Efficacy Analysis of In Situ Synthesis of Nanogold via Copper/Iodonium/Amine/Gold System under a Visible Light
Abstract
:1. Introduction
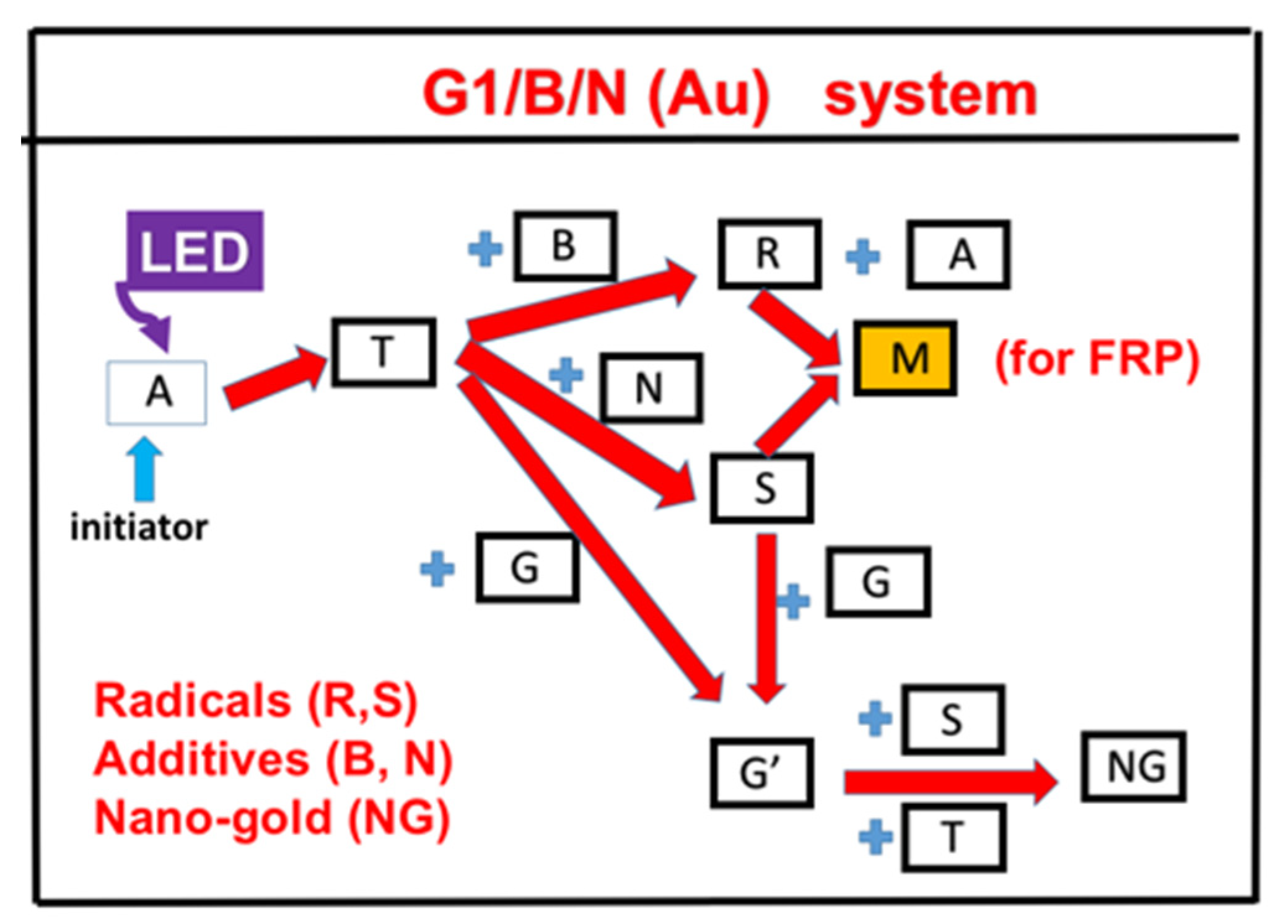
2. Methods and Modeling Systems
2.1. Photochemical Kinetics
2.2. The Rate Equations
3. Results and Discussion
3.1. Analytic Results
3.2. General Features and New Findings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fouassier, J.P.; Lalevée, J. Photoinitiators for Polymer Synthesis: Scope, Reactivity and Efficiency; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Kelly, B.E.; Bhattacharya, I.; Heidari, H.; Shusteff, M.; Spadaccini, C.M.; Taylor, H.K. Volumetric additive manufacturing via tomographic reconstruction. Science 2019, 363, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- de Beer, M.P.; van der Laan, H.L.; Cole, M.A.; Whelan, R.J.; Burns, M.A.; Scott, T.F. Rapid, continuous additive manufacturing by volumetric polymerization inhibition patterning. Sci. Adv. 2019, 5, eaau8723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Laan, H.L.; Burns, M.A.; Scott, T.F. Volumetric Photopolymerization Confinement through Dual-Wavelength Photoinitiation and Photoinhibition. ACS Macro Lett. 2019, 8, 899–904. [Google Scholar] [CrossRef]
- Lin, J.T.; Chen, K.T.; Cheng, D.C.; Liu, H.W. Dual-wavelength (UV and Blue) controlled photopolymerization confinement for 3D-printing: Modeling and analysis of measurements. Polymers 2019, 11, 1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.T.; Liu, H.W.; Chen, K.T.; Cheng, D.C. 3-wavelength (UV, blue, red) controlled photopolymerization: Improved conversion and confinement in 3D-printing. IEEE Access 2020, 8, 49353–49362. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Cheng, D.C.; Lin, J.T.; Chen, K.T.; Liu, H.W. Dual-function enhancer for near-infrared photopolymerization: Kinetic modeling for improved efficacy by suppressed oxygen inhibition. IEEE Access 2020, 8, 83465–83471. [Google Scholar] [CrossRef]
- Lin, J.T.; Chen, K.T.; Cheng, D.C.; Liu, H.W. Enhancing blue-light-initiated photopolymerization in a three-component system: Kinetic and modeling of conversion strategies. J. Polym. Res. 2021, 28, 2. [Google Scholar] [CrossRef]
- Lin, J.; Cheng, D.; Chen, K.; Chiu, Y.; Liu, H. Enhancing UV Photopolymerization by a Red-light Preirradiation: Kinetics and Modeling Strategies for Reduced Oxygen Inhibition. J. Appl. Polym. Sci. 2020, 58, 683–691. [Google Scholar] [CrossRef]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Brunel, D.; Dumur, F. Recent advances on push–pull organic dyes as visible light photoinitiators of polymerization. Eur. Polym. J. 2020, 133, 109797. [Google Scholar] [CrossRef]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Redox two-component initiated free radical and cationic polymerizations: Concepts, reactions and applications. Prog. Polym. Sci. 2019, 94, 33–56. [Google Scholar] [CrossRef]
- Noirbent, G.; Dumur, F. Recent Advances on Copper Complexes as Visible Light Photoinitiators and (Photo) Redox Initiators of Polymerization. Catalysts 2020, 10, 953. [Google Scholar] [CrossRef]
- Mokbel, H.; Anderson, D.; Plenderleith, R.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.; Lalevée, J. Simultaneous initiation of radical and cationic polymerization reactions using the “G1” copper complex as photoredox catalyst: Applications of free radical/cationic hybrid photopolymerization in the composites and 3D printing fields. Prog. Org. Coat. 2019, 132, 50–61. [Google Scholar] [CrossRef]
- Rahal, M.; Mokbel, H.; Graff, B.; Toufaily, J.; Hamieh, T.; Dumur, F.; Lalevée, J. Mono vs. Difunctional Coumarin as Photoinitiators in Photocomposite Synthesis and 3D Printing. Catalyst 2020, 10, 1202. [Google Scholar] [CrossRef]
- Rahal, M.; Graff, B.; Toufaily, J.; Hamieh, T.; Noirbent, G.; Gigmes, D.; Dumur, F.; Lalevée, J. 3-Carboxylic Acid and Formyl-Derived Coumarins as Photoinitiators in Photo-Oxidation or Photo-Reduction Processes for Photopolymerization upon Visible Light: Photocomposite Synthesis and 3D Printing Applications. Molecules 2021, 26, 1753. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-T.; Lalevee, J.; Cheng, D.-C. A Critical Review for Synergic Kinetics and Strategies for Enhanced Photopolymerizations for 3D-Printing and Additive Manufacturing. Polymers 2021, 13, 2325. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Hijazi, A.; Lin, J.-T.; Graff, B.; Dumur, F.; Lalevée, J. Coumarin Derivatives as Photoinitiators in Photo-Oxidation and Photo-Reduction Processes and a Kinetic Model for Simulations of the Associated Polymerization Profiles. ACS Appl. Polym. Mater. 2020, 2, 2769–2780. [Google Scholar] [CrossRef]
- Tar, H.; Kashar, T.I.; Kouki, N.; Aldawas, R.; Graff, B.; Lalevée, J. Novel Copper Photoredox Catalysts for Polymerization: An In Situ Synthesis of Metal Nanoparticles. Polymers 2020, 12, 2293. [Google Scholar] [CrossRef]
- Lin, J.-T. Modeling the scaling law of surface plasmon resonance in gold spherical nanoshells. J. Nanophotonics 2010, 4, 049507. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.-T. Nonlinear optical theory and figure of merit of surface plasmon resonance of gold nanorods. J. Nanophotonics 2011, 5, 051506. [Google Scholar] [CrossRef]
- Lin, J.T. Scaling law and figure of merit of biosensor using gold nanoshells. J. Nanophotonics 2010, 4, 049507. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.-T.; Cheng, D.-C. Modeling the efficacy profiles of UV-light activated corneal collagen crosslinking. PLoS ONE 2017, 12, e0175002. [Google Scholar] [CrossRef]
- Lin, J.-T. Kinetics of Enhancement for Corneal Cross-linking: Proposed Model for a Two-initiator System. Ophthalmol. Res. Int. J. 2019, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.-T.; Liu, H.-W.; Chen, K.-T.; Cheng, D.-C. Modeling the Kinetics, Curing Depth, and Efficacy of Radical-Mediated Photopolymerization: The Role of Oxygen Inhibition, Viscosity, and Dynamic Light Intensity. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, H.-W.; Chen, K.-T.; Cheng, D.-C. Modeling the Optimal Conditions for Improved Efficacy and Crosslink Depth of Photo-Initiated Polymerization. Polymers 2019, 11, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
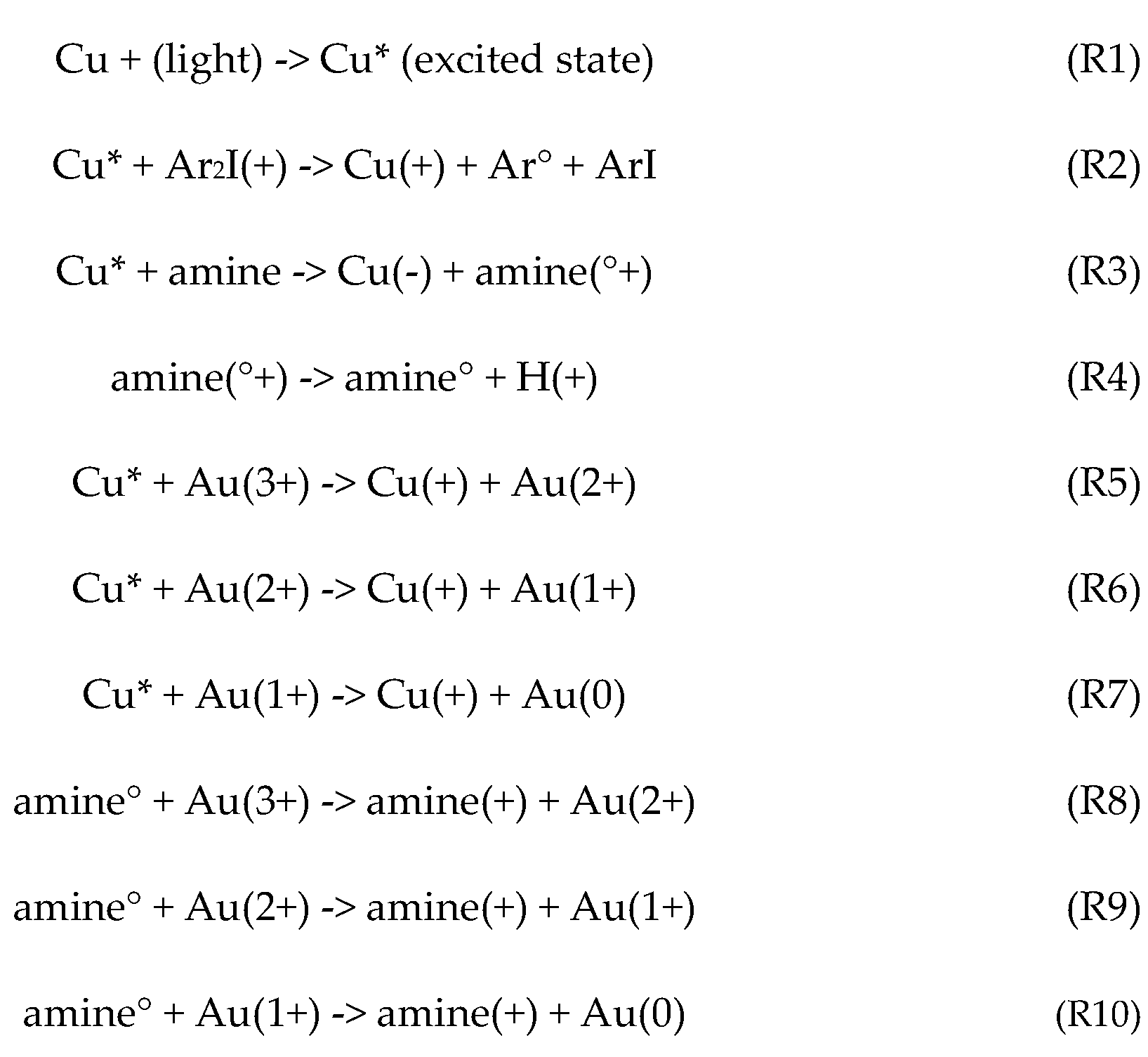
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-T.; Lalevee, J.; Liu, H.-W. Efficacy Analysis of In Situ Synthesis of Nanogold via Copper/Iodonium/Amine/Gold System under a Visible Light. Polymers 2021, 13, 4013. https://doi.org/10.3390/polym13224013
Lin J-T, Lalevee J, Liu H-W. Efficacy Analysis of In Situ Synthesis of Nanogold via Copper/Iodonium/Amine/Gold System under a Visible Light. Polymers. 2021; 13(22):4013. https://doi.org/10.3390/polym13224013
Chicago/Turabian StyleLin, Jui-Teng, Jacques Lalevee, and Hsia-Wei Liu. 2021. "Efficacy Analysis of In Situ Synthesis of Nanogold via Copper/Iodonium/Amine/Gold System under a Visible Light" Polymers 13, no. 22: 4013. https://doi.org/10.3390/polym13224013
APA StyleLin, J.-T., Lalevee, J., & Liu, H.-W. (2021). Efficacy Analysis of In Situ Synthesis of Nanogold via Copper/Iodonium/Amine/Gold System under a Visible Light. Polymers, 13(22), 4013. https://doi.org/10.3390/polym13224013






