Synthesis of Low Temperature Resistant Hydrogenated Nitrile Rubber Based on Esterification Reaction
Abstract
:1. Introduction
2. Materials & Methods
2.1. Materials
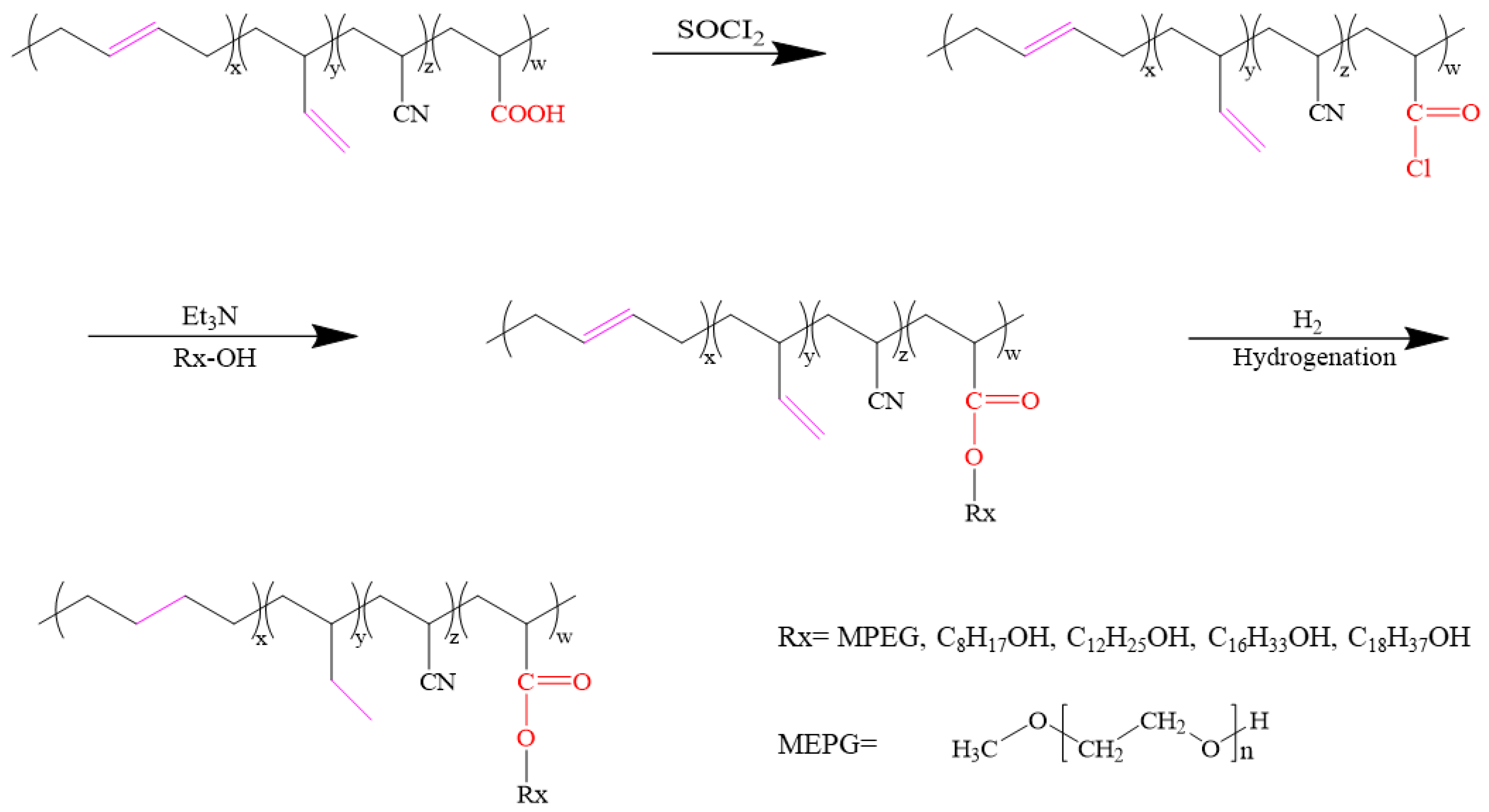
2.2. Preparation of Grafted XNBR with Different Side Groups
2.3. Hydrogenation of Grafted XNBR with Different Side Groups
2.4. Characterization
3. Results and Discussion
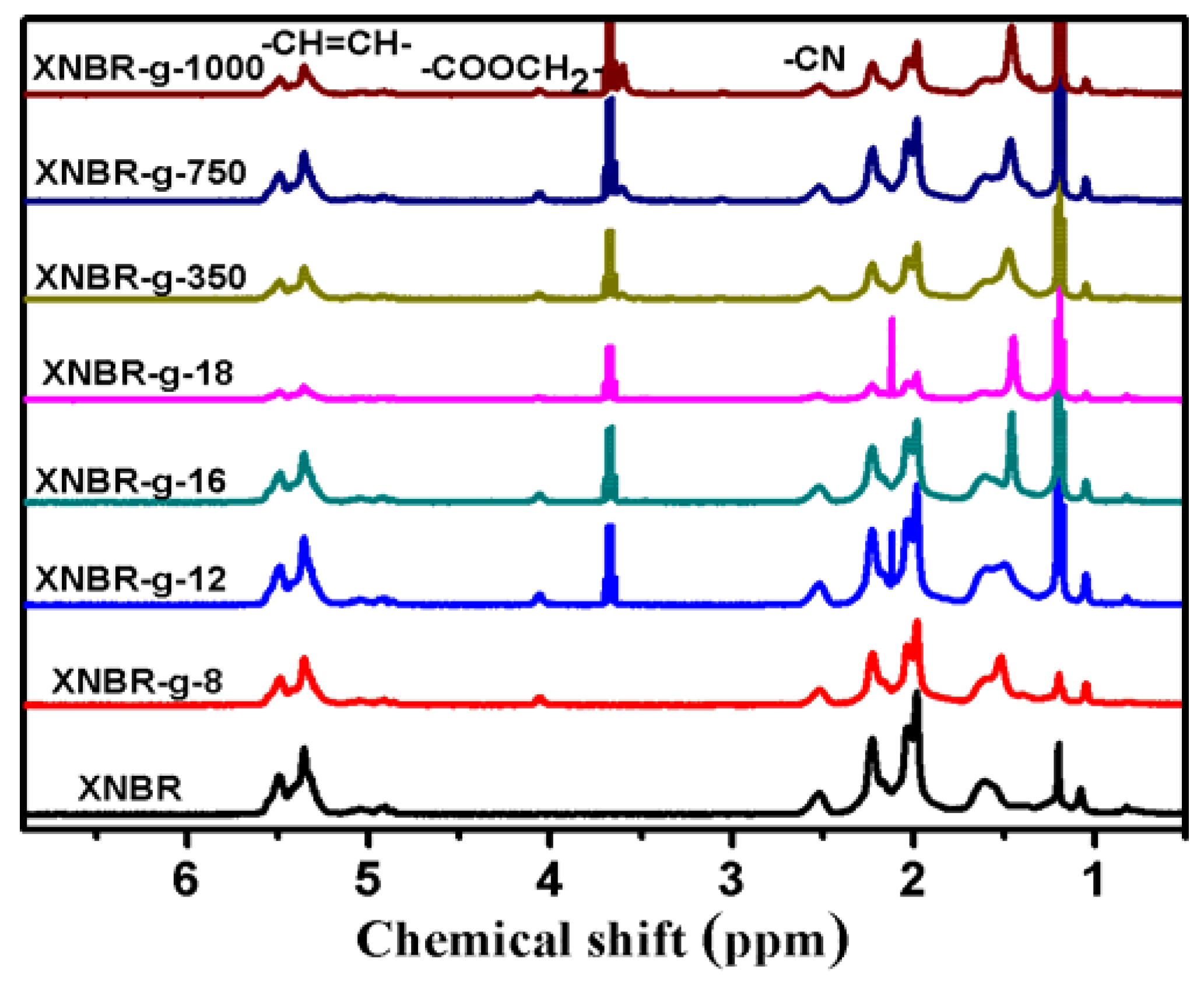
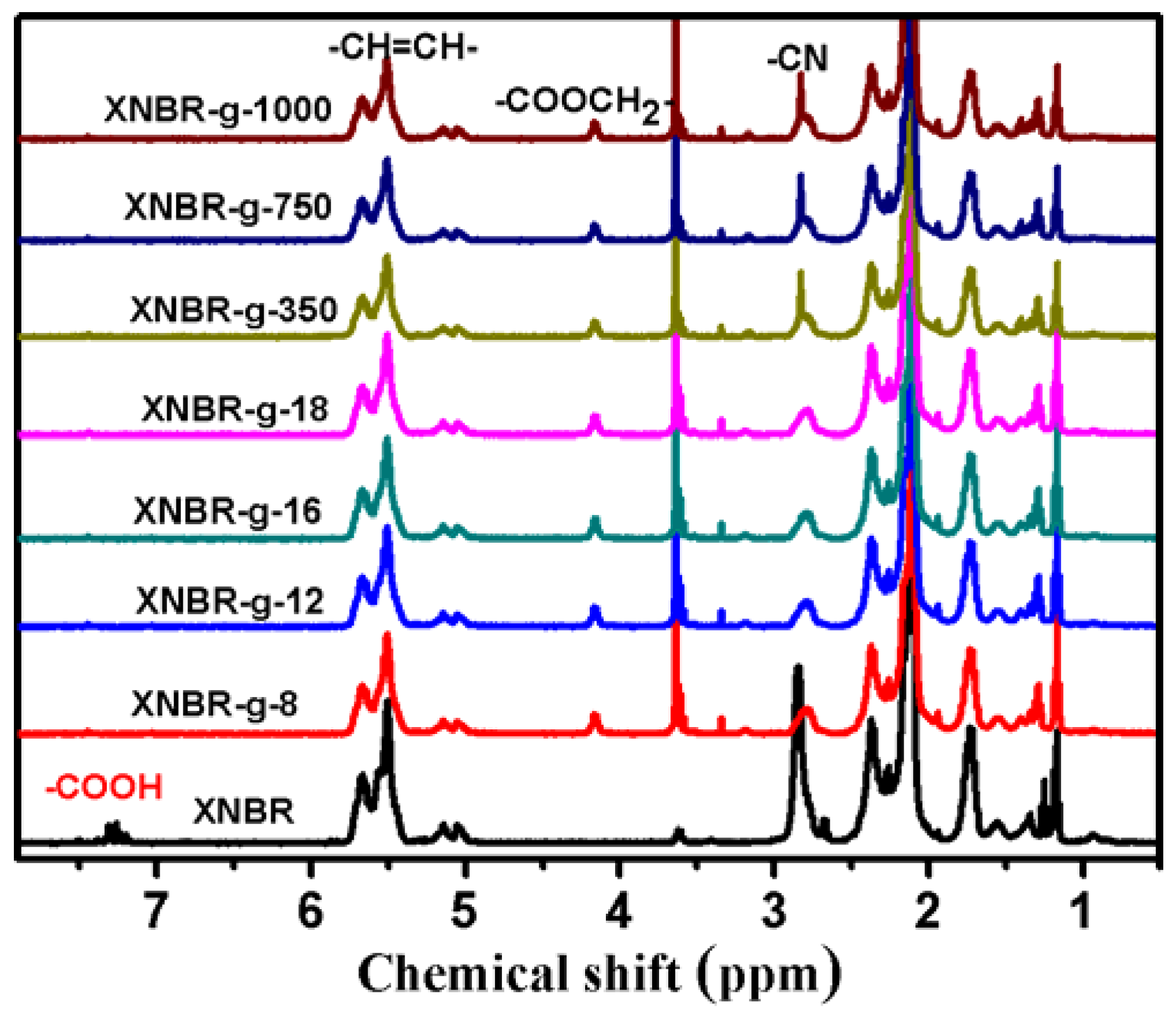
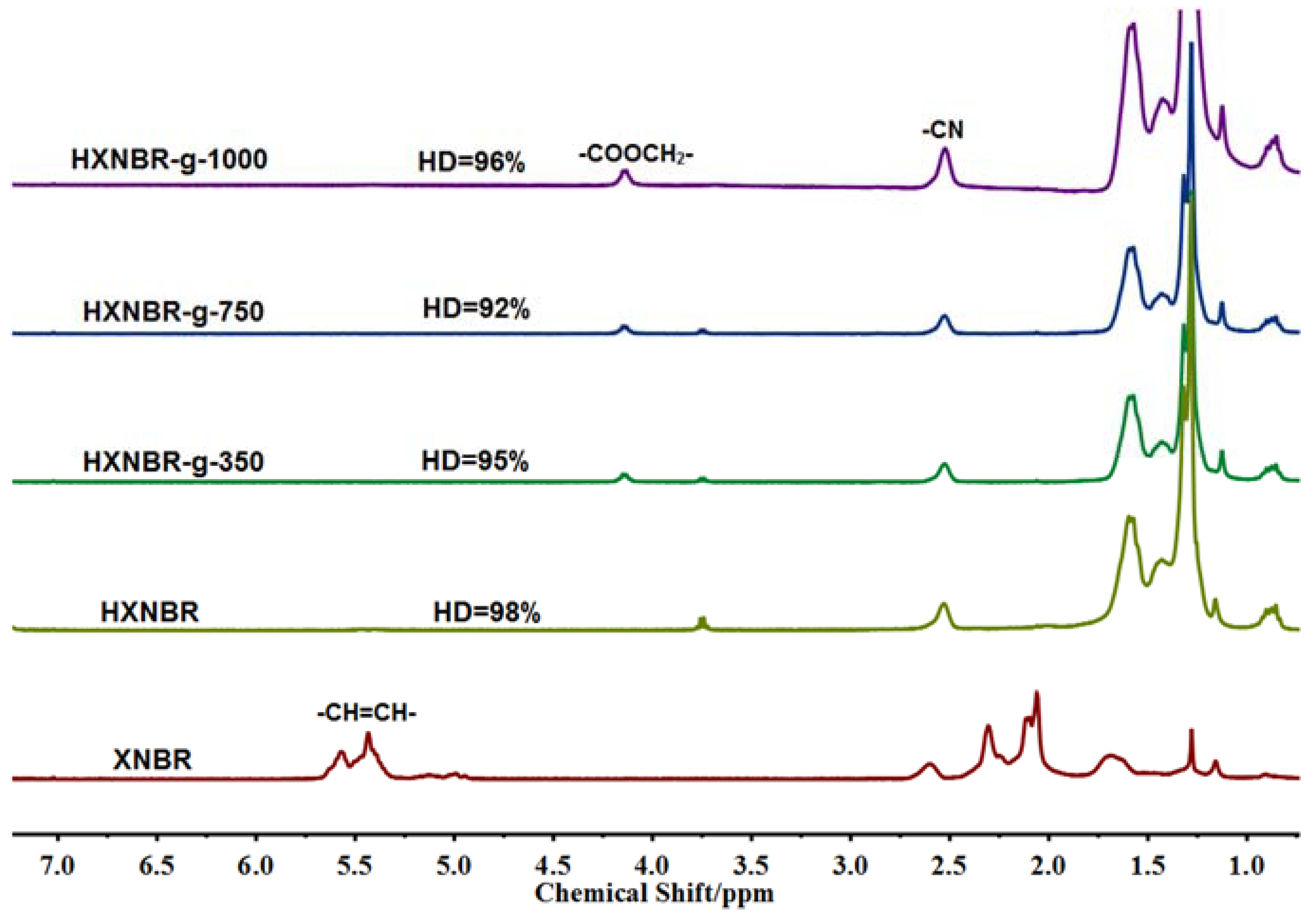
3.1. Structures of Grafted XNBR
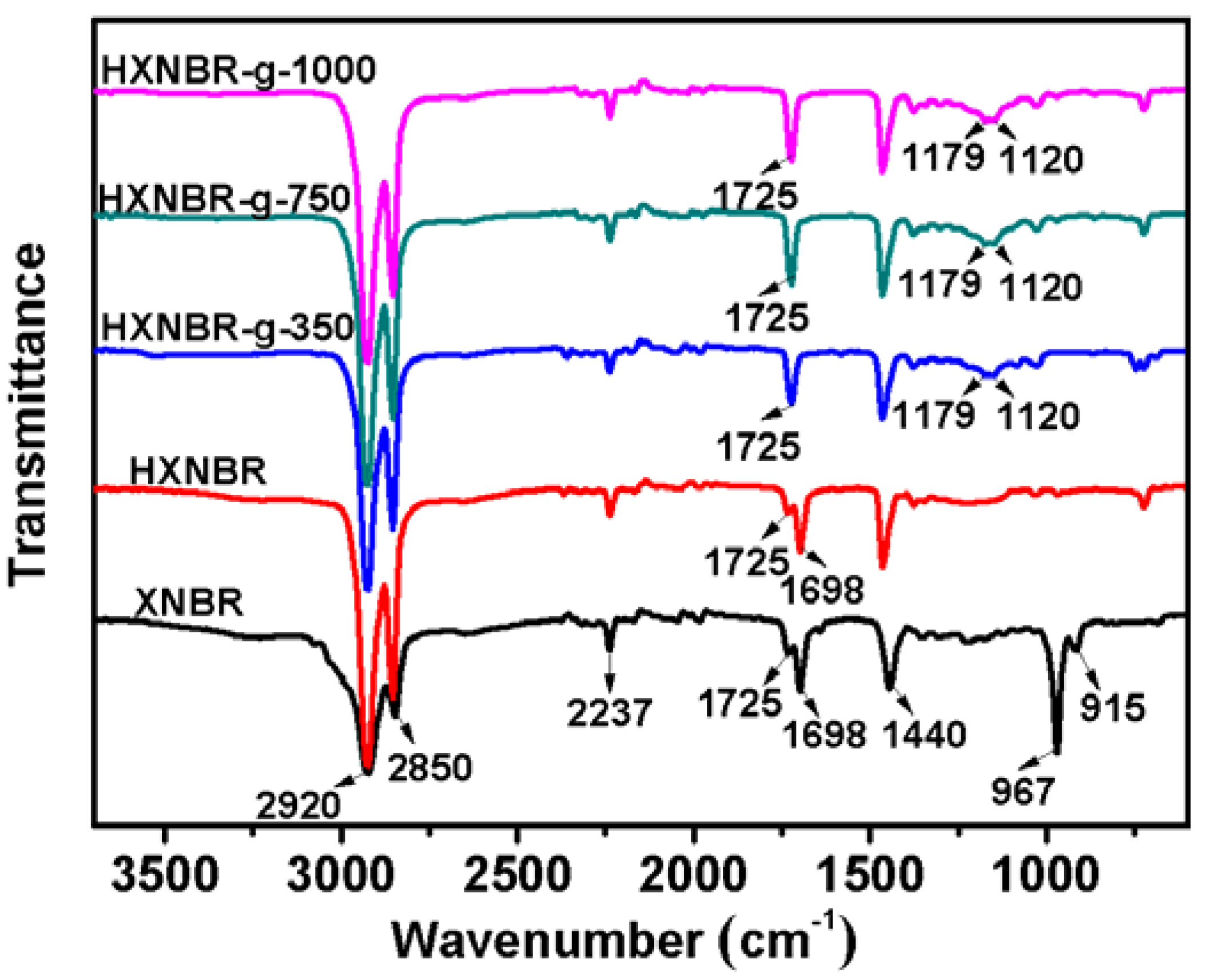
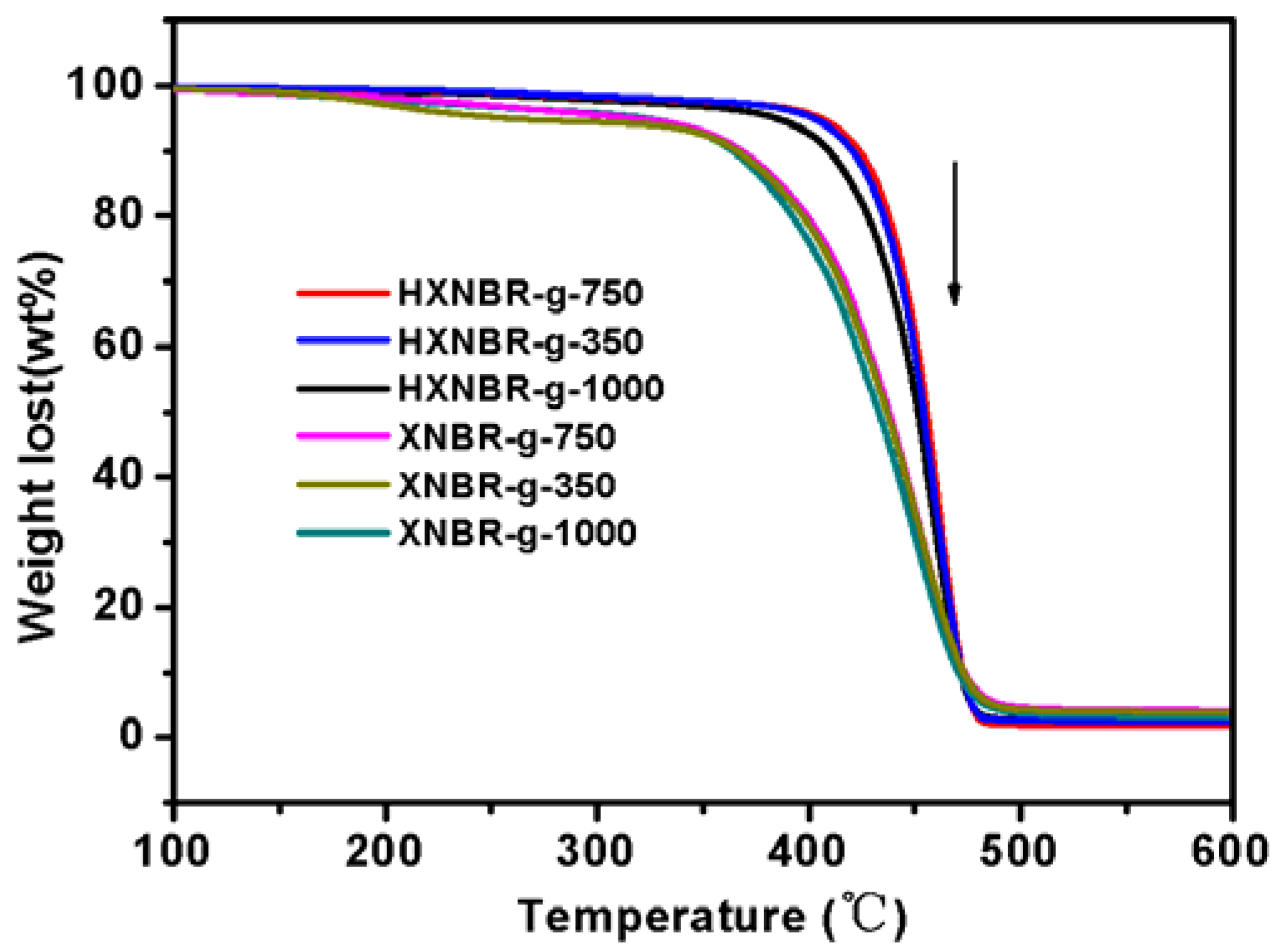
3.2. Structures of Grafted HXNBR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, J.; Bai, G.; Chen, W. Lifetime estimation of nitrile butadiene rubber O-rings under storage conditions using time-varying copula. Proc. Inst. Mech. Eng. Part O J. Risk Reliab. 2018, 232, 635–646. [Google Scholar] [CrossRef]
- Li, G.; Qiu, Z.M.; Zhu, A.B.; Liu, Z.W. Preparation on Low-Temperature Resistance Nitrile-Butadiene Rubber Coat-Metal Sealing Gasket. Adv. Mater. Res. 2010, 152–153, 572–579. [Google Scholar] [CrossRef]
- Nihmath, A.; Ramesan, M.T. Comparative evaluation of oil resistance, dielectric properties, AC conductivity, and transport properties of nitrile rubber and chlorinated nitrile rubber. Prog. Rubber Plast. Recycl. Technol. 2020, 37, 131–147. [Google Scholar] [CrossRef]
- Chang, X.; Yin, H.; Lyu, Y.; Shi, X.; Hoch, M. HNBR-based composite for seals used in coolant fluids: Swelling related to different silicates at high temperature. Polymer 2019, 178, 121691. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Zhang, Z.; Yang, H.; Nie, X. One-step Synthesis of End-Functionalized Hydrogenated Nitrile-Butadiene Rubber by Combining the Functional Metathesis with Hydrogenation. ChemistryOpen 2020, 9, 374–380. [Google Scholar] [CrossRef]
- Wang, S.; Ge, B.; Yin, Y.; Wu, X.; Zhu, H.; Yue, Y.; Bai, Z.; Bao, X.; Yuan, P. Solvent Effect in Heterogeneous Catalytic Selective Hydrogenation of Nitrile Butadiene Rubber: Relationship between Reaction Activity and Solvents with Density Functional Theory Analysis. ChemCatChem 2019, 12, 663–672. [Google Scholar] [CrossRef]
- Ai, C.; Gong, G.; Zhao, X.; Liu, P. Ureido-modified macroporous hollow silica microspheres for recovery of Wilkinson’s catalyst in hydrogenated nitrile butadiene rubber. Powder Technol. 2017, 318, 501–506. [Google Scholar] [CrossRef]
- Alcock, B.; Olafsen, K.; Huse, J.; Grytten, F. The low temperature crystallization of hydrogenated nitrile butadiene rubber (HNBR). Polym. Test. 2018, 66, 228–234. [Google Scholar] [CrossRef]
- Changjie, Y.; Zhang, Q.; Junwei, G.; Junping, Z.; Youqiang, S.; Yuhang, W. Cure characteristics and mechanical properties of styrene-butadiene rubber/hydrogenated acrylonitrile-butadiene rubber/silica composites. J. Polym. Res. 2011, 18, 2487–2494. [Google Scholar] [CrossRef]
- Akulichev, A.G.; Alcock, B.; Krauklis, A.E.; Tiwari, A.; Echtermeyer, A.T. Long-term ISO 23936-2 sweet oil ageing of HNBR. Polym. Test. 2021, 102, 107343. [Google Scholar] [CrossRef]
- Luo, M.; Putnam, Z.A.; Incavo, J.; Huang, M.Y.; McLaughlin, J.B.; Krishnan, S. Molecular Simulations and Experimental Characterization of Fluorinated Nitrile Butadiene Elastomers with Low H2S Permeability. Ind. Eng. Chem. Res. 2019, 58, 14823–14838. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, G.H.; Nam, G.M.; Kang, D.G.; Seo, K.H. Oil resistance and low-temperature characteristics of plasticized nitrile butadiene rubber compounds. J. Appl. Polym. Sci. 2019, 136, 47851. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Zhao, Y. Improving performance of low-temperature hydrogenated acrylonitrile butadiene rubber nanocomposites by using nano-clays. Mater. Des. 2013, 50, 322–331. [Google Scholar] [CrossRef]
- Pavlova, V.V.; Sokolova, M.D.; Fedorova, A.F. Influence of the Content and Nature of the Plasticizer on the Properties of Butadiene-Nitrile Rubber. Journal of Siberian Federal University. Eng. Technol. 2021, 14, 222–232. [Google Scholar]
- Laskowska, A.; Marzec, A.; Boiteux, G.; Zaborski, M.; Gain, O.; Serghei, A. Effect of imidazolium ionic liquid type on the properties of nitrile rubber composites. Polym. Int. 2013, 62, 1575–1582. [Google Scholar] [CrossRef]
- Liang, L.; Dong, J.; Yue, D. Branched EHNBR and its properties with enhanced low-temperature performance and oil resistance. RSC Adv. 2019, 9, 32130–32136. [Google Scholar] [CrossRef] [Green Version]
- Krzemińska, S.M.; Smejda-Krzewicka, A.A.; Leniart, A.; Lipińska, L.; Woluntarski, M. Effects of curing agents and modified graphene oxide on the properties of XNBR composites. Polym. Test. 2020, 83, 106368. [Google Scholar] [CrossRef]
- Paran SM, R.; Naderi, G.; Movahedifar, E.; Jouyandeh, M.; Formela, K.; Colom, X.; Cañavate, J.; Saeb, M.R. Isothermal Vulcanization and Non-Isothermal Degradation Kinetics of XNBR/Epoxy/XNBR-g-Halloysite Nanotubes (HNT) Nanocomposites. Materials 2021, 14, 2872. [Google Scholar] [CrossRef]
- Pal, K.; Pal, S.K.; Das, C.K.; Kim, J.K. Effect of fillers on morphological properties and wear characteristics of XNBR/NR blends. J. Appl. Polym. Sci. 2011, 120, 710–718. [Google Scholar] [CrossRef]
- Severe, G.; White, J.L. Dynamically vulcanized blends of oil-resistant elastomers with HNBR. J. Appl. Polym. Sci. 2005, 95, 2–5. [Google Scholar] [CrossRef]
- Paran SM, R.; Naderi, G.; Mosallanezhad, H.; Movahedifar, E.; Formela, K.; Saeb, M.R. Microstructure and Mechanical Properties of Carboxylated Nitrile Butadiene Rubber/Epoxy/XNBR-grafted Halloysite Nanotubes Nanocomposites. Polymers 2020, 12, 1192. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, X.; Jia, H.; Yin, Q.; Wang, J.; Yin, B.; Xu, Z. High mechanical properties, thermal conductivity and solvent resistance in graphene oxide/styrene-butadiene rubber nanocomposites by engineering carboxylated acrylonitrile-butadiene rubber. Compos. Part B Eng. 2017, 130, 257–266. [Google Scholar] [CrossRef]
- Azizli, M.J.; Barghamadi, M.; Rezaeeparto, K.; Mokhtary, M.; Parham, S.; Darabi, M.J. Theoretical and experimental analyses of rheological, compatibility and mechanical properties of PVMQ/XNBR-g GMA/XNBR/GO ternary hybrid nanocomposites. Iran. Polym. J. 2021, 30, 1001–1018. [Google Scholar] [CrossRef]
- Aliabadi, M.M.; Naderi, G.; Shahtaheri, S.J.; Forushani, A.R.; Mohammadfam, I.; Jahangiri, M. Mechanical and barrier properties of XNBR-clay nanocomposite: A promising material for protective gloves. Iran. Polym. J. 2014, 23, 289–296. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Rempel, G.L. Homogeneous Hydrogenation Art of Nitrile Butadiene Rubber: A Review. Polym. Rev. 2013, 53, 192–239. [Google Scholar] [CrossRef]
- Nihmath, A.; Ramesan, M.T. Synthesis, characterization, processability, mechanical properties, flame retardant, and oil resistance of chlorinated acrylonitrile butadiene rubber. Polym. Adv. Technol. 2018, 29, 2165–2173. [Google Scholar] [CrossRef]
- Wang, W.; Song, Q.; Liu, Q.; Zheng, H.; Li, C.; Yan, Y.; Zhang, Q. A Novel Reprocessable and Recyclable Acrylonitrile-Butadiene Rubber Based on Dynamic Oxime-Carbamate Bond. Macromol. Rapid Commun. 2019, 40, e1800733. [Google Scholar] [CrossRef]
- Yimmut, K.; Homchoo, K.; Hinchiranan, N. Poly(butyl acrylate-co-fluorinated acrylate)-graft-natural rubber: Synthesis and application as compatibilizer for natural rubber/poly(butyl acrylate-co-fluorinated acrylate) films. Colloids Surfaces A Physicochem. Eng. Aspect 2018, 540, 11–22. [Google Scholar] [CrossRef]
- Gui, Q.; Pan, C.; Shen, S.; Xie, P.; Pei, M.; Liu, P. Facile fluorination of nitrile-butadiene rubber via olefin cross metathesis. Polymer 2021, 217, 123455. [Google Scholar] [CrossRef]
- Gui, Q.; Pan, C.; Shen, S.; Liu, P. Controllable synthesis of fluorinated liquid nitrile-butadiene rubber via facile metathesis degradation strategy. J. Mol. Liquids 2021, 326, 115269. [Google Scholar] [CrossRef]




| Samples | C [%] | N [%] | O [%] | H [%] |
|---|---|---|---|---|
| XNBR | 79.84 | 6.68 | 4.10 | 9.38 |
| XNBR-g-8 | 76.83 | 6.67 | 6.85 | 9.65 |
| XNBR-g-12 | 77.75 | 6.61 | 5.93 | 9.71 |
| XNBR-g-16 | 79.45 | 6.54 | 4.35 | 9.66 |
| XNBR-g-18 | 79.06 | 6.49 | 4.76 | 9.69 |
| XNBR-g-350 | 78.15 | 6.47 | 5.73 | 9.65 |
| XNBR-g-750 | 75.01 | 6.36 | 9.19 | 9.44 |
| XNBR-g-1000 | 72.13 | 6.23 | 12.36 | 9.28 |
| Brand | Acrylonitrile (wt%) | HD (%) | Tg (°C) |
|---|---|---|---|
| HXNBR-g-1000 | 28 | 96 | −29.8 |
| H2865C | 28 | 98 | −23.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Ni, Y.; Qi, X.; Zhang, L.; Yue, D. Synthesis of Low Temperature Resistant Hydrogenated Nitrile Rubber Based on Esterification Reaction. Polymers 2021, 13, 4096. https://doi.org/10.3390/polym13234096
Wang L, Ni Y, Qi X, Zhang L, Yue D. Synthesis of Low Temperature Resistant Hydrogenated Nitrile Rubber Based on Esterification Reaction. Polymers. 2021; 13(23):4096. https://doi.org/10.3390/polym13234096
Chicago/Turabian StyleWang, Lin, Yanqiang Ni, Xin Qi, Liqun Zhang, and Dongmei Yue. 2021. "Synthesis of Low Temperature Resistant Hydrogenated Nitrile Rubber Based on Esterification Reaction" Polymers 13, no. 23: 4096. https://doi.org/10.3390/polym13234096
APA StyleWang, L., Ni, Y., Qi, X., Zhang, L., & Yue, D. (2021). Synthesis of Low Temperature Resistant Hydrogenated Nitrile Rubber Based on Esterification Reaction. Polymers, 13(23), 4096. https://doi.org/10.3390/polym13234096







