Elastic Nanofibrous Membranes for Medical and Personal Protection Applications: Manufacturing, Anti-COVID-19, and Anti-Colistin Resistant Bacteria Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis of CuO Nanoparticles
2.1.2. Manufacturing of Nanofibrous Membranes
2.1.3. Fiber Morphological and Physical Characterizations
2.1.4. Mechanical Characterization
2.2. Antibacterial Activity
2.2.1. Microorganisms
2.2.2. Antibacterial Activity of the Prepared Nanofibers against Colistin Resistant Bacteria
2.2.3. Metal Oxides Release from the Nanofiber and the Corresponding Antibacterial Activity
2.2.4. The Mechanism of Action of the Antibacterial Activity
2.3. Antiviral Activity of the Prepared Nanofibers
3. Results
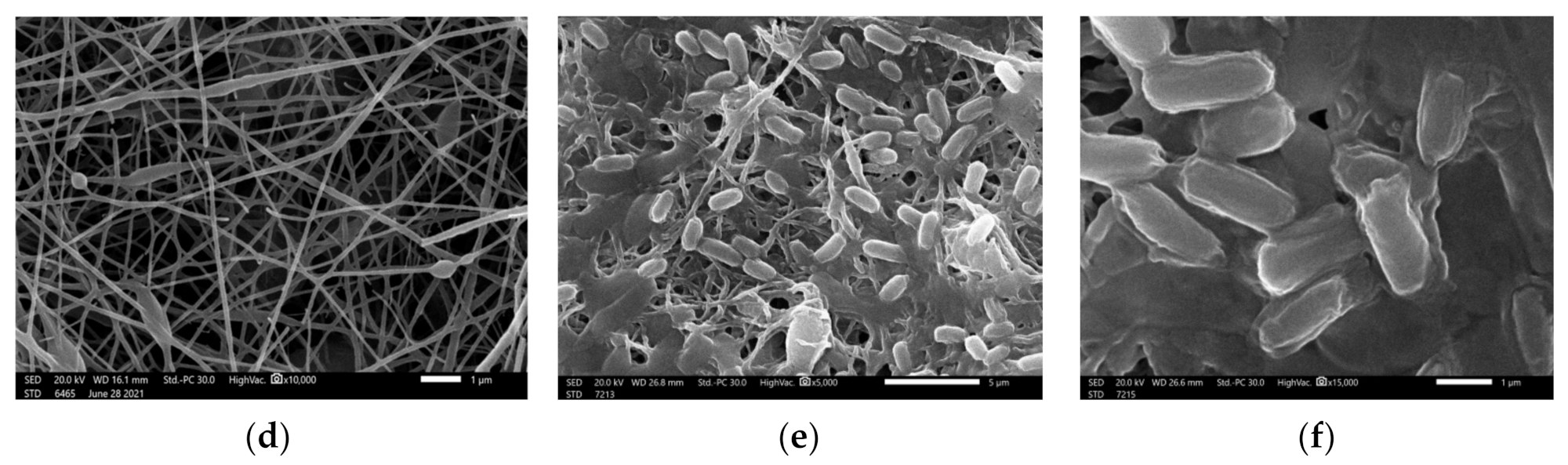
3.1. Morphological Characterization of Nanofibers
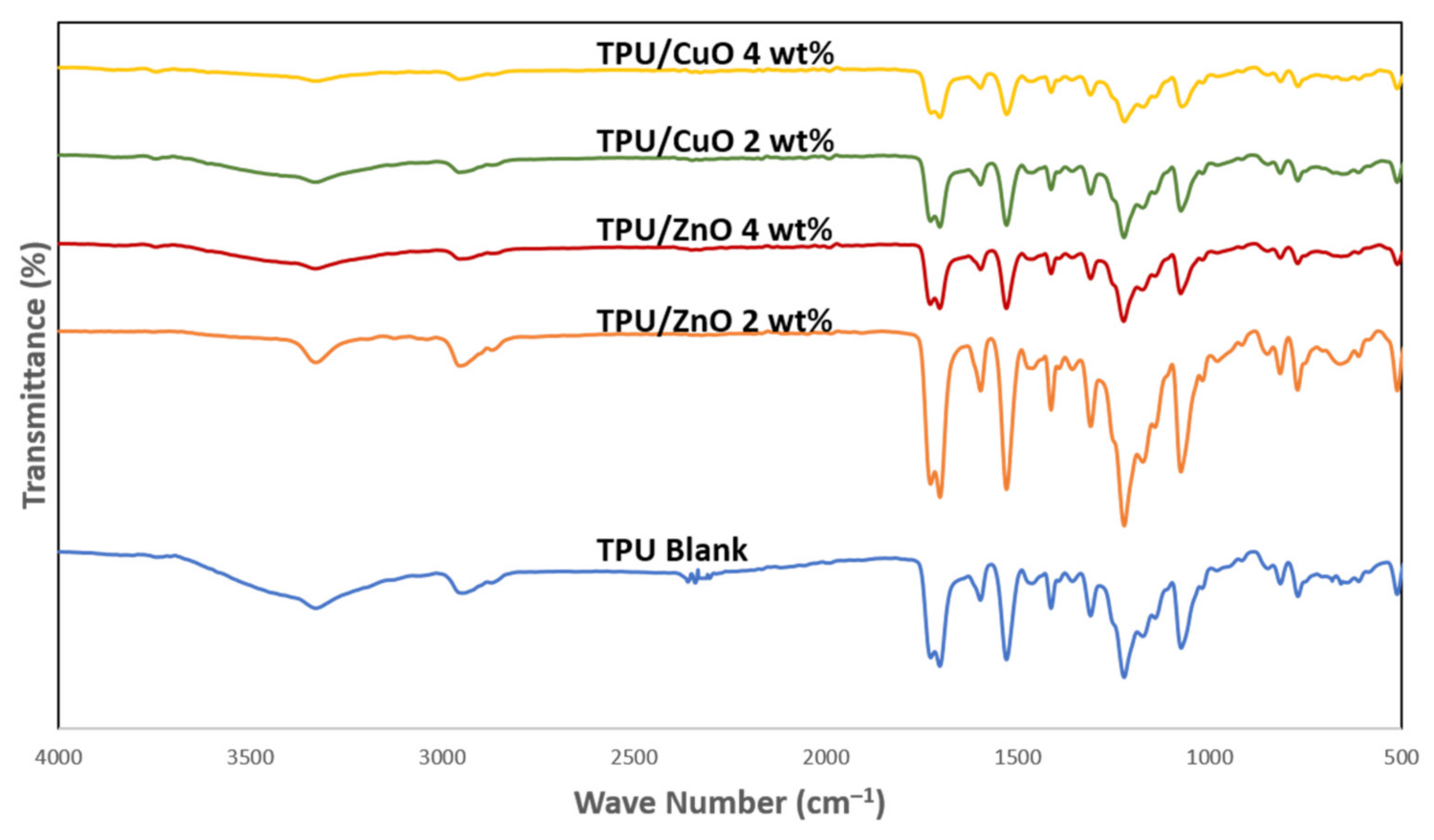
3.2. Physicochemical Characterization of Nanofibers
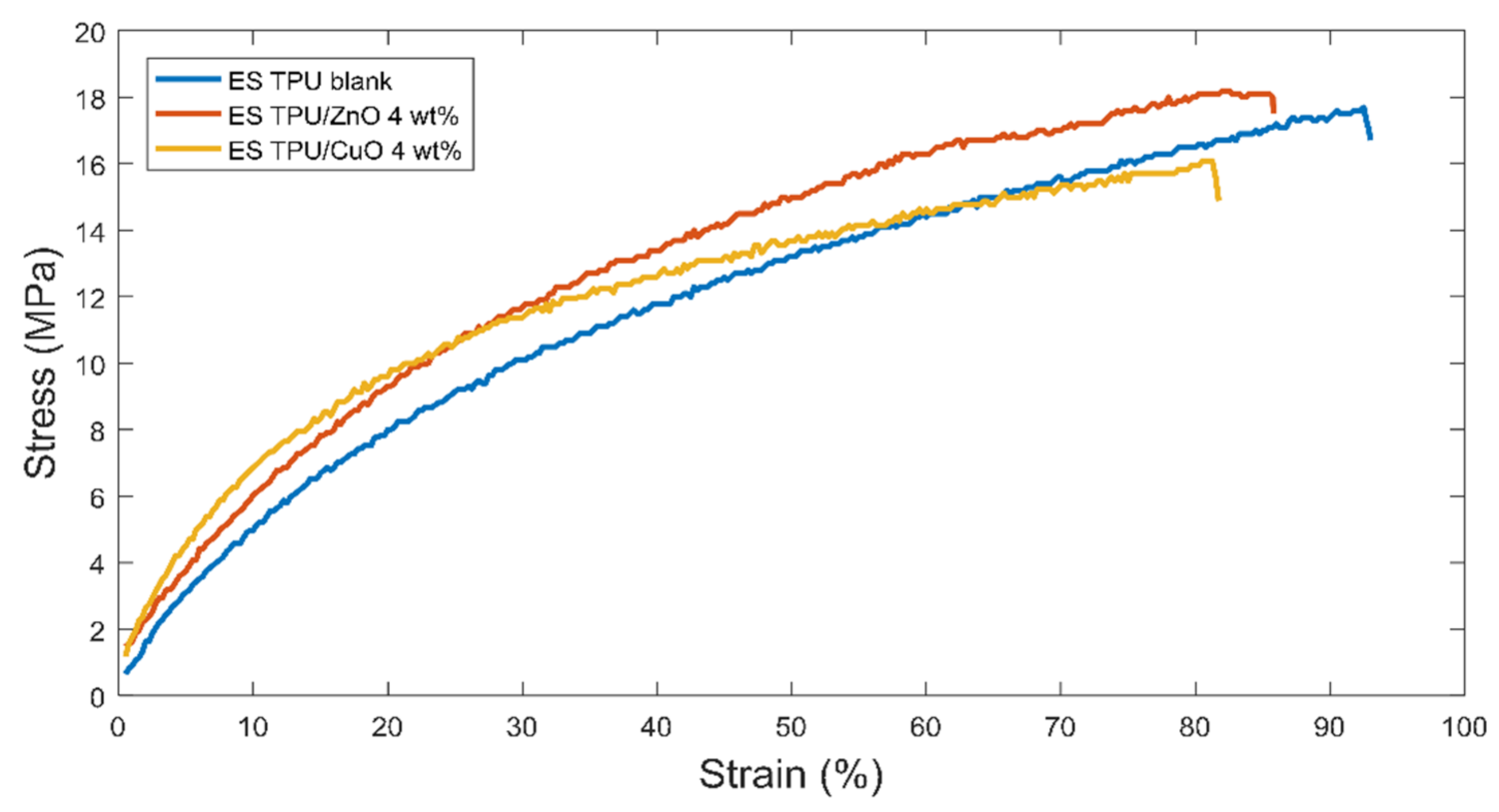
3.3. Mechanical Analysis
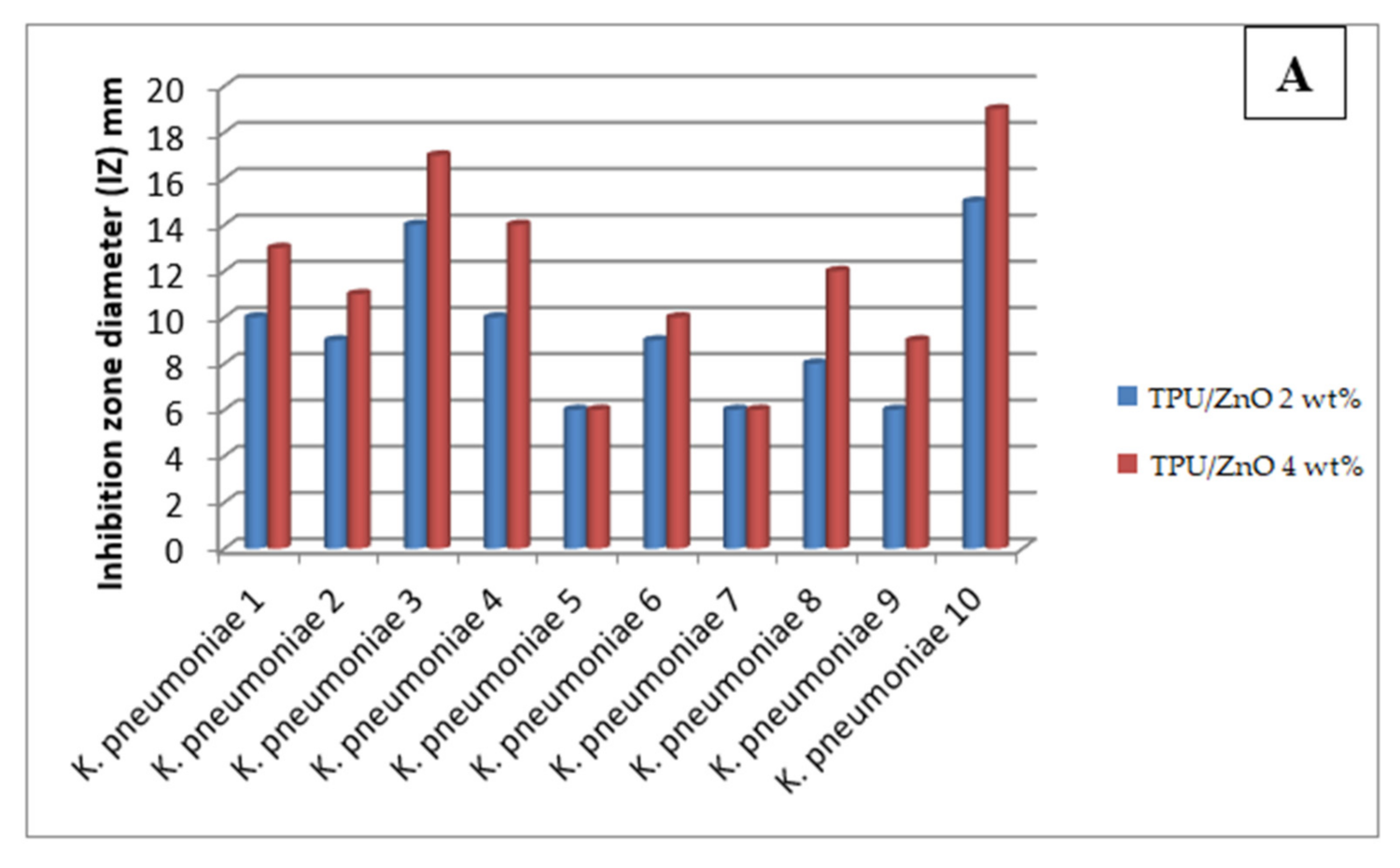
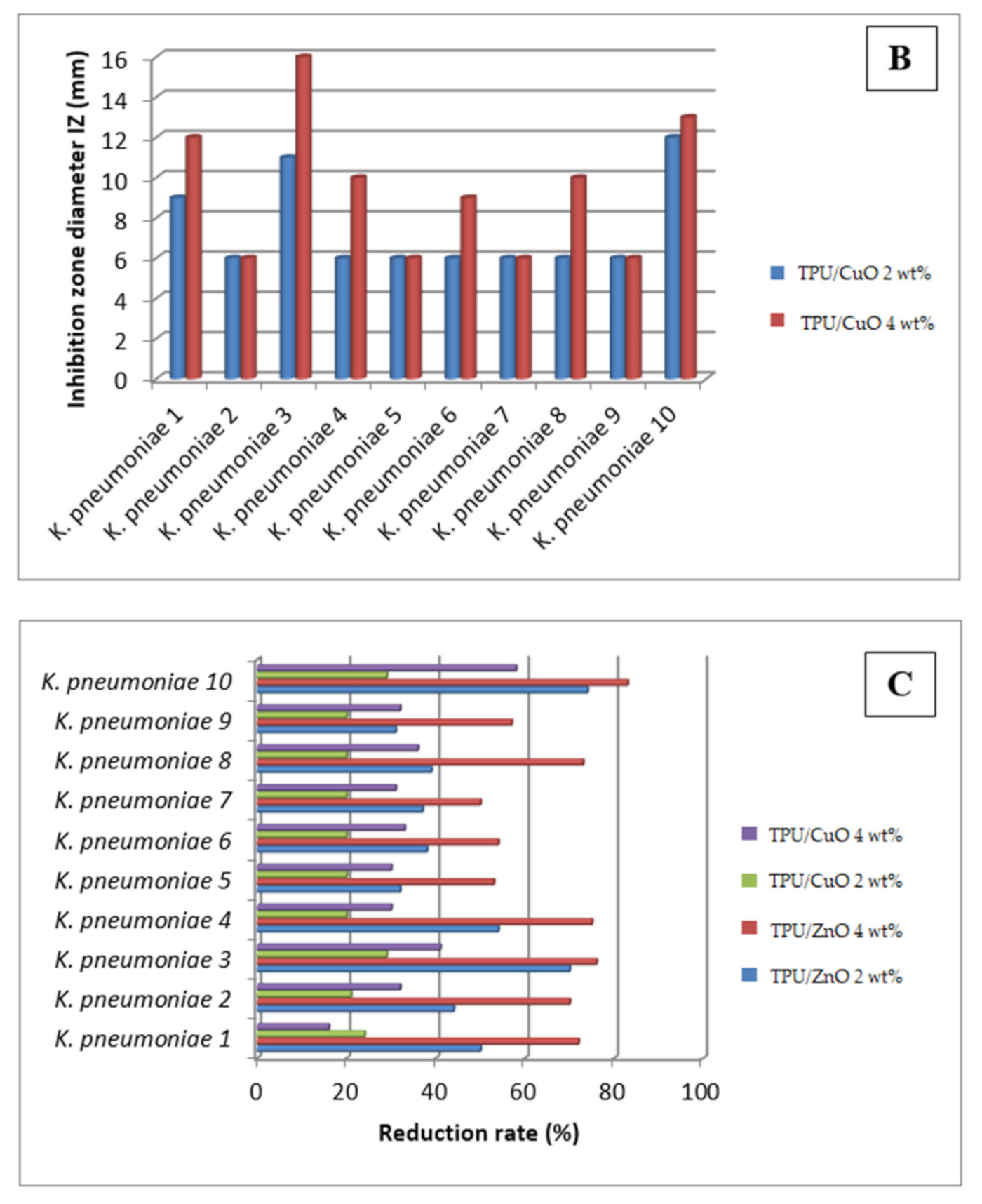
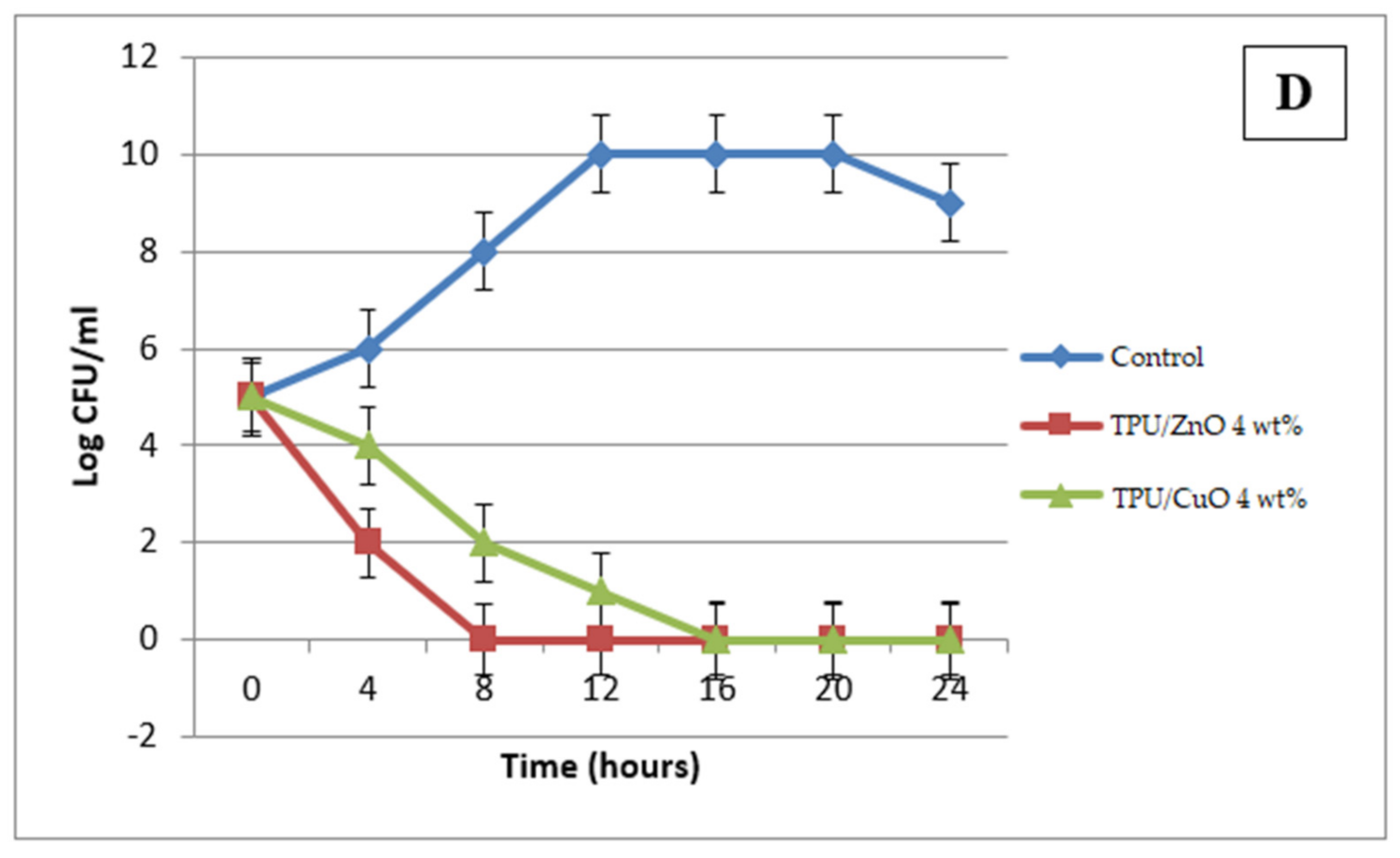
3.4. Antibacterial Activity
3.5. Antiviral Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Livingston, E.; Desai, A.; Berkwits, M.J.J. Sourcing personal protective equipment during the COVID-19 pandemic. JAMA 2020, 323, 1912–1914. [Google Scholar] [CrossRef] [Green Version]
- Bauchner, H.; Fontanarosa, P.B.; Livingston, E.H. Conserving supply of personal protective equipment—A call for ideas. JAMA 2020, 323, 1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowden, E.; Campanile, C.; Golding, B. Worker at NYC Hospital Where Nurses Wear Trash Bags as Protection Dies from Coronavirus. N. Y. Post 2020, 3, 25. Available online: https://nypost.com/2020/03/25/worker-at-nyc-hospital-where-nurses-wear-trash-bags-as-protection-dies-from-coronavirus/ (accessed on 25 August 2021).
- Chris, M. Some Amazon Prime shipments won’t arrive for a month due to coronavirus. Fortune, 23 March 2020. Available online: https://fortune.com/2020/03/23/amazon-prime-delays-coronavirus/(accessed on 25 August 2021).
- Trexler, P.; Tiffany, T. Strategic National Stockpile fails to quench Ohio’s need for medical supplies. In MSN, 2020th ed.; 26 March 2020; Available online: ttps://www.wkyc.com/article/news/health/coronavirus/strategic-nation-stockpile-fails-to-quench-ohio-need-for-medical-supplies/95-9c05ef80-5152-4951-b8f0-210786b23d33 (accessed on 25 August 2021).
- Harris, A.D.; Pineles, L.; Belton, B.; Johnson, J.K.; Shardell, M.; Loeb, M.; Newhouse, R.; Dembry, L.; Braun, B.; Perencevich, E.N.; et al. Universal Glove and Gown Use and Acquisition of Antibi-otic-Resistant Bacteria in the ICU: A Randomized Trial. JAMA 2013, 310, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Bachman, M.A.; Microbiology, I. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front. Cell Infect. Microbiol. 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, K.; Gupta, A.; Shrivastava, D. The last resort antibiotics: Carbapenems. Int. J. Adv. Res. 2017, 5, 1410–1413. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International consensus guidelines for the optimal use of the polymyxins: Endorsed by the American college of clinical pharmacy (ACCP), European society of clinical microbiology and infectious diseases (ESCMID), infectious diseases society of America (IDSA), international society for anti-infective Pharmacology (ISAP), society of critical care medicine (SCCM), and society of infectious diseases pharmacists (SIDP). Pharmacother. J. Hum. Pharmacol. Drug Ther. 2019, 39, 10–39. [Google Scholar]
- Brown, P.; Dawson, M.J. Development of new polymyxin derivatives for multi-drug resistant Gram-negative infections. J. Antibiot. 2017, 70, 386–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spapen, H.; Jacobs, R.; Van Gorp, V.; Troubleyn, J.; Honoré, P.M. Renal and neurological side effects of colistin in critically ill patients. Ann. Intensiv. Care 2011, 1, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacGowan, A.; Macnaughton, E. Antimicrobial therapy: Principles of use. Medcine 2017, 45, 614–621. [Google Scholar] [CrossRef]
- Fratini, S.; Nikolka, M.; Salleo, A.; Schweicher, G.; Sirringhaus, H. Charge transport in high-mobility conjugated polymers and molecular semiconductors. Nat. Mater. 2020, 19, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Ogunseitan, O.A. The Materials Genome and COVID-19 Pandemic. Jom 2020, 72, 1–3. [Google Scholar]
- Vozzola, E.; Overcash, M.; Griffing, E. Environmental considerations in the selection of isolation gowns: A life cycle assessment of reusable and disposable alternatives. Am. J. Infect. Control. 2018, 46, 881–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.A.; Samore, M.H.; Harris, A.D. The importance of contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. JAMA 2018, 319, 863–864. [Google Scholar] [CrossRef]
- Gutarowska, B.; Skóra, J.; Nowak, E.; Łysiak, I.; Wdówka, M. Antimicrobial Activity and Filtration Effectiveness of Nonwovens with Sanitized for Respiratory Protective Equipment. 2014. Available online: http://www.fibtex.lodz.pl/pliki/Fibtex_(lkzi8pi6xun6202m).pdf (accessed on 27 August 2021).
- Longano, D.; Ditaranto, N.; Sabbatini, L.; Torsi, L.; Cioffi, N. Synthesis and antimicrobial activity of copper nanomaterials. In Nano-Antimicrobials; Springer: Berlin/Heidelberg, Germany, 2012; pp. 85–117. [Google Scholar]
- Karim, N.; Afroj, S.; Lloyd, K.; Oaten, L.C.; Andreeva, D.V.; Carr, C.; Farmery, A.D.; Kim, I.-D.; Novoselov, K.S. Sustainable personal protective clothing for healthcare applications: A review. ACS Nano 2020, 14, 12313–12340. [Google Scholar] [CrossRef] [PubMed]
- Alshabanah, L.A.; Hagar, M.; Al-Mutabagani, L.A.; Abozaid, G.M.; Abdallah, S.M.; Ahmed, H.; Hassanin, A.H.; Shehata, N. Biodegradable Nanofibrous Membranes for Medical and Personal Protection Applications: Manufacturing, Anti-COVID-19 and Anti-Multidrug Resistant Bacteria Evaluation. Materials 2021, 14, 3862. [Google Scholar] [CrossRef] [PubMed]
- Alshabanah, L.A.; Hagar, M.; Al-Mutabagani, L.A.; Abozaid, G.M.; Abdallah, S.M.; Shehata, N.; Ahmed, H.; Hassanin, A.H. Hybrid Nanofibrous Membranes as a Promising Functional Layer for Personal Protection Equipment: Manufacturing and Antiviral/Antibacterial Assessments. Polymers 2021, 13, 1776. [Google Scholar] [CrossRef] [PubMed]
- Sportelli, M.C.; Izzi, M.; Kukushkina, E.A.; Hossain, S.I.; Picca, R.A.; Ditaranto, N.; Cioffi, N. Can nanotechnology and materials science help the fight against SARS-CoV-2? Nanomaterials 2020, 10, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M. Nanoparticle-based Antimicrobial Paper as Spread-breaker for Coronavirus. Pap. Technol. Int. 2020, 62, 20–25. [Google Scholar]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J. Multistate point-prevalence survey of health care–associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [Green Version]
- Żakowska, Z. Microbial biodegradation and biodeterioration of technical materials. In Proceedings of the IV International Scientific Conference, Conference Materials, Łódź, Poland, 2006; pp. 75–84. [Google Scholar]
- Abo-Shosha, M.; Hashem, A.; El-Hosamy, M.; El-Nagar, A. Easy care finishing of knitted cotton fabric in presence of a reactive-type antibacterial agent. J. Ind. Text. 2008, 38, 103–126. [Google Scholar] [CrossRef]
- Chen, R.; Morsi, Y.; Patel, S.; Ke, Q.-F.; Mo, X.-M. A novel approach via combination of electrospinning and FDM for tri-leaflet heart valve scaffold fabrication. Front. Mater. Sci. China 2009, 3, 359–366. [Google Scholar] [CrossRef]
- Fallahiarezoudar, E.; Ahmadipourroudposht, M.; Yusof, N.M.; Idris, A.; Ngadiman, N. 3D biofabrication of thermoplastic polyurethane (TPU)/poly-L-lactic acid (PLLA) electrospun nanofibers containing maghemite (γ-Fe2O3) for tissue engineering aortic heart valve. Polymer 2017, 9, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Jang, J.U.; Yin, W.B.; Lee, B.; Lee, K. Synthesis of highly functionalized thermoplastic polyurethanes and their potential applications. Polymer 2017, 116, 287–294. [Google Scholar] [CrossRef]
- Hattig, J.; Drube, W. Wide area of applications: Upward trend for thermoplastic polyurethanes. Kunstst.-Plast Eur. 2003, 93, BA28. [Google Scholar]
- Kharbas, H.A.; Ellingham, T.; Manitiu, M.; Scholz, G.; Turng, L.-S. Effect of a cross-linking agent on the foamability of microcellular injection molded thermoplastic polyurethane. J. Cell. Plast. 2017, 53, 407–423. [Google Scholar] [CrossRef]
- Bazmara, B.; Tahersima, M.; Behravan, A.J.C.; Materials, B. Influence of thermoplastic polyurethane and synthesized polyurethane additive in performance of asphalt pavements. Constr. Build. Mater. 2018, 166, 1–11. [Google Scholar] [CrossRef]
- Dong, M.; Li, Q.; Liu, H.; Liu, C.; Wujcik, E.K.; Shao, Q.; Ding, T.; Mai, X.; Shen, C.; Guo, Z. Thermoplastic polyurethane-carbon black nanocomposite coating: Fabrication and solid particle erosion resistance. Polymer 2018, 158, 381–390. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, B.; Zheng, G.; Liu, X.; Li, T.; Yan, C.; Cheng, C.; Dai, K.; Liu, C.; Shen, C. Continuously prepared highly conductive and stretchable SWNT/MWNT synergistically composited electrospun thermoplastic polyurethane yarns for wearable sensing. J. Mater. Chem. C 2018, 6, 2258–2269. [Google Scholar] [CrossRef]
- Ke, K.; Bonab, V.S.; Yuan, D.; Manas-Zloczower, I. Piezoresistive thermoplastic polyurethane nanocomposites with carbon nanostructures. Carbon 2018, 139, 52–58. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, X.; Wang, P.; Xie, K.; Jian, J.; Zhang, Y.; Zhang, J.; Yuan, Y.; Na, P.; Yi, M. Transparent thermoplastic polyurethane air filters for efficient electrostatic capture of particulate matter pollutants. Nanotechnology 2018, 30, 015703. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhou, Y.; Wang, Y.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. Highly stretchable and durable strain sensor based on carbon nanotubes decorated thermoplastic polyurethane fibrous network with aligned wave-like structure. Chem. Eng. J. 2019, 360, 762–777. [Google Scholar] [CrossRef]
- Rubin Pedrazzo, A.; Cecone, C.; Morandi, S.; Manzoli, M.; Bracco, P.; Zanetti, M. Nanosized SnO2 Prepared by Electrospinning: Influence of the Polymer on Both Morphology and Microstructure. Polymers 2021, 13, 977. [Google Scholar] [CrossRef] [PubMed]
- Thamer, B.M.; Aldalbahi, A.; Moydeen, A.M.; Rahaman, M.; El-Newehy, M.H. Modified Electrospun Polymeric Nanofibers and Their Nanocomposites as Nanoadsorbents for Toxic Dye Removal from Contaminated Waters: A Review. Polymers 2021, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, D.; Li, J.; Li, S.; Chen, Z.; Yu, D.-G.; Liu, Z.; Guo, J.Z. Electrospun Janus zein–PVP nanofibers provide a two-stage controlled release of poorly water-soluble drugs. Mater. Des. 2020, 196, 109075. [Google Scholar] [CrossRef]
- Zhao, K.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Electrospun Functional Nanofiber Membrane for Antibiotic Removal in Water. Polymers 2021, 13, 226. [Google Scholar] [CrossRef]
- Deng, C.; Seidi, F.; Yong, Q.; Jin, X.; Li, C.; Zhang, X.; Han, J.; Liu, Y.; Huang, Y.; Wang, Y.; et al. Antiviral/antibacterial biodegradable cellulose nonwovens as environmentally friendly and bioprotective materials with potential to minimize microplastic pollution. J. Hazard. Mater. 2022, 424, 127391. [Google Scholar] [CrossRef]
- Abdoluosefi, H.E.; Honarasa, G.; Abdoluosefi, H.E. Fabrication of polyurethane and thermoplastic polyurethane nanofiber by controlling the electrospinning parameters. Mater. Res. Express 2017, 4, 105308. [Google Scholar] [CrossRef]
- Pedicini, A.; Farris, R.J. Mechanical behavior of electrospun polyurethane. Polymer 2003, 44, 6857–6862. [Google Scholar] [CrossRef]
- Lee, K.; Lee, B.; Kim, C.; Kim, H.; Kim, K.; Nah, C. Stress-strain behavior of the electrospun thermoplastic polyurethane elastomer fiber mats. Macromol. Res. 2005, 13, 441–445. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Liu, J.; Zhang, H.; Wang, K. Preparation and antibacterial property of polyethersulfone ultrafiltration hybrid membrane containing halloysite nanotubes loaded with copper ions. Chem. Eng. J. 2012, 210, 298–308. [Google Scholar] [CrossRef]
- Stafford, S.L.; Bokil, N.J.; Achard, M.E.; Kapetanovic, R.; Schembri, M.A.; McEwan, A.G.; Sweet, M.J. Metal ions in macrophage antimicrobial pathways: Emerging roles for zinc and copper. Biosci. Rep. 2013, 33, e00049. [Google Scholar] [CrossRef] [PubMed]
- Radovanović, Ž.; Jokić, B.; Veljović, D.; Dimitrijević, S.; Kojić, V.; Petrović, R.; Janaćković, D. Antimicrobial activity and biocompatibility of Ag+-and Cu2+-doped biphasic hydroxyapatite/α-tricalcium phosphate obtained from hydrothermally synthesized Ag+-and Cu2+-doped hydroxyapatite. Appl. Surf. Sci. 2014, 307, 513–519. [Google Scholar] [CrossRef]
- Malwal, D.; Gopinath, P. Efficient adsorption and antibacterial properties of electrospun CuO-ZnO composite nanofibers for water remediation. J. Hazard. Mater. 2017, 321, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res. Part A 2006, 78, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Bužarovska, A.; Dinescu, S.; Lazar, A.D.; Serban, M.; Pircalabioru, G.G.; Costache, M.; Gualandi, C.; Avérous, L. Nanocomposite foams based on flexible biobased thermoplastic polyurethane and ZnO nanoparticles as potential wound dressing materials. Mater. Sci. Eng. C 2019, 104, 109893. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.K.H. Wastewater Disinfection by Synthesized Copper Oxide Nanoparticles Stabilized with Surfactant. 2013. Available online: https://repository.najah.edu/bitstream/handle/20.500.11888/8493/muath%20mousa.pdf?sequence=1&isAllowed=y (accessed on 28 August 2021).
- Cheng, Z.; Zhang, F.; Liu, W.; Cui, L.; Kang, L. A novel preparation for a PVA/L-histidine/AgNPs membrane and its antibacterial property. RSC Adv. 2015, 5, 54182–54187. [Google Scholar] [CrossRef]
- Santiago-Morales, J.; Amariei, G.; Letón, P.; Rosal, R.J.C.; Biointerfaces, S.B. Antimicrobial activity of poly (vinyl alcohol)-poly (acrylic acid) electrospun nanofibers. Colloids Surf. B Biointerfaces 2016, 146, 144–151. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; Elwakil, B.H.; Elshewemi, S.S.; El-Naggar, M.Y.; Bekhit, A.A.; Olama, Z.A. Novel Siwa propolis and colistin-integrated chitosan nanoparticles: Elaboration; in vitro and in vivo appraisal. Nanomedicine 2020, 15, 1269–1284. [Google Scholar] [CrossRef] [PubMed]
- Mokhena, T.C.; Luyt, A.S. Electrospun alginate nanofibres impregnated with silver nanoparticles: Preparation, morphology and antibacterial properties. Carbohydr. Polym. 2017, 165, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.H.; Cheng, W.; Goh, S.S.; Kong, J.; Li, B.; Lim, J.Y.; Mao, L.; Wang, S.; Xue, K.; Yang, L.J. Face masks in the new COVID-19 normal: Materials, testing, and perspectives. Research 2020, 2020, 7286735. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Xu, Y.; Li, X.; Wang, X.-X.; Zhang, H.-D.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Transparent polyurethane nanofiber air filter for high-efficiency PM2.5 capture. Nanoscale Res. Lett. 2019, 14, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velayutham, T.; Abd Majid, W.H.; Gan, W.; Khorsand Zak, A.; Gan, S.N. Theoretical and experimental approach on dielectric properties of ZnO nanoparticles and polyurethane/ZnO nanocomposites. J. Appl. Phys. 2012, 112, 054106. [Google Scholar] [CrossRef] [Green Version]
- Nirmala, R.; Jeon, K.S.; Lim, B.H.; Navamathavan, R.; Kim, H.Y. Preparation and characterization of copper oxide particles incorporated polyurethane composite nanofibers by electrospinning. Ceram. Int. 2013, 39, 9651–9658. [Google Scholar] [CrossRef]
- Elnabawy, E.; Hassanain, A.H.; Shehata, N.; Popelka, A.; Nair, R.; Yousef, S.; Kandas, I. Piezoelastic PVDF/TPU nanofibrous composite membrane: Fabrication and characterization. Polymers 2019, 11, 1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, Z.; Wang, J.; Liu, J.; Li, C. Preparation and properties of TPU micro/nanofibers by a laser melt-electrospinning system. Polym. Eng. Sci. 2014, 54, 1412–1417. [Google Scholar] [CrossRef]





| Nanofiber Membrane | Nanoparticles Concentration (%) | Average Fiber Diameter (nm) |
|---|---|---|
| TPU | 0 wt% | 116 ± 39 |
| TPU/ZnO | 2 wt% | 102 ± 36 |
| 4 wt% | 89 ± 30 | |
| TPU/CuO | 2 wt% | 66 ± 21 |
| 4 wt% | 68 ± 20 |
| Sample | Max. Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| TPU Blank | 17.7 ± 1.2 | 92.5 ± 20.4 |
| TPU/ZnO 4 wt% | 18.1 ± 0.9 | 85.5 ± 10.2 |
| TPU/CuO 4 wt% | 16.08 ± 2.3 | 81.25 ± 18.9 |
| Tested Strains | TPU/ZnO 2 wt% | TPU/ZnO 4 wt% | TPU/CuO 2 wt% | TPU/CuO 4 wt% | ||||
|---|---|---|---|---|---|---|---|---|
| IZ (mm) | Reduction Rate (%) | IZ (mm) | Reduction Rate (%) | IZ (mm) | Reduction Rate (%) | IZ (mm) | Reduction Rate (%) | |
| K. pneumoniae 1 | 10 | 50 | 13 | 72 | 9 | 24 | 12 | 16 |
| K. pneumoniae 2 | 9 | 44 | 11 | 70 | 6 | 21 | 6 | 32 |
| K. pneumoniae 3 | 14 | 70 | 17 | 76 | 11 | 29 | 16 | 41 |
| K. pneumoniae 4 | 10 | 54 | 14 | 75 | 6 | 20 | 10 | 30 |
| K. pneumoniae 5 | 6 | 32 | 6 | 53 | 6 | 20 | 6 | 30 |
| K. pneumoniae 6 | 9 | 38 | 10 | 54 | 6 | 20 | 9 | 33 |
| K. pneumoniae 7 | 6 | 37 | 6 | 50 | 6 | 20 | 6 | 31 |
| K. pneumoniae 8 | 8 | 39 | 12 | 73 | 6 | 20 | 10 | 36 |
| K. pneumoniae 9 | 6 | 31 | 9 | 57 | 6 | 20 | 6 | 32 |
| K. pneumoniae 10 | 15 | 74 | 19 | 83 | 12 | 29 | 13 | 58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshabanah, L.A.; Omran, N.; Elwakil, B.H.; Hamed, M.T.; Abdallah, S.M.; Al-Mutabagani, L.A.; Wang, D.; Liu, Q.; Shehata, N.; Hassanin, A.H.; et al. Elastic Nanofibrous Membranes for Medical and Personal Protection Applications: Manufacturing, Anti-COVID-19, and Anti-Colistin Resistant Bacteria Evaluation. Polymers 2021, 13, 3987. https://doi.org/10.3390/polym13223987
Alshabanah LA, Omran N, Elwakil BH, Hamed MT, Abdallah SM, Al-Mutabagani LA, Wang D, Liu Q, Shehata N, Hassanin AH, et al. Elastic Nanofibrous Membranes for Medical and Personal Protection Applications: Manufacturing, Anti-COVID-19, and Anti-Colistin Resistant Bacteria Evaluation. Polymers. 2021; 13(22):3987. https://doi.org/10.3390/polym13223987
Chicago/Turabian StyleAlshabanah, Latifah Abdullah, Nada Omran, Bassma H. Elwakil, Moaaz T. Hamed, Salwa M. Abdallah, Laila A. Al-Mutabagani, Dong Wang, Qiongzhen Liu, Nader Shehata, Ahmed H. Hassanin, and et al. 2021. "Elastic Nanofibrous Membranes for Medical and Personal Protection Applications: Manufacturing, Anti-COVID-19, and Anti-Colistin Resistant Bacteria Evaluation" Polymers 13, no. 22: 3987. https://doi.org/10.3390/polym13223987
APA StyleAlshabanah, L. A., Omran, N., Elwakil, B. H., Hamed, M. T., Abdallah, S. M., Al-Mutabagani, L. A., Wang, D., Liu, Q., Shehata, N., Hassanin, A. H., & Hagar, M. (2021). Elastic Nanofibrous Membranes for Medical and Personal Protection Applications: Manufacturing, Anti-COVID-19, and Anti-Colistin Resistant Bacteria Evaluation. Polymers, 13(22), 3987. https://doi.org/10.3390/polym13223987








