanent hydrophilic modification of a BOPP film for high quality printing with water-based ink. RSC Adv. 2015, 5, 87963–87970. [Google Scholar] [CrossRef]
- Darvish, F.; Mostofi Sarkari, N.; Khani, M.; Eslami, E.; Shokri, B.; Mohseni, M.; Ebrahimi, M.; Alizadeh, M.; Dee, C.F. Direct plasma treatment approach based on non-thermal gliding arc for surface modification of biaxially-oriented polypropylene with post-exposure hydrophilicity improvement and minus aging effects. Appl. Surf. Sci. 2020, 509, 144815. [Google Scholar] [CrossRef]
- Wang, C.; He, X. Polypropylene surface modification model in atmospheric pressure dielectric barrier discharge. Surf. Coat. Technol. 2006, 201, 3377–3384. [Google Scholar] [CrossRef]
- Gim, S.; Lee, I.; Park, J.Y.; Lee, J.L. Spontaneously Embedded Scattering Structures in a Flexible Substrate for Light Extraction. Small 2017, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Morgan, H. Controlling the Wettability of Hierarchically Structured Thermoplastics. Langmuir 2012, 28, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Dorai, R.; Kushner, M.J. A model for plasma modification of polypropylene using atmospheric pressure discharges. J. Phys. D Appl. Phys. 2003, 36, 666–685. [Google Scholar] [CrossRef]
- Borcia, G.; Anderson, C.A.; Brown, N.M.D. The surface oxidation of selected polymers using an atmospheric pressure air dielectric barrier discharge: Part I. Appl. Surf. Sci. 2004, 221, 203–214. [Google Scholar] [CrossRef]
- Hanusová, J.; Kováčik, D.; Stupavská, M.; Černák, M.; Novák, I. Atmospheric pressure plasma treatment of polyamide-12 foils. Open Chem. 2015, 13, 382–388. [Google Scholar] [CrossRef]
- Bhat, N.V.; Upadhyay, D.J. Plasma-induced surface modification and adhesion enhancement of polypropylene surface. J. Appl. Polym. Sci. 2002, 86, 925–936. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Arabi, H.; Salem, A.; Asiaban, S. Effect of low-pressure O2 and Ar plasma treatments on the wettability and morphology of biaxial-oriented polypropylene (BOPP) film. Prog. Org. Coat. 2007, 60, 105–111. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Comparison between XPS- And FTIR-analysis of plasma-treated polypropylene film surfaces. Surf. Interface Anal. 2008, 40, 597–600. [Google Scholar] [CrossRef]
- Navaneetha Pandiyaraj, K.; Selvarajan, V.; Deshmukh, R.R.; Gao, C. Adhesive properties of polypropylene (PP) and polyethylene terephthalate (PET) film surfaces treated by DC glow discharge plasma. Vacuum 2008, 83, 332–339. [Google Scholar] [CrossRef]
- Leroux, F.; Campagne, C.; Perwuelz, A.; Gengembre, L. Polypropylene film chemical and physical modifications by dielectric barrier discharge plasma treatment at atmospheric pressure. J. Colloid Interface Sci. 2008, 328, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhang, X.; Wang, Y. Study on the Behavior of BOPP Film Treated by Corona Discharge. Coatings 2020, 10, 1195. [Google Scholar] [CrossRef]
| Atomic Concentration [%] 1 | O/C Ratio | Functional Groups Concentration [%] 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | N | C–C/C-H | C–O | C=O | O–C=O | |||
| 284.8 eV | 285.9 eV | 287.5 eV | 289.3 eV | ||||||
| REF | 95 | 5 | - | 0.05 | 94.2 | 5.8 | - | - | |
| DCSBD | 1 s | 80 | 20 | <1 | 0.25 | 76.5 | 15.1 | 6.7 | 1.7 |
| 3 s | 74 | 24 | 1.8 | 0.33 | 71.5 | 14.3 | 8.1 | 6.1 | |
| 5 s | 73 | 24 | 2.2 | 0.33 | 69.2 | 14.2 | 9.1 | 7.5 | |
| 10 s | 72 | 26 | 1.7 | 0.36 | 65.8 | 15.9 | 9.8 | 8.6 | |
| VDBD | 1 s | 79 | 20 | <1 | 0.26 | 73.7 | 14.0 | 7.1 | 5.3 |
| 3 s | 74 | 25 | 1.0 | 0.33 | 66.5 | 15.3 | 9.3 | 8.9 | |
| 5 s | 73 | 26 | 1.1 | 0.35 | 65.7 | 15.2 | 9.1 | 10.0 | |
| 10 s | 71 | 28 | 1.2 | 0.39 | 62.3 | 15.3 | 9.6 | 12.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šrámková, P.; Kelar Tučeková, Z.; Fleischer, M.; Kelar, J.; Kováčik, D. Changes in Surface Characteristics of BOPP Foil after Treatment by Ambient Air Plasma Generated by Coplanar and Volume Dielectric Barrier Discharge. Polymers 2021, 13, 4173. https://doi.org/10.3390/polym13234173
Šrámková P, Kelar Tučeková Z, Fleischer M, Kelar J, Kováčik D. Changes in Surface Characteristics of BOPP Foil after Treatment by Ambient Air Plasma Generated by Coplanar and Volume Dielectric Barrier Discharge. Polymers. 2021; 13(23):4173. https://doi.org/10.3390/polym13234173
Chicago/Turabian StyleŠrámková, Petra, Zlata Kelar Tučeková, Michal Fleischer, Jakub Kelar, and Dušan Kováčik. 2021. "Changes in Surface Characteristics of BOPP Foil after Treatment by Ambient Air Plasma Generated by Coplanar and Volume Dielectric Barrier Discharge" Polymers 13, no. 23: 4173. https://doi.org/10.3390/polym13234173
APA StyleŠrámková, P., Kelar Tučeková, Z., Fleischer, M., Kelar, J., & Kováčik, D. (2021). Changes in Surface Characteristics of BOPP Foil after Treatment by Ambient Air Plasma Generated by Coplanar and Volume Dielectric Barrier Discharge. Polymers, 13(23), 4173. https://doi.org/10.3390/polym13234173