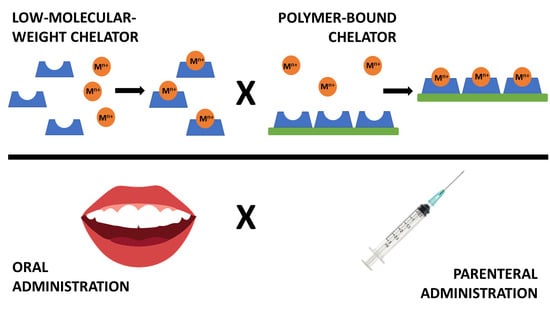
Chelators for Treatment of Iron and Copper Overload: Shift from Low-Molecular-Weight Compounds to Polymers
Abstract
1. Introduction
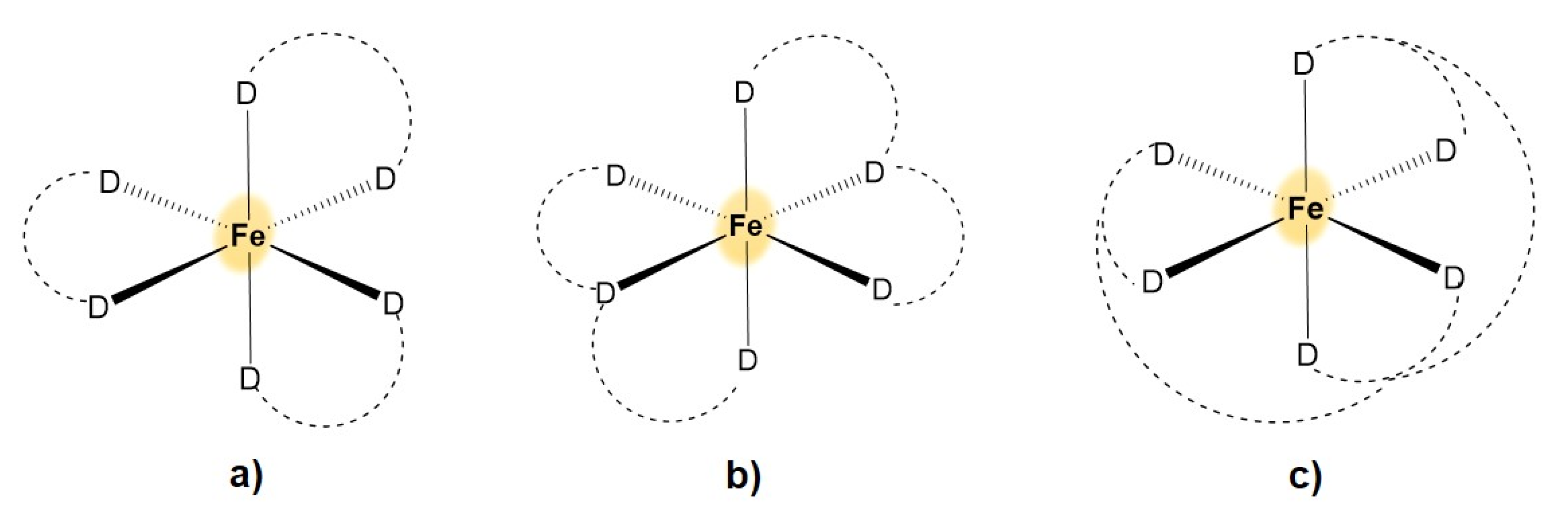
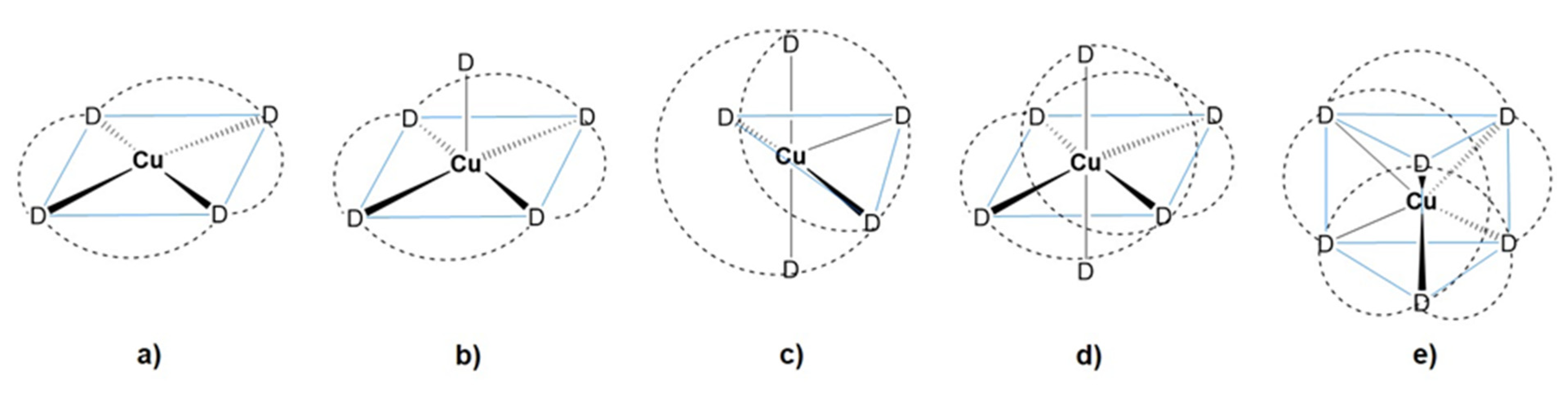
2. Coordination Chemistry of Iron and Copper
3. Iron Uptake and Metabolism, Diseases Connected with Iron Overload and Current Treatment Strategies
4. Copper Metabolism, Diseases Connected with Its Overload and Current Treatment
5. Polymer Iron and Copper Chelators for Metal Overload Treatment
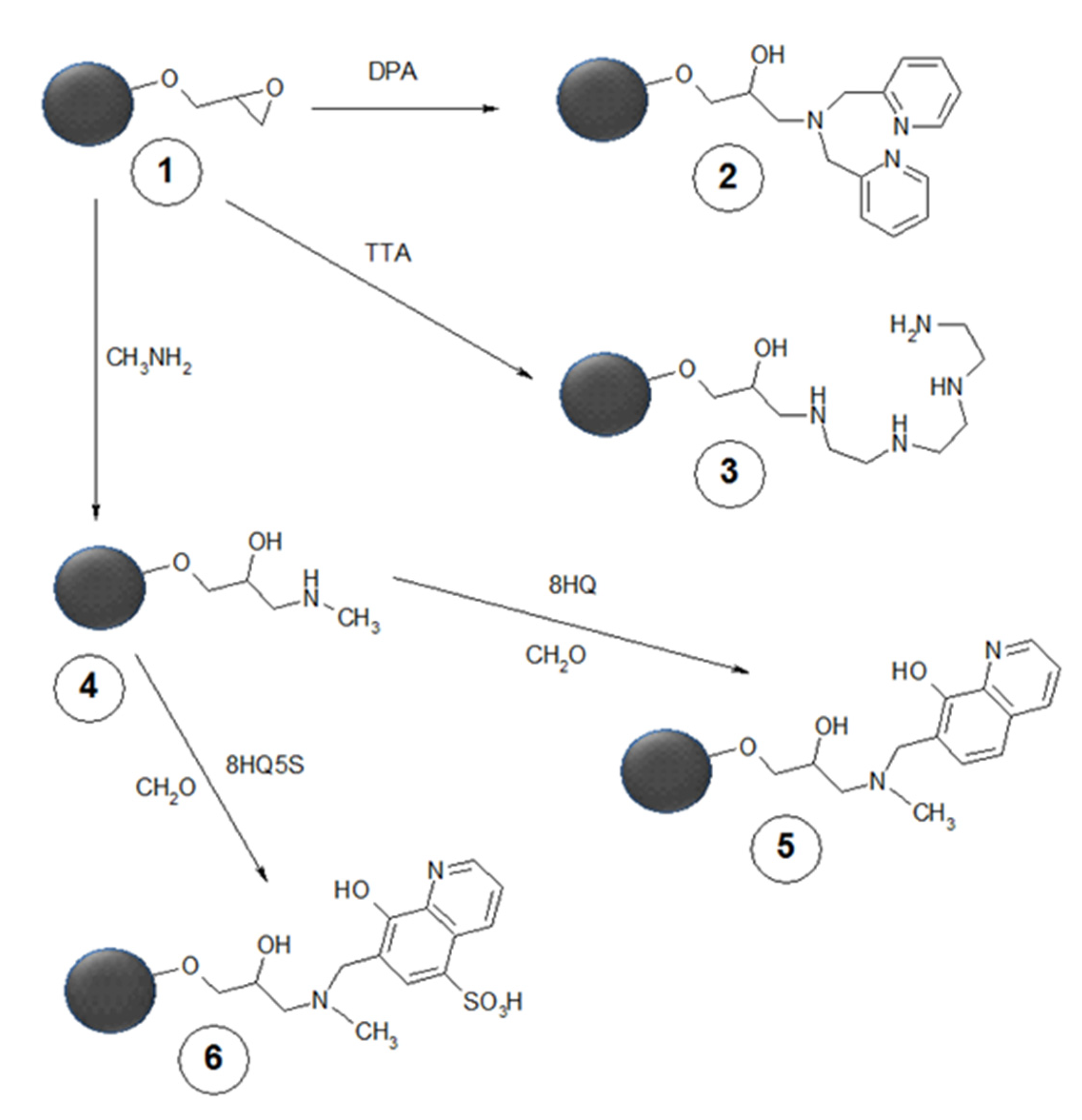
5.1. Polymers and Nanospecies with Bound Chelators to Be Applied Parenterally
5.2. Polymers with Bound Chelators to Be Applied Orally
6. Conclusions and Further Challenges in Polymer Iron- and Copper-Chelating Therapeutics
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vest, K.E.; Paskavitz, A.L.; Lee, J.B.; Padilla-Benavides, T. Dynamic changes in copper homeostasis and post-transcriptional regulation ofAtp7aduring myogenic differentiation. Metallomics 2018, 10, 309–322. [Google Scholar] [CrossRef]
- Cadet, E.; Gadenne, M.; Capron, D.; Rochette, J. Advances in iron metabolism: A transition state. Rev. Med. Internet 2005, 26, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Rishi, G.; Subramaniam, V.N. The liver in regulation of iron homeostasis. Am. J. Physiol. Liver Physiol. 2017, 313, G157–G165. [Google Scholar] [CrossRef] [PubMed]
- Vallerio, L.G. Mammalian iron metabolism. Toxicol. Mech. Methods 2007, 17, 497–517. [Google Scholar] [CrossRef] [PubMed]
- Saboor, M.; Zehra, A.; Hamali, H.; Mobarki, A. Revisiting Iron Metabolism, Iron Homeostasis and Iron Deficiency Anemia. Clin. Lab. 2021, 67. [Google Scholar] [CrossRef]
- Bi, Y.; Ajoolabady, A.; Demillard, L.J.; Yu, W.; Hilaire, M.L.; Zhang, Y.; Ren, J. Dysregulation of iron metabolism in cardiovascular diseases: From iron deficiency to iron overload. Biochem. Pharmacol. 2021, 190, 114661. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Sadler, P.J. Exploration of the medical periodic table: Towards new targets. Chem. Commun. 2013, 49, 5106–5131. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Spinello, A.; Saha, A.; Magistrato, A. Frontiers of metal-coordinating drug design. Expert Opin. Drug Discov. 2020, 16, 497–511. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Loginova, N.V.; Harbatsevich, H.I.; Osipovich, N.P.; Ksendzova, G.A.; Koval’Chuk, T.V.; Polozov, G.I. Metal Complexes as Promising Agents for Biomedical Applications. Curr. Med. Chem. 2020, 27, 5213–5249. [Google Scholar] [CrossRef] [PubMed]
- Werner, A. Beitrag zur Konstitution anorganischer Verbindungen. Z. Anorg. Chem. 1893, 3, 267–330. [Google Scholar] [CrossRef]
- Pearson, R.G. The HSAB Principle—more quantitative aspects. Inorganica Chim. Acta 1995, 240, 93–98. [Google Scholar] [CrossRef]
- Schwarzenbach, G. DER CHELATEFFEKT. Helv. Chim. Acta 1952, 35, 2344–2363. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, T.; Kong, X.; Hider, R. Chelating Agents for the Treatment of Systemic Iron Overload. Curr. Med. Chem. 2012, 19, 2816–2827. [Google Scholar] [CrossRef] [PubMed]
- Grady, R.W.; Graziano, J.H.; Akers, H.A.; Cerami, A. The development of new iron-chelating drugs. J. Pharmacol. Exp. Ther. 1976, 196. [Google Scholar]
- Nunez, M.T.; Chana-Cuevas, P. New Perspectives in Iron Chelation Therapy for the Treatment of Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 109. [Google Scholar] [CrossRef]
- Turnquis, T.D.; Sandell, E.B. Stability constants of iron(III)-8-hydroxyquinoline complexes. Anal. Chim. Acta 1968, 42, 239–245. [Google Scholar] [CrossRef]
- Zhou, T.; Ma, Y.; Kong, X.; Hider, R.C. Design of iron chelators with therapeutic application. Dalton Trans. 2012, 41, 6371–6389. [Google Scholar] [CrossRef] [PubMed]
- Prachayasittikul, V.; Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S. 8-Hydroxyquinolines: A review of their metal chelating properties and medicinal applications. Drug Des. Dev. Ther. 2013, 7, 1157–1178. [Google Scholar] [CrossRef] [PubMed]
- Bareggi, S.R.; Cornelli, U. Clioquinol: Review of its Mechanisms of Action and Clinical Uses in Neurodegenerative Disorders. CNS Neurosci. Ther. 2010, 18, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.W.; Bush, A.I.; Mackinnon, A.; Macfarlane, S.; Mastwyk, M.; MacGregor, L.; Kiers, L.; Cherny, R.; Li, Q.X.; Tammer, A.; et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting A beta amyloid deposition and toxicity in Alzheimer disease—A pilot phase 2 clinical trial. Arch. Neurol. 2003, 60, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Lannfelt, L.; Blennow, K.; Zetterberg, H.; Batsman, S.; Ames, D.; Hrrison, J.; Masters, C.L.; Targum, S.; Bush, A.I.; Murdoch, R.; et al. Safety, efficacy, and biomarker findings of PBT2 in targeting A beta as a modifying therapy for Alzheimer’s disease: A phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008, 7, 779–786. [Google Scholar] [CrossRef]
- Kim, J.-J.; Kim, Y.-S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elements Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Nurchi, V.M.; Crisponi, G.; Pivetta, T.; Donatoni, M.; Remelli, M. Potentiometric, spectrophotometric and calorimetric study on iron(III) and copper(II) complexes with 1,2-dimethyl-3-hydroxy-4-pyridinone. J. Inorg. Biochem. 2008, 102, 684–692. [Google Scholar] [CrossRef]
- Cilibrizzi, A.; Abbate, V.; Chen, Y.-L.; Ma, Y.; Zhou, T.; Hider, R. Hydroxypyridinone Journey into Metal Chelation. Chem. Rev. 2018, 118, 7657–7701. [Google Scholar] [CrossRef] [PubMed]
- Hershko, C.; Konijn, A.M.; Nick, H.P.; Breuer, W.; Cabantchik, Z.I.; Link, G. ICL670A: A new synthetic oral chelator: Evalu-ation in hypertransfused rats with selective radioiron probes of hepatocellular and reticuloendothelial iron stores and in iron-loaded rat heart cells in culture. Blood 2001, 97, 1115–1122. [Google Scholar] [CrossRef]
- Steinhauser, S.; Heinz, U.; Bartholomä, M.; Weyhermüller, T.; Nick, H.; Hegetschweiler, K. Complex Formation of ICL670 and Related Ligands with FeIII and FeII. Eur. J. Inorg. Chem. 2005, 2005, 2262. [Google Scholar] [CrossRef]
- Dhungana, S.; White, P.S.; Crumbliss, A.L. Crystal structure of ferrioxamine B: A comparative analysis and implications for molecular recognition. JBIC J. Biol. Inorg. Chem. 2001, 6, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Copper chelation chemistry and its role in copper radiopharmaceu-ticals. Curr. Pharm. Design 2007, 13, 3–16. [Google Scholar] [CrossRef]
- Jahn, H.A.; Teller, E. Stability of polyatomic molecules in degenerate electronic states. I. Orbital degeneracy. Proc. R. Soc. Lond. A-Math. Phys. Sci. 1937, 161, 220–235. [Google Scholar]
- Meares, C.F.; Wensel, T. Metal chelates as probes of biological systems. Accounts Chem. Res. 1984, 17, 202–209. [Google Scholar] [CrossRef]
- Hnatowich, D.J.; Layne, W.W.; Childs, R.L.; Lanteigne, D.; Davis, M.A.; Griffin, T.W.; Doherty, P.W. Radioactive Labeling of Antibody: A Simple and Efficient Method. Science 1983, 220, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Felix, V.; Lima, L.M.P.; Price, D.W. Metal complexes of cyclen and cyclam derivatives useful for medical appli-cations: A discussion based on thermodynamic stability constants and structural data. Dalton Trans. 2007, 26, 2734–2745. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Kubeil, M.; Nsubuga, A.; Singh, G.; Gasser, G.; Stephan, H. Harnessing the Coordination Chemistry of 1,4,7-Triazacyclononane for Biomimicry and Radiopharmaceutical Applications. ChemPlusChem 2018, 83, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Parker, D. Tumor targeting with radiolabeled antibody conjugates. Chem. Soc. Rev. 1990, 19, 271–291. [Google Scholar] [CrossRef]
- Donnelly, P.S. The role of coordination chemistry in the development of copper and rhenium radiopharmaceuticals. Dalton Trans. 2011, 40, 999–1010. [Google Scholar] [CrossRef]
- Anderson, C.; Welch, M.J. Radiometal-Labeled Agents (Non-Technetium) for Diagnostic Imaging. Chem. Rev. 1999, 99, 2219–2234. [Google Scholar] [CrossRef] [PubMed]
- Lukes, I.; Kotek, J.; Vojtisek, P.; Hermann, P. Complexes of tetraazacycles bearing methylphosphinic/phosphonic acid pendant arms with copper(II), zinc(II) and lanthanides(III). A comparison with their acetic acid analogues. Coord. Chem. Rev. 2001, 216, 287–312. [Google Scholar] [CrossRef]
- Sargeson, A.M. Developments in the synthesis and reactivity of encapsulated metal ions. Pure Appl. Chem. 1986, 58, 1511–1522. [Google Scholar] [CrossRef]
- Voloshin, Y.Z.; Novikov, V.V.; Nelyubina, Y.V. Recent advances in biological applications of cage metal complexes. RSC Adv. 2015, 5, 72621–72637. [Google Scholar] [CrossRef]
- Hancock, R.D. The pyridyl group in ligand design for selective metal ion complexation and sensing. Chem. Soc. Rev. 2012, 42, 1500–1524. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Lu, F.; Overstreet, T.; E Milenic, D.; Brechbiel, M.W. Novel chelating agents for potential clinical applications of copper. Nucl. Med. Biol. 2002, 29, 91–105. [Google Scholar] [CrossRef]
- Gasser, G.; Tjioe, L.; Graham, B.; Belousoff, M.; Juran, S.; Walther, M.; Künstler, J.-U.; Bergmann, R.; Stephan, H.; Spiccia, L. Synthesis, Copper(II) Complexation, 64Cu-Labeling, and Bioconjugation of a New Bis(2-pyridylmethyl) Derivative of 1,4,7-Triazacyclononane. Bioconjugate Chem. 2008, 19, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Przyborowska, A.M.; Ye, N.; Tsoupas, N.M.; Bauer, C.B.; Broker, G.A.; Rogers, R.D.; Brechbiel, M.W.; Planalp, R.P. Steric effects caused by N-alkylation of the tripodal chelator N,N′,N″-tris(2-pyridylmethyl)-cis,cis-1,3,5-triaminocyclohexane (tachpyr): Structural and electronic properties of the Mn(ii), Co(ii), Ni(ii), Cu(ii) and Zn(ii) complexes. Dalton Trans. 2002, 318–324. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2013, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Ramogida, C.F.; Orvig, C. Tumour targeting with radiometals for diagnosis and therapy. Chem. Commun. 2013, 49, 4720–4739. [Google Scholar] [CrossRef]
- Boros, E.; Packard, A.B. Radioactive Transition Metals for Imaging and Therapy. Chem. Rev. 2018, 119, 870–901. [Google Scholar] [CrossRef] [PubMed]
- Heroux, K.J.; Woodin, K.S.; Tranchemontagne, D.J.; Widger, P.C.B.; Southwick, E.; Wong, E.H.; Weisman, G.R.; Tomellini, S.A.; Wadas, T.J.; Anderson, C.J.; et al. The long and short of it: The influence of N-carboxyethyl versusN-carboxymethyl pendant arms on in vitro and in vivo behavior of copper complexes of cross-bridged tetraamine macrocycles. Dalton Trans. 2007, 2150–2162. [Google Scholar] [CrossRef]
- Boswell, C.A.; Sun, X.; Niu, W.; Weisman, G.R.; Wong, E.H.; Rheingold, A.L.; Anderson, C.J. Comparative in Vivo Stability of Copper-64-Labeled Cross-Bridged and Conventional Tetraazamacrocyclic Complexes. J. Med. Chem. 2004, 47, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Juran, S.; Walther, M.; Stephan, H.; Bergmann, R.; Steinbach, J.; Kraus, W.; Emmerling, F.; Comba, P. Hexadentate Bispidine Derivatives as Versatile Bifunctional Chelate Agents for Copper(II) Radioisotopes. Bioconjugate Chem. 2009, 20, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Bleiholder, C.; Börzel, H.; Comba, P.; Ferrari, R.; Heydt, M.; Kerscher, M.; Kuwata, S.; Laurenczy, G.; Lawrance, G.A.; Lienke, A.; et al. Coordination Chemistry of a New Rigid, Hexadentate Bispidine-Based Bis(amine)tetrakis(pyridine) Ligand. Inorg. Chem. 2005, 44, 8145–8155. [Google Scholar] [CrossRef] [PubMed]
- Stephan, H.; Walther, M.; Fähnemann, S.; Ceroni, P.; Molloy, J.K.; Bergamini, G.; Heisig, F.; Müller, C.E.; Kraus, W.; Comba, P. Bispidines for Dual Imaging. Chem.—A Eur. J. 2014, 20, 17011–17018. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Zarschler, K.; Hunoldt, S.; Martinez, I.I.S.; Ruehl, C.L.; Matterna, M.; Bergmann, R.; Mathe, D.; Hegedus, N.; Bachmann, M.; et al. Versatile Bispidine-Based Bifunctional Chelators for Cu-64(II)-Labelling of Biomolecules. Chem. Eur. J. 2020, 26, 1989–2001. [Google Scholar] [CrossRef] [PubMed]
- Comba, P.; Kerscher, M.; Schiek, W. Bispidine Coordination Chemistry. Prog. Inorg. Chem. 2007, 613–704. [Google Scholar] [CrossRef]
- Southcott, L.; Wang, X.Z.; Choudhary, N.; Wharton, L.; Patrick, B.O.; Yang, H.; Zarschler, K.; Kubeil, M.; Stephan, H.; Jara-quemada-Pelaez, M.D.; et al. H(2)pyhox—Octadentate Bis(pyridyloxine). Inorg. Chem. 2021, 60, 12186–12196. [Google Scholar] [CrossRef] [PubMed]
- Keramidas, K.G.; Rentzeperis, P.I. The crystal structure of triethylenetetraamine copper(II) fluorophosphate, Cu(TRIEN)(PF6). Z. Kristallogr. 1992, 201, 171–176. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Crisponi, G.; Crespo-Alonso, M.; Lachowicz, J.I.; Szewczuk, Z.; Cooper, G.J.S. Complex formation equilibria of Cu-II and Zn-II with triethylenetetramine and its mono- and di-acetyl metabolites. Dalton Trans. 2013, 42, 6161–6170. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.X.; Hong, M.C.; Cao, R.; Liu, H.Q. Synthesis and characterization of (Et(4)N)(4) MoS4Cu10Cl12: A polynuclear mo-lybdenum-copper cluster containing a central tetrahedral MoS4 encapsulated by octahedral Cu-6 and tetrahedral Cu-4 arrays. Inorg. Chem. 1996, 35, 1080–1082. [Google Scholar] [CrossRef] [PubMed]
- Birker, P.J.M.W.L.; Freeman, H.C. Metal-binding in chelation therapy: X-ray crystal structure of a copper(I)–copper(II) complex ofD-penicillamine. J. Chem. Soc. Chem. Commun. 1976, 9, 312–313. [Google Scholar] [CrossRef]
- Milman, N.T. Managing Genetic Hemochromatosis: An Overview of Dietary Measures, Which May Reduce Intestinal Iron Absorption in Persons with Iron Overload. Gastroenterol. Res. 2021, 14, 66–80. [Google Scholar] [CrossRef]
- Yanatori, I.; Kishi, F. DMT1 and iron transport. Free. Radic. Biol. Med. 2018, 133, 55–63. [Google Scholar] [CrossRef]
- Shayeghi, M.; Latunde-Dada, G.O.; Oakhill, J.; Laftah, A.H.; Takeuchi, K.; Halliday, N.; Khan, Y.; Warley, A.; McCann, F.; Hider, R.; et al. Identification of an Intestinal Heme Transporter. Cell 2005, 122, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, G.; Yoshida, T.; Noguchi, M. Heme oxygenase and heme degradation. Biochem. Biophys. Res. Commun. 2005, 338, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Engle-Stone, R.; Yeung, A.; Welch, R.; Glahn, R. Meat and Ascorbic Acid Can Promote Fe Availability from Fe−Phytate but Not from Fe−Tannic Acid Complexes. J. Agric. Food Chem. 2005, 53, 10276–10284. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, X.; Ding, K.; Li, Y.; Li, W. Ascorbic Acid can Reverse the Inhibition of Phytic Acid, Sodium Oxalate and Sodium Silicate on Iron Absorption in Caco-2 cells. Int. J. Vitam. Nutr. Res. 2018, 88, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Casal, M.N.; Leets, I.; Layrisse, M. beta-carotene and inhibitors of iron absorption modify iron uptake by Caco-2 cells. J. Nutr. 2000, 130, 5–9. [Google Scholar] [CrossRef]
- Christides, T.; Amagloh, F.K.; Coad, J. Iron Bioavailability and Provitamin A from Sweet Potato- and Cereal-Based Comple-mentary Foods. Foods 2015, 4, 463–476. [Google Scholar] [CrossRef]
- Kristan, A.; Gaspersic, J.; Rezen, T.; Kunej, T.; Kolic, R.; Vuga, A.; Fink, M.; Zula, S.; Doma, S.A.; Zupan, I.P.; et al. Genetic analysis of 39 erythrocytosis and hereditary hemochromatosis-associated genes in the Slove-nian family with idiopathic erythrocytosis. J. Clin. Lab. Anal. 2021, 35, 1–10. [Google Scholar] [CrossRef]
- Roemhild, K.; von Maltzahn, F.; Weiskirchen, R.; Knüchel, R.; von Stillfried, S.; Lammers, T. Iron metabolism: Pathophysiology and pharmacology. Trends Pharmacol. Sci. 2021, 42, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Murphree, C.R.; Nguyen, N.N.; Raghunathan, V.; Olson, S.R.; Deloughery, T.; Shatzel, J.J. Diagnosis and management of hereditary haemochromatosis. Vox Sang. 2020, 115, 255–262. [Google Scholar] [CrossRef]
- Milman, N.T.; Schioedt, F.V.; Junker, A.E.; Magnussen, K. Diagnosis and Treatment of Genetic HFE-Hemochromatosis: The Danish Aspect. Gastroenterol. Res. 2019, 12, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.H.; Besser, M.; Bowden, D.J.; A Kelly, D. Juvenile haemochromatosis. Lancet Child Adolesc. Health 2021, 5, 524–530. [Google Scholar] [CrossRef]
- Wu, L.Y.; Song, Z.Y.; Li, Q.H.; Mou, L.J.; Yu, Y.Y.; Shen, S.S.; Song, X.X. Iron chelators reverse organ damage in type 4B hereditary hemochromatosis Case reports. Medicine 2021, 100, e25258. [Google Scholar] [CrossRef] [PubMed]
- Brissot, P.; Loreal, O. Iron metabolism and related genetic diseases: A cleared land, keeping mysteries. J. Hepatol. 2016, 64, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- E Radford-Smith, D.; Powell, E.E.; Powell, L.W. Haemochromatosis: A clinical update for the practising physician. Intern. Med. J. 2018, 48, 509–516. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, M.C.; Roque, M.E. Physiological focus on the erythropoietin-hepcidin-ferroportin axis. Can. J. Physiol. Pharmacol. 2013, 91, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Grech, L.; Borg, K.; Borg, J. Novel therapies in beta-thalassaemia. Br. J. Clinic. Pharmaco. 2021, 2021, 1–16. [Google Scholar]
- Franchini, M.; Forni, G.L.; Liumbruno, G.M. Is there a standard-of-care for transfusion therapy in thalassemia? Curr. Opin. Hematol. 2017, 24, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.T.; Saliba, A.; Harb, A.R. Iron chelation therapy in transfusion-dependent thalassemia patients: Current strategies and future directions. J. Blood Med. 2015, 6, 197–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Espinoza, A.; Le Blanc, S.; Olivares, M.; Pizarro, F.; Ruz, M.; Arredondo, M. Iron, Copper, and Zinc Transport: Inhibition of Divalent Metal Transporter 1 (DMT1) and Human Copper Transporter 1 (hCTR1) by shRNA. Biol. Trace Element Res. 2011, 146, 281–286. [Google Scholar] [CrossRef]
- Wang, X.; Flores, S.R.; Ha, J.-H.; Doguer, C.; Woloshun, R.R.; Xiang, P.; Grosche, A.; Vidyasagar, S.; Collins, J.F. Intestinal DMT1 Is Essential for Optimal Assimilation of Dietary Copper in Male and Female Mice with Iron-Deficiency Anemia. J. Nutr. 2018, 148, 1244–1252. [Google Scholar] [CrossRef]
- Linder, M.C. Copper Homeostasis in Mammals, with Emphasis on Secretion and Excretion. A Review. Int. J. Mol. Sci. 2020, 21, 4932. [Google Scholar] [CrossRef] [PubMed]
- Vetrik, M.; Mattova, J.; Mackova, H.; Kucka, J.; Pouckova, P.; Kukackova, O.; Brus, J.; Eigner-Henke, S.; Sedlacek, O.; Sefc, L.; et al. Biopolymer strategy for the treatment of Wilson’s disease. J. Control. Release 2018, 273, 131–138. [Google Scholar] [CrossRef]
- Poujois, A.; Woimant, F. Wilson’s disease: A 2017 update. Clin. Res. Hepatol. Gas. 2018, 42, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luan, J.; Zhou, X.Y.; Cui, Y.Z.; Han, J.X. Epidemiology, diagnosis, and treatment of Wilson’s disease. Intract. Rare Disease. Res. 2017, 6, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Maung, M.T.; Carlson, A.; Olea-Flores, M.; Elkhadragy, L.; Schachtschneider, K.M.; Navarro-Tito, N.; Padilla-Benavides, T. The molecular and cellular basis of copper dysregulation and its relationship with human pathologies. FASEB J. 2021, 35, e21810. [Google Scholar] [CrossRef] [PubMed]
- Hedera, P. Update on the clinical management of Wilson’s disease. Appl. Clini. Gen. 2017, 10, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Di Rocco, G.; Toietta, G. Current Biomedical Use of Copper Chelation Therapy. Int. J. Mol. Sci. 2020, 21, 1069. [Google Scholar] [CrossRef]
- Vilensky, J.A.; Redman, K. British anti-Lewisite (dimercaprol): An amazing history. Ann. Emerg. Med. 2003, 41, 378–383. [Google Scholar] [CrossRef]
- Ni, W.; Dong, Q.-Y.; Zhang, Y.; Wu, Z.-Y. Zinc Monotherapy and a Low-copper Diet are Beneficial in Patients with Wilson Disease After Liver Transplantation. CNS Neurosci. Ther. 2013, 19, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R.; Siotto, M.; Polimanti, R. Low-copper diet as a preventive strategy for Alzheimer’s disease. Neurobiol. Aging 2014, 35, S40–S50. [Google Scholar] [CrossRef] [PubMed]
- Kissel, M.; Peschke, P.; Subr, V.; Ulbrich, K.; Schuhmacher, J.; Debus, J.; Friedrich, E. Synthetic macromolecular drug carriers: Biodistribution of poly[(N-2-hydroxypropyl)methacrylamide] copolymers and their accumulation in solid rat tumors. PDA J. Pharm. Sci. Technol. 2001, 55, 191–201. [Google Scholar]
- Jagur-Grodzinski, J. Polymers for targeted and/or sustained drug delivery. Polym. Adv. Technol. 2008, 20, 595–606. [Google Scholar] [CrossRef]
- Fox, M.E.; Szoka, F.C.; Fréchet, J.M.J. Soluble Polymer Carriers for the Treatment of Cancer: The Importance of Molecular Architecture. Accounts Chem. Res. 2009, 42, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Abbina, S.; Abbasi, U.; Gill, A.; Wong, K.; Kalathottukaren, M.T.; Kizhakkedathu, J.N. Design of Safe Nanotherapeutics for the Excretion of Excess Systemic Toxic Iron. ACS Central Sci. 2019, 5, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Ul-Haq, M.I.; Hamilton, J.L.; Lai, B.F.L.; Shenoi, R.A.; Horte, S.; Constantinescu, I.; Leitch, H.A.; Kizhakkedathu, J.N. Design of Long Circulating Nontoxic Dendritic Polymers for the Removal of Iron in Vivo. ACS Nano 2013, 7, 10704–10716. [Google Scholar] [CrossRef]
- Rossi, N.A.; Mustafa, I.; Jackson, J.K.; Burt, H.M.; Horte, S.A.; Scott, M.D.; Kizhakkedathu, J.N. In vitro chelating, cytotoxicity, and blood compatibility of degradable poly(ethylene glycol)-based macromolecular iron chelators. Biomaterials 2009, 30, 638–648. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Ul-Haq, M.I.; Abbina, S.; Kalathottukaren, M.T.; Lai, B.F.; Hatef, A.; Unniappan, S.; Kizhakkedathu, J.N. In vivo efficacy, toxicity and biodistribution of ultra-long circulating desferrioxamine based polymeric iron chelator. Biomaterials 2016, 102, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Chen, X.; Li, H.; Ma, L.; Gu, Z.P.; Qi, X.; Li, X.; Tan, H.; You, C. Long-term and oxidative-responsive algi-nate-deferoxamine conjugates with a low toxicity for iron overload. RSC Adv. 2016, 6, 32471–32479. [Google Scholar] [CrossRef]
- Tian, M.; Chen, X.; Gu, Z.P.; Li, H.; Ma, L.; Qi, X.; Tan, H.; You, C. Synthesis and evaluation of oxidation-responsive algi-nate-deferoxamine conjugates with increased stability and lowtoxicity. Carbohydr. Polym. 2016, 144, 522–530. [Google Scholar] [CrossRef]
- Jones, G.; Goswami, S.K.; Kang, H.M.; Choi, H.S.; Kim, J. Combating iron overload: A case for deferoxamine-based nano-chelators. Nanomedicine 2020, 15, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, T.-M.; Purro, M.; Xiong, M.P. Enzymatically Biodegradable Polyrotaxane–Deferoxamine Conjugates for Iron Chelation. ACS Appl. Mater. Interfaces 2016, 8, 25788–25797. [Google Scholar] [CrossRef] [PubMed]
- Hallaway, P.E.; Eaton, J.W.; Panter, S.S.; Hedlund, B.E. Modulation of deferoxamine toxicity and clearance by covalent at-tachment to biocompatible polymers. Proc. Natl. Acad. Sci. USA 1989, 86, 10108–10112. [Google Scholar] [CrossRef]
- Dragsten, P.R.; Hallaway, P.E.; Hanson, G.J.; Berger, A.E.; Bernard, B.; Hedlund, B.E. First human studies with a high-molecular-weight iron chelator. J. Lab. Clin. Med. 2000, 135, 57–65. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Ul-Haq, M.I.; Creagh, A.L.; Haynes, C.A.; Kizhakkedathu, J.N. Iron Binding and Iron Removal Efficiency of Desferrioxamine Based Polymeric Iron Chelators: Influence of Molecular Size and Chelator Density. Macromol. Biosci. 2016, 17, 1600244. [Google Scholar] [CrossRef]
- Guo, S.; Liu, G.; Frazer, D.M.; Liu, T.; You, L.; Xu, J.; Wang, Y.; Anderson, G.J.; Nie, G. Polymeric Nanoparticles Enhance the Ability of Deferoxamine to Deplete Hepatic and Systemic Iron. Nano Lett. 2018, 18, 5782–5790. [Google Scholar] [CrossRef] [PubMed]
- Harmatz, P.; Grady, R.W.; Dragsten, P.; Vichinsky, E.; Giardina, P.; Madden, J.; Jeng, M.; Miller, B.; Hanson, G.; Hedlund, B. Phase Ib clinical trial of starch-conjugated deferoxamine (40SD02): A novel long-acting iron chelator. Br. J. Haematol. 2007, 138, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.T.C.; Gumbau-Brisa, R.; Allan, D.S.; McDonald, R.; Ferguson, M.J.; Holbein, B.E.; Bierenstiel, M. DIBI, a 3-hydroxypyridin-4-one chelator iron-binding polymer with enhanced antimicrobial activity. MedChemComm 2018, 9, 1206–1212. [Google Scholar] [CrossRef]
- Kang, H.; Han, M.; Xue, J.; Baek, Y.; Chang, J.; Hu, S.; Nam, H.; Jo, M.J.; El Fakhri, G.; Hutchens, M.P.; et al. Renal clearable nanochelators for iron overload therapy. Nat. Commun. 2019, 10, 5134. [Google Scholar] [CrossRef]
- Zhou, T.; Le Kong, X.; Liu, Z.D.; Liu, D.Y.; Hider, R.C. Synthesis and Iron(III)-Chelating Properties of Novel 3-Hydroxypyridin-4-one Hexadentate Ligand-Containing Copolymers. Biomacromolecules 2008, 9, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Skodova, M.; Hruby, M.; Filippov, S.K.; Karlsson, G.; Mackova, H.; Spirkova, M.; Kankova, D.; Steinhart, M.; Stepanek, P.; Ulbrich, K. Novel Polymeric Nanoparticles Assembled by Metal Ion Addition. Macromol. Chem. Phys. 2011, 212, 2339–2348. [Google Scholar] [CrossRef]
- Skodova, M.; Cernoch, P.; Stepanek, P.; Chanova, E.; Kucka, J.; Kalalova, Z.; Kankova, D.; Hruby, M. Self-Assembled Poly-meric Chelate Nanoparticles as Potential Theranostic Agents. ChemPhysChem 2012, 13, 4244–4250. [Google Scholar] [CrossRef] [PubMed]
- Winston, A.; Varaprasad, D.V.; Metterville, J.J.; Rosenkrantz, H. Evaluation of polymeric hydroxamic acid iron chelators for treatment of iron overload. J. Pharmacol. Exp. Ther. 1985, 232, 644–649. [Google Scholar]
- Lim, J.; Venditto, V.J.; Simanek, E.E. Synthesis and characterization of a triazine dendrimer that sequesters iron(III) using 12 desferrioxamine B groups. Bioorganic Med. Chem. 2010, 18, 5749–5753. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Z.; Wang, Y.; Purro, M.; Xiong, M.P. Oxidation-Induced Degradable Nanogels for Iron Chelation. Sci. Rep. 2016, 6, 20923. [Google Scholar] [CrossRef] [PubMed]
- Ergun, B.; Baydemir, G.; Andac, M.; Yavuz, H.; Denizli, A. Ion imprinted beads embedded cryogels for in vitro removal of iron from beta-thalassemic human plasma. J. Appl. Polym. Sci. 2012, 125, 254–262. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Lin, T.M.; Chanana, S.; Xiong, M.P. Nanogel-DFO conjugates as a model to investigate pharmacokinetics, biodistribution, and iron chelation in vivo. Int. J. Pharm. 2018, 538, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qiao, J.; Nagy, T.; Xiong, M.P. ROS-triggered degradable iron-chelating nanogels: Safely improving iron elimination in vivo. J. Control. Release 2018, 283, 84–93. [Google Scholar] [CrossRef]
- Golenser, J.; Domb, A.J.; Teomim, D.; Tsafack, A.; Nisim, O.; Ponka, P.; Eling, W.; I Cabantchik, Z. The treatment of animal models of malaria with iron chelators by use of a novel polymeric device for slow drug release. J. Pharmacol. Exp. Ther. 1997, 281. [Google Scholar]
- Tran, D.T.; Hayes, M.E.; O Noble, C.; Dai, Z.; Working, P.K.; Szoka, F.C. Twice Monthly Liposome Encapsulated Deferoxamine (LDFO) Has a High Molar Efficiency in Removing Total Body Iron in an Iron Dextran-Overloaded Mouse Model. Blood 2016, 128, 2322. [Google Scholar] [CrossRef]
- Kozempel, J.; Hruby, M.; Novakova, M.; Kucka, J.; Leseticky, L.; Lebeda, O. Novel polymer vectors of Cu-Radiochim. Acta 2009, 97, 747–752. [Google Scholar]
- Niculae, D.; Dusman, R.; Leonte, R.A.; Chilug, L.E.; Dragoi, C.M.; Nicolae, A.; Serban, R.M.; Niculae, D.A.; Dumitrescu, I.B.; Draganescu, D. Biological Pathways as Substantiation of the Use of Copper Radioisotopes in Cancer Theranostics. Front. Phys. 2021, 8. [Google Scholar] [CrossRef]
- Pant, K.; Sedlacek, O.; Nadar, R.A.; Hruby, M.; Stephan, H. Radiolabelled Polymeric Materials for Imaging and Treatment of Cancer: Quo Vadis? Adv. Healthc. Mater. 2017, 6, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, G.; Varani, M.; Lauri, C.; Franchi, G.; Pizzichini, P.; Signore, A. Copper-64 labeled nanoparticles for positron emis-sion tomography imaging: A review of the recent literature. Q. J. Nucl. Med. Mol. Im. 2020, 64, 346–355. [Google Scholar]
- Mendonca, P.V.; Serra, A.C.; Silva, C.L.; Simoes, S.; Coelho, J.F.J. Polymeric bile acid sequestrants-Synthesis Using conven-tional methods and new approaches based on “controlled”/living radical polymerization. Progr. Polym. Sci. 2013, 38, 445–461. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Q.; Ou, G.; Yang, M.; Du, L. Bile acid sequestrants: A review of mechanism and design. J. Pharm. Pharmacol. 2021, 73, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J.; Nurchi, V.M.; Andersen, O. Medical Therapy of Patients Contaminated with Radioactive Cesium or Iodine. Biomolecules 2019, 9, 856. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Eracleous, E.; Economides, C.; Kolnagou, A. Advances in Iron Overload Therapies. Prospects for Effective Use of Deferiprone (L1), Deferoxamine, the New Experimental Chelators ICL670, GT56-252, L1NAll and their Combinations. Curr. Med. Chem. 2005, 12, 2663–2681. [Google Scholar] [CrossRef]
- Zhou, T.; Neubert, H.; Liu, D.Y.; Liu, Z.D.; Ma, Y.M.; Le Kong, X.; Luo, W.; Mark, A.S.; Hider, R.C. Iron Binding Dendrimers: A Novel Approach for the Treatment of Haemochromatosis. J. Med. Chem. 2006, 49, 4171–4182. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Adamczyk, B.; Simon, J.; Kitunen, V.; Adamczyk, S.; Smolander, A. Tannins and Their Complex Interaction with Different Organic Nitrogen Compounds and Enzymes: Old Paradigms versus Recent Advances. ChemistryOpen 2017, 6, 610–614. [Google Scholar] [CrossRef]
- Florez, I.D.; Sierra, J.M.; Niño-Serna, L.F. Gelatin tannate for acute diarrhoea and gastroenteritis in children: A systematic review and meta-analysis. Arch. Dis. Child. 2019, 105, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Groborz, O.; Poláková, L.; Kolouchová, K.; Švec, P.; Loukotová, L.; Miriyala, V.M.; Francová, P.; Kučka, J.; Krijt, J.; Páral, P.; et al. Chelating Polymers for Hereditary Hemochromatosis Treatment. Macromol. Biosci. 2020, 20, 2000254. [Google Scholar] [CrossRef] [PubMed]
- Brzonova, I.; Steiner, W.; Zankel, A.; Nyanhongo, G.S.; Guebitz, G.M. Enzymatic synthesis of catechol and hydroxyl-carboxic acid functionalized chitosan microspheres for iron overload therapy. Eur. J. Pharm. Biopharm. 2011, 79, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Polomoscanik, S.C.; Cannon, C.P.; Neenan, T.X.; Holmes-Farley, S.R.; Mandeville, W.H.; Dhal, P.K. Hydroxamic acidcontaining hydrogels for nonabsorbed iron chelation therapy: Synthesis, characterization, and biological evaluation. Biomacromolecules 2005, 6, 2946–2953. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Sullivan, B.P.; Peterson, S.J.; Berkland, C. Nonabsorbable Iron Binding Polymers Prevent Dietary Iron Absorption for the Treatment of Iron Overload. ACS Macro Lett. 2017, 6, 350–353. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Xie, S.-X.; Peltier, E.; Veisi, M.; Berkland, C. Enhancing the selectivity of an iron binding hydrogel. Eur. Polym. J. 2011, 47, 1485–1488. [Google Scholar] [CrossRef]
- Huang, X.L.; Lu, D.; Ma, Y.M.; Zhang, L.M.; Wang, L.N.; Deng, J.; Wang, Z.; Zhao, Y.J. From small deferiprone to macro-molecular micelles: Self-assembly enhances iron chelation. J. Colloid Interface Sci. 2019, 533, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Mattová, J.; Poučková, P.; Kucka, J.; Skodova, M.; Vetrik, M.; Štěpánek, P.; Urbánek, P.; Petrik, M.; Novy, Z.; Hrubý, M. Chelating polymeric beads as potential therapeutics for Wilson’s disease. Eur. J. Pharm. Sci. 2014, 62, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Skodova, M.; Kucka, J.; Vetrik, M.; Skopal, J.; Walterová, Z.; Sedláček, O.; Štěpánek, P.; Mattová, J.; Poučková, P.; Urbánek, P.; et al. Chelating polymeric particles intended for the therapy of Wilson’s disease. React. Funct. Polym. 2013, 73, 1426–1431. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Xie, S.-X.; Golub, A.L.; Gehrke, S.H.; Berkland, C. Siderophore-Mimetic hydrogel for iron chelation therapy. J. Appl. Polym. Sci. 2011, 121, 1384–1392. [Google Scholar] [CrossRef]
- Ghisalberti, C.A.; Falletta, E.; Lammi, C.; Facchetti, G.; Bucci, R.; Erba, E.; Pellegrino, S. Nonabsorbable Iron(III) binding polymers: Synthesis and evaluation of the chelating properties. Polym. Test. 2020, 90, 106693. [Google Scholar] [CrossRef]
- Zhou, T.; Winkelmann, G.; Dai, Z.-Y.; Hider, R.C. Design of clinically useful macromolecular iron chelators. J. Pharm. Pharmacol. 2011, 63, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Saghaie, L.; Liu, D.; Hider, R.C. Synthesis of polymers containing 3-hydroxypyridin-4-one bidentate ligands for treatment of iron overload. Res. Pharm. Sci. 2015, 10, 364–377. [Google Scholar] [PubMed]
- Andrews, M.; Briones, L.; Jaramillo, A.; Pizarro, F.; Arredondo, M. Effect of Calcium, Tannic Acid, Phytic Acid and Pectin over Iron Uptake in an In Vitro Caco-2 Cell Model. Biol. Trace Element Res. 2014, 158, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.T. A Review of Nutrients and Compounds, Which Promote or Inhibit Intestinal Iron Absorption: Making a Platform for Dietary Measures That Can Reduce Iron Uptake in Patients with Genetic Haemochromatosis. J. Nutr. Metab. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Zhang, H.; Onning, G.; Oste, R.; Gramatkovski, E.; Hulthen, L. Improved iron bioavailability in an oat-based beverage: The combined effect of citric acid addition, dephytinization and iron supplementation. Eur. J. Nutr. 2007, 46, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-De-Leyva, A.; Gonçalves-Araújo, T.; Daza, V.; Caraballo, I. A new deferiprone controlled release system obtained by ultrasound-assisted compression. Pharm. Dev. Technol. 2013, 19, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Inman, R.S.; Coughlan, M.M.; Wesslingresnick, M. Extracellular ferrireductase activity of K562 cells is coupled to transfer-rin-dependent iron transport. Biochemistry 1994, 33, 11850–11857. [Google Scholar] [CrossRef]
- Manatschal, C.; Pujol-Giménez, J.; Poirier, M.; Reymond, J.-L.; A Hediger, M.; Dutzler, R. Author response: Mechanistic basis of the inhibition of SLC11/NRAMP-mediated metal ion transport by bis-isothiourea substituted compounds. Elife 2019, 8, e51913. [Google Scholar] [CrossRef] [PubMed]
- Coraça-Huber, D.C.; Dichtl, S.; Steixner, S.; Nogler, M.; Weiss, G. Iron chelation destabilizes bacterial biofilms and potentiates the antimicrobial activity of antibiotics against coagulase-negative Staphylococci. Pathog. Dis. 2018, 76, fty052. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.K.; Li, X.; Huang, X.P.; Liang, S.Y.; Cai, P.G.; Wang, Y.H.; Cui, Y.M.; Chen, W.; Dong, X.W. Development of lacto-bionic acid conjugated-copper chelators as anticancer candidates for hepatocellular carcinoma. Arab. J. Chem. 2021, 14, 103241. [Google Scholar] [CrossRef]
- Argenziano, M.; Di Paola, A.; Tortora, C.; Di Pinto, D.; Pota, E.; Di Martino, M.; Perrotta, S.; Rossi, F.; Punzo, F. Effects of Iron Chelation in Osteosarcoma. Curr. Cancer Drug Targets 2020, 20, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B.; Pretorius, E. Iron-Induced Fibrin in Cardiovascular Disease. Curr. Neurovascular Res. 2013, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B.; Pretorius, E. Novel pathway of iron-induced blood coagulation: Implications for diabetes mellitus and its com-plications. Pol. Arch. Med. Wewn. 2012, 122, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G. Albumin Infusion in Critically Ill COVID-19 Patients: Hemodilution and Anticoagulation. Int. J. Mol. Sci. 2021, 22, 7126. [Google Scholar] [CrossRef] [PubMed]



| Important Iron Chelator Types | |||
|---|---|---|---|
| Chemistry | Donor Atoms | Examples | References |
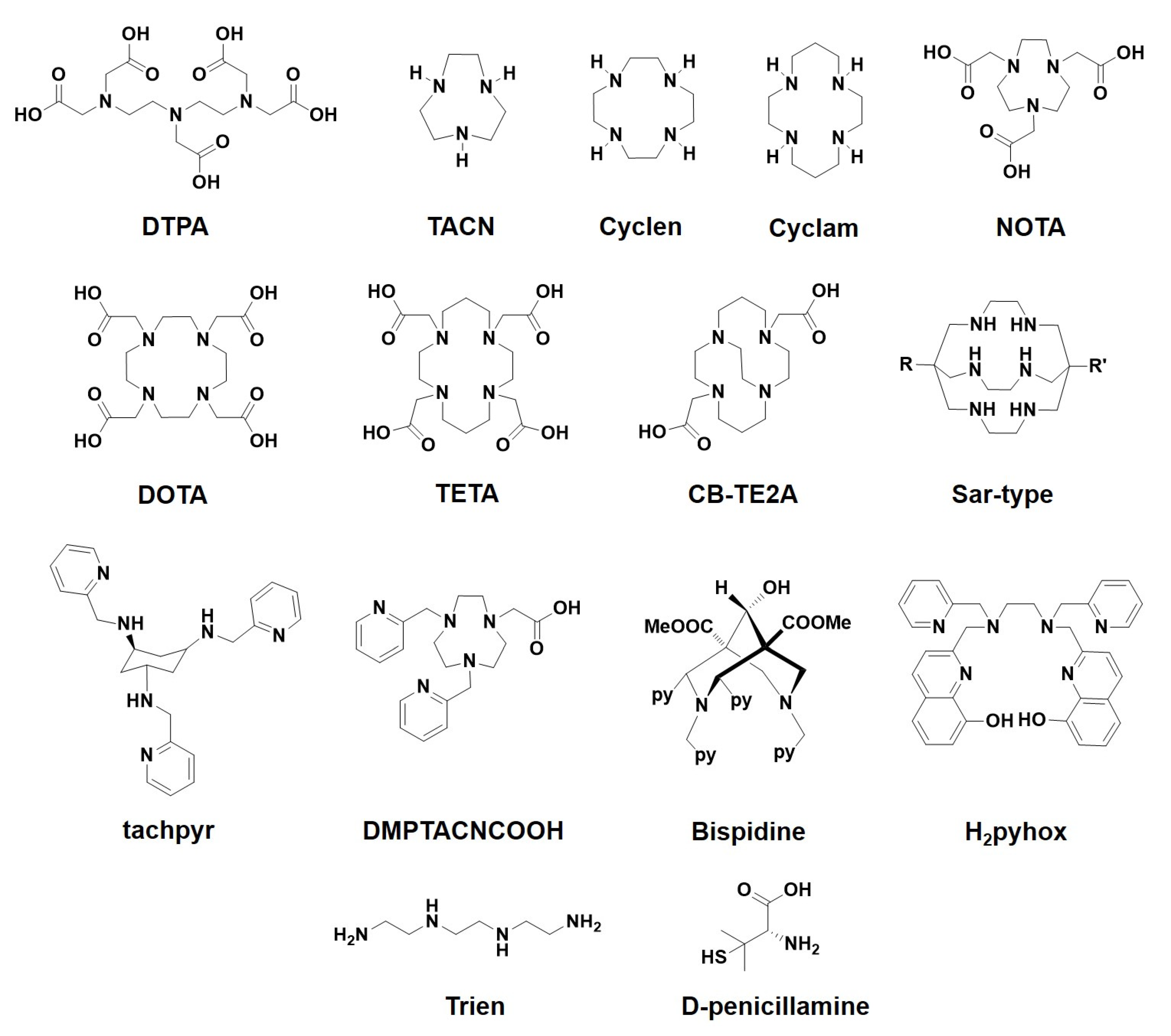
| Hydroxamate type | N,O | DFO | [28] |
| Hydroxypyridone type | O,O | Diferiprone, DIBI, 3,4-HOPO | [14,24,25] |
| 8-Hydroxyquinoline type | N,O | Clioquinol, PBT2, HLA20, M30, VK-28 | [18,19,20,21,22,23] |
| 1,2,4-Triazol type | N,O | Deferasirox | [26,27] |
| Important Copper Chelator Types | |||
| Chemistry | Donor Atoms | Examples | References |
| Open-chain polyamines | N | Trien | [9,23,56,57] |
| Open-chain polyaminocarboxylates | N,O | EDTA, DTPA | [31,32] |
| Azamacrocycles | N | TACN, cyclen, cyclam | [33,34,35] |
| Macrocyclic polyaminocarboxylates | N,O | DOTA, NOTA, TETA | [36,37,38] |
| Caged azamacrocyclic systems | N | CB-TE2A, Sarcophagine | [39,40,48,49] |
| Pyridine ligands | N | tachpyr, dipicolylamine, DMPTACNCOOH, bispydine | [41,42,43,44,45,46,47,50,51,52,53,54] |
| 8-Hydroxyquinoline type | N,O | H2Pyhox | [55] |
| Thiol-amine type | S,N | D-penicillamine | [58,59] |
| Tetrathiomolybdate | S | Tetrathiomolybdate | [9,23,58,59] |
| Administration Route | System Architecture | Biodegradable | References |
|---|---|---|---|
| Parenteral | Linear | No | [98] |
| Parenteral | Linear | Yes | [100,101,104,105,109,110] |
| Parenteral | Hyperbranched | No | [97,99] |
| Parenteral | Crosslinked | Yes | [96] |
| Parenteral | Polyrotaxane | Yes | [102,103] |
| Parenteral | Nanogel | No | [111,112,113,114,116,117,118,119] |
| Parenteral | Dendrimer | No | [106,115] |
| Sustained release of free drug | Device | No | [120] |
| Sustained release of free drug | Liposome | Yes | [121] |
| Oral | Linear | No | [129] |
| Oral | Dendrimer | No | [130] |
| Oral | Hydrogel | No | [84,135,136,137,138] |
| Oral | Micelles | Yes | [112,113,139] |
| Oral | Crosslinked, imprinted | No | [117] |
| Oral | Macroreticular | No | [84,134,140,141] |
| Sustained release of free drug | Device | No | [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hruby, M.; Martínez, I.I.S.; Stephan, H.; Pouckova, P.; Benes, J.; Stepanek, P. Chelators for Treatment of Iron and Copper Overload: Shift from Low-Molecular-Weight Compounds to Polymers. Polymers 2021, 13, 3969. https://doi.org/10.3390/polym13223969
Hruby M, Martínez IIS, Stephan H, Pouckova P, Benes J, Stepanek P. Chelators for Treatment of Iron and Copper Overload: Shift from Low-Molecular-Weight Compounds to Polymers. Polymers. 2021; 13(22):3969. https://doi.org/10.3390/polym13223969
Chicago/Turabian StyleHruby, Martin, Irma Ivette Santana Martínez, Holger Stephan, Pavla Pouckova, Jiri Benes, and Petr Stepanek. 2021. "Chelators for Treatment of Iron and Copper Overload: Shift from Low-Molecular-Weight Compounds to Polymers" Polymers 13, no. 22: 3969. https://doi.org/10.3390/polym13223969
APA StyleHruby, M., Martínez, I. I. S., Stephan, H., Pouckova, P., Benes, J., & Stepanek, P. (2021). Chelators for Treatment of Iron and Copper Overload: Shift from Low-Molecular-Weight Compounds to Polymers. Polymers, 13(22), 3969. https://doi.org/10.3390/polym13223969