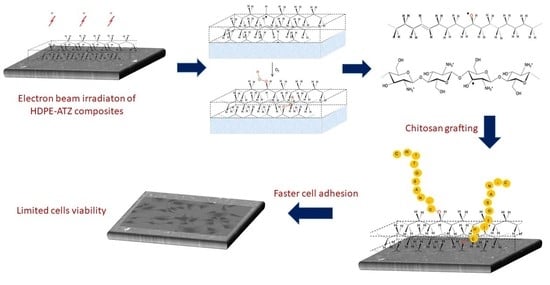
Electron-Beam-Induced Grafting of Chitosan onto HDPE/ATZ Composites for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composites Preparation
2.3. Irradiation and Grafting Process
2.4. Characterisation
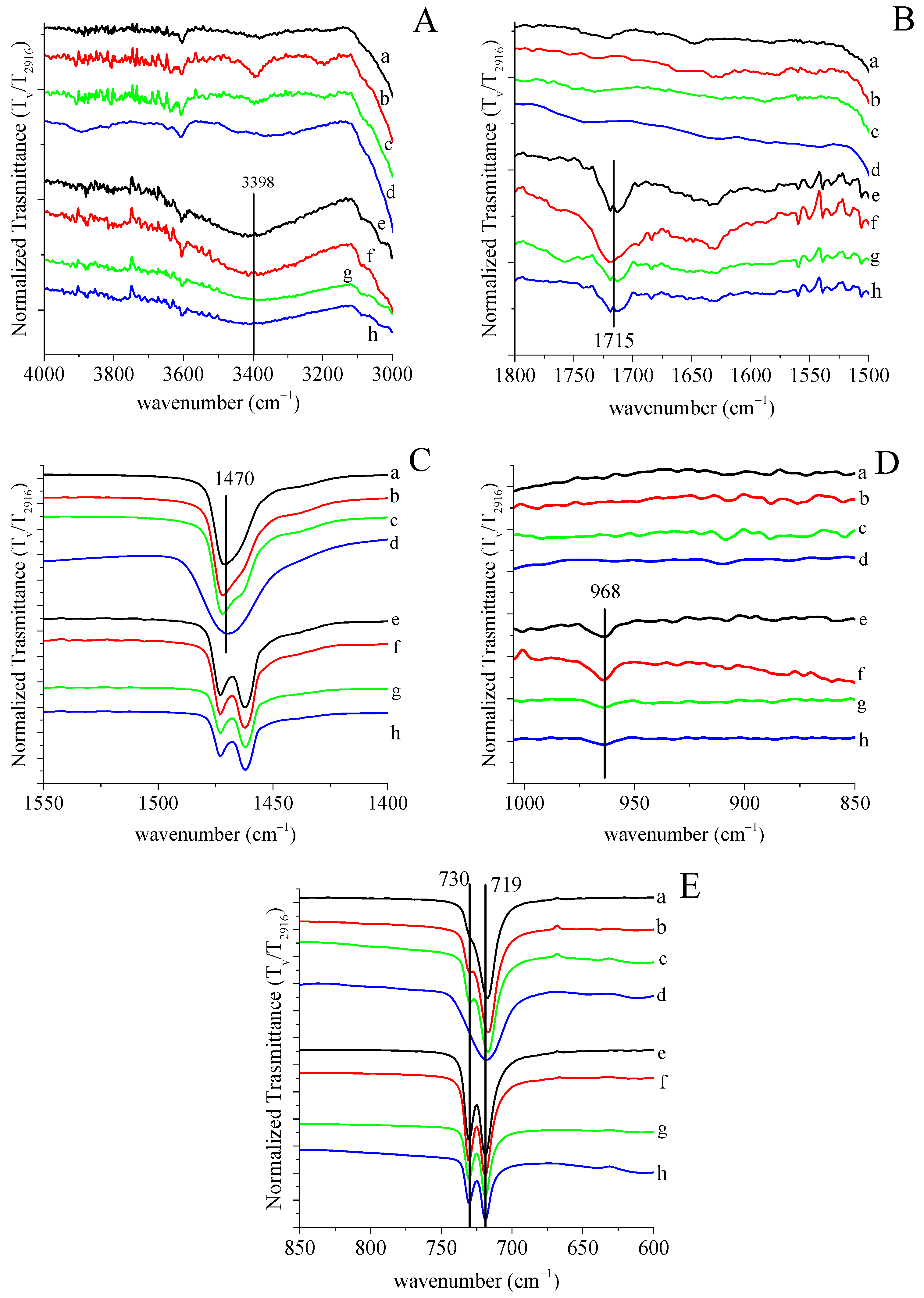
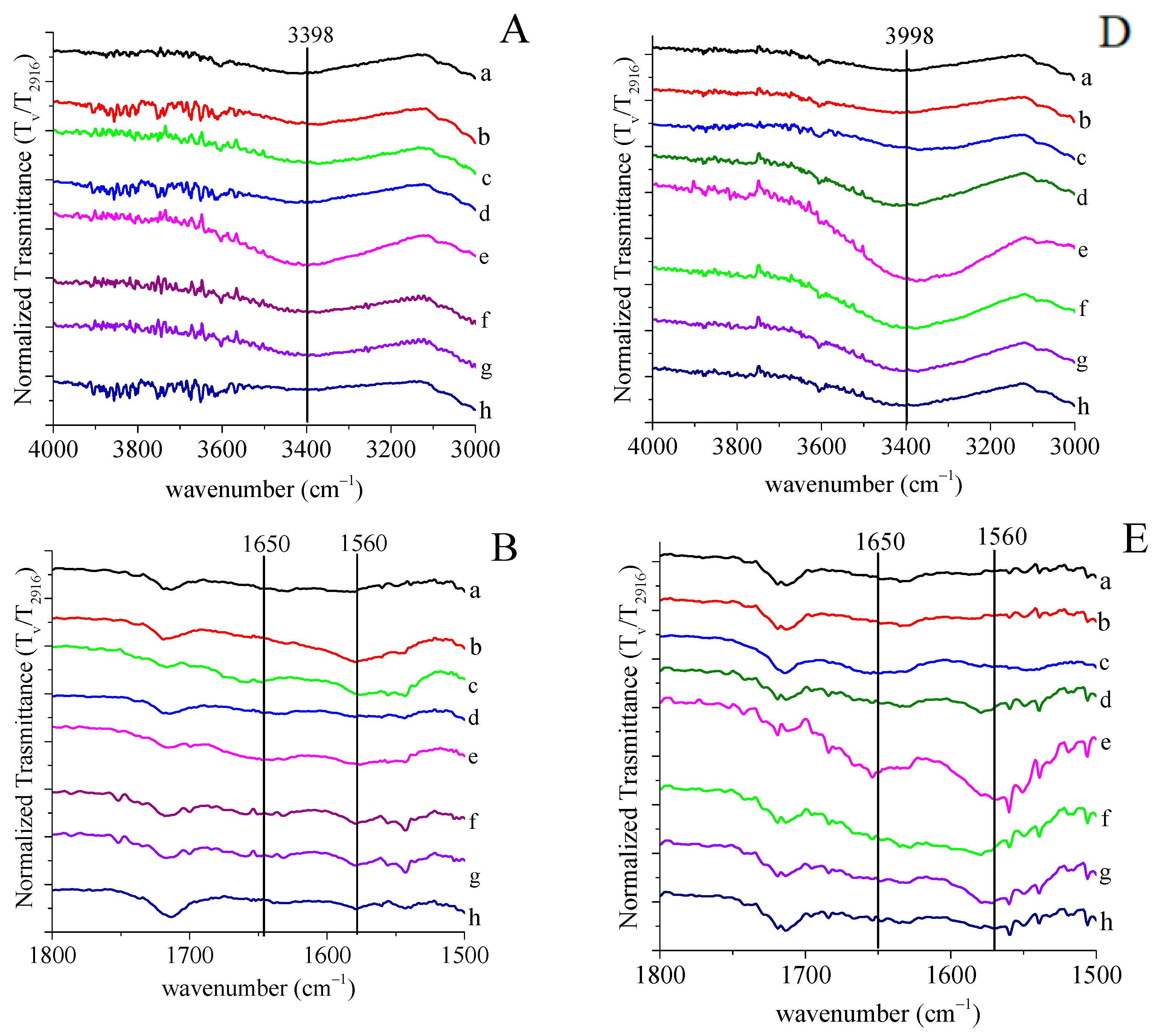
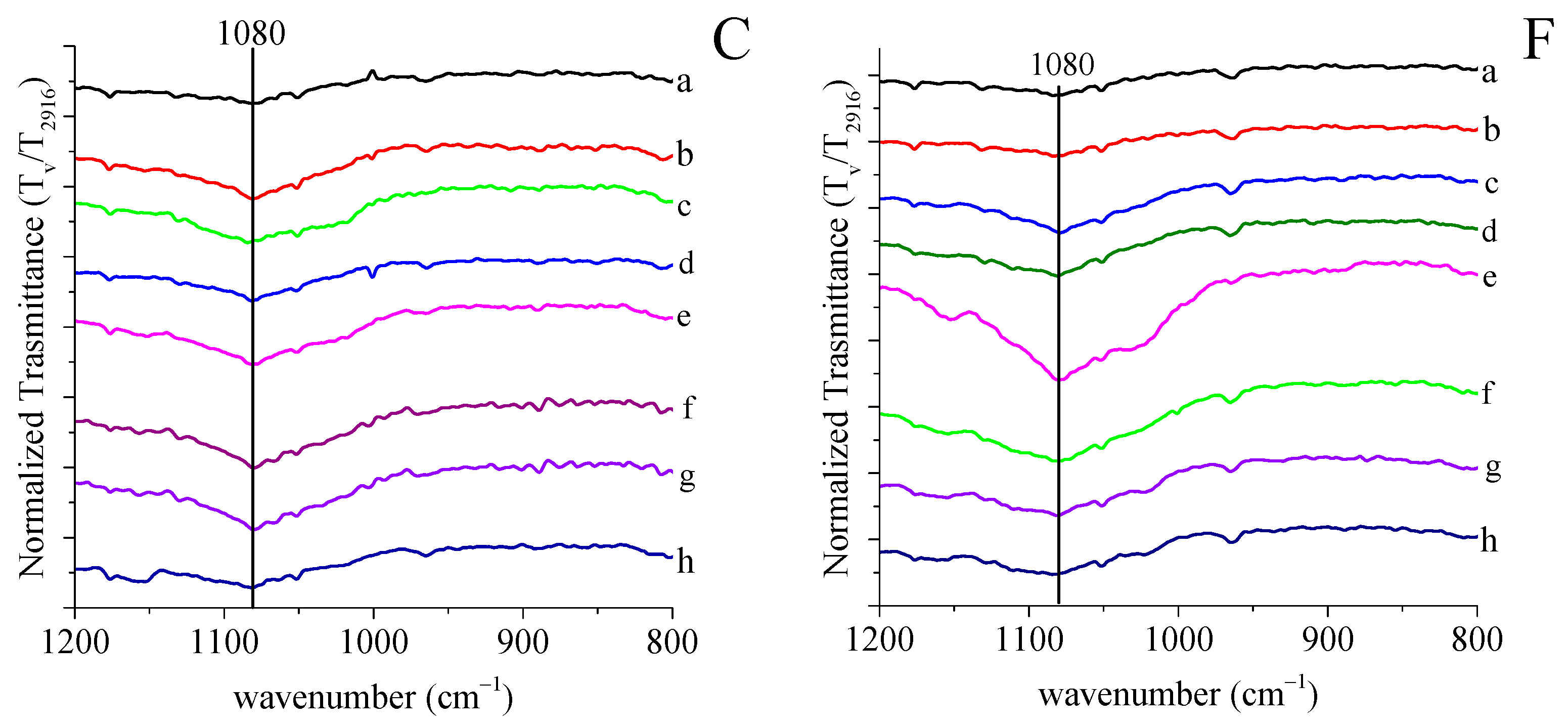
2.4.1. FTIR Spectroscopy
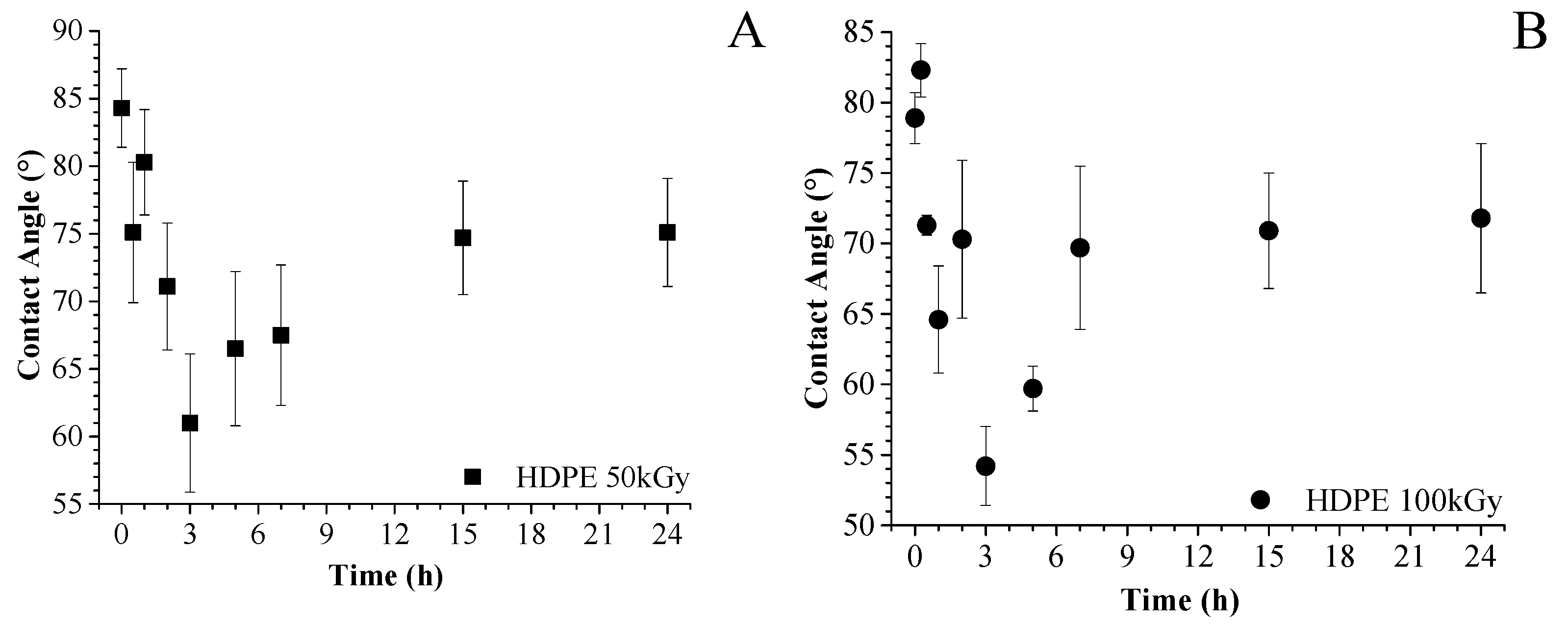
2.4.2. Surface Wettability
2.4.3. Nano-Roughness
2.4.4. Wide-Angle X-ray Powder Diffraction
2.4.5. Tensile Properties
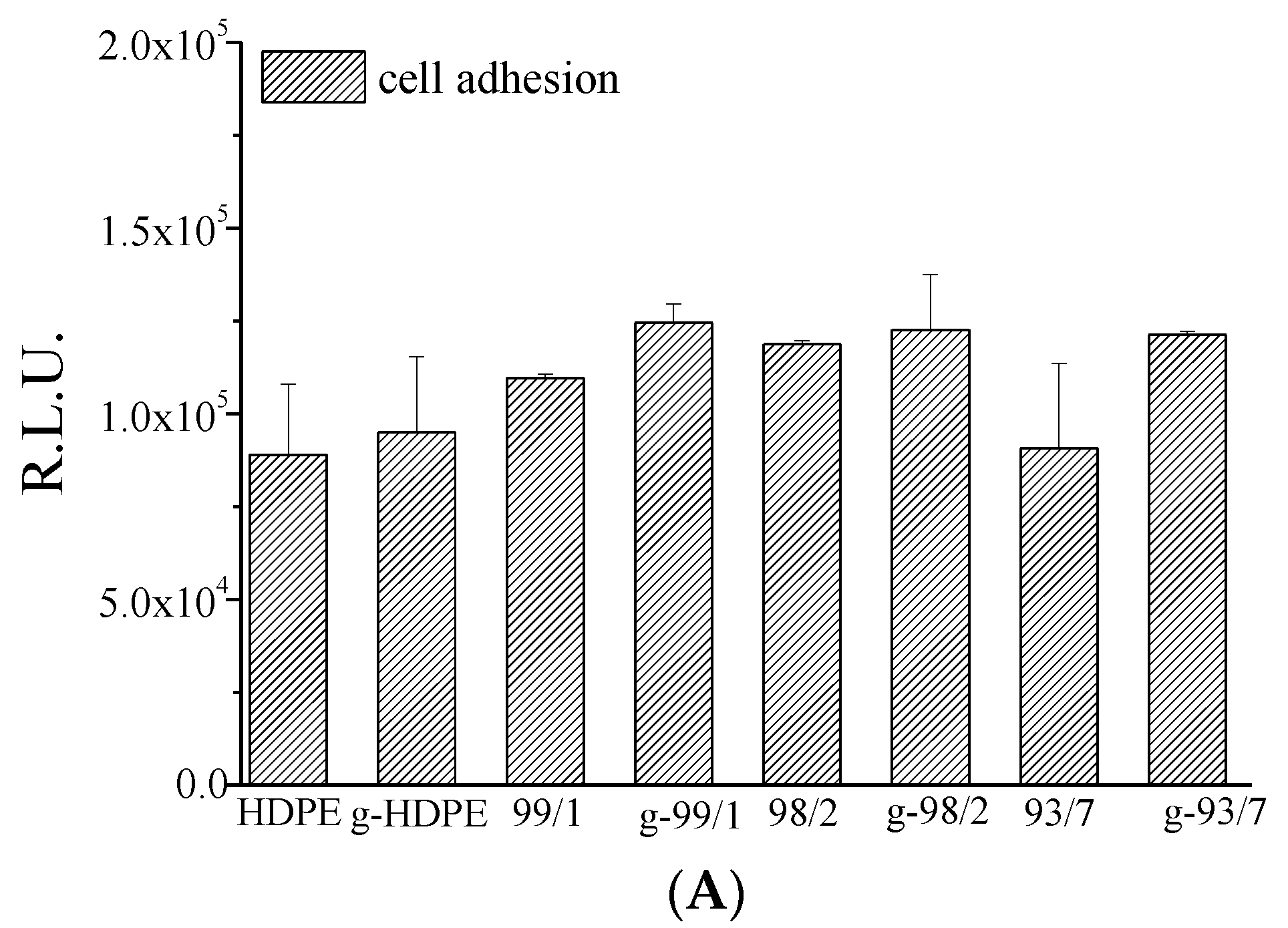
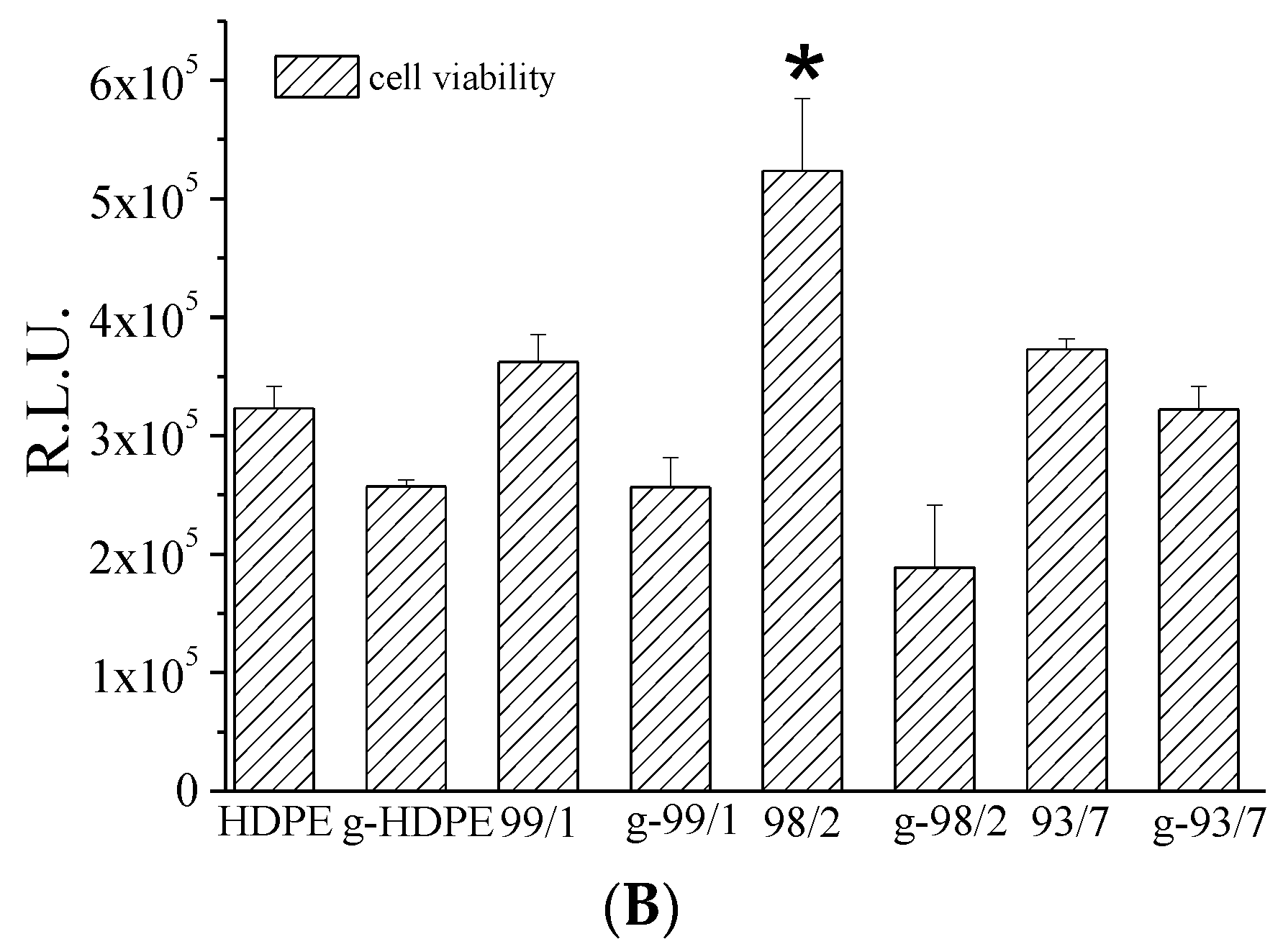
2.4.6. Biological Assays
3. Results
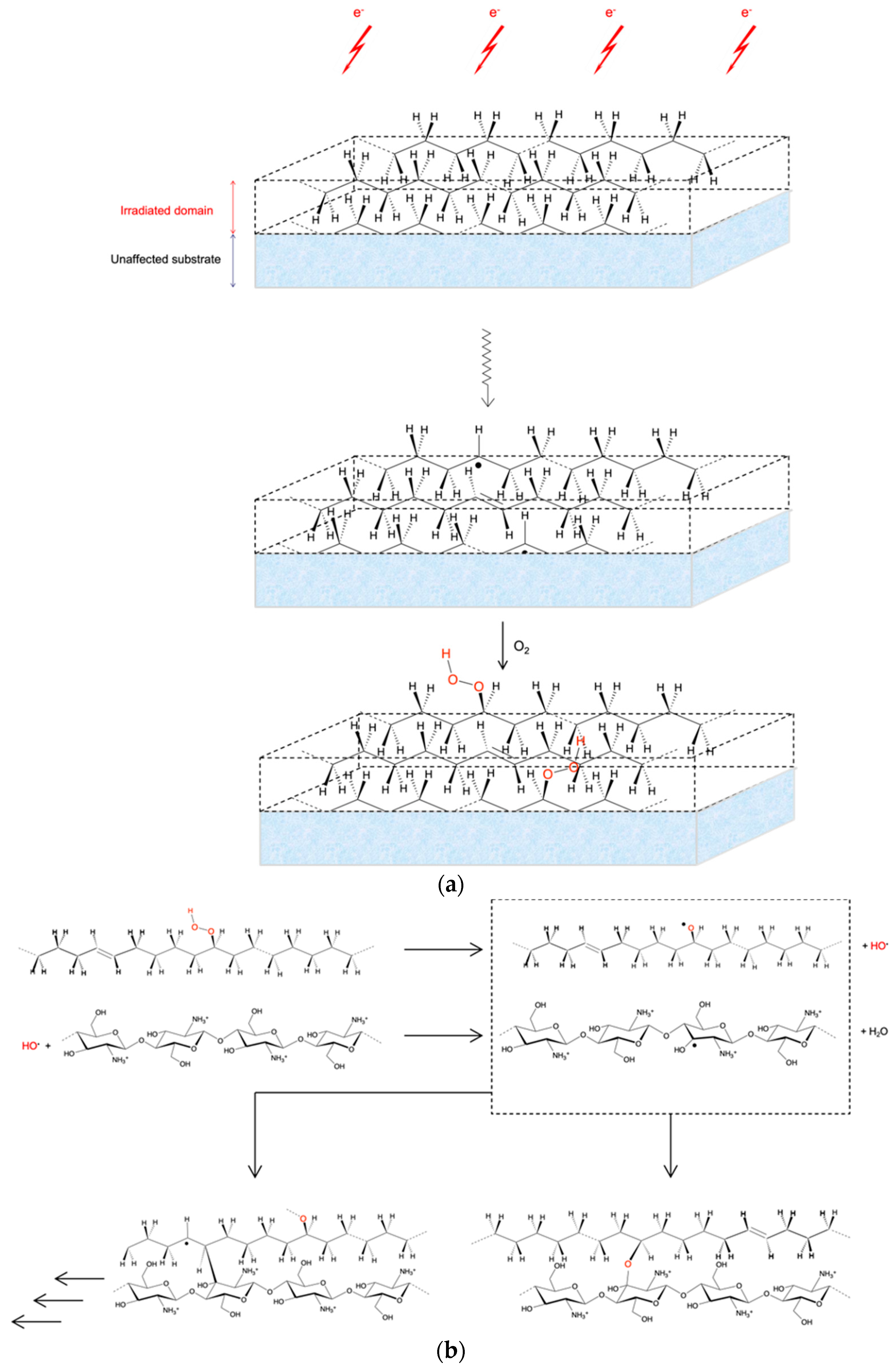
3.1. Grafting by Radiation-Induced Peroxidation of HDPE-Based Substrates
3.2. Chitosan Grafting Reaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayakumar, R.; Nwe, N.; Tokura, S.; Tamura, H. Sulfated chitin and Chitosan as novel biomaterials. Int. J. Biol. Macromol. 2007, 40, 175–181. [Google Scholar] [CrossRef]
- Muzzarelli, A.A. Natural Chelating Polymers; Alginic Acid, Chitin, and Chitosan; Pergamon Press Oxford: New York, NY, USA, 1973; p. 254. [Google Scholar]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Oner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef] [Green Version]
- Kadouche, S.; Farhat, M.; Lounici, H.; Fiallo, M.; Sharrock, P.; Mecherri, M.; Hadioui, M. Low Cost Chitosan Biopolymer for Environmental Use Made from Abundant Shrimp Wastes. Waste Biomass Valor. 2017, 8, 401–406. [Google Scholar] [CrossRef]
- Roshini Yadav, L.; Viji Chandran, S.; Lavanya, K.; Selvamurugan, N. Chitosan-based 3D-printed scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2021, 183, 1925–1938. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Kim, J.-S.; Kim, J.-E.; Lee, M.-H.; Jeon, J.-G.; Park, I.-S.; Yi, H.-K. Nanoparticle mediated PPARγ gene delivery on dental implants improves osseointegration via mitochondrial biogenesis in diabetes mellitus rat model. Nanomedicine 2017, 13, 1821–1832. [Google Scholar] [CrossRef]
- Bhattarai, G.; Lee, Y.H.; Lee, M.H.; Yi, H.K. Gene delivery of c-myb increases bone formation surrounding oral implants. J. Dent. Res. 2013, 92, 840–845. [Google Scholar] [CrossRef]
- De la Riva, B.; Nowak, C.; Sánchez, E.; Hernández, A.; Schulz-Siegmund, M.; Pec, M.K.; Delgado, A.; Evora, C. VEGF-controlled release within a bone defect from alginate/chitosan/PLA-H scaffolds. Eur. J. Pharm. Biopharm. 2009, 73, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Di Maro, M.; Faga, M.G.; Malucelli, G.; Mussano, F.D.; Genova, T.; Morsi, R.E.; Hamdy, A.; Duraccio, D. Influence of chitosan on the mechanical and biological properties of HDPE for biomedical applications. Polym. Test. 2020, 91, 106610. [Google Scholar] [CrossRef]
- Pon-On, W.; Charoenphandhu, N.; Teerapornpuntakit, J.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.-M. Mechanical properties, biological activity and protein controlled release by poly(vinyl alcohol)-bioglass/chitosan-collagen composite scaffolds: A bone tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 63–72. [Google Scholar] [CrossRef]
- Pino-Ramos, V.H.; Melendez-Ortiz, H.I.; Ramos-Ballesteros, A.; Bucio, E. Radiation grafting of biopolymers and synthetic polymers: Synthesis and biomedical applications. In Biopolymer Grafting: Applications; Thakur, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 205–250. [Google Scholar]
- Paxton, C.; Allenby, M.C.; Lewis, P.M.; Woodruff, M.A. Biomedical applications of polyethylene. Eur. Polym. J. 2019, 118, 412–428. [Google Scholar] [CrossRef]
- Ginting, S.I.; Yoichi, A.; Shin-ichi, K. Polyethylene film surface functionalized with chitosan via γ-ray irradiation in aqueous system: An approach to induce copper (II) ion adsorptivity on PE. Eur. Polyp. J. 2004, 40, 171–179. [Google Scholar]
- Ginting, S.I.; Shin-ichi, K.; Hitoshi, K.; Takashi, K. Effect of monomer concentration on characteristics of methacrylic acid-grafted polyethylene film prepared by photografting. Eur. Polym. J. 2000, 38, 1145–1150. [Google Scholar]
- Fayek, S.A.; El-Sayed, S.M.; El-Arnaouty, M.B. Study the effect of gamma irradiation on optical and morphological properties of grafted low density polyethylene. Polym. Test. 2000, 21, 19435–19443. [Google Scholar] [CrossRef]
- Di Maro, M.; Duraccio, D.; Malucelli, G.; Faga, M.G. High density polyethylene composites containing alumina-toughened zirconia particles: Mechanical and tribological behavior. Compos. Part B Eng. 2021, 217, 108892. [Google Scholar] [CrossRef]
- dos Santos, D.S.; Goulet, P.J.G.; Pieczonka, N.P.W.; Oliveira, O.N.; Aroca, R.F. Gold nanoparticle embedded, self-sustained chitosan films as substrates for surface-enhanced Raman scattering. Langmuir 2004, 20, 10273–10277. [Google Scholar] [CrossRef]
- Zenkiewicz, M.; Rauchfleisz, M.; Czupryńska, J.; Polański, J.; Karasiewicz, T.; Engelgard, W. Effects of electron-beam irradiation on surface oxidation of polymer composites. Appl. Surf. Sci. 2007, 253, 8992–8999. [Google Scholar] [CrossRef]
- Mizera, A.; Manas, M.; Holik, Z.; Manas, D.; Stanek, M.; Cerny, J.; Bednarik, M.; Ovsik, M. Properties of HDPE after Radiation Cross-linking. Int. J. Math. Comput. Simul. 2012, 16, 597. [Google Scholar]
- Tretinnikov, O.N.; Sakae, O.; Ikada, Y. Surface crosslinking of polyethylene by electron beam irradiation in air. Polymer 1998, 39, 6115–6120. [Google Scholar] [CrossRef]
- Duraccio, D.; Strongone, V.; Malucelli, G.; Auriemma, F.; De Rosa, C.; Mussano, F.D.; Genova, T.; Faga, M.G. The role of alumina-zirconia loading on the mechanical and biological properties of UHMWPE for biomedical applications. Compos. Part B 2019, 164, 800–808. [Google Scholar] [CrossRef]
- Pino-Ramos, V.H.; Ramos-Ballesteros, A.; López-Saucedo, F.; López-Barriguete, J.E.; Varca, G.H.C.; Bucio, E. Radiation grafting for the functionalization and development of smart polymeric materials. Top. Curr. Chem. 2016, 374, 63. [Google Scholar] [CrossRef]
- Ashfaq, A.; Clochard, M.C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulanski, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef]
- Ferry, M.; Ngono-Ravache, Y.; Aymes-Chodur, C.; Clochard, M.C.; Coqueret, X.; Cortella, L.; Pellizzi, E.; Rouif, S.; Esnouf, S. Ionizing radiation effects in polymers. In Reference Module in Materials Science and Materials Engineering; Hashmi, S., Ed.; Elsevier: Oxford, UK, 2016. [Google Scholar]
- Rugg, F.M.; Smith, J.J.; Bacon, R.C. Infrared spectrophotometric studies on polyethylene. II. Oxidation. J. Polym. Sci. 1954, 13, 535–582. [Google Scholar] [CrossRef]
- Lacoste, J.; Deslandes, Y.; Black, P.; Carlsson, D.J. Surface and bulk analyses of the oxidation of polyolefins. Polym. Degrad. Stab. 1995, 49, 21–28. [Google Scholar] [CrossRef]
- Lacoste, J.; Carlsson, D.J.; Falicki, S.; Wiles, D.M. Polyethylene hydroperoxide decomposition products. Polym. Degrad. Stab. 1991, 34, 309–323. [Google Scholar] [CrossRef]
- Al-Assaf, S.; Coqueret, X.; Zaman, H.M.K.; Sen, M.; Ulanski, P. The Radiation Chemistry of Polysaccharides; IAEA: Vienna, Austria, 2016. [Google Scholar]
- Albert, C.F.; Busfield, W.K.J. Identifying crosslinks in polyethylene following gamma-irradiation in acetylene: A model compound study. Polym. Sci. Part A Polym. Chem. 1997, 35, 1549. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, K.-J.; Seo, Y.; Kwak, S.; Koh, S.-K. HDPE Surface Functionalization by Low-Energy Ion-Beam Irradiation under a Reactive O2 Environment and Its Effect on the HDPE/Nylon 66 Blend. Macromolecules 2001, 34, 2546–2558. [Google Scholar] [CrossRef]
- Krimm, S.; Liang, C.Y.; Sutherland, G.B.B. Infrared Spectra of High Polymers. II. Polyethylene. J. Chem. Phys. 1956, 25, 549. [Google Scholar] [CrossRef] [Green Version]
- Bower, D.I.; Maddams, W.F. The Vibrational Spectroscopy of Polymers; Cambridge University Press: New York, NY, USA, 1989. [Google Scholar]
- Abdel-Fattah, A.A.; Ebraheem, S.; Ali, Z.I.; Abdel-Rehim, F. Ultraviolet and infrared spectral analysis of irradiated polyethylene films: Correlation and possible application for large-dose radiation dosimetry. J. Appl. Polym. Sci. 1998, 67, 1837. [Google Scholar] [CrossRef]
- Smith, D.J.; Mc Cartney, M.R.; Bursill, L.A. The electron-beam-induced reduction of transition metal oxide surfaces to metallic lower oxides. Ultramicroscopy 1987, 23, 299–303. [Google Scholar] [CrossRef]
- Nathawat, R.; Kumar, A.; Acharya, N.K.; Vijay, Y.K. XPS and AFM surface study of PMMA irradiated by electron beam. Surf. Coat. Technol. 2009, 203, 2600–2604. [Google Scholar] [CrossRef]
- Feulner, R.; Brocka, Z.; Seefried, A.; Kobes, M.O.; Hülder, G.; Osswald, T.A. The effects of e-beam irradiation induced cross linking on the friction and wear of polyamide 66 in sliding contact. Wear 2010, 268, 905–910. [Google Scholar] [CrossRef]
- Duraccio, D.; Strongone, V.; Faga, M.G.; Auriemma, F.; Mussano, F.D.; Genova, T.; Malucelli, G. The role of different dry-mixing techniques on the mechanical and biological behavior of UHMWPE/alumina-zirconia composites for biomedical applications. Eur. Polym. J. 2019, 120, 109274. [Google Scholar] [CrossRef]
- Bhateja, S.K.; Andrews, E.H.; Young, R.J. Radiation-Induced crystallinity changes in linear polyethylene. J. Polym. Sci. Polymer Phys. Ed. 1983, 21, 523–536. [Google Scholar] [CrossRef]
- Thome, T.; Braga, D.; Blaise, G. Effect of current density on electron beam induced charging in sapphire and yttria-stabilized zirconia. J. App. Phys. 2004, 95, 2619–2624. [Google Scholar] [CrossRef]
- Gheysari, D.j.; Behjat, A.; Haji-Saeid, M. The effect of high-energy electron beam on mechanical and thermal properties of LDPE and HDPE. Eur. Polym. J. 2001, 37, 295–302. [Google Scholar] [CrossRef]
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Biopolymer/Clay nanocomposites based on chitosan intercalated in montmorillonite. Chem. Mat. 2003, 15, 3774–3780. [Google Scholar] [CrossRef]
- Amaral, I.F.; Granja, P.L.; Barbosa, M.A. Chemical modification of chitosan by phosphorylation: An XPS, FT-IR and SEM study. J. Biomat. Sci. Polym. Ed. 2005, 16, 1575–1593. [Google Scholar] [CrossRef]
- Sha, X.; Xu, X.; Sohlberg, K.; Lollb, P.J.; Penn, L.S. Evidence that three-regime kinetics is inherent to formation of a polymer brush by a grafting-to approach. RSC Adv. 2014, 4, 42122. [Google Scholar] [CrossRef]
- Hsu, S.-C.; Don, T.-M.; Chiu, W.-Y. Free radical degradation of chitosan with potassium persulfate. Polym. Degrad. Stab. 2002, 75, 73–83. [Google Scholar] [CrossRef]
- Hadjicharalambous, C.; Flouraki, C.; Narain, R.; Chatzinikolaidou, M.; Vamvakaki, M. Controlling pre-osteoblastic cell adhesion and spreading on glycopolymer brushes of variable film thickness. J. Mater. Sci. Mater. Med. 2018, 26, 98–108. [Google Scholar] [CrossRef]
- Suh, J.K.F.; Matthew, H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [PubMed]
- Chuang, W.Y.; Young, T.H.; Yao, C.H.; Chiu, W.Y. Properties of the poly(vinyl alcohol)/chitosan composite and its effect on the culture of fibroblast in vitro. Biomaterals 1999, 20, 1479–1487. [Google Scholar] [CrossRef]
- Moysan, A.; Marquis, I.; Gaboriau, F.; Santus, R.; Dubertret, L.; Morliére, P. Ultraviolet A-induced lipid peroxidation and antioxidant defence systems in cultured human skin fibroblasts. J. Investig. Dermatol. 1993, 100, 692–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Untreated Sample | Irradiated Sample | |||||
|---|---|---|---|---|---|---|
| ra (nm) | θ (°) | xc (%) | ra (nm) | θ (°) | xc (%) | |
| HDPE | 73 | 96 ± 2 | 69 ± 1 | 151 | 79 ± 3 | 72 ± 1 |
| HDPE/ATZ 99/1 | 58 | 95 ± 2 | 71 ± 1 | 96 | 72 ± 3 | 74 ± 1 |
| HDPE/ATZ 93/7 | 74 | 96 ± 2 | 75 ± 1 | 83 | 69 ± 3 | 85 ± 1 |
| Young’s Modulus (E) (MPa) | |||
|---|---|---|---|
| Irradiation dose (kGy) | 0 | 50 | 100 |
| HDPE | 969 ± 17 | 1147 ± 80 | 1052 ± 75 |
| HDPE/ATZ 99/1 | 1189 ± 34 | 1090 ± 18 | 1202 ± 65 |
| HDPE/ATZ 98/2 | 1139 ± 16 | 1210 ± 18 | 1152 ± 50 |
| HDPE/ATZ 97/3 | 1088 ± 53 | 1245 ± 36 | 1271 ± 24 |
| Tensile Strength (σb) (MPa) | |||
| HDPE | 13.7 ± 0.4 | 16.4 ± 0.4 | 15.3 ± 0.6 |
| HDPE/ATZ 99/1 | 14.3 ± 0.3 | 12.0 ± 0.4 | 13.9 ± 0.5 |
| HDPE/ATZ 98/2 | 15.4 ± 0.5 | 14.2 ± 0.9 | 13.7 ± 1.5 |
| HDPE/ATZ 97/3 | 12.5 ± 1.5 | 13.9 ± 0.7 | 13.0 ± 0.9 |
| Elongation at Break (εb) (%) | |||
| HDPE | 320 ± 100 | 355 ± 150 | 79 ± 81 |
| HDPE/ATZ 99/1 | 647 ± 28 | 138 ± 118 | 97 ± 105 |
| HDPE/ATZ 98/2 | 704 ± 85 | 131 ± 58 | 154 ± 70 |
| HDPE/ATZ 97/3 | 365 ± 145 | 92 ± 65 | 100 ± 6 |
| Before Grafting | After Grafting | |
|---|---|---|
| θ (°) | θ (°) | |
| HDPE | 79 ± 2 | 66 ± 1 |
| HDPE/ATZ 99/1 | 72 ± 2 | 66 ± 1 |
| HDPE/ATZ 98/2 | 72 ± 4 | 73 ± 3 |
| HDPE/ATZ 93/7 | 69 ± 4 | 70 ± 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faga, M.G.; Duraccio, D.; Di Maro, M.; Kowandy, C.; Malucelli, G.; Mussano, F.D.; Genova, T.; Coqueret, X. Electron-Beam-Induced Grafting of Chitosan onto HDPE/ATZ Composites for Biomedical Applications. Polymers 2021, 13, 4016. https://doi.org/10.3390/polym13224016
Faga MG, Duraccio D, Di Maro M, Kowandy C, Malucelli G, Mussano FD, Genova T, Coqueret X. Electron-Beam-Induced Grafting of Chitosan onto HDPE/ATZ Composites for Biomedical Applications. Polymers. 2021; 13(22):4016. https://doi.org/10.3390/polym13224016
Chicago/Turabian StyleFaga, Maria Giulia, Donatella Duraccio, Mattia Di Maro, Christelle Kowandy, Giulio Malucelli, Federico Davide Mussano, Tullio Genova, and Xavier Coqueret. 2021. "Electron-Beam-Induced Grafting of Chitosan onto HDPE/ATZ Composites for Biomedical Applications" Polymers 13, no. 22: 4016. https://doi.org/10.3390/polym13224016
APA StyleFaga, M. G., Duraccio, D., Di Maro, M., Kowandy, C., Malucelli, G., Mussano, F. D., Genova, T., & Coqueret, X. (2021). Electron-Beam-Induced Grafting of Chitosan onto HDPE/ATZ Composites for Biomedical Applications. Polymers, 13(22), 4016. https://doi.org/10.3390/polym13224016