Abstract
Hyaluronic acid, together with collagen, vitamins or plant extracts, is a part of many cosmetic and food preparations. For example, this polysaccharide is used in formulation of many food supplements due to its protective effects on human health. In this work, the screening of the chemical composition of three chosen dietary supplements (powder, tablets and capsules) containing hyaluronic acid was carried out using Fourier-transform infrared spectroscopy. Because of the low amount of analyte in all these samples, it was isolated or concentrated prior to the analysis using a suitable sequential fractionation protocol. Individual isolation procedures were established for each sample based on their declared composition. Firstly, the major components such as collagen or vitamins were removed to obtain polysaccharide fractions by the enzymatic treatment and/or washing out with the appropriate solvents. In some cases, the water insoluble part was removed from the rest dissolved in water. Then, hyaluronic acid was precipitated with copper(II) cations and thus separated from the other polysaccharides. Finally, the analyte was identified in the enriched fractions by the characteristic vibrational bands. The amount of hyaluronic acid in the purified fractions was determined in three ways: gravimetrically, spectrophotometrically, and using isotachophoresis. The combination of the appropriate preparative and analytical steps led to the successful evaluation of chemical composition, finding and quantification of hyaluronic acid in all the studied samples.
1. Introduction
Hyaluronic acid is an acidic polysaccharide of animal and bacterial origin that serves various physiological purposes, for example, in connective tissues of vertebrates and bacterial capsules [1,2,3,4]. It is an alternating co-polymer of 1,4-linked β-d-glucuronic acid and 1,3-linked N-acetyl-β-d-glucosamine. Hyaluronic acid is the only non-sulfated glycosaminoglycan with unique physicochemical and biological properties [5]. It is a highly hygroscopic polymer that can hold water molecules and create a gel-like environment [6,7,8]. Even at low concentrations in an aqueous medium, hyaluronic acid and its salts, hyaluronates, behave like viscoelastic systems [9,10,11,12]. A number of charged and polar groups allow hyaluronic acid to hold water molecules and participate in many biologically important polar interactions. On the other hand, regularly repeating N-acetyl groups promote interaction with cell membranes and hydrophobic regions of membrane proteins, which is important for ensuring cell motility [13]. Thus, the combination of hydrophilic and hydrophobic characteristics is an integral part of the basic structure of this polysaccharide [14,15,16].
Hyaluronic acid is involved in protective and other physiological processes, including healing of wound and burns [17,18,19,20,21], tissue regeneration [22,23], cell differentiation, morphogenesis, angiogenesis, and inflammation [23,24,25]. Biocompatibility and high affinity for water make it possible to use hyaluronic acid in various fields of medicine. Natural hyaluronic acid itself or healing systems containing this polysaccharide are used in surgery, pharmacology, ophthalmology, dermatology and cosmetology [26,27,28,29]. It is a component of synovial fluid substitutes as a medium for ocular surgery, preservation and cell transfer. This polysaccharide has been used in various nutritional supplements and cosmetics.
Determination of hyaluronic acid in food supplements is difficult due to the low content of this analyte, as well as the presence of many other components such as proteins (collagen), other polysaccharides, vitamins or plant extracts. Moreover, these substances are often present in larger quantities than the target compound, which further complicates the analysis. Hyaluronic acid has been determined as intact polymeric chains in food supplements and medicinal preparations by separation methods including capillary zone electrophoresis (CZE), isotachophoresis (ITP) and high-performance liquid chromatography (HPLC) [30,31,32,33,34]. Another approach is based on a combination of complete or partial hydrolysis using mineral acids or enzymes, followed by quantitative analysis of the hydrolysate by photometry, separation or electromigration methods [35,36,37]. Both approaches have a number of disadvantages associated primarily with large differences in molecular weight or with incomplete hydrolysis of hyaluronic acid macromolecules. In addition, the presence of various substances in the samples can interfere with the determination, which means that they must be removed before analysis. This presupposes an individual approach to each sample, depending on their composition declared by the manufacturer. As an alternative approach, we can offer sequential purification of the sample using suitable extraction and precipitation steps, and the composition and purity of the resulting fractions can be assessed using Fourier-transform infrared (FT-IR) spectroscopy as a fast, structure-sensitive and non-invasive method that has been used in structural and conformational analysis of hyaluronic acid, hyaluronates and derived oligomers [38,39,40,41,42,43,44]. In addition to this, FT-IR spectroscopy can be used as screening method to evaluate the composition of food supplements and thus detect not only hyaluronic acid but also the other organic and some inorganic components, which are not transparent in the middle infrared region.
The disadvantage of FT-IR spectroscopy compared to spectrophotometry and separation methods such as HPLC or CZE is its low sensitivity, so it is well suited only for the determination of major components. Therefore, in order to detect hyaluronic acid in dietary supplements, it is necessary to concentrate it through suitable purification steps. The water-insoluble part can be easily separated from the aqueous solution of the remaining compounds, small molecules can be washed out using suitable solvents, and the proteins must be pre-hydrolyzed with proteases, and then the resulting peptides must be removed in the same way as other small molecules. A difficult situation can arise if, as a result of all the above stages, hyaluronic acid remains in a mixture with other polysaccharides that can be added together with it to the formulation, for example, with starch or starch modification products. In this case, precipitation with metal counter cations can be used to separate hyaluronic acid from neutral polysaccharides.
Multivalent metal cations are known as effective cross-linkers for biopolymers including anionic polysaccharides [45]. When treated with such metal cations, polysaccharide gels and precipitates can form. Precipitation by metal cations has been used for preparative and analytical purposes as a method for the rapid separation of macromolecules depending on their charge. For example, precipitation with copper(II) cations has been used for the purification of pectins, anionic plant cell wall polysaccharides [46,47,48,49]. This approach has been also used for determination of pectin content by photometry [50]. The precipitation of pectins by metal cations is based on the selective formation of insoluble complexes. Compared to precipitation with ethanol, the use of metal counter cations in the precipitation of apple pectins led to an increase in galacturonic acid content [46]. Metal precipitation avoids co-precipitation of other polysaccharides, which is observed in the ethanol precipitation [47]. Copper(II) cations selectively bind anionic regions of pectin chains, thereby separating pectic polysaccharides from oligosaccharides and proteins [48]. Consequently, counter cations have a certain affinity for negatively charged structural regions of macromolecules, thereby selectively precipitating polyuronic acids, while neutral polysaccharides remain in solution. Similarly to pectins, copper(II) cations can be used for the precipitation of other polyuronides including hyaluronates. However, unlike pectins, the precipitation of hyaluronic acid with copper(II) cations has not been sufficiently studied and widely used, although copper(II) hyaluronates have been prepared and described [51,52,53,54,55]. Copper(II) cations are coordinated with hyaluronate through the carboxylate group and anomeric oxygen of the β-d-glucuronic acid units, while the N-acetamide group of the N-acetyl-β-d-glucosamine units is not involved in coordination. Therefore, as in the case of pectins, copper(II) cations are able to crosslink hyaluronic acid macromolecules through carboxylate groups and therefore precipitate copper(II) hyaluronate from a solution in which neutral macromolecules remain. Precipitation of copper(II) hyaluronate occurs under neutral and weak alkaline conditions, while under weakly acidic conditions, the complex is stable in solution [51], possibly due to incomplete dissociation of carboxylic groups [49].
This work is devoted to the screening analysis of chemical composition, identification and quantification of hyaluronic acid in three dietary supplements of different dosage forms (powder doze, tablet and capsule) and composition declared by manufacturers using a combination of suitable purification steps, including precipitation with copper(II) cations, and FT-IR spectroscopy of the fractions obtained. For each sample, individual isolation procedures for analyte enrichment were chosen in relation to sample composition and analyte content in raw material. Quantification was carried out gravimetrically by the mass of the final fractions identified by FT-IR as hyaluronic acid, as well as by spectrophotometry and isotachophoresis. This approach is suitable for the detailed characterization of food supplements and similar samples containing hyaluronic acid together with an excess of other components (collagen, polysaccharides, vitamins, etc.).
2. Materials and Methods
2.1. Samples and Chemicals
The purpose of the study was to identify the hyaluronic acid in three samples of dietary supplements with different composition obtained from the manufacturers and/or from the domestic market (Table 1). Expected amount of hyaluronic acid in these supplements was in the range of ~0.8–5.9% w/w.

Table 1.
Composition of food supplements 1–3 declared by manufacturers.
Following chemicals were used in this work:
- Hyaluronic acid, glycine, 2-hydroxyethylcellulose, pepsin, copper(II) chloride dihydrate (Sigma-Aldrich, Saint Louis, MO, USA)
- Citric acid (Lach-Ner Ltd., Neratovice, Czech Republic)
- Ethanol 96%, ethyl acetate, hexane 99%, sodium hydroxide, hydrochloric acid, sodium carbonate, sodium hydrogen carbonate (PENTA s.r.o., Prague, Czech Republic)
- Potassium bromide for IR spectroscopy (Merck KGaA, Darmstadt, Germany)
A carbonate-bicarbonate buffer was prepared by dissolving 129 g of Na2CO3 and 50 g of NaHCO3 in 1 L of distilled water [56]. All chemicals were of analytical grade. Deionized water of Milli-Q quality (electrical resistivity 18.2 MΩ·cm) was used for the electrolyte, standard and sample preparations.
2.2. Preparative Procedures
Due to the low amount of the analyte in all food supplements, the appropriate isolation procedures were made to obtain fractions rich of hyaluronic acid. For each supplement sample, a specific isolation procedure was developed based on a combination of extraction and precipitation steps. An individual hyaluronic acid isolation procedure was chosen for each sample in relation to the sample composition and analyte content. The presence of target polysaccharide in the enriched fractions was confirmed using FT-IR spectroscopy. Isolation procedures for supplements 1–3 are illustrated in Figure 1, Figure 2 and Figure 3.
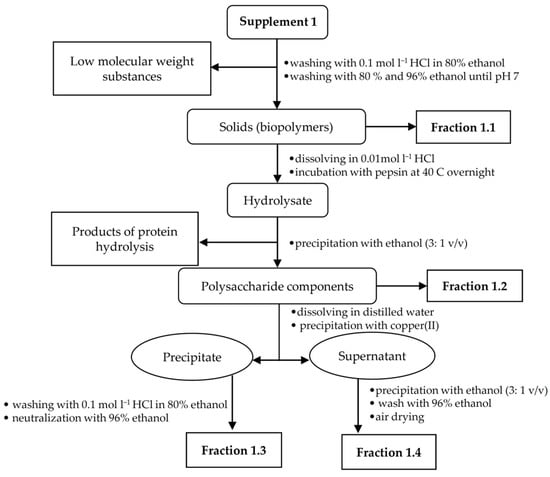
Figure 1.
Isolation scheme for supplement 1.
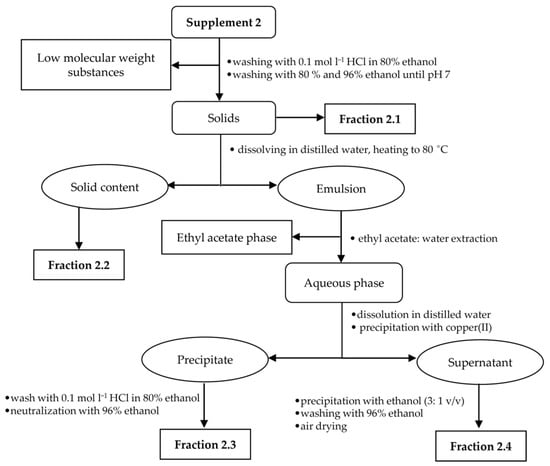
Figure 2.
Isolation scheme for supplement 2.
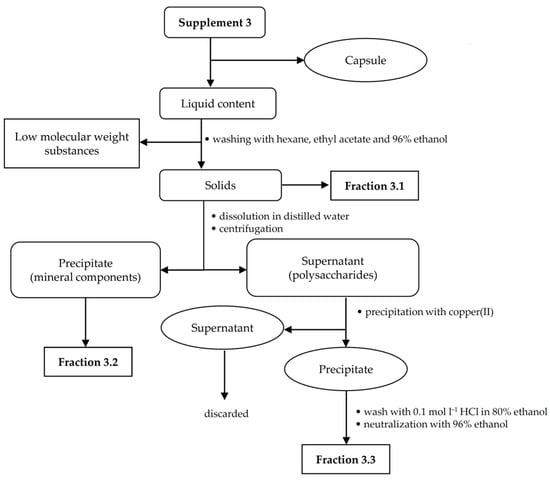
Figure 3.
Isolation scheme for supplement 3.
2.2.1. Fractionation of Supplement 1
The powder sample was weighed (13.2 g) and successively washed with 96% ethanol, acidified ethanol (0.1 mol L−1 HCl in 80% ethanol) and then again with 96% ethanol. The ethanolic suspensions were stirred on a magnetic stirrer for 30 min. Then, the mixture was filtered through filter paper and the solids assigned as fraction 1.1 were dried on a watch glass. The dried solids were dissolved in 50 mL of 0.01 mol L−1 hydrochloric acid, and 4 g of pepsin was added to the solution. Next, the solution was stirred for 30 min on a magnetic stirrer C-MAG HS7 (IKA-Werke, Staufen, Germany), and after dissolving the enzyme; it was placed in a thermostat BT 120 (LABsystem Praha s.r.o., Prague, Czech Republic) at 40 °C overnight. The enzyme was then denatured by heating the solution at 90 °C for 10–15 min on magnetic stirrer and filtrated. The solution was cooled and solids were precipitated with an excess of 96% ethanol (3: 1 v/v) and then filtered off. The resulting precipitate (0.65 g) was air dried and designated as fraction 1.2. The dried precipitate was ground in a powder mortar and then dissolved in 20 mL of distilled water. A small amount of copper(II) chloride was added with stirring. The pH value was adjusted to 7.0 by adding a small volume of 0.1 mol L−1 sodium hydroxide solution. The resulting precipitate, designated as fraction 1.3, was centrifuged and washed with acidified ethanol to remove copper(II) cations that were detected by visual decolonization of the precipitate. Finally, the precipitate was washed with 96% ethanol to pH ~7.0. The supernatant was precipitated with excess ethanol and the resulting precipitate, i.e., fraction 1.4, was washed in the same way as fraction 1.3.
2.2.2. Fractionation of Supplement 2
The sample (30 tablets) was weighed and then washed with 96% ethanol, acidified ethanol and then again with 96% ethanol yielding fraction 2.1. Cellulose was separated from the other polysaccharides by suspending this fraction in 150 mL of distilled water and heating to 80 °C. The mixture was filtered through filter paper. The solids, assigned as fraction 2.2, contained cellulose, which is insoluble in water, while the filtrate contained water-soluble polysaccharides including hyaluronic acid. The filtrate was orange due to the presence of β-carotene, which was then removed by washing with ethyl acetate, and a small amount of copper(II) chloride was added to the clear solution with stirring. The pH was adjusted to 7.0 by adding of sodium hydroxide. The resulting precipitate, i.e., fraction 2.3, containing hyaluronic acid, was washed with acidified ethanol and further with 96% ethanol to remove copper(II) cations from the sample. The neutral polysaccharides were precipitated from supernatant with ethanol excess, and the resulting precipitate, i.e., fraction 2.4, was washed with 96% ethanol.
2.2.3. Fractionation of Supplement 3
Firstly, the shells of 10 capsules were removed, and the internal liquid content (emulsion) was poured onto a watch glass. Then, the emulsion (3.4 g) was decomposed and decolorized by successive washing with hexane, ethyl acetate and 96% ethanol. As a result, insoluble powdered fraction 3.1 was obtained. The presence of insoluble minerals such as zinc(II) oxide has been declared by the manufacturer (see Table 1). To remove these mineral components, the obtained powder was dissolved in a small amount of distilled water and further centrifuged using Sigma 2-16K centrifuge (Sigma-Aldrich, Saint Louis, MO, USA). The precipitate, assigned as fraction 3.2, was dried on a watch glass. The supernatant, contained the expected polysaccharide components, was used for further fractionation. A small amount of copper(II) chloride was added with stirring and the pH was adjusted to 7.0 by adding 0.1 mol L−1 sodium hydroxide. The precipitate formed was washed with acidified ethanol and further with 96% ethanol to remove copper(II) cations and then centrifuged. These solids containing hyaluronic acid were assigned as fraction 3.3. The supernatant was treated with excess ethanol, but no precipitate was obtained; therefore, the sample did not contain neutral polysaccharides.
2.3. FT-IR Spectroscopy
FT-IR spectra (400–4000 cm−1) of the supplements and isolated fractions were recorded in KBr pellets by Nicolet 6700 FT-IR spectrometer using Omnic 8.0 software (ThermoFisher Scientific, Waltham, MA, USA); 64 scans were accumulated with a spectral resolution of 2.0 cm−1. The spectra were smoothed by 11 points, baseline corrected, and exported in ASCII format to Origin 6.0 (Microcal Origin, Northampton, MA, USA) software for preparation of graphs. The position of shoulders was determined using the 2nd derivative algorithm.
2.4. Isotachophoresis
Isotachophoretic measurement of hyaluronic acid in partially purified dietary supplements was recorded using a hand-held column electrophoretic analyzer EA 101 with contact conductivity detectors and a UV detector operating at 254 nm (Villa-Labeco, Spišská Nová Ves, Slovak Republic) [57,58]. The separation section of the analyzer consisted of a pre-separating FEP capillary (90 mm × 0.8 mm i.d) in conjunction with an analytical FEP capillary (90 mm × 0.3 mm i.d) and was controlled by a PC and ITPPro32 software (KasComp Ltd., Bratislava, Slovak Republic). The device works with a hydrodynamically closed separation system, i.e., the electroosmotic flow is suppressed. For anionic analysis, a mixture of 5 mmol L−1 HCl + 10 mmol L−1 glycine + 0.05% 2-hydroxyethylcellulose was used as the main electrolyte, and the 10 mmol L−1 citric acid solution served as the terminal electrolyte. The method of adding an internal standard of hyaluronic acid was used. The analyte was detected using a conductivity detectorof EA 101 analyzer. The results were presented in the form of isotachopherograms.
2.5. Photometry
The content of hyaluronic acid in dietary supplements was determined by photometry according to the method which has been proposed for the determination of pectin [50], with some modifications. The method was adapted for analysis of hyaluronic acid and is based on the precipitation of copper(II) hyaluronates by the treatment with copper(II) chloride and estimation of the remaining content of copper(II) ions as a complex with carbonate. One and a half milliliters of the filtrate after precipitation of copper(II) hyaluronate was mixed with 2 mL of the carbonate buffer solution, and the absorbance of the mixtures was recorded at 712 nm with a UV-vis spectrophotometer Specord 50 Plus (Analytic Jena, Jena, Germany). Standard aqueous solutions of copper(II) chloride (2–100 mmol L−1) in mixture with carbonate buffer (1: 4 v/v) were used for calibration. For quantitative determination, a standard solution of hyaluronic acid was used.
3. Results and Discussion
3.1. Description of the Isolated Fractions
Because of the low amount of analyte in the samples, it was decided to isolate hyaluronic acid from food supplements and subsequently confirm its presence in the enriched fractions by FT-IR spectroscopy. The first step was the removal of major components such as collagen and low-molecular substances, in particular various vitamins, to obtain polysaccharide fractions for further purification. Enzymatic hydrolysis by pepsin led to removal of already partially hydrolyzed collagen. The low-molecular components were washed out by suitable organic solvents. Then, hyaluronic acid was separated from the other polysaccharides present in the fractions, including cellulose and maltodextrins. Soluble polysaccharides, including hyaluronic acid, were separated from cellulose by dissolving in water. Finally, hyaluronic acid was precipitated with copper(II) cations and thus separated from other maltodextrins. The presence of hyaluronic acid was confirmed by FT-IR spectroscopy based on the assignment of the band’s characteristic for this polysaccharide and comparison with the literature. The combination of the described preparative and analytical steps and methods led to the successful finding of hyaluronic acid in all the studied samples.
The chosen procedure for the isolation of hyaluronic acid from the starting material should meet two basic requirements. First, the process should be as efficient as possible, along with minimal losses during the isolation process. If the sample contains low molecular weight hyaluronic acid or its fragments (oligosaccharides), which can dissolve in dilute aqueous ethanol, there is a possibility of analyte loss. To prevent this, precipitation with excess ethanol should be replaced by a combination of dialysis through suitable membranes and lyophilization. Second, there should be no structural degradation of the polysaccharide during the isolation process. It is essential that the sample does not decompose during operation by chemicals or enzymes used to remove unwanted components.
Table 2 shows the yields of the individual fractions (% w/w) of each sample and their expected composition. Supplement 1 contained mainly biopolymer components (collagen, maltodextrins) in addition to hyaluronic acid. Enzymatic hydrolysis with pepsin was chosen to remove collagen. Supplements 2 and 3 contained a large amount of low molecular weight substances that had to be removed first.

Table 2.
Yields and composition of the obtained fractions.
The next step was to separate the individual polysaccharides based on their solubility or ability to form complexes with copper(II) cations. Thus, hyaluronic acid was separated from cellulose and maltodextrins. Fractions 1.3 and 2.3, which allegedly contained hyaluronic acid, were determined in the case of supplement 2 to less than a tenth of a percent. On the contrary, the respective fraction 3.3 of supplement 3 (capsule content) had a content in the order of units of percent, which corresponds to a larger amount of this polysaccharide in the sample, as declared by the manufacturer (see Table 1). It was also found based on the comparison of FT-IR spectra of the above fractions that the largest amount of analyte was present in fraction 3.3 of supplement 3, which is an advantage for proving its presence.
3.2. FT-IR Spectra
3.2.1. Raw Supplements
The FT-IR spectra of food supplements 1, 2 and 3 where the presence of hyaluronic acid was declared are shown in Figure 4. The FT-IR spectrum of supplement 1 has wide, distinct bands of biopolymer components and their derivatives—partially hydrolyzed collagen and maltodextrins. Two intense bands of amide vibrations at 1650 and 1545 cm−1 indicate the presence of proteins [59,60], i.e., modified collagen, while several less pronounced bands at 1154, 1080 and 1030 cm−1 originated mainly from COC, CO and CC stretching vibrations in carbohydrates which are typical for starch and its oligomers dextrins [61,62]. By contrast, there are much narrower bands of low molecular weight components in the spectra of supplements 2 and 3. In both cases, the most of intense bands corresponded to vibrations of the major components, i.e., l-methionine (~46 % w/w) and l-ascorbic acid (~46% w/w), respectively, as it has been declared by manufacturers (Table 1). Indeed, the intense bands of l-methionine at 2917 cm−1 (CH2 symmetric stretching), 1613 and 1512 cm−1 (NH3+ bending), 1584 and 1409 cm−1 (COO– stretching), 1447 and 1352 cm−1 (CH2 bending), 1318 cm−1 (SCH and CH3 bending), 1275 and 1243 cm−1 (CCH and SCH bending), 1027 (CC stretching), 543 and 418 cm−1 (CCO and CCN bending) as well as many other bands of this amino acid were found in the FT-IR spectrum of supplement 2 [63,64]. Similarly, the bands of l-ascorbic acid at 3525–3215 cm−1 (OH stretching), 1673 cm−1 (C=C stretching), 1660, 1457 and 1246 cm−1 (CH and CH2 deformation modes), 1364, 1321 and 1276 cm−1 (in-plane ring deformation), 1142–821 cm−1 (CO and CC stretching), 756 (in-plane OH twisting), 722 and 685 cm−1 (C=O deformation modes) and skeletal ring vibrations at 629–448 cm−1 [65] were found to be the most intense in the FT-IR spectrum of supplement 3. In addition, the IR band around 1748 cm−1 indicates stretching C=O vibrations of organic acids and esters, and together with other bands at 2925, 2855, 1466 and 721 cm−1 (vibration of CH2 groups) confirmed the presence of fats [66]. In contrast, the broad bands in the region of 3600–3200 cm−1 were assigned to the stretching vibrations of OH and NH bonds. However, characteristic bands of hyaluronic acid were not pronounced in FT-IR spectra of all raw supplements 1–3 because of low amounts of this polysaccharide.
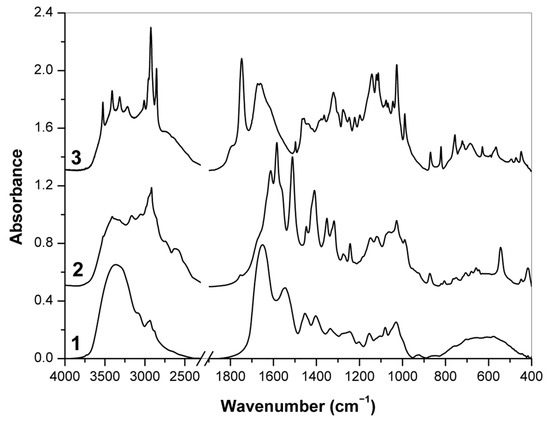
Figure 4.
FT-IR spectra of supplements 1, 2 and 3 (in the latter case, capsule content).
3.2.2. Fractionation of Supplement 1
The FT-IR spectra of fractions 1.1–1.4 isolated from food supplement 1 are shown in Figure 5a. The spectrum of fraction 1.1, obtained by washing with acidified ethanol, did not differ significantly from the spectrum of the initial supplement, which indicates that the sample contained few low-molecular-weight substances. In the spectrum of fraction 1.2, after enzymatic removal of proteins, the protein bands at 1800–1500 cm−1 (amide I and amide II vibrations) significantly decreased [59,60]. In contrast, the polysaccharide bands in the 1200–950 cm−1 region were much more intense. These results confirm that as a result of enzymatic hydrolysis, the collagen content decreased, and at the same time, the proportion of polysaccharides, mainly maltodextrins, increased. In the FT-IR spectrum of fraction 1.3 obtained by precipitation with copper(II), bands at 1738, 1617 and 1560 cm−1 indicate the presence of carboxyl and amide groups of hyaluronic acid [39,40]. On the contrary, fraction 1.4 contained mainly maltodextrins, which do not form copper (II) complexes in a neutral medium, which is confirmed by several strong bands at 1154, 1080 and 1030 cm−1 (stretching vibrations of CO and CC), as well as weaker bands of skeletal vibrations in the region of 930–445 cm−1 [61,62].
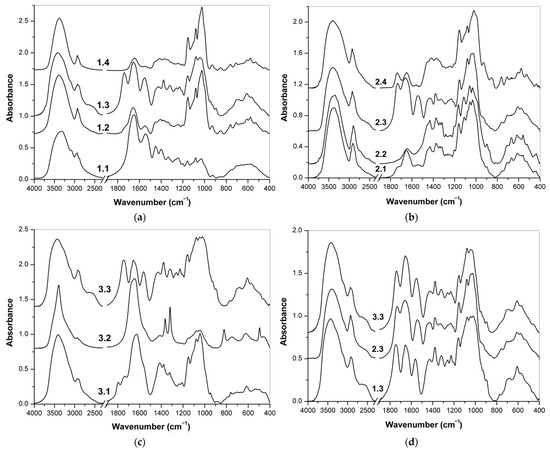
Figure 5.
FT-IR spectra of the fractions obtained from: (a) supplement 1; (b) supplement 2; (c) supplement 3; (d) FT-IR spectra of the fractions 1.3, 2.3 and 3.3 with an expected high content of hyaluronic acid obtained from supplements 1, 2 and 3, respectively.
3.2.3. Fractionation of Supplement 2
The FT-IR spectra of fractions 2.1–2.4 isolated from additive 2 are shown in Figure 5b. The spectrum of fraction 2.1 after washing with acidified ethanol showed significant differences from the spectrum of the original sample due to the removal of l-methionine and other low molecular weight substances. The spectrum contains a number of bands at 3346, 2902, 1432, 1373, 1337, 1319, 1165, 1113, 1059, 1032, 897, 710, 668, 617, 560, 519 and 436 cm−1 characteristics of cellulose [67,68]. This polysaccharide was probably not added to the preparation as a pure substance but apparently was included in the plant extracts. The FT-IR spectrum of the water-insoluble part 2.2 showed much more pronounced cellulose bands. This fraction was defined as almost pure cellulose, separated from water-soluble polysaccharides, including hyaluronic acid. Two more fractions were formed by precipitation of copper(II) complexes from the water-soluble part. Hyaluronic acid was observed in precipitate 2.3 based on characteristic IR bands. Finally, the FT-IR spectrum of supernatant 2.4 showed intense bands of starch originating from plant extracts [61] and less pronounced bands of carboxyl and amide groups compared to the previous fraction. This means that there was an incomplete separation of polysaccharides and proteins with a larger proportion of hyaluronic acid entering fraction 2.3.
3.2.4. Fractionation of Supplement 3
The last supplement was in the form of gelatin capsules with a liquid mixture of active ingredients inside. The liquid contents of the capsules were isolated from the shells and thus separated from the solid gelatin. The obtained FT-IR spectra of fractions 3.1, 3.2 and 3.3 are shown in Figure 5c. The spectrum of fraction 3.1 no longer contained bands of l-ascorbic acid, fats and other low molecular weight components, and various bands in the areas 1800–1500 cm−1 and 1200-950 cm−1 indicated the presence of hyaluronic acid, but there are a number of other bands. The water-insoluble part of 3.2 had a very different FT-IR spectrum. This fraction probably contained insoluble inorganic substances (metal oxides, etc.). Finally, fraction 3.3 was precipitated with copper(II) cations and then purified. This fraction contained mainly hyaluronic acid, which was confirmed by a number of characteristic bands, including vibrations of amide and carboxyl groups (see Table 3).

Table 3.
Assignment of IR bands of hyaluronic acid for fractions 1.3, 2.3 and 3.3 [38,39,40,41,42,43,44].
3.3. Detection and Quantification of Hyaluronic Acid
The FT-IR spectra of fractions 1.3, 2.3 and 3.3 derived from supplements 1–3 and allegedly containing hyaluronic acid are shown in Figure 5d. A number of characteristic bands summarized and assigned in Table 3 confirmed the presence of this polysaccharide in acidic form. All these fractions evidently contained this polysaccharide as the major part, and the characteristic bands of hyaluronic acid were sufficiently prominent for identification. However, fraction 2.3 also contained some proteins because the amide I and amide II bands are evidently much more intense than the bands of C=O stretching vibration in carboxylic groups at 1732 cm−1. In addition, the band of amide II vibration is shifted to 1545 cm−1, which is typical for proteins, so as the positions of some other amide bands. Due to the importance of the amide and carboxyl groups of hyaluronic acid, the characteristic IR bands of these groups are key markers for the identification of this polysaccharide [39]. Bands at 3275–3277, 3094–3098, 1657, 1552–1563 and 1318–1320 cm−1 are indicative for the amide groups in N-acetyl-β-d-glucosamine units, and the bands at ~2660, 1732–1745, 1260 and 923–930 cm−1 are assigned to the vibrations of the carboxylic groups in β-d-glucuronic acid units [38,39,40]. In the case of salts (hyaluronates), these bands of carboxylic vibrations do not occur in the spectra; instead, two bands around 1618 and 1411 cm−1 corresponding to the antisymmetric and symmetric valence vibration COO– are present [39,40].
Since fractions 1.3, 2.3 and 3.3 were identified as hyaluronic acid by FT-IR, their relative masses were used to quantify this analyte gravimetrically in supplements 1, 2 and 3, respectively. Moreover, the level of hyaluronic acids in the supplements was determined by spectrophotometry and isotachophoresis. The isotachopherograms of the standard solution of hyaluronic acid and the purified fraction of supplement 1 are shown in Figure 6a,b; the arrow indicates the zone of hyaluronic acid. The height of the zone corresponds to a certain anionic compound (analyte) in accordance with its mobility in an electric field, and the length of the zone is proportional to the concentration of this analyte. The absorption spectra of the copper(II) chloride solutions and the calibration curve for quantification of copper(II) cations are represented in Figure 7a,b. The wavelength of absorption maximum at 718 nm was used for the analysis; this value is much closed to that in the literature (712 nm) [50]. The obtained results are summarized in Table 4 in comparison with the corresponding declared values represented in % w/w. It can be seen from this table that the values obtained by the three methods coincide with each other and are quite close to the declared values. For the spectrophotometry, the relative standard deviations (RSD) in three measurements were less than 3%, indicating good reliability for determination of hyaluronic acid in food supplements. These values were similar to those obtained previously for the determination of pectin [50] and for the earlier reported isotachophoretic method [57,58]. In any case, all of the analytical methods used in this work can be recommended for the analysis of food supplements containing hyaluronic acid, but each of them has its own advantages and disadvantages, which are summarized in Table 5. Besides, effective fractionation allows for removal of impurities that interfere with the analysis, for example, proteins and their fragments (peptides) that can be precipitated by copper(II) cations together with hyaluronic acid. Partially, FTIR and isotachophoresis are of interest as polyanalytical methods that can be used to analyze many other components, not only hyaluronic acid. FTIR is able to evaluate the composition of individual fractions and confirm or deny the presence of the declared substances. For example, in the current study, the presence of undeclared cellulose was proven for the water-insoluble fraction 2.2 isolated from supplement 2. Both methods also allow for the determination of other glycosaminoglycans [43,57,58,69,70,71], which is of particular interest since chondroitin sulfate is often used in dietary supplements to support cartilage along with collagen and hyaluronic acid. On the other hand, the spectrophotometric method for the determination of copper(II) as the carbonate complex is quite sensitive and allows determination over a wide range of concentrations. However, care should be taken to ensure that the pH value is optimal and does not lead to alkalization and precipitation of copper(II) hydroxide. On the contrary, upon acidification, the copper(II) complex with hyaluronic acid may remain in solution due to insufficient ionization of polysaccharide macromolecules [49,51,52,53]. Finally, although gravimetry is not structure-sensitive, FT-IR can be used to determine if the precipitate formed is actually hyaluronic acid, with an assessment of possible impurities.
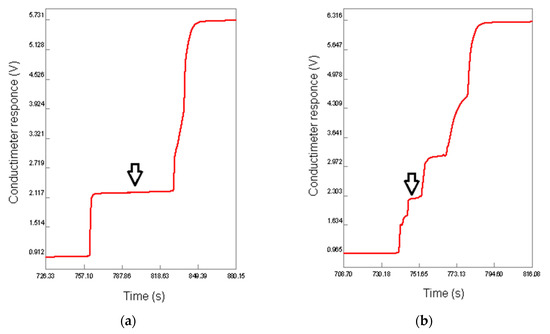
Figure 6.
Isotachopherograms of the standard solution of hyaluronic acid (a) and supplement 1 (b). Arrow indicates the analyte zone.
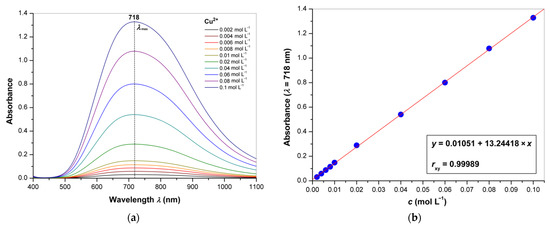
Figure 7.
(a) Absorption spectra of copper(II) chloride (0.002–0.1 mol L−1) dissolved in the carbonate buffer; (b) calibration curve for determination of copper(II).

Table 4.
Content of hyaluronic acid (% w/w) in dietary supplements obtained by gravimetry, photometry and isotachophoresis and declared by the manufacturer.

Table 5.
Advantages and disadvantages of analytical methods used in the analysis.
4. Conclusions
The aim of this work was to identify hyaluronic acid in three food supplements that differ in dosage form and composition. For each sample, a specific isolation procedure for the isolation and concentration of the analyte was proposed. The first step was to remove low molecular weight substances by washing the samples with suitable solvents. The obtained biopolymeric fractions, in addition to hyaluronic acid itself, contained proteins (partially hydrolyzed collagen) as well as various oligo- or polysaccharides such as maltodextrins, starch, and cellulose. The second step was to remove proteins by enzymatic hydrolysis. This step has proven to be very effective in removing collagen that was initially partially hydrolyzed. The separation of cellulose or other water-insoluble components was achieved by dissolving in hot water followed by filtration. Then, hyaluronic acid was precipitated by the addition of copper(II) cations, which form insoluble complexes with anionic polysaccharides. Neutral polysaccharides such as starch and maltodextrins do not form such complexes and remain in solution. The effectiveness of each of these steps was confirmed by Fourier-transform infrared spectroscopy, which is suitable for the structural analysis of organic and some inorganic compounds. The conclusion of this work is the confirmation of the presence of hyaluronic acid in all of the analyzed samples, and the content of this analyte was determined by three analytical methods that gave similar results. On the example of three food additives, the effectiveness of the selected preparative and analytical procedures was demonstrated in assessing the composition of the obtained fractions and quantitative estimation of hyaluronic acid. The approach discussed in this paper can be used to analyze various dietary supplements, as well as medicinal and cosmetic preparations containing hyaluronic acid, for example, as an active substance for the regeneration of cartilage tissue, skin softening or wound healing.
Author Contributions
Conceptualization and methodology, J.Č., F.K. and A.S.; software and validation, T.M., F.K. and R.B.; formal analysis, T.M. and F.K.; investigation, T.M. and A.S.; resources, A.S. and J.Č.; writing—original draft preparation, review and editing, T.M. and A.S.; visualization, T.M., R.B. and J.Č.; supervision, A.S., J.Č. and R.B.; project administration, J.Č.; funding acquisition, A.S. and J.Č. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grant of Specific university research of UCT Prague, project number 21-SVV/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rosa, C.S.D.; Tovar, A.F.; Mourão, P.; Pereira, R.; Barreto, P.; Beirão, L.H. Purification and characterization of hyaluronic acid from chicken combs. Ciência Rural 2012, 42, 1682–1687. [Google Scholar] [CrossRef] [Green Version]
- Chahuki, F.F.; Aminzadeh, S.; Jafarian, V.; Tabandeh, F.; Khodabandeh, M. Hyaluronic acid production enhancement via genetically modification and culture medium optimization in Lactobacillus acidophilus. Int. J. Biol. Macromol. 2019, 121, 870–881. [Google Scholar] [CrossRef]
- Widner, B.; Behr, R.; Von Dollen, S.; Tang, M.; Heu, T.; Sloma, A.; Sternberg, D.; DeAngelis, P.L.; Weigel, P.H.; Brown, S. Hyaluronic Acid Production in Bacillus subtilis. Appl. Environ. Microbiol. 2005, 71, 3747–3752. [Google Scholar] [CrossRef] [Green Version]
- Kogan, G.; Šoltés, L.; Stern, R.; Schiller, J.; Mendichi, R. Hyaluronic Acid: Its Function and Degradation in in vivo Systems. Bioact. Nat. Prod. (Part L) 2008, 34, 789–882. [Google Scholar] [CrossRef]
- Kripotou, S.; Zafeiris, K.; Culebras-Martínez, M.; Gallego-Ferrer, G.; Kyritsis, A. Dynamics of hydration water in gelatin and hyaluronic acid hydrogels. Eur. Phys. J. E 2019, 42, 109. [Google Scholar] [CrossRef]
- Hahn, S.K.; Park, J.K.; Tomimatsu, T.; Shimoboji, T. Synthesis and degradation test of hyaluronic acid hydrogels. Int. J. Biol. Macromol. 2007, 40, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulou, A.; Molina, J.V.; Kyritsis, A.; Pradas, M.M.; Lluch, A.V.; Ferrer, G.G.; Pissis, P. Glass Transition and Water Dynamics in Hyaluronic Acid Hydrogels. Food Biophys. 2013, 8, 192–202. [Google Scholar] [CrossRef]
- Salwowska, N.M.; Bebenek, K.A.; Żądło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical properties and application of hyaluronic acid: A systematic review. J Cosmet. Derm. 2016, 15, 520–526. [Google Scholar] [CrossRef]
- Vorvolakos, K.; Coburn, J.C.; Saylor, D.M. Dynamic interfacial behavior of viscoelastic aqueous hyaluronic acid: Effects of molecular weight, concentration and interfacial velocity. Soft Matter 2014, 10, 2304–2312. [Google Scholar] [CrossRef]
- Zhang, Z.; Christopher, G.F. The nonlinear viscoelasticity of hyaluronic acid and its role in joint lubrication. Soft Matter 2015, 11, 2596–2603. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Okamoto, A.; Nishinari, K. Viscoelasticity of hyaluronic acid with different molecular weights. Biorheology 1994, 31, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Fusco, S.; Borzacchiello, A.; Miccio, L.; Pesce, G.; Rusciano, G.; Sasso, A.; Netti, P. High frequency viscoelastic behaviour of low molecular weight hyaluronic acid water solutions. Biorheology 2007, 44, 403–418. [Google Scholar]
- Tammi, M.I.; Day, A.; Turley, E.A. Hyaluronan and Homeostasis: A Balancing Act. J. Biol. Chem. 2002, 277, 4581–4584. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.W.; Johns, M.R.; Greenfield, P.F. Hyaluronic acid-a versatile biopolymer. Aust. J. Biotechnol. 1990, 4, 38–43. [Google Scholar]
- Mráček, A.; Varhaníková, J.; Lehocký, M.; Gřundělová, L.; Pokopcová, A.; Velebný, V. The Influence of Hofmeister Series Ions on Hyaluronan Swelling and Viscosity. Molecules 2008, 13, 1025–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spagnoli, C.; Korniakov, A.; Ulman, A.; Balazs, E.A.; Lyubchenko, Y.L.; Cowman, M.K. Hyaluronan conformations on surfaces: Effect of surface charge and hydrophobicity. Carbohydr. Res. 2005, 340, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef] [PubMed]
- Longinotti, C. The use of hyaluronic acid based dressings to treat burns: A review. Burn. Trauma 2014, 2, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Schneider, H.P.; Landsman, A. Preclinical and Clinical Studies of Hyaluronic Acid in Wound Care: A Case Series and Literature Review. Wounds 2019, 31, 41–48. [Google Scholar]
- Neuman, M.G.; Nanau, R.M.; Oruña-Sanchez, L.; Coto, G. Hyaluronic Acid and Wound Healing. J. Pharm. Pharm. Sci. 2015, 18, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Price, R.D.; Myers, S.; Leigh, I.M.; Navsaria, H.A. The Role of Hyaluronic Acid in Wound Healing. Am. J. Clin. Dermatol. 2005, 6, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar]
- Ahmadian, E.; Dizaj, S.M.; Eftekhari, A.; Dalir, E.; Vahedi, P.; Hasanzadeh, A.; Samiei, M. The Potential Applications of Hyaluronic Acid Hydrogels in Biomedicine. Drug Res. 2020, 70, 6–11. [Google Scholar] [CrossRef]
- Cooper, C.A.; Brown, K.K.; Meletis, C.D.; Zabriskie, N. Inflammation and Hyaluronic Acid. Altern. Complement. Ther. 2008, 14, 78–84. [Google Scholar] [CrossRef] [Green Version]
- How, K.N.; Yap, W.H.; Lim, C.L.H.; Goh, B.H.; Lai, Z.W. Hyaluronic Acid-Mediated Drug Delivery System Targeting for Inflammatory Skin Diseases: A Mini Review. Front. Pharmacol. 2020, 11, 1105. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Price, R.D.; Berry, M.; Navsaria, H.A. Hyaluronic acid: The scientific and clinical evidence. J. Plast. Reconstr. Aesthetic Surg. 2007, 60, 1110–1119. [Google Scholar] [CrossRef]
- Sionkowska, A.; Gadomska, M.; Musiał, K.; Piątek, J. Hyaluronic Acid as a Component of Natural Polymer Blends for Biomedical Applications: A Review. Molecules 2020, 25, 4035. [Google Scholar] [CrossRef]
- Restaino, O.F.; De Rosa, M.; Schiraldi, C. High-performance capillary electrophoresis to determine intact keratan sulfate and hyaluronic acid in animal origin chondroitin sulfate samples and food supplements. Electrophoresis 2020, 41, 1740–1748. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, W.J. Development of validated HPLC method for the determination of hyaluronic acid in dietary supplement formulations. Bull. Korean Chem. Soc. 2015, 36, 1270–1273. [Google Scholar] [CrossRef]
- Chindaphan, K.; Wongravee, K.; Nhujak, T.; Dissayabutra, T.; Srisa-Art, M. Online preconcentration and determination of chondroitin sulfate, dermatan sulfate and hyaluronic acid in biological and cosmetic samples using capillary electrophoresis. J. Sep. Sci. 2019, 42, 2867–2874. [Google Scholar] [CrossRef]
- Alkrad, J.A.; Merstani, Y.; Neubert, R.H. New approaches for quantifying hyaluronic acid in pharmaceutical semisolid formulations using HPLC and CZE. J. Pharm. Biomed. Anal. 2002, 30, 913–919. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ohta, T.; Morikawa, Y. Electrophoretic behavior of sodium hyaluronate by capillary-tube isotachophoresis. Bunseki Kagaku 1982, 31, 557–561. [Google Scholar] [CrossRef]
- Kašparová, J.; Arnoldová, K.; Korecká, L.; Česlová, L. Determination of hyaluronic acid in pharmaceutical products by spectrophotometry and HPLC coupled to fluorescence or mass spectrometric detection. Sci. Pap. Univ. Pardubice Ser. A Fac. Chem. Technol. 2018, 24, 39–47. [Google Scholar]
- Pepeliaev, S.; Hrudíková, R.; Jílková, J.; Pavlík, J.; Smirnou, D.; Černý, Z. Colorimetric enzyme-coupled assay for hyaluronic acid determination in complex samples. Eur. Polym. J. 2017, 94, 460–470. [Google Scholar] [CrossRef]
- Yang, Y.; Breadmore, M.; Thormann, W. Analysis of the disaccharides derived from hyaluronic acid and chondroitin sulfate by capillary electrophoresis with sample stacking. J. Sep. Sci. 2005, 28, 2381–2389. [Google Scholar] [CrossRef] [Green Version]
- Kovács, A.; Nyerges, B.; Izvekov, V. Vibrational Analysis of N-Acetyl-α-d-glucosamine and β-d-Glucuronic Acid. J. Phys. Chem. B 2008, 112, 5728–5735. [Google Scholar] [CrossRef] [PubMed]
- Gilli, R.; Kacuráková, M.; Mathlouthi, M.; Navarini, L.; Paoletti, S. FTIR studies of sodium hyaluronate and its oligomers in the amorphous solid phase and in aqueous solution. Carbohydr. Res. 1994, 263, 315–326. [Google Scholar] [CrossRef]
- Haxaire, K.; Maréchal, Y.; Milas, M.; Rinaudo, M. Hydration of polysaccharide hyaluronan observed by IR spectrometry. I. Preliminary experiments and band assignments. Biopolymers 2003, 72, 10–20. [Google Scholar] [CrossRef]
- Maréchal, Y.; Milas, M.; Rinaudo, M. Hydration of hyaluronan polysaccharide observed by IR spectrometry. III. Structure and mechanism of hydration. Biopolym. Orig. Res. Biomol. 2003, 72, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Servaty, R.; Schiller, J.; Binder, H.; Arnold, K. Hydration of polymeric components of cartilage—An infrared spectroscopic study on hyaluronic acid and chondroitin sulfate. Int. J. Biol. Macromol. 2001, 28, 121–127. [Google Scholar] [CrossRef]
- Mainreck, N.; Brézillon, S.; Sockalingum, G.D.; Maquart, F.-X.; Manfait, M.; Wegrowski, Y. Rapid Characterization of Glycosaminoglycans Using a Combined Approach by Infrared and Raman Microspectroscopies. J. Pharm. Sci. 2011, 100, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Al-Sibani, M.; Al-Harrasi, A.; Neubert, R.H.H. Characterization of linear and chemically cross-linked hyaluronic acid using various analytical techniques including FTIR, ESI-MS, H1 NMR, and SEM. J. Biochem. Anal. Stud. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Wurm, F.; Rietzler, B.; Pham, T.; Bechtold, T. Multivalent Ions as Reactive Crosslinkers for Biopolymers—A Review. Molecules 2020, 25, 1840. [Google Scholar] [CrossRef]
- Hwang, J.; Roshdy, T.; Kontominas, M.; Kokini, J. Comparison of Dialysis and Metal Precipitation Effects on Apple Pectins. J. Food Sci. 1992, 57, 1180–1184. [Google Scholar] [CrossRef]
- Yapo, B.M. Pectin quantity, composition and physicochemical behaviour as influenced by the purification process. Food Res. Int. 2009, 42, 1197–1202. [Google Scholar] [CrossRef]
- Guo, X.; Meng, H.; Zhu, S.; Zhang, T.; Yu, S. Purifying sugar beet pectins from non-pectic components by means of metal precipitation. Food Hydrocoll. 2015, 51, 69–75. [Google Scholar] [CrossRef]
- Kohn, R.; Tibenský, V. Determination of the carboxyl groups of pectin by the precipitation of insoluble copper pectates and pectinates. Chem. Pap. 1965, 19, 98–106. [Google Scholar]
- Wang, F.; Du, C.; Chen, J.; Shi, L.; Li, H. A New Method for Determination of Pectin Content Using Spectrophotometry. Polymers 2021, 13, 2847. [Google Scholar] [CrossRef]
- Pirc, E.T.; Arčon, I.; Bukovec, P.; Kodre, A. Preparation and characterisation of copper(II) hyaluronate. Carbohydr. Res. 2000, 324, 275–282. [Google Scholar] [CrossRef]
- Pirc, E.T.; Zidar, J.; Bukovec, P. A Computational Study of Calcium(II) and Copper(II) Ion Binding to the Hyaluronate Molecule. Int. J. Mol. Sci. 2012, 13, 12036–12045. [Google Scholar] [CrossRef] [Green Version]
- Lapčík, Ľ.; Dammer, C.; Valko, M. Hyaluronic acid-copper(II) complexes: Spectroscopic characterization. Colloid Polym. Sci. 1992, 270, 1049–1052. [Google Scholar] [CrossRef]
- Nagy, L.; Yamashita, S.; Yamaguchi, T.; Sipos, P.; Wakita, H.; Nomura, M. The local structures of Cu(II) and Zn(II) complexes of hyaluronate. J. Inorg. Biochem. 1998, 72, 49–55. [Google Scholar] [CrossRef]
- Sterk, H.; Braun, M.; Schmut, O.; Feichtinger, H. Investigation of the hyaluronic acid-copper complex by N.M.R. spectroscopy. Carbohydr. Res. 1985, 145, 1–11. [Google Scholar] [CrossRef]
- Chai, X.-S.; Zhang, D.; Hou, Q.; Yoon, S.-H. Spectrophotometric determination of reducing aldehyde groups in bleached chemical pulps. J. Ind. Eng. Chem. 2007, 13, 597–601. [Google Scholar]
- Vaclavikova, E.; Kvasnicka, F. Isotachophoretic determination of glucosamine and chondroitin sulphate in dietary supplements. Czech J. Food Sci. 2013, 31, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Václavíková, E.; Kvasnička, F. Quality control of chondroitin sulphate used in dietary supplements. Czech J. Food Sci. 2016, 33, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Glassford, S.E.; Byrne, B.; Kazarian, S. Recent applications of ATR FTIR spectroscopy and imaging to proteins. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2013, 1834, 2849–2858. [Google Scholar] [CrossRef] [Green Version]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.; Restrepo, I. Study of the structural order of native starch granules using combined FTIR and XRD analysis. J. Polym. Res. 2018, 25, 266. [Google Scholar] [CrossRef]
- Beaussart, A.; Petrone, L.; Mierczynska-Vasilev, A.; McQuillan, A.J.; Beattie, D.A. In Situ ATR FTIR Study of Dextrin Adsorption on Anatase TiO2. Langmuir 2012, 28, 4233–4240. [Google Scholar] [CrossRef]
- Ramachandran, E.; Natarajan, S. Gel growth and characterization of β-DL-methionine. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 2006, 41, 411–415. [Google Scholar] [CrossRef]
- Grunenberg, A.; Bougeard, D. Vibrational spectra and conformational phase transition of crystalline l-methionine. J. Mol. Struct. 1987, 160, 27–36. [Google Scholar] [CrossRef]
- Bichara, L.C.; Lanús, H.E.; Nieto-Peñalver, C.; Brandán, S.A. Density Functional Theory Calculations of the Molecular Force Field of l-Ascorbic Acid, Vitamin C. J. Phys. Chem. A 2010, 114, 4997–5004. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Cabo, N. Infrared spectroscopy in the study of edible oils and fats. J. Sci. Food Agric. 1997, 75, 1–11. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Zhbankov, R.G. Infrared Spectra of Cellulose and Its Derivatives; Springer: Boston, MA, USA, 1995. [Google Scholar]
- Jaravel, L.; Schindler, B.; Randon, J.; Compagnon, I.; Demesmay, C.; Dugas, V. Off-line coupling of capillary isotachophoresis separation to IRMPD spectroscopy for glycosaminoglycans analysis: Application to the chondroitin sulfate disaccharides model solutes. J. Chromatogr. A 2019, 1617, 460782. [Google Scholar] [CrossRef]
- Sundaresan, G.; Abraham, R.J.J.; Rao, V.A.; Babu, R.N.; Govind, V.; Meti, M.F. Established method of chondroitin sulphate extraction from buffalo (Bubalus bubalis) cartilages and its identification by FTIR. J. Food Sci. Technol. 2018, 55, 3439–3445. [Google Scholar] [CrossRef] [Green Version]
- Devlin, A.; Mauri, L.; Guerrini, M.; Yates, E.A.; Skidmore, M.A. The use of ATR-FTIR spectroscopy to characterise crude heparin samples by composition and structural features. BioRxiv 2019, 744532. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).