Nanostructured Plasma Polymerized Fluorocarbon Films for Drop Coating Deposition Raman Spectroscopy (DCDRS) of Liposomes
Abstract
:1. Introduction
2. Materials and Methods
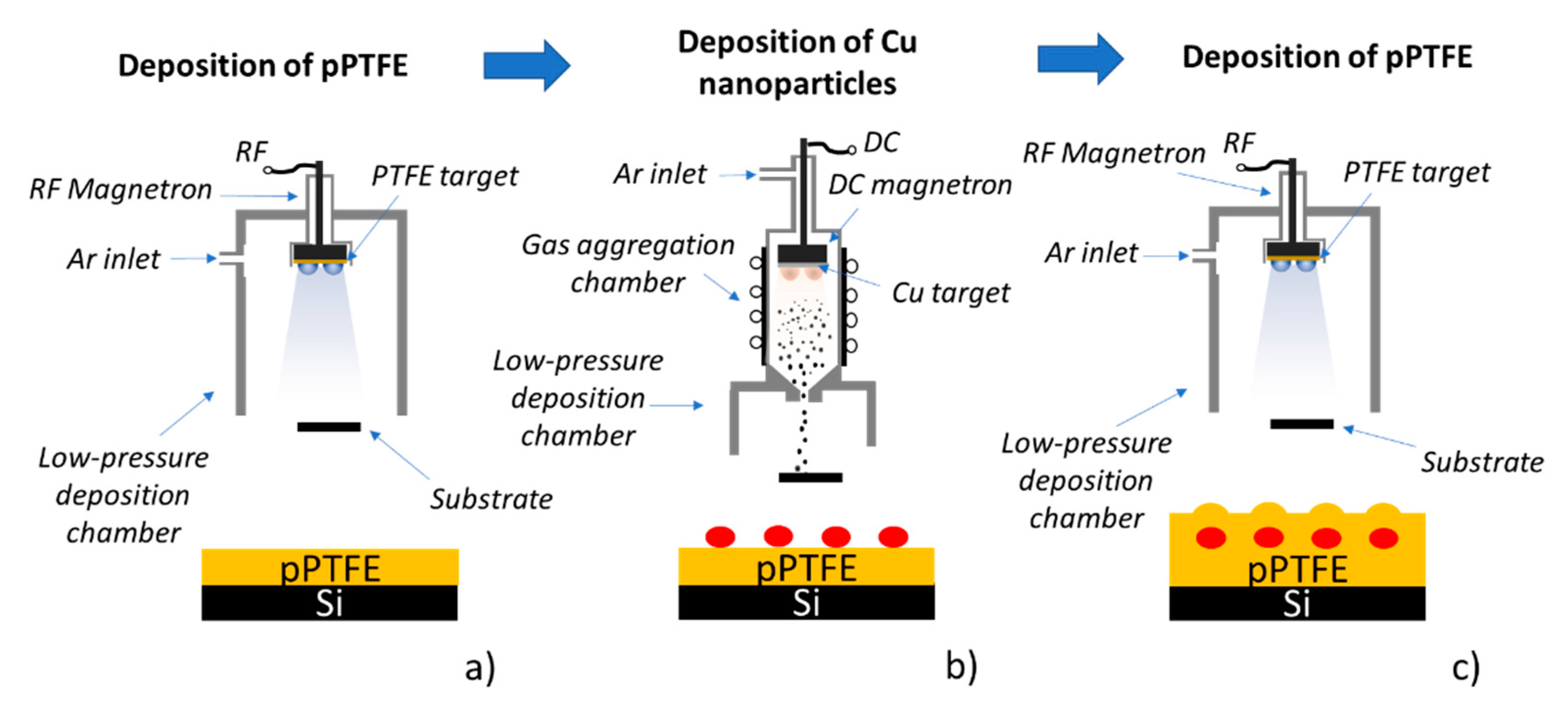
2.1. Fabrication of DCDRS Substrates and Their Characterization
2.2. DCDRS Measurements
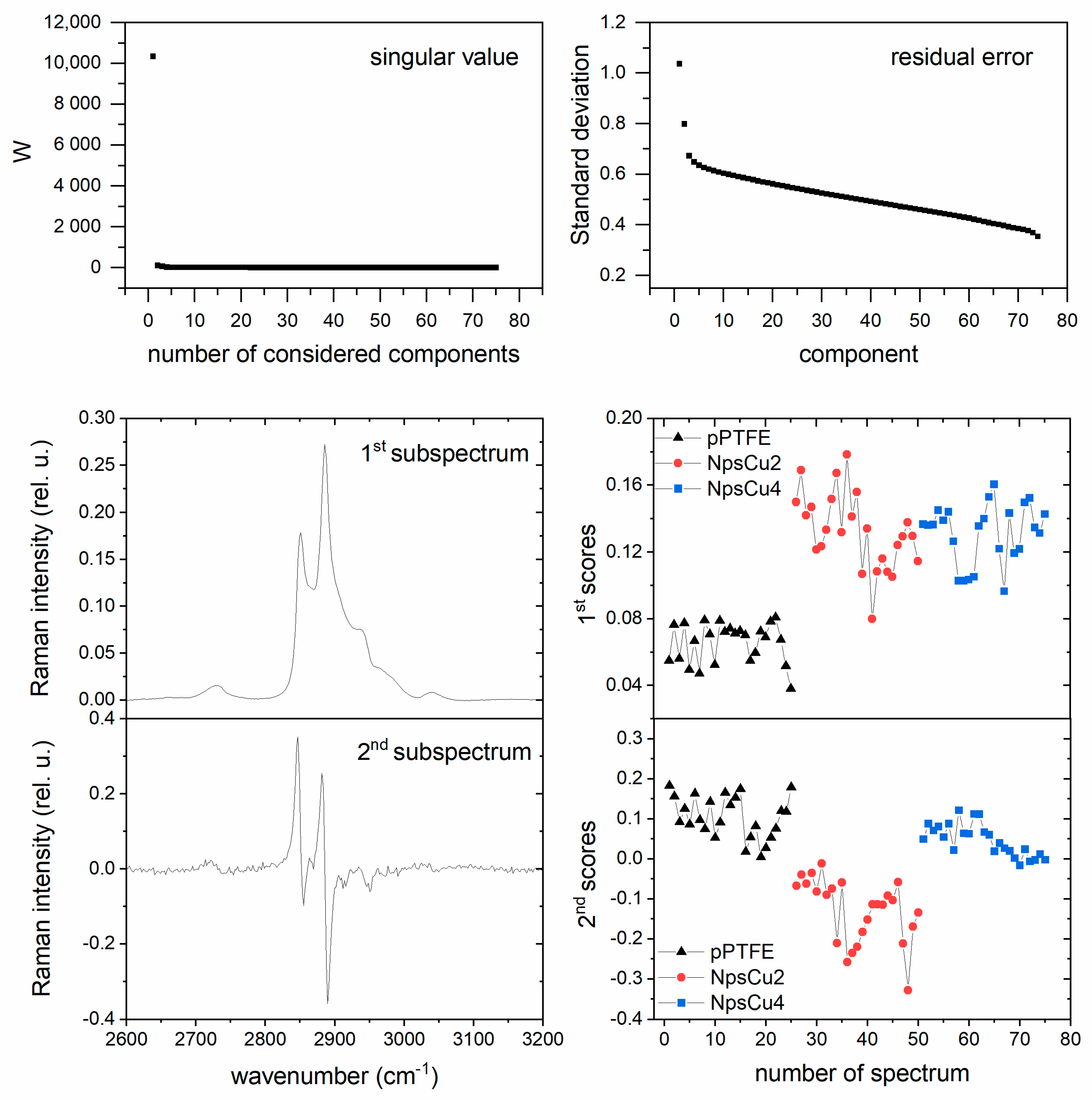
2.3. Treatment of Measured Spectra by Factor Analysis (FA)
3. Results
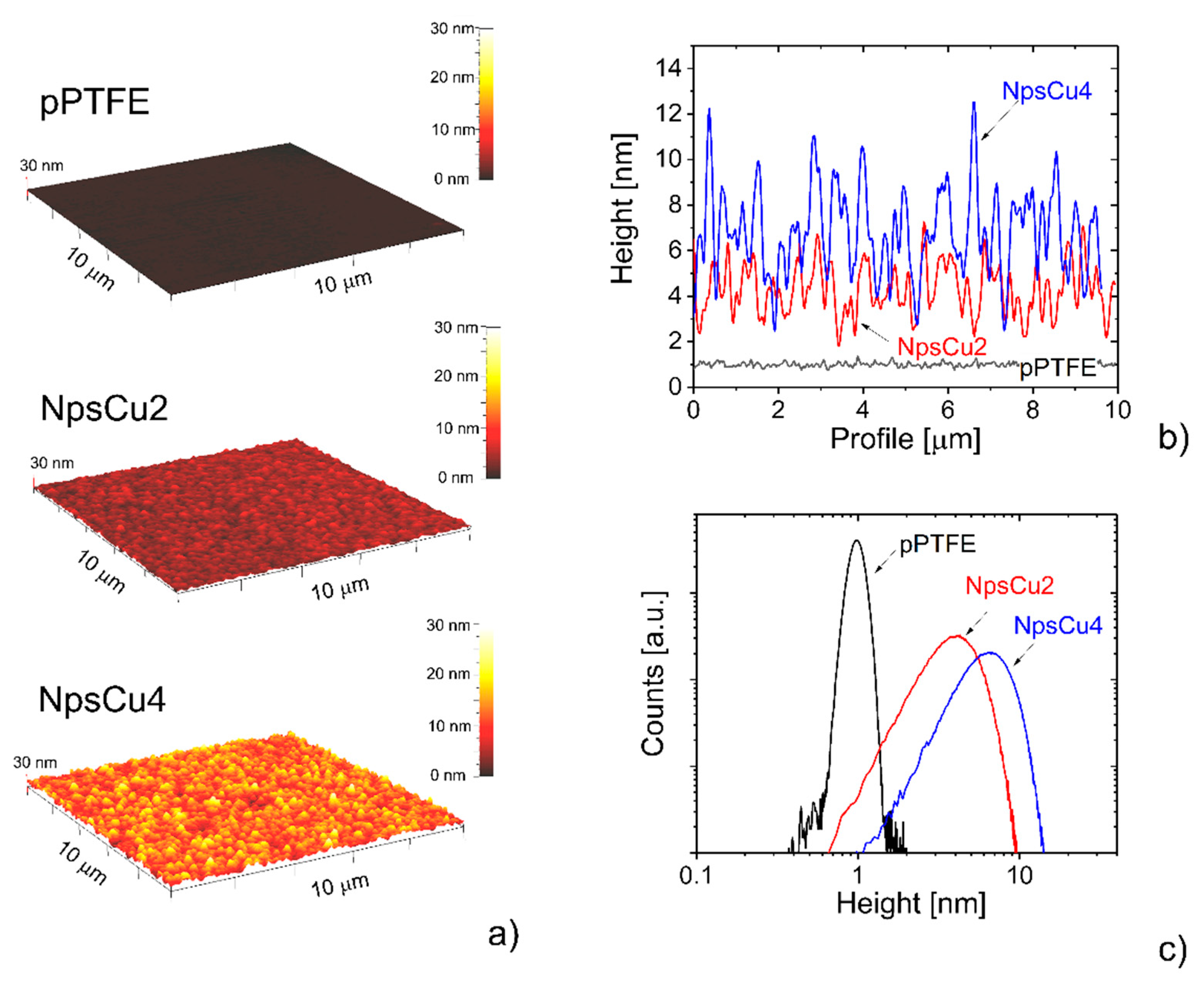
3.1. Characterization of C:F Substrates
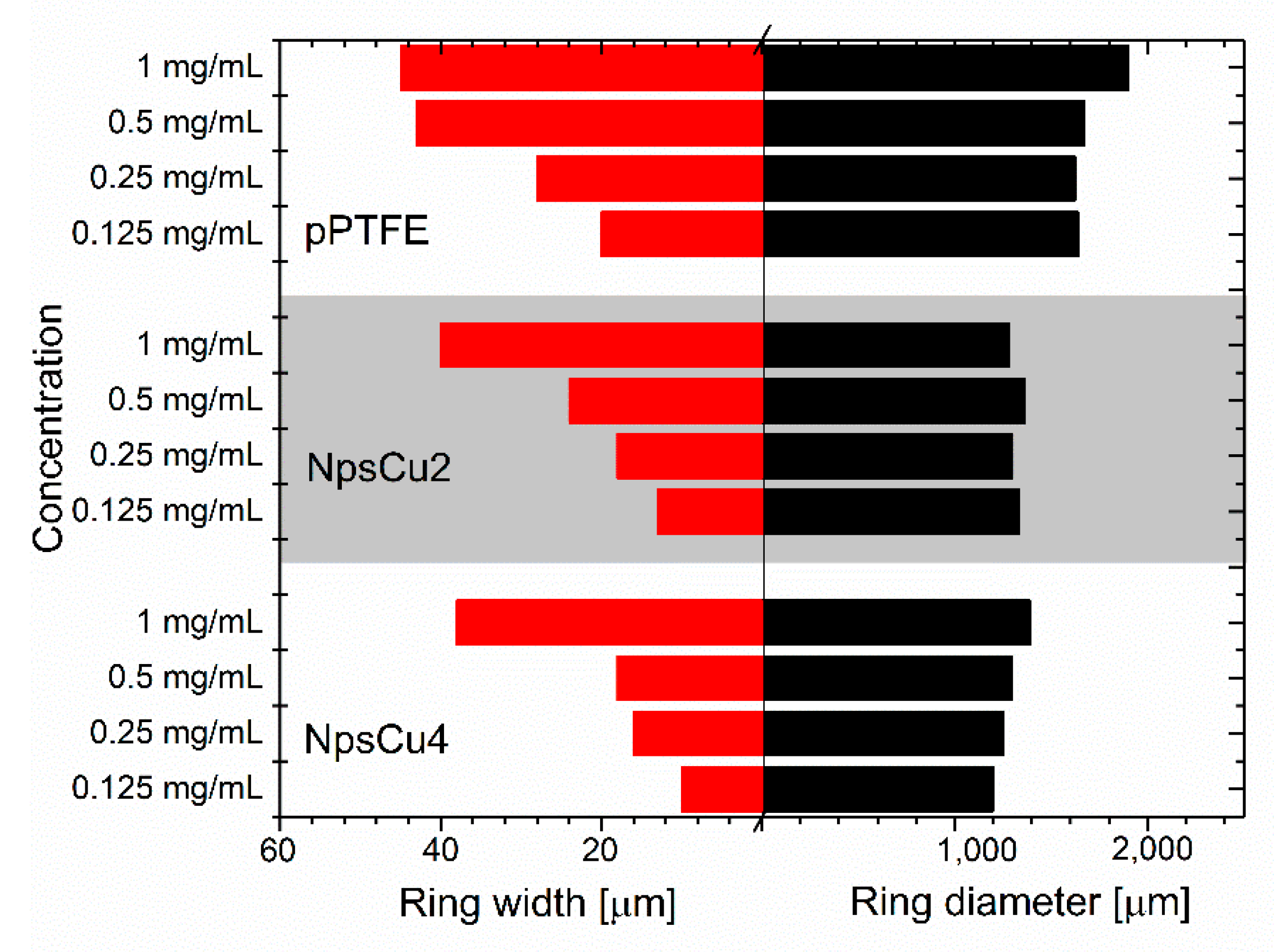
3.2. Wettability and Drying of Liposome Suspension
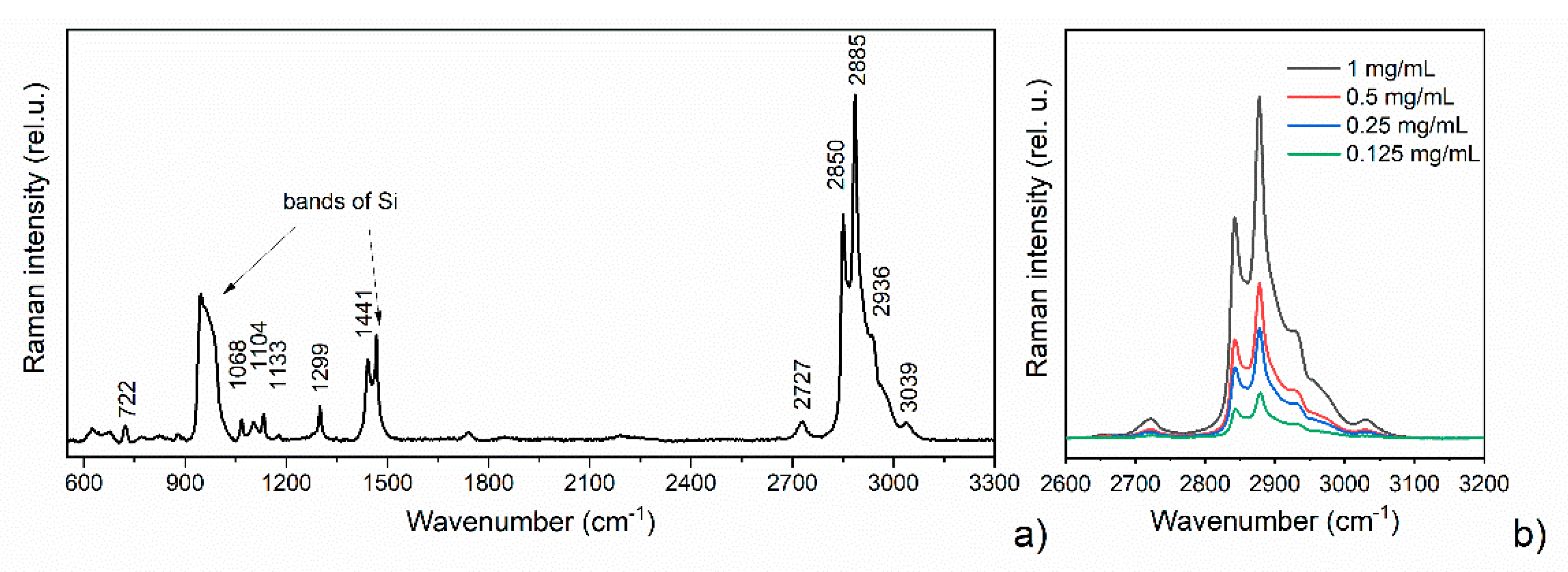
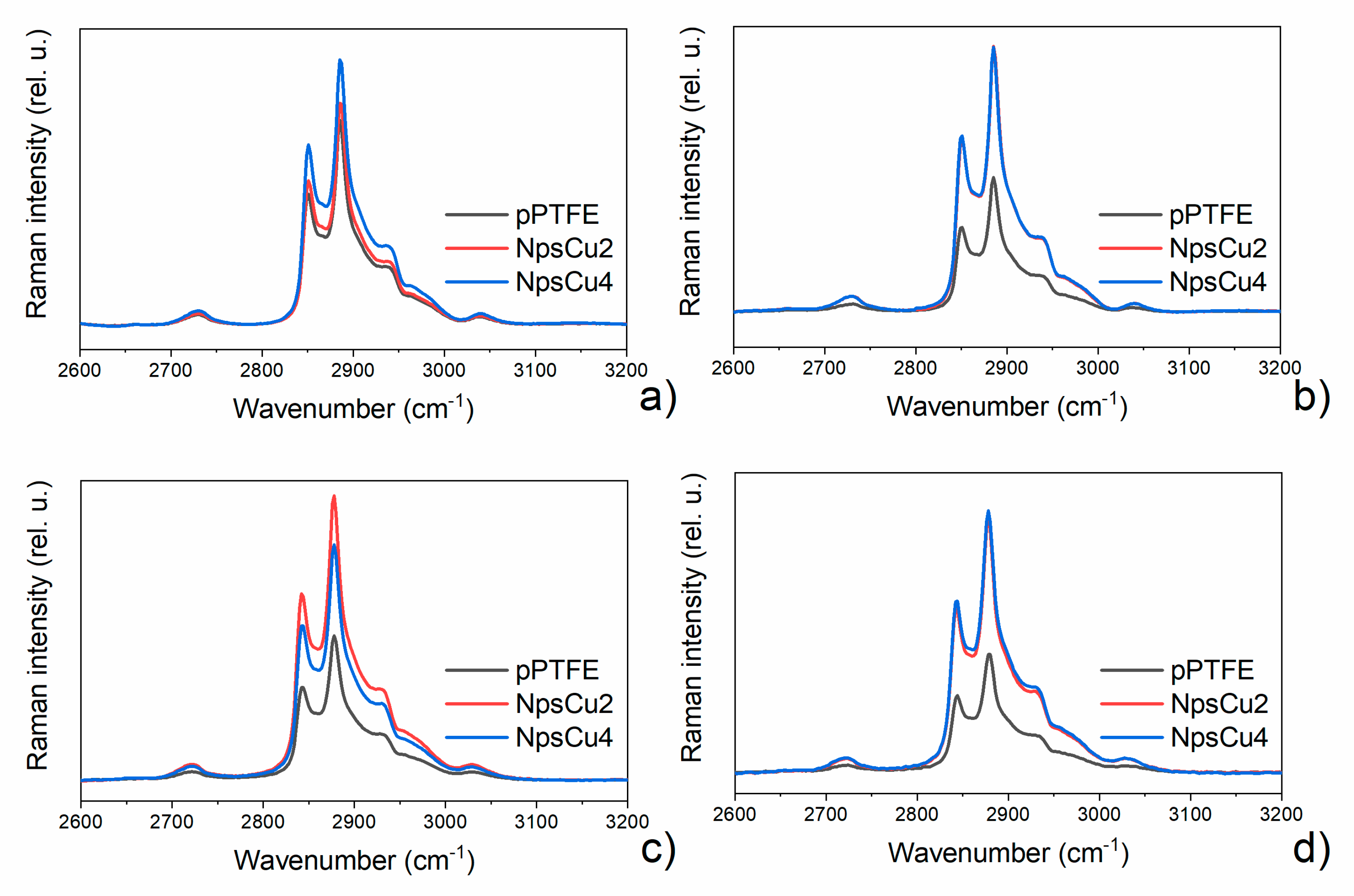
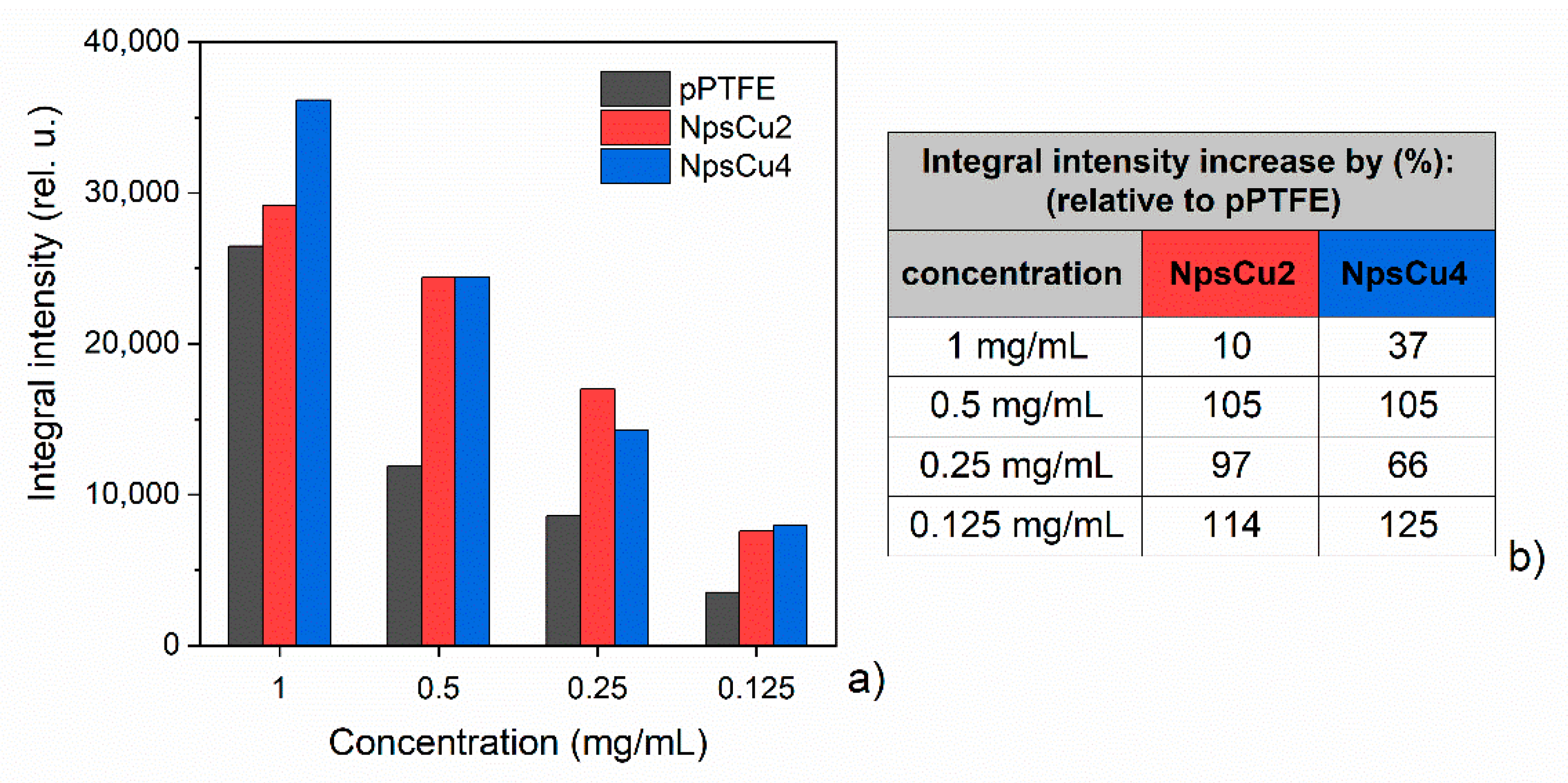
3.3. DCDRS Performance
4. Conclusions
- The smooth C:F surfaces deposited by magnetron sputtering were found to be suitable for the DCDRS detection of liposomes. This is due to the hydrophobic character of C:F coatings that forces liquid liposome suspensions to form coffee-ring structures after the complete evaporation of the liquid phase, at least at a higher DPPC concentration.
- As it is shown, the DCDRS measurements might be significantly improved when the fluorocarbon coatings are nanostructured. This led not only to the pinning of the drying droplet that allowed for the formation of well-defined rings also in the case of lower DPPC concentration but also to the lowering the diameter of the resulting rings. The latter, in turn, made it possible to reach a higher concentration of liposomes in the rings and, hence, to get two times higher Raman signal intensity as compared to smooth fluorocarbon films without compromising the profile of recorded Raman spectra.
- These findings may pave the way for the development of cheap and disposable platforms for efficient DCDRS-based biodetection.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. A review on special wettability textiles: Theoretical models, fabrication technologies and multifunctional applications. J. Mater. Chem. A 2017, 5, 31–55. [Google Scholar] [CrossRef] [Green Version]
- van Osch, T.H.J.; Perelaer, J.; de Laat, A.W.M.; Schubert, U.S. Inkjet Printing of Narrow Conductive Tracks on Untreated Polymeric Substrates. Adv. Mater. 2008, 20, 343–345. [Google Scholar] [CrossRef]
- Su, X.; Li, X.; Ong, C.Y.A.; Herng, T.S.; Wang, Y.; Peng, E.; Ding, J. Metallization of 3D Printed Polymers and Their Application as a Fully Functional Water-Splitting System. Adv. Sci. 2019, 6, 1801670. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, P.; Alves, P.; Coimbra, P.; Gil, M.H. Improving polymeric surfaces for biomedical applications: A review. J. Coat. Technol. Res. 2015, 12, 463–475. [Google Scholar] [CrossRef]
- Kim, M.S.; Khang, G.; Lee, H.B. Gradient polymer surfaces for biomedical applications. Prog. Polym. Sci. 2008, 33, 138–164. [Google Scholar] [CrossRef]
- van Wachem, P.B.; Beugeling, T.; Feijen, J.; Bantjes, A.; Detmers, J.P.; van Aken, W.G. Interaction of cultured human endothelial cells with polymeric surfaces of different wettabilities. Biomaterials 1985, 6, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Khang, G.; Lee, J.W.; Lee, H.B. Interaction of Different Types of Cells on Polymer Surfaces with Wettability Gradient. J. Colloid Interface Sci. 1998, 205, 323–330. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Mampallil, D.; Eral, H.B. A review on suppression and utilization of the coffee-ring effect. Adv. Colloid Interface Sci. 2018, 252, 38–54. [Google Scholar] [CrossRef]
- Parsa, M.; Harmand, S.; Sefiane, K. Mechanisms of pattern formation from dried sessile drops. Adv. Colloid Interface Sci. 2018, 254, 22–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, D.; Tarafdar, S.; Tarasevich, Y.Y.; Dutta Choudhury, M.; Dutta, T. Evaporation of a Droplet: From physics to applications. Phys. Rep. 2019, 804, 1–56. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Zhang, D.; Xie, Y.; Mrozek, M.F.; Ortiz, C.; Davisson, V.J.; Ben-Amotz, D. Raman Detection of Proteomic Analytes. Anal. Chem. 2003, 75, 5703–5709. [Google Scholar] [CrossRef]
- Kopecký, V.; Baumruk, V. Structure of the ring in drop coating deposited proteins and its implication for Raman spectroscopy of biomolecules. Vib. Spectrosc. 2006, 42, 184–187. [Google Scholar] [CrossRef]
- Kočišová, E.; Procházka, M. Drop-coating deposition Raman spectroscopy of porphyrins. J. Raman Spectrosc. 2015, 46, 280–282. [Google Scholar] [CrossRef]
- Kočišová, E.; Procházka, M. Drop-coating deposition Raman spectroscopy of liposomes. J. Raman Spectrosc. 2011, 42, 1606–1610. [Google Scholar] [CrossRef]
- Šimáková, P.; Kocisová, E.; Procházka, M. Sensitive Raman spectroscopy of lipids based on drop deposition using DCDR and SERS. J. Raman Spectrosc. 2013, 44, 1479–1482. [Google Scholar] [CrossRef]
- Kočišová, E.; Procházka, M. Drop coating deposition Raman spectroscopy of dipicolinic acid. J. Raman Spectrosc. 2018, 49, 2050–2052. [Google Scholar] [CrossRef]
- Mrozek, M.F.; Zhang, D.; Ben-Amotz, D. Oligosaccharide identification and mixture quantification using Raman spectroscopy and chemometric analysis. Carbohydr. Res. 2004, 339, 141–145. [Google Scholar] [CrossRef]
- Kuižová, A.; Přikryl, M.; Procházka, M.; Kočišová, E. Drop coating deposition Raman (DCDR) spectroscopy of contaminants. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120109. [Google Scholar] [CrossRef] [PubMed]
- Filik, J.; Stone, N. Analysis of human tear fluid by Raman spectroscopy. Anal. Chim. Acta 2008, 616, 177–184. [Google Scholar] [CrossRef]
- Filik, J.; Stone, N. Investigation into the protein composition of human tear fluid using centrifugal filters and drop coating deposition Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 218–224. [Google Scholar] [CrossRef]
- Hu, P.; Zheng, X.-S.; Zong, C.; Li, M.-H.; Zhang, L.-Y.; Li, W.; Ren, B. Drop-coating deposition and surface-enhanced Raman spectroscopies (DCDRS and SERS) provide complementary information of whole human tears. J. Raman Spectrosc. 2014, 45, 565–573. [Google Scholar] [CrossRef]
- Dingari, N.C.; Horowitz, G.L.; Kang, J.W.; Dasari, R.R.; Barman, I. Raman Spectroscopy Provides a Powerful Diagnostic Tool for Accurate Determination of Albumin Glycation. PLoS ONE 2012, 7, e32406. [Google Scholar] [CrossRef] [Green Version]
- Abdolahzadeh, S.; Boyle, N.M.; Draksharapu, A.; Dennis, A.C.; Hage, R.; de Boer, J.W.; Browne, W.R. Off-line reaction monitoring of the oxidation of alkenes in water using drop coating deposition Raman (DCDR) spectroscopy. Analyst 2013, 138, 3163–3171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Jiang, Y.; Ben-Amotz, D. Detection of amino acid and peptide phosphate protonation using Raman spectroscopy. Anal. Biochem. 2005, 343, 223–230. [Google Scholar] [CrossRef]
- Kočišová, E.; Procházka, M.; Vaculčiaková, L. Drop-Coating Deposition Raman (DCDR) Spectroscopy as a Tool for Membrane Interaction Studies: Liposome–Porphyrin Complex. Appl. Spectrosc. 2015, 69, 939–945. [Google Scholar] [CrossRef]
- Zhang, D.; Mrozek, M.F.; Xie, Y.; Ben-Amotz, D. Chemical Segregation and Reduction of Raman Background Interference Using Drop Coating Deposition. Appl. Spectrosc. 2004, 58, 929–933. [Google Scholar] [CrossRef]
- Filik, J.; Stone, N. Drop coating deposition Raman spectroscopy of protein mixtures. Analyst 2007, 132, 544–550. [Google Scholar] [CrossRef]
- Prochazka, M. Surface-Enhanced Raman Spectroscopy. Bioanalytical, Biomolecular and Medical Applications, 1st ed.; Springer: Cham, Switzerland, 2016; pp. 1–221. [Google Scholar]
- Mosier-Boss, P.A. Review of SERS Substrates for Chemical Sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocisova, E.; Petr, M.; Sipova, H.; Kylian, O.; Prochazka, M. Drop coating deposition of a liposome suspension on surfaces with different wettabilities: “Coffee ring” formation and suspension preconcentration. Phys. Chem. Chem. Phys. 2017, 19, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Kylián, O.; Polonskyi, O.; Kratochvíl, J.; Artemenko, A.; Choukourov, A.; Drábik, M.; Solař, P.; Slavínská, D.; Biederman, H. Control of Wettability of Plasma Polymers by Application of Ti Nano-Clusters. Plasma Process. Polym. 2012, 9, 180–187. [Google Scholar] [CrossRef]
- Kylián, O.; Petr, M.; Serov, A.; Solař, P.; Polonskyi, O.; Hanuš, J.; Choukourov, A.; Biederman, H. Hydrophobic and super-hydrophobic coatings based on nanoparticles overcoated by fluorocarbon plasma polymer. Vacuum 2014, 100, 57–60. [Google Scholar] [CrossRef]
- Kratochvíl, J.; Kuzminova, A.; Solař, P.; Hanuš, J.; Kylián, O.; Biederman, H. Wetting and drying on gradient-nanostructured C:F surfaces synthesized using a gas aggregation source of nanoparticles combined with magnetron sputtering of polytetrafluoroethylene. Vacuum 2019, 166, 50–56. [Google Scholar] [CrossRef]
- Haberland, H.; Karrais, M.; Mall, M.; Thurner, Y. Thin films from energetic cluster impact: A feasibility study. J. Vac. Sci. Technol. A 1992, 10, 3266–3271. [Google Scholar] [CrossRef]
- MacDonald, R.; Macdonald, R.; Menco, B.; Takeshita, K.; Subbarao, N.; Hu, L.-r. Small-Volume Extrusion Apparatus for Preparation of Large, Unilamellar Vesicles. Biochim. Biophys. Acta 1991, 1061, 297–303. [Google Scholar] [CrossRef]
- Palacký, J.; Mojzeš, P.; Bok, J. SVD-based method for intensity normalization, background correction and solvent subtraction in Raman spectroscopy exploiting the properties of water stretching vibrations. J. Raman Spectrosc. 2011, 42, 1528–1539. [Google Scholar] [CrossRef]
- Malinowski, E.R. Factor Analysis in Chemistry, 3rd ed.; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Choukourov, A.; Kylian, O.; Petr, M.; Vaidulych, M.; Nikitin, D.; Hanus, J.; Artemenko, A.; Shelemin, A.; Gordeev, I.; Kolska, Z.; et al. RMS roughness-independent tuning of surface wettability by tailoring silver nanoparticles with a fluorocarbon plasma polymer. Nanoscale 2017, 9, 2616–2625. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Pittoni, P.G.; Chang, C.-C.; Yu, T.-S.; Lin, S.-Y. Evaporation of water drops on polymer surfaces: Pinning, depinning and dynamics of the triple line. Colloids Surf. A Physicochem. Eng. Asp. 2013, 432, 89–98. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Levin, I.W. Vibrational spectroscopy of membrane assemblies. In Advances in Infrared and Raman Sepctroscopy, 1st ed.; Clark, R.J.H., Hester, R.E., Eds.; Wiley: New York, NY, USA, 1984; Volume 12, p. 48. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuižová, A.; Kuzminova, A.; Kylián, O.; Kočišová, E. Nanostructured Plasma Polymerized Fluorocarbon Films for Drop Coating Deposition Raman Spectroscopy (DCDRS) of Liposomes. Polymers 2021, 13, 4023. https://doi.org/10.3390/polym13224023
Kuižová A, Kuzminova A, Kylián O, Kočišová E. Nanostructured Plasma Polymerized Fluorocarbon Films for Drop Coating Deposition Raman Spectroscopy (DCDRS) of Liposomes. Polymers. 2021; 13(22):4023. https://doi.org/10.3390/polym13224023
Chicago/Turabian StyleKuižová, Alžbeta, Anna Kuzminova, Ondřej Kylián, and Eva Kočišová. 2021. "Nanostructured Plasma Polymerized Fluorocarbon Films for Drop Coating Deposition Raman Spectroscopy (DCDRS) of Liposomes" Polymers 13, no. 22: 4023. https://doi.org/10.3390/polym13224023
APA StyleKuižová, A., Kuzminova, A., Kylián, O., & Kočišová, E. (2021). Nanostructured Plasma Polymerized Fluorocarbon Films for Drop Coating Deposition Raman Spectroscopy (DCDRS) of Liposomes. Polymers, 13(22), 4023. https://doi.org/10.3390/polym13224023






