Study on Selected Metal-Organic Framework-Based Catalysts for Cycloaddition Reaction of CO2 with Epoxides: A Highly Economic Solution for Carbon Capture and Utilization
Abstract
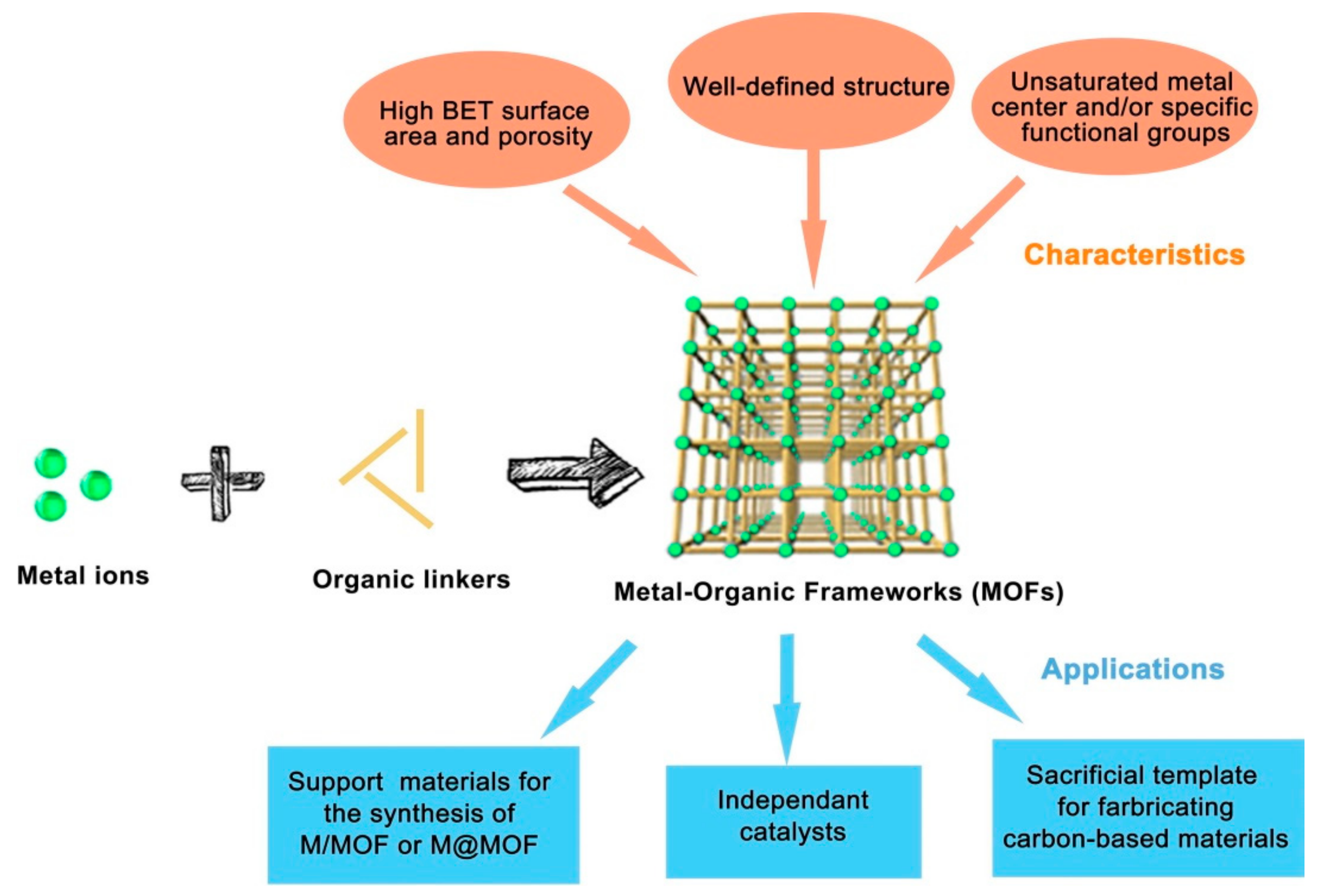
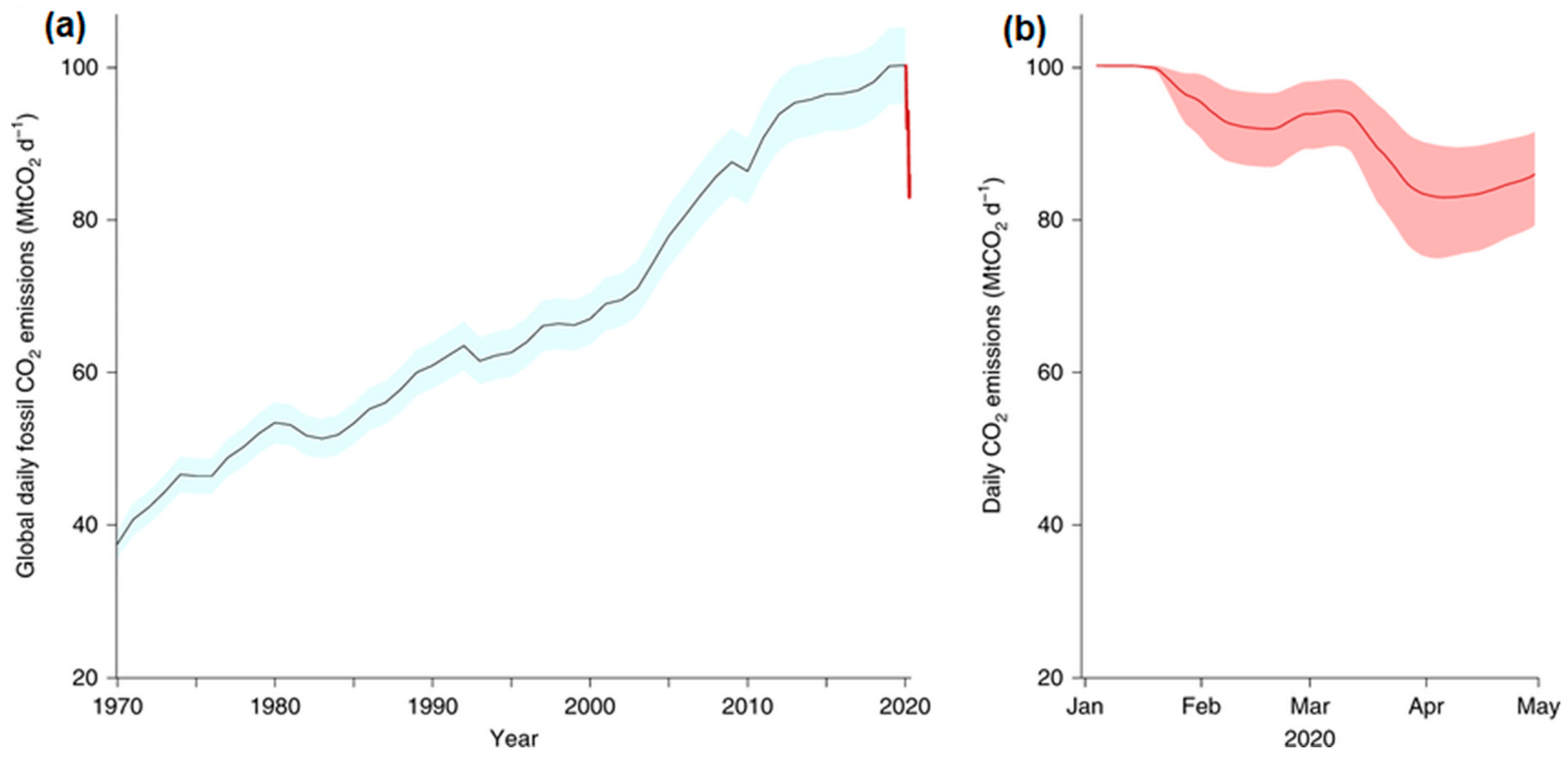
:1. Introduction
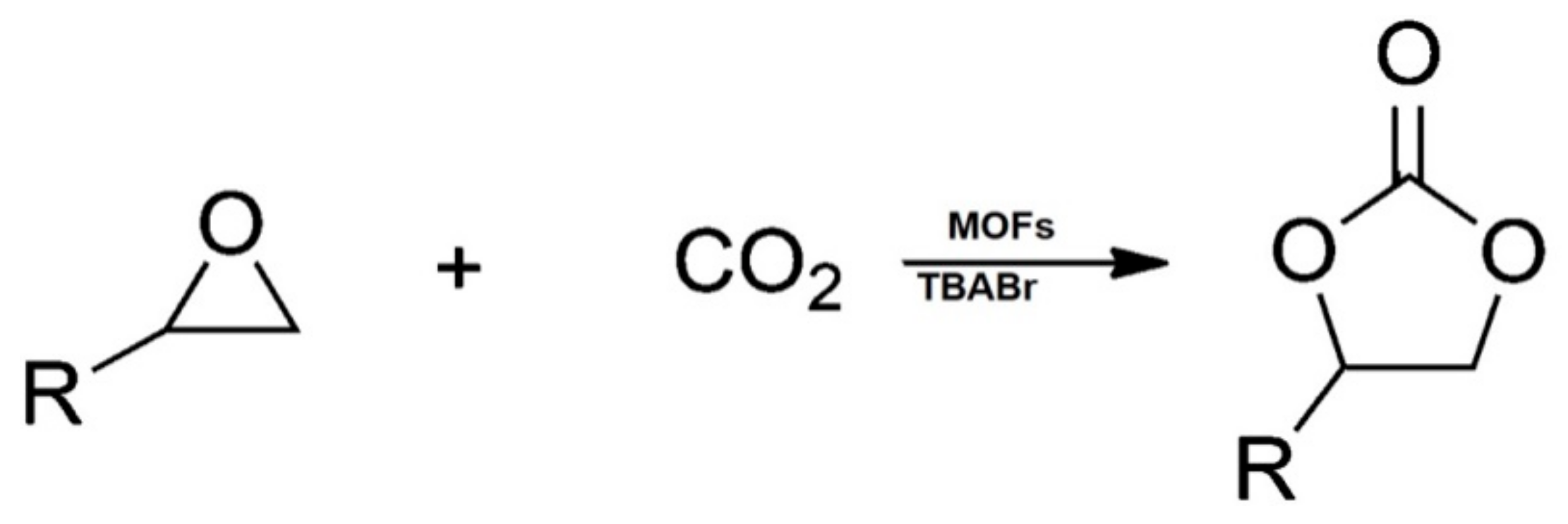
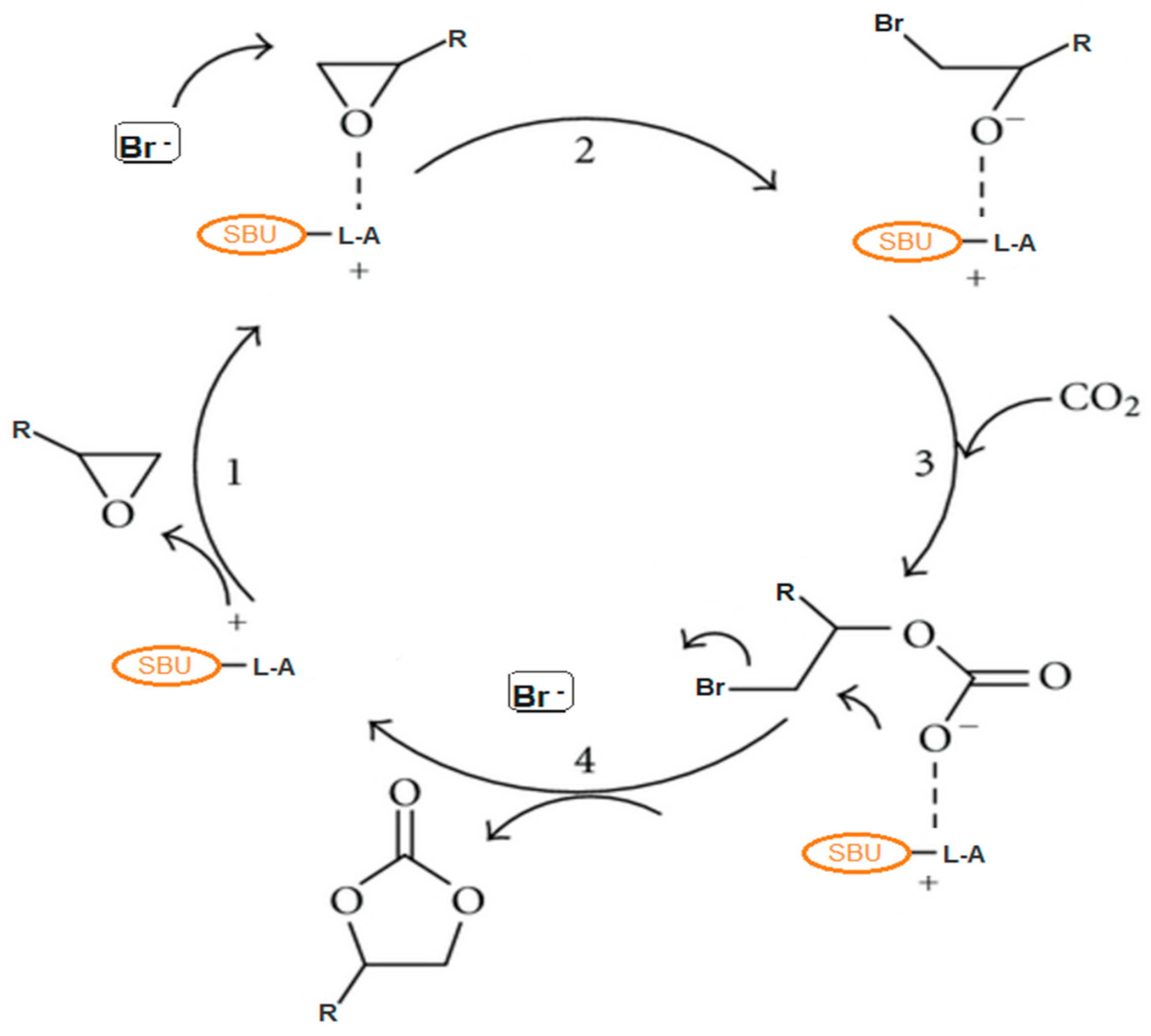
2. Reaction Mechanism for the Production of Cyclic Carbonates from CO2 and Epoxides
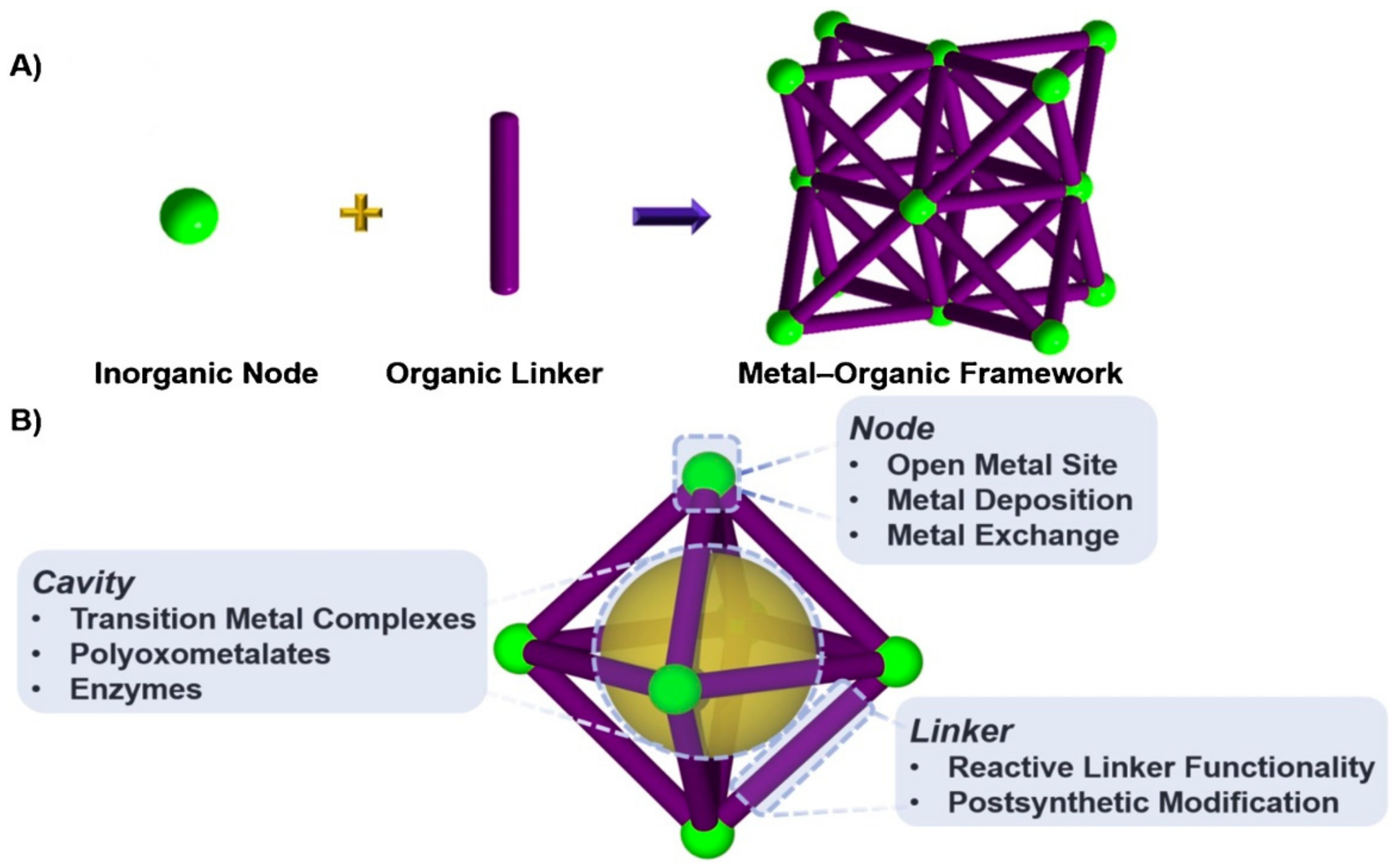
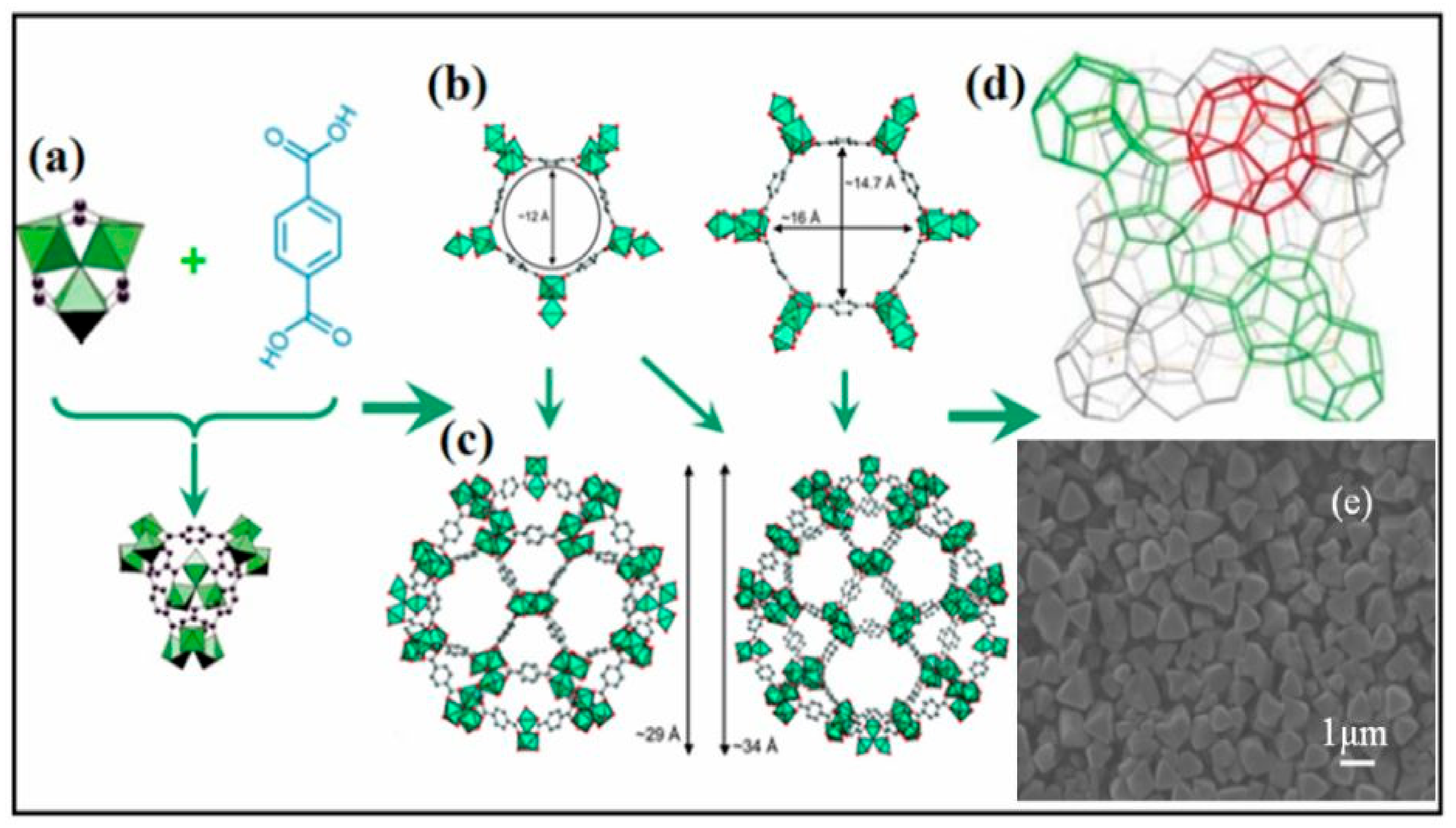
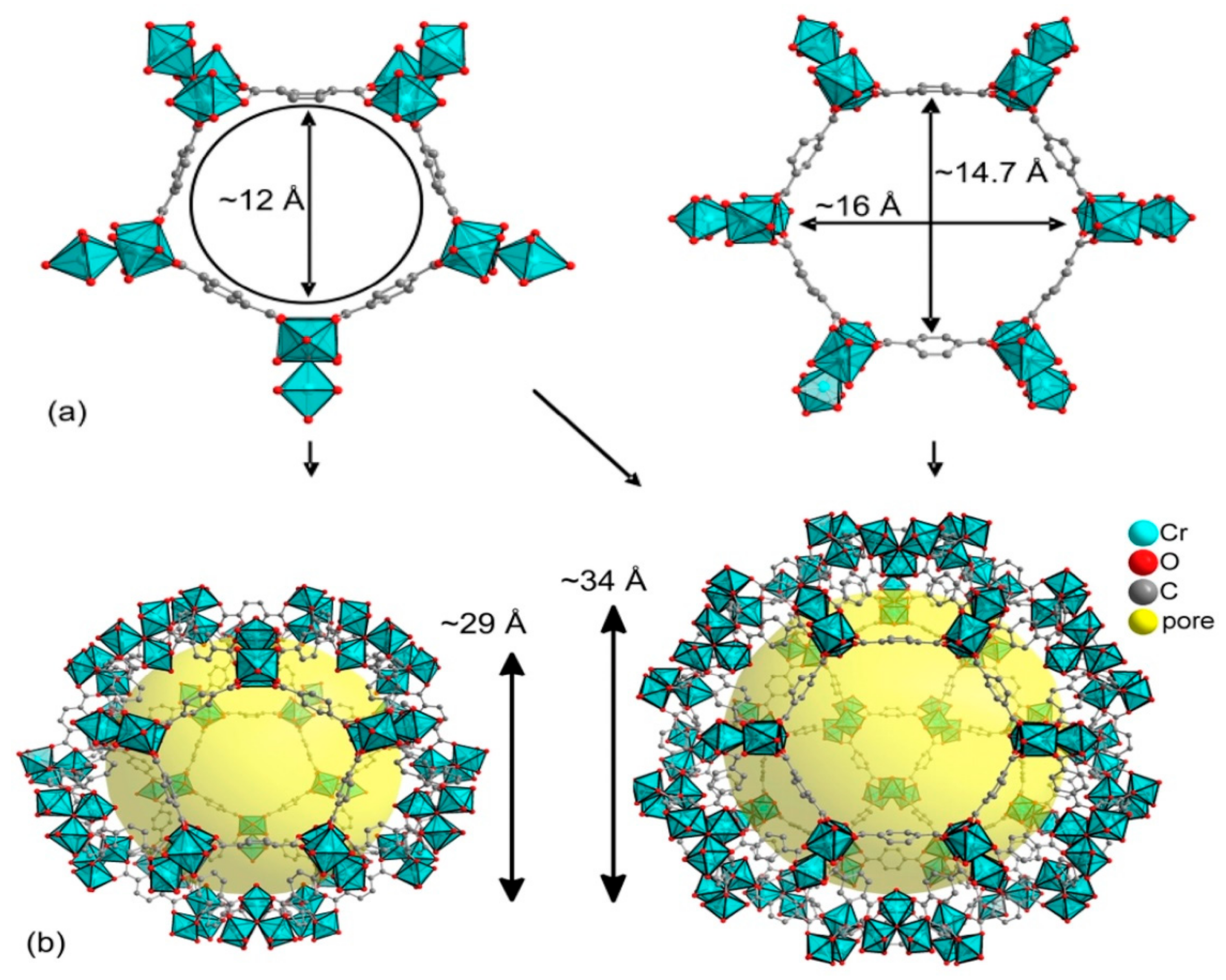
3. Metal-Organic Frameworks in CO2 Cycloaddition with Epoxides
| Entry | MOF Material | Co-Catalyst | Catalyst: Cocatalyst Loading (mol%) | SBET (m2/g) | Epoxide | Press (atm) | Temp. (°C) | Time (h) | Selectivity (%) | Yield (%) | Isosteric Heat Qst (Kj/Mol) | Reusability | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Al(OH) (O2C–CH=CH–CO2)∙nH2O | TBABr | 0.02:0.002 | 1169 | ECH | 10 | 50 | 6 | 97 | 95 | 23 | 4 cycles | [134] |
| 2 | Zn2(Py)(Atz)2∙DMF∙2H2O | TBABr | 0.1:0.1 | 764.5 | PO | 15 | 60 | 4 | 98 | 92 | 27.7 | 6 cycles | [118] |
| 3 | [In2(L)(OH)2]·2DMF·2H2O | TBABr | 0.5:0.2 | 1022 | EBH | 1 | 70 | 12 | 89 | 99 | - | 5 cycles | [83] |
| 4 | F-Mn-MOF-74 | TBABr | 0.1:0.031 | 20.83 | SO | 10 | 100 | 6 | 99 | 99 | - | 7 cycles | [135] |
| 5 | PCN-222(Co)@MTTB | TBABr | 0.1:0.216 | PO/ECH | 1 | 50 | 20 | 98 | >98 | - | 3 cycles | [126] | |
| 6 | rho-ZMOF | TBABr | 0.1:1.4 | 871 | ECH | 10 | 40 | 3 | 98 | 98 | - | 5 cycles | [129] |
| 7 | Co-MOF-2 {[Co(BDC)(L)]·2H2O.xG}n | TBABr | 1.8:2.5 | 6.8 | SO/ECH | 1 | 40 | 12 | 99 | 99 | 35.0 | 6 cycles | [119] |
| 8 | {[Zn(H2O)(HL)]⋅(DMF)2 (H2O)2}n | TBABr | 0.25:0.232 | 945 | PO | 1 | RT | 48 | - | 76 | - | - | [123] |
| 9 | MOF-5-MIX | TBABr | 0.5:0.5 | 357 | ECH | 12 | 50 | 6 | 99 | 98 | - | 5 cycles | [63] |
| 10 | Ce-NU-1008 | TBABr | 0.02:0.002 | SO | 1 | RT | 20 | 95 | - | 3 cycles | [57] | ||
| 11 | Co-MOF-2 {[Co(BDC)(L)]·2H2O·xG}n | KI | 5.0:0.2 | 6.8 | SEO | 1 | 40 | 8 | 99 | 99 | 35.0 | 6 cycles | [56] |
| 12 | {[Ni3HL(μ3-OH)(H2O)2]∙3(H2O)∙DMA}n | TBABr | 0.025:1.5 | 743.5 | ECH | 10 | 100 | 6 | - | >99 | - | 5 cycles | [33] |
| 13 | [(Cu2 BPDSDC∙4DMF)∙2DMF]n | TBABr | 0.05:0.1 | - | PO | 25 | 80 | 5 | 98 | 99 | - | 4 cycles | [136] |
| 14 | {[Co6(OH)2(H2O)4 (cpt)9](NO3)(DMF)13} | TBABr | 0.1:2 | 873 | PO | 1 | 40 | 48 | 97 | 97 | 32 | 4 cycles | [137] |
| 15 | InDCPN-Cl | TBABr | 0.05:5.00 | 997 | SO | 1 | 80 | 24 | 98 | 93 | 30 | 5 cycles | [96] |
| 16 | Ce-NU-1008 | TBABr | 0.002:0.02 | 910 | SO | 1 | RT | 20 | 95 | - | - | [57] | |
| 17 | MOF-5@Imidazolium iodide | - | - | 277.9 | SO | 10 | 110 | 8 | - | 92 | - | 4 cycles | [138] |
| 18 | [(CH3)2NH2][M(COOH)3] | - | 13.1 | 13.11 | PO | 20 | 120 | 6 | 100 | 98 | - | 3 cycles | [139] |
| 19 | Im-MnF [C3H5N2][Mn(COOH)3] | - | - | 81.57 | ECH | 15 | 100 | 6 | 99 | 95 | - | - | [140] |
| 20 | Pt/Mg-MOF-74 | - | 513 | PO | 17.5 | 150 | 4 | 77 | 44 | - | 3 cycles | [72] |
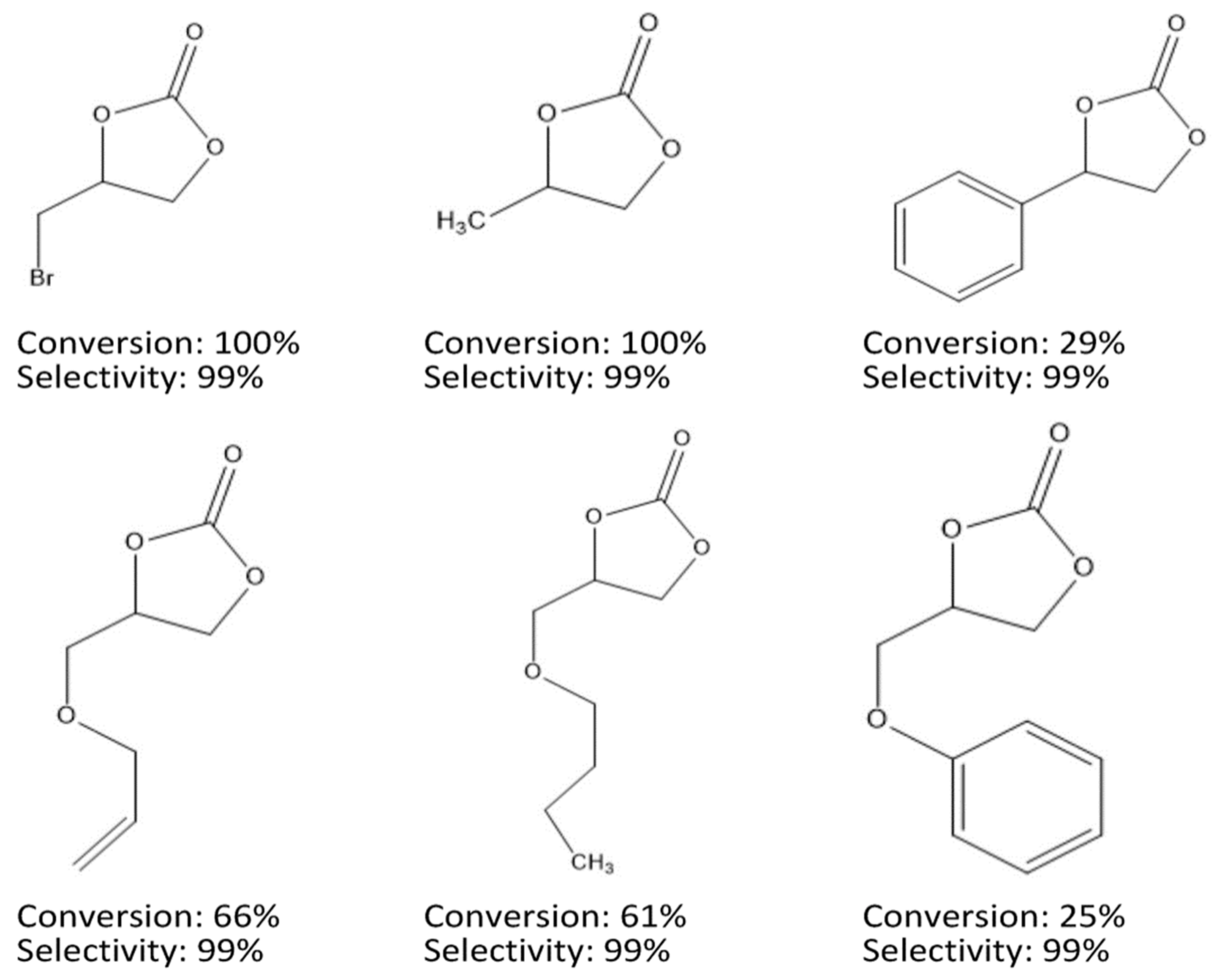
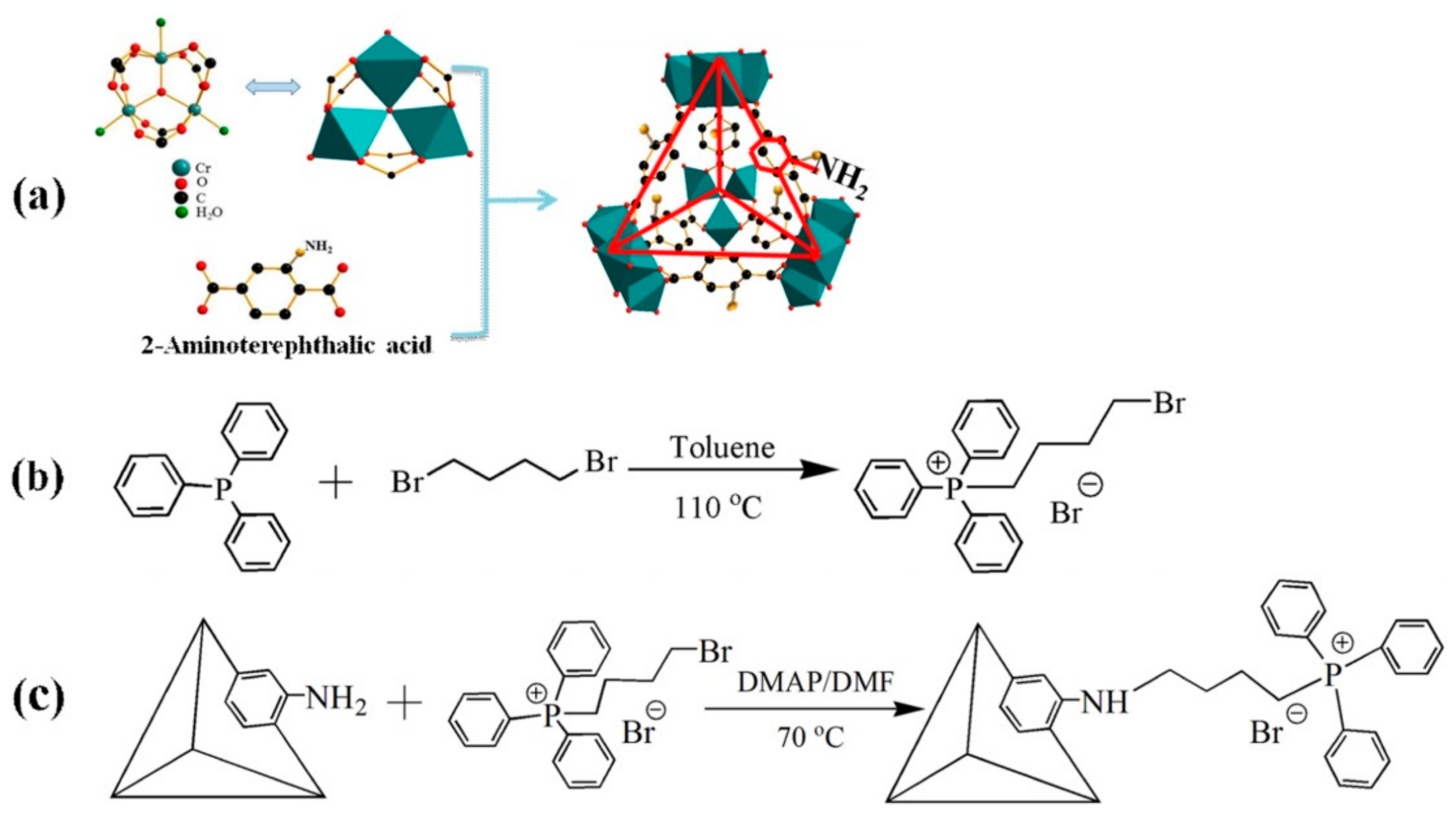
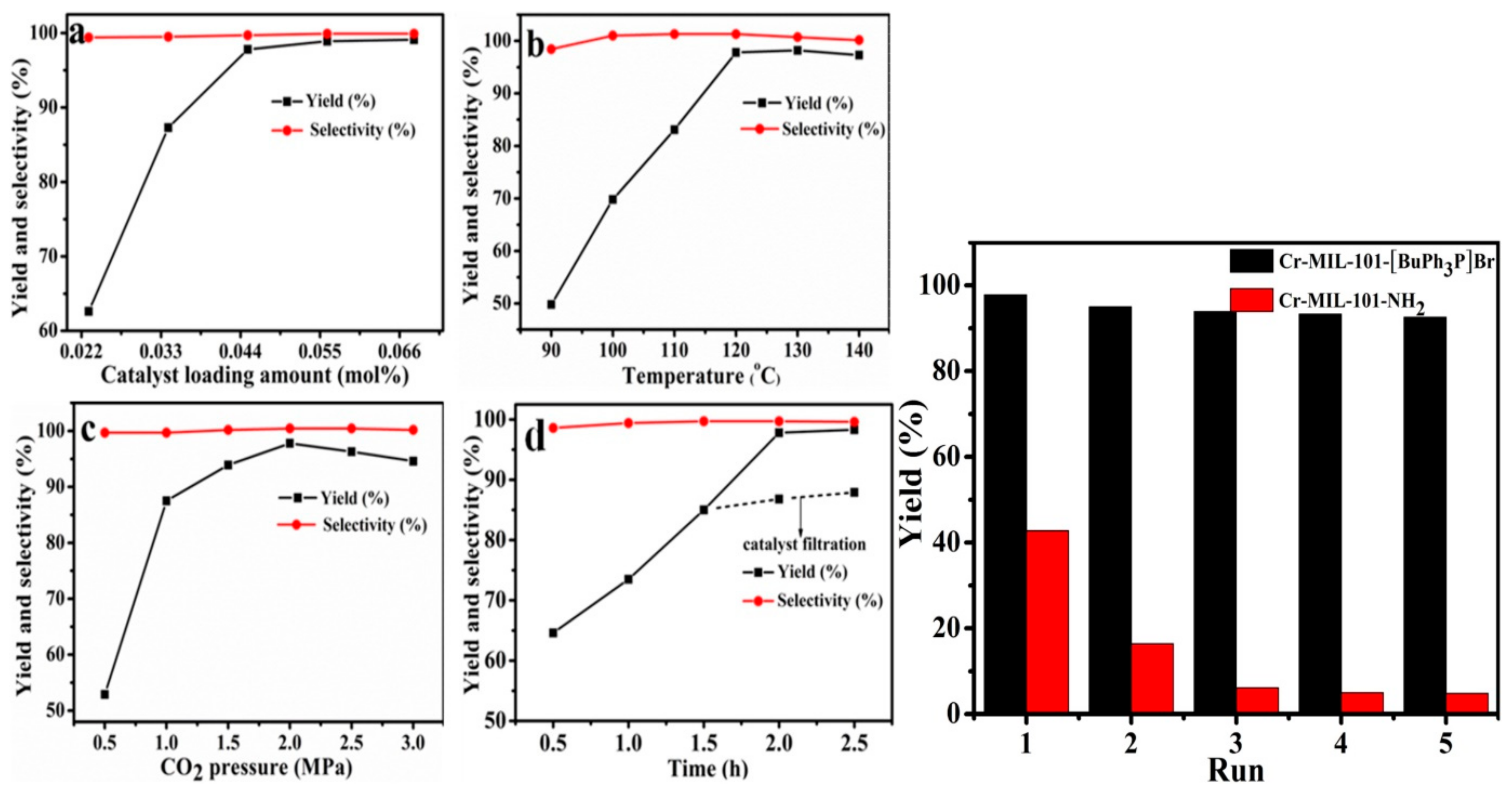
4. MIL-101 Based MOFs in CO2 Cycloaddition with Epoxides
5. HKUST-1 for CO2 Cycloaddition with Epoxides
6. Summary and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MOFs | Metal-organic frameworks |
| TBABr | Tetrabutyl ammonium bromide |
| SBU | Secondary Building Unit |
| SO | Styrene Oxide |
| PO | Propylene oxide |
| PC | propylene Carbonate |
| ECH | Epichlorohydrin |
| EBH | Epibromohydrin |
| SEO | Spiro-Epoxy Oxindole |
| BPE | 1,2-bis(4-pyridyl)ethylene ligand |
| BTC | 1,3,5-benzenetricarboxylate |
| HKUST | Hong Kong University of Science and Technology |
| RT | Room temperature |
| KI | potassium iodide |
| Å | Aperture |
| CTAB | Cetyltrimethylammonium bromide |
References
- Ma, Y.; Wang, Z.; Xu, X.; Wang, J. Review on porous nanomaterials for adsorption and photocatalytic conversion of CO2. Chin. J. Catal. 2017, 38, 1956–1969. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Adam, F. A facile and efficient synthesis of styrene carbonate via cycloaddition of CO2 to styrene oxide over ordered mesoporous MCM-41-Imi/Br catalyst. Appl. Catal. B Environ. 2013, 136–137, 150–159. [Google Scholar] [CrossRef]
- Schoedel, A.; Ji, Z.; Yaghi, O.M. The role of metal–organic frameworks in a carbon-neutral energy cycle. Nat. Energy 2016, 1, 16034. [Google Scholar] [CrossRef]
- Gao, W.-Y.; Chen, Y.; Niu, Y.; Williams, K.; Cash, L.; Perez, P.J.; Wojtas, L.; Cai, J.; Chen, Y.-S.; Ma, S. Crystal Engineering of an nbo Topology Metal-Organic Framework for Chemical Fixation of CO2 under Ambient Conditions. Angew. Chem. Int. Ed. 2014, 53, 2615–2619. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.-S.; Shi, Y.; Xu, H.; Zhao, B. A multifunctional MOF as a recyclable catalyst for the fixation of CO2 with aziridines or epoxides and as a luminescent probe of Cr(vi). Dalton Trans. 2018, 47, 4545–4553. [Google Scholar] [CrossRef] [PubMed]
- Mario, R.; Yuan, Z.; Eden, M.R.; Gani, R. Toward the Development and Deployment of Large-Scale Carbon Dioxide Capture and Conversion Processes. Ind. Eng. Chem. Res. 2016, 55, 3383–3419. [Google Scholar] [CrossRef] [Green Version]
- Raganati, F.; Gargiulo, V.; Ammendola, P.; Alfe, M.; Chirone, R. CO2 capture performance of HKUST-1 in a sound assisted fluidized bed. Chem. Eng. J. 2014, 239, 75–86. [Google Scholar] [CrossRef]
- Beyzavi, M.H.; Stephenson, C.J.; Eliu, Y.; Ekaragiaridi, O.; Hupp, J.T.; Farha, O.K. Metal-Organic Framework-Based Catalysts: Chemical Fixation of CO2 with Epoxides Leading to Cyclic Organic Carbonates. Front. Energy Res. 2015, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Wu, L.; Bu, Z.; Jie, S.; Li, B.-G. Polyethyleneimine-Modified UiO-66-NH2(Zr) Metal–Organic Frameworks: Preparation and Enhanced CO2 Selective Adsorption. ACS Omega 2019, 4, 3188–3197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashim, M.Z.; Tsegab, H.; Rahmani, O.; Abu Bakar, Z.A.; Aminpour, S.M. Reaction Mechanism of Wollastonite In Situ Mineral Carbonation for CO2 Sequestration: Effects of Saline Conditions, Temperature, and Pressure. ACS Omega 2020, 5, 28942–28954. [Google Scholar] [CrossRef]
- Rahmani, O. Siderite precipitation using by-product red gypsum for CO2 sequestration. J. CO2 Util. 2018, 24, 321–327. [Google Scholar] [CrossRef]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Kim, J.; Ahn, W.-S. CO2 capture by amine-functionalized nanoporous materials: A review. Korean J. Chem. Eng. 2014, 31, 1919–1934. [Google Scholar] [CrossRef]
- Pentyala, V.; Davydovskaya, P.; Pohle, R.; Urban, G.; Yurchenko, O. Mg-MOF74 and Co-MOF74 as Sensing Layers for CO2 Detection. Procedia Eng. 2014, 87, 1071–1074. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef] [PubMed]
- Huh, S. Direct Catalytic Conversion of CO2 to Cyclic Organic Carbonates under Mild Reaction Conditions by Metal—Organic Frameworks. Catalysts 2019, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Song, X.; Li, S.; Zhang, J.; Yang, X.; Shen, P.; Gao, L.; Wei, R.; Zhang, J.; Xiao, G. 3D-monoclinic M–BTC MOF (M = Mn, Co, Ni) as highly efficient catalysts for chemical fixation of CO2 into cyclic carbonates. J. Ind. Eng. Chem. 2018, 58, 296–303. [Google Scholar] [CrossRef]
- Liang, J.; Xie, Y.-Q.; Wu, Q.; Wang, X.-Y.; Liu, T.-T.; Li, H.-F.; Huang, Y.-B.; Cao, R. Zinc Porphyrin/Imidazolium Integrated Multivariate Zirconium Metal–Organic Frameworks for Transformation of CO2 into Cyclic Carbonates. Inorg. Chem. 2018, 57, 2584–2593. [Google Scholar] [CrossRef]
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. Catalytic Strategies for the Cycloaddition of Pure, Diluted, and Waste CO2 to Epoxides under Ambient Conditions. ACS Catal. 2018, 8, 419–450. [Google Scholar] [CrossRef]
- Le Quéré, C.; Jackson, R.B.; Jones, M.W.; Smith, A.J.P.; Abernethy, S.; Andrew, R.M.; De-Gol, A.J.; Willis, D.R.; Shan, Y.; Canadell, J.G.; et al. Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nat. Clim. Chang. 2020, 10, 647–653. [Google Scholar] [CrossRef]
- Carreon, M.A. Metal Organic Frameworks as Catalysts in the Conversion of CO2 to Cyclic Carbonates. Indian J. Chem. Sect. A 2012, 51, 1306–1314. [Google Scholar]
- Beyzavi, M.H.; Klet, R.C.; Tussupbayev, S.; Borycz, J.; Vermeulen, N.A.; Cramer, C.J.; Stoddart, J.F.; Hupp, J.T.; Farha, O.K. A Hafnium-Based Metal–Organic Framework as an Efficient and Multifunctional Catalyst for Facile CO2 Fixation and Regioselective and Enantioretentive Epoxide Activation. J. Am. Chem. Soc. 2014, 136, 15861–15864. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, V.; Dong, H.; Rossini, A.J.; Widdifield, C.M.; Vummaleti, S.V.C.; Minenkov, Y.; Poater, A.; Abou-Hamad, E.; Pelletier, J.D.A.; Cavallo, L.; et al. Cooperative Effect of Monopodal Silica-Supported Niobium Complex Pairs Enhancing Catalytic Cyclic Carbonate Production. J. Am. Chem. Soc. 2015, 137, 7728–7739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Usov, P.M.; Xu, W.; Celis-Salazar, P.J.; Lin, S.; Kessinger, M.C.; Landaverde-Alvarado, C.; Cai, M.; May, A.M.; Slebodnick, C.; et al. A New Class of Metal-Cyclam-Based Zirconium Metal-Organic Frameworks for CO2 Adsorption and Chemical Fixation. J. Am. Chem. Soc. 2018, 140, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, C.; Jiang, F.; Chen, Q.; Zhang, L.; Xue, H.; Jiang, H.-L.; Qian, J.; Yuan, D.; Hong, M. Carbon dioxide capture and conversion by an acid-base resistant metal-organic framework. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Ravi, S.; Puthiaraj, P.; Ahn, W.-S. Cyclic carbonate synthesis from CO2 and epoxides over diamine-functionalized porous organic frameworks. J. CO2 Util. 2017, 21, 450–458. [Google Scholar] [CrossRef]
- Kim, H.-G.; Lim, C.-S.; Kim, D.-W.; Cho, D.-H.; Lee, D.-K.; Chung, J.S. Multifunctional alkanolamine as a catalyst for CO2 and propylene oxide cycloaddition. Mol. Catal. 2017, 438, 121–129. [Google Scholar] [CrossRef]
- Bondarenko, G.N.; Dvurechenskaya, E.G.; Ganina, O.G.; Alonso, F.; Beletskaya, I.P. Solvent-free synthesis of cyclic carbonates from CO2 and epoxides catalyzed by reusable alumina-supported zinc dichloride. Appl. Catal. B Environ. 2019, 254, 380–390. [Google Scholar] [CrossRef]
- Luo, R.; Zhang, W.; Yang, Z.; Zhou, X.; Ji, H. Synthesis of cyclic carbonates from epoxides over bifunctional salen aluminum oligomers as a CO2-philic catalyst: Catalytic and kinetic investigation. J. CO2 Util. 2017, 19, 257–265. [Google Scholar] [CrossRef]
- Fu, X.; Zhou, D.; Wang, K.; Jing, H. Pd/C as a high efficient and reusable catalyst for cycloaddition of CO2 to epoxides. J. CO2 Util. 2016, 14, 31–36. [Google Scholar] [CrossRef]
- Ma, D.; Zheng, H.; Wan, H.-M.; Chen, Y.; Xu, J.; Xue, B. Nitrogen-containing ordered mesoporous carbon grafted by alkyl bromide: Simple synthesis and its catalytic application in solvent-free cycloaddition of CO2. Microporous Mesoporous Mater. 2018, 258, 244–250. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.; Puthiaraj, P.; Ahn, W.-S. MgFeAl layered double hydroxide prepared from recycled industrial solid wastes for CO2 fixation by cycloaddition to epoxides. J. CO2 Util. 2019, 34, 395–403. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Z.; Chen, X.-H.; Liu, J. A Ni3O-cluster based porous MOF for catalytic conversion of CO2 to cyclic carbonates. J. Solid State Chem. 2019, 276, 190–193. [Google Scholar] [CrossRef]
- Ahmed, M.; Sakthivel, A. Preparation of cyclic carbonate via cycloaddition of CO2 on epoxide using amine-functionalized SAPO-34 as catalyst. J. CO2 Util. 2017, 22, 392–399. [Google Scholar] [CrossRef]
- Jayakumar, S.; Li, H.; Tao, L.; Li, C.; Liu, L.; Chen, J.; Yang, Q. Cationic Zn-Porphyrin Immobilized in Mesoporous Silicas as Bifunctional Catalyst for CO2 Cycloaddition Reaction under Cocatalyst Free Conditions. ACS Sustain. Chem. Eng. 2018, 6, 9237–9245. [Google Scholar] [CrossRef]
- Fu, X.; Jing, X.; Jin, L.; Zhang, L.; Zhang, X.; Hu, B.; Jing, H. Chiral basket-handle porphyrin-Co complexes for the catalyzed asymmetric cycloaddition of CO2 to epoxides. Chin. J. Catal. 2018, 39, 997–1003. [Google Scholar] [CrossRef]
- Jiang, X.; Gou, F.; Fu, X.; Jing, H. Ionic liquids-functionalized porphyrins as bifunctional catalysts for cycloaddition of carbon dioxide to epoxides. J. CO2 Util. 2016, 16, 264–271. [Google Scholar] [CrossRef]
- Dai, W.; Yang, W.; Zhang, Y.; Wang, D.; Luo, X.; Tu, X. Novel isothiouronium ionic liquid as efficient catalysts for the synthesis of cyclic carbonates from CO2 and epoxides. J. CO2 Util. 2017, 17, 256–262. [Google Scholar] [CrossRef]
- Mao, P.; Dai, W.; Yang, W.; Luo, S.; Zhang, Y.; Mao, J.; Luo, X.; Zou, J. Polymer nanoparticles grafted zinc-containing ionic liquids: A highly efficient and recyclable catalyst for cooperative cycloaddition of CO2 with epoxides. J. CO2 Util. 2018, 28, 96–106. [Google Scholar] [CrossRef]
- Pawar, A.A.; Kim, H. Reaction parameters dependence of the CO2/epoxide coupling reaction catalyzed by tunable ionic liquids, optimization of comonomer-alternating enhancement pathway. J. CO2 Util. 2019, 33, 500–512. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, L.; Jiang, J.; Liu, F.; Zhang, J. Effect of cluster of protic pyrazolium ionic liquids or epoxides on the cycloaddition of CO2. J. Mol. Liq. 2019, 295, 111652. [Google Scholar] [CrossRef]
- Wang, T.; Ma, Y.; Jiang, J.; Zhu, X.; Fan, B.; Yu, G.; Li, N.; Wang, S.; Ren, T.; Wang, L.; et al. Hydroxyl-functionalized pyrazolium ionic liquids to catalyze chemical fixation of CO2: Further benign reaction condition for the single-component catalyst. J. Mol. Liq. 2019, 293, 111479. [Google Scholar] [CrossRef]
- Guglielmero, L.; Mezzetta, A.; Pomelli, C.S.; Chiappe, C.; Guazzelli, L. Evaluation of the effect of the dicationic ionic liquid structure on the cycloaddition of CO2 to epoxides. J. CO2 Util. 2019, 34, 437–445. [Google Scholar] [CrossRef]
- Liu, D.; Li, G.; Liu, J.; Wei, Y.; Guo, H. Mesoporous Titanium-Silicalite Zeolite Containing Organic Templates as a Bifunctional Catalyst for Cycloaddition of CO2 and Epoxides. ACS Appl. Mater. Interfaces 2018, 10, 22119–22129. [Google Scholar] [CrossRef]
- Liu, D.; Li, G.; Liu, J.; Yi, Y. Organic-inorganic hybrid mesoporous titanium silica material as bi-functional heterogeneous catalyst for the CO2 cycloaddition. Fuel 2019, 244, 196–206. [Google Scholar] [CrossRef]
- Kim, H.-G.; Seo, B.; Lim, C.-S. Metal- and halide-free catalysts supported on silica and their applications to CO2 cycloaddition reactions. J. Ind. Eng. Chem. 2019, 75, 202–210. [Google Scholar] [CrossRef]
- Hu, L.; Yan, Z.; Mo, X.; Peng, X.; Chen, L. Hierarchical Co/ZIF-8 as an efficient catalyst for cycloaddition of CO2 and epoxide. Microporous Mesoporous Mater. 2020, 294, 109917. [Google Scholar] [CrossRef]
- Bhin, K.M.; Tharun, J.; Roshan, K.R.; Kim, D.-W.; Chung, Y.; Park, D.-W. Catalytic performance of zeolitic imidazolate framework ZIF-95 for the solventless synthesis of cyclic carbonates from CO2 and epoxides. J. CO2 Util. 2017, 17, 112–118. [Google Scholar] [CrossRef]
- Della Monica, F.; Leone, M.; Buonerba, A.; Grassi, A.; Milione, S.; Capacchione, C. CO2 cycloaddition to epoxides promoted by bis-thioether-phenolate Fe(II) and Fe(III) complexes. Mol. Catal. 2018, 460, 46–52. [Google Scholar] [CrossRef]
- Cuesta-Aluja, L.; Campos-Carrasco, A.; Castilla, J.; Reguero, M.; Masdeu-Bultó, A.M.; Aghmiz, A. Highly active and selective Zn(II)-NN′O Schiff base catalysts for the cycloaddition of CO2 to epoxides. J. CO2 Util. 2016, 14, 10–22. [Google Scholar] [CrossRef]
- Ullah, H.; Mousavi, B.; Younus, H.A.; Khattak, Z.A.; Suleman, S.; Jan, M.T.; Yu, B.; Chaemchuen, S.; Verpoort, F. ONO pincer type ligand complexes of Al(III) as efficient catalyst for chemical fixation of CO2 to epoxides at atmospheric pressure. J. Catal. 2019, 377, 190–198. [Google Scholar] [CrossRef]
- Qu, L.; del Rosal, I.; Li, Q.; Wang, Y.; Yuan, D.; Yao, Y.; Maron, L. Efficient CO2 transformation under ambient condition by heterobimetallic rare earth complexes: Experimental and computational evidences of a synergistic effect. J. CO2 Util. 2019, 33, 413–418. [Google Scholar] [CrossRef]
- Pal, T.K.; De, D.; Bharadwaj, P.K. Metal–organic frameworks for the chemical fixation of CO2 into cyclic carbonates. Coord. Chem. Rev. 2020, 408, 213173. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, Z.; Chen, C.; Bai, J.; He, C.; Duan, C. New rht-Type Metal–Organic Frameworks Decorated with Acylamide Groups for Efficient Carbon Dioxide Capture and Chemical Fixation from Raw Power Plant Flue Gas. ACS Appl. Mater. Interfaces 2016, 8, 31746–31756. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.-N.; Jang, H.-G.; Seo, G.; Ahn, W.-S. CO2 cycloaddition of styrene oxide over MOF catalysts. Appl. Catal. A Gen. 2013, 453, 175–180. [Google Scholar] [CrossRef]
- Parmar, B.; Patel, P.; Pillai, R.S.; Tak, R.K.; Kureshy, R.I.; Khan, N.-U.H.; Suresh, E. Cycloaddition of CO2 with an Epoxide-Bearing Oxindole Scaffold by a Metal–Organic Framework-Based Heterogeneous Catalyst under Ambient Conditions. Inorg. Chem. 2019, 58, 10084–10096. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Zhang, X.; Li, P.; Wang, X.; Buru, C.T.; Bai, P.; Guo, X.; Farha, O.K. Exploring the Role of Hexanuclear Clusters as Lewis Acidic Sites in Isostructural Metal–Organic Frameworks. Chem. Mater. 2019, 31, 4166–4172. [Google Scholar] [CrossRef]
- Rani, D.; Kumar, R.; Kumar, V.; Singh, M. High yield cycloaddition of carbon dioxide to epoxides catalyzed by metal–organic frameworks. Mater. Today Sustain. 2019, 5, 100021. [Google Scholar] [CrossRef]
- Noh, J.; Kim, Y.; Park, H.; Lee, J.; Yoon, M.; Park, M.H.; Kim, Y.; Kim, M. Functional group effects on a metal-organic framework catalyst for CO2 cycloaddition. J. Ind. Eng. Chem. 2018, 64, 478–483. [Google Scholar] [CrossRef]
- Xue, Z.; Jiang, J.; Ma, M.-G.; Li, M.-F.; Mu, T. Gadolinium-Based Metal–Organic Framework as an Efficient and Heterogeneous Catalyst to Activate Epoxides for Cycloaddition of CO2 and Alcoholysis. ACS Sustain. Chem. Eng. 2017, 5, 2623–2631. [Google Scholar] [CrossRef]
- Kim, S.-H.; Babu, R.; Kim, D.-W.; Lee, W.; Park, D.-W. Cycloaddition of CO2 and propylene oxide by using M (HBTC)(4,4′-bipy)·3DMF (M = Ni, Co, Zn) metal-organic frameworks. Chin. J. Catal. 2018, 39, 1311–1319. [Google Scholar] [CrossRef]
- Li, X.; Cheetham, A.K.; Jiang, J. CO2 cycloaddition with propylene oxide to form propylene carbonate on a copper metal-organic framework: A density functional theory study. Mol. Catal. 2019, 463, 37–44. [Google Scholar] [CrossRef]
- Kurisingal, J.F.; Rachuri, Y.; Gu, Y.; Choe, Y.; Park, D.-W. Multi-variate metal organic framework as efficient catalyst for the cycloaddition of CO2 and epoxides in a gas-liquid-solid reactor. Chem. Eng. J. 2020, 386, 121700. [Google Scholar] [CrossRef]
- Kurisingal, J.F.; Rachuri, Y.; Gu, Y.; Kim, G.-H.; Park, D.-W. Binary metal-organic frameworks: Catalysts for the efficient solvent-free CO2 fixation reaction via cyclic carbonates synthesis. Appl. Catal. A Gen. 2019, 571, 1–11. [Google Scholar] [CrossRef]
- Lu, B.-B.; Jiang, W.; Yang, J.; Liu, Y.-Y.; Ma, J.-F. Resorcin [4]arene-Based Microporous Metal–Organic Framework as an Efficient Catalyst for CO2 Cycloaddition with Epoxides and Highly Selective Luminescent Sensing of Cr2O72−. ACS Appl. Mater. Interfaces 2017, 9, 39441–39449. [Google Scholar] [CrossRef] [PubMed]
- Olajire, A.A. Synthesis chemistry of metal-organic frameworks for CO2 capture and conversion for sustainable energy future. Renew. Sustain. Energy Rev. 2018, 92, 570–607. [Google Scholar] [CrossRef]
- Hu, T.-D.; Sun, Y.-W.; Ding, Y.-H. A quantum-chemical insight on chemical fixation carbon dioxide with epoxides co-catalyzed by MIL-101 and tetrabutylammonium bromide. J. CO2 Util. 2018, 28, 200–206. [Google Scholar] [CrossRef]
- Babu, R.; Kim, S.-H.; Kurisingal, J.F.; Kim, H.-J.; Choi, G.-G.; Park, D.-W. A room temperature synthesizable zeolitic imidazolium framework catalyst for the solvent-free synthesis of cyclic carbonates. J. CO2 Util. 2018, 25, 6–13. [Google Scholar] [CrossRef]
- Zou, B.; Hu, C. Halogen-free processes for organic carbonate synthesis from CO2. Curr. Opin. Green Sustain. Chem. 2017, 3, 11–16. [Google Scholar] [CrossRef]
- Sang, Y.; Huang, J. Benzimidazole-based hyper-cross-linked poly(ionic liquid)s for efficient CO2 capture and conversion. Chem. Eng. J. 2020, 385, 123973. [Google Scholar] [CrossRef]
- Li, M.; Abdolmohammadi, S.; Hoseininezhad-Namin, M.S.; Behmagham, F.; Vessally, E. Carboxylative cyclization of propargylic alcohols with carbon dioxide: A facile and Green route to α-methylene cyclic carbonates. J. CO2 Util. 2020, 38, 220–231. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Gardner, T.H.; Kababji, A.H.; Fan, Y. Catalytic conversion of CO2 to propylene carbonate over Pt-decorated Mg-substituted metal organic framework. Appl. Catal. A Gen. 2019, 586, 117225. [Google Scholar] [CrossRef]
- Peng, J.; Wang, S.; Yang, H.-J.; Ban, B.; Wei, Z.; Wang, L.; Bo, L. Chemical fixation of CO2 to cyclic carbonate catalyzed by new environmental-friendly bifunctional bis-β-cyclodextrin derivatives. Catal. Today 2019, 330, 76–84. [Google Scholar] [CrossRef]
- Li, P.; Liu, Y.; Mi, L.; Shi, X.-L.; Duan, P.; Cao, J.; Zhang, W. Bifunctionalized polyacrylonitrile fibers as highly efficient and selective heterogeneous catalysts for cycloaddition of CO2 with epichlorohydrin under mild conditions. Catal. Today 2019, 355, 162–170. [Google Scholar] [CrossRef]
- Yu, W.; Gu, S.; Fu, Y.; Xiong, S.; Pan, C.; Liu, Y.; Yu, G. Carbazole-decorated covalent triazine frameworks: Novel nonmetal catalysts for carbon dioxide fixation and oxygen reduction reaction. J. Catal. 2018, 362, 1–9. [Google Scholar] [CrossRef]
- Hu, L.; Chen, L.; Peng, X.; Zhang, J.; Mo, X.; Liu, Y.; Yan, Z. Bifunctional metal-doped ZIF-8: A highly efficient catalyst for the synthesis of cyclic carbonates from CO2 cycloaddition. Microporous Mesoporous Mater. 2020, 299, 110123. [Google Scholar] [CrossRef]
- Wu, X.; Wang, M.; Xie, Y.; Chen, C.; Li, K.; Yuan, M.; Zhao, X.; Hou, Z. Carboxymethyl cellulose supported ionic liquid as a heterogeneous catalyst for the cycloaddition of CO2 to cyclic carbonate. Appl. Catal. A Gen. 2016, 519, 146–154. [Google Scholar] [CrossRef]
- Rehman, A.; Fernández, A.M.L.; Resul, M.G.; Harvey, A. Kinetic investigations of styrene carbonate synthesis from styrene oxide and CO2 using a continuous flow tube-in-tube gas-liquid reactor. J. CO2 Util. 2018, 24, 341–349. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.-M.; Han, Z.-B.; Ding, M.; Yuan, D.-Q.; Jiang, H.-L. Exceptionally Robust In-Based Metal–Organic Framework for Highly Efficient Carbon Dioxide Capture and Conversion. Inorg. Chem. 2016, 55, 3558–3565. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Zhang, X.; Chang, F.; Xue, N.; Yang, H. Incorporation of flexible ionic polymers into a Lewis acid-functionalized mesoporous silica for cooperative conversion of CO2 to cyclic carbonates. Chin. J. Catal. 2019, 40, 1874–1883. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Lu, C.; Wang, F.; Ma, C.; Chen, Z.; Yang, G. Imidazolium-based polymeric ionic liquids for heterogeneous catalytic conversion of CO2 into cyclic carbonates. Microporous Mesoporous Mater. 2020, 292, 109751. [Google Scholar] [CrossRef]
- Lan, J.; Qu, Y.; Xu, P.; Sun, J. Novel HBD-Containing Zn (dobdc) (datz) as efficiently heterogeneous catalyst for CO2 chemical conversion under mild conditions. Green Energy Environ. 2020, 6, 66–74. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, P.-Y.; Ma, L.-N.; Liu, B.; Hou, L.; Wang, Y.-Y. Two Robust In(III)-Based Metal–Organic Frameworks with Higher Gas Separation, Efficient Carbon Dioxide Conversion, and Rapid Detection of Antibiotics. Inorg. Chem. 2020, 59, 5231–5239. [Google Scholar] [CrossRef]
- Dai, Z.; Sun, Q.; Liu, X.; Bian, C.; Wu, Q.; Pan, S.; Wang, L.; Meng, X.; Deng, F.; Xiao, F.-S. Metalated porous porphyrin polymers as efficient heterogeneous catalysts for cycloaddition of epoxides with CO2 under ambient conditions. J. Catal. 2016, 338, 202–209. [Google Scholar] [CrossRef]
- Sankar, M.; Tarte, N.; Manikandan, P. Effective catalytic system of zinc-substituted polyoxometalate for cycloaddition of CO2 to epoxides. Appl. Catal. A Gen. 2004, 276, 217–222. [Google Scholar] [CrossRef]
- Alivand, M.S.; Shafiei-Alavijeh, M.; Tehrani, N.H.M.H.; Ghasemy, E.; Rashidi, A.; Fakhraie, S. Facile and high-yield synthesis of improved MIL-101(Cr) metal-organic framework with exceptional CO2 and H2S uptake; the impact of excess ligand-cluster. Microporous Mesoporous Mater. 2019, 279, 153–164. [Google Scholar] [CrossRef]
- Niknam, E.; Panahi, F.; Daneshgar, F.; Bahrami, F.; Khalafi-Nezhad, A. Metal–Organic Framework MIL-101(Cr) as an Efficient Heterogeneous Catalyst for Clean Synthesis of Benzoazoles. ACS Omega 2018, 3, 17135–17144. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhang, J.; Xue, Z.; Han, B.; Sang, X.; Liu, C.; Yang, G. Highly mesoporous metal–organic framework assembled in a switchable solvent. Nat. Commun. 2014, 5, 4465. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-S.; Adhikari, A.K.; Ku, C.-N.; Chiang, C.-L.; Kuo, H. Synthesis and characterization of porous HKUST-1 metal organic frameworks for hydrogen storage. Int. J. Hydrogen Energy 2012, 37, 13865–13871. [Google Scholar] [CrossRef]
- Tufa, R.A.; Chanda, D.; Ma, M.; Aili, D.; Demissie, T.B.; Vaes, J.; Li, Q.; Liu, S.; Pant, D. Towards highly efficient electrochemical CO2 reduction: Cell designs, membranes and electrocatalysts. Appl. Energy 2020, 277, 115557. [Google Scholar] [CrossRef]
- Maina, J.W.; Pozo-Gonzalo, C.; Kong, L.; Schütz, J.; Hill, M.; Dumée, L.F. Metal Organic Framework Based Catalysts for CO2 Conversion. Mater. Horizons 2017, 4, 345–361. [Google Scholar] [CrossRef]
- Connolly, B.M.; Mehta, J.P.; Moghadam, P.Z.; Wheatley, A.E.; Fairen-Jimenez, D. From synthesis to applications: Metal–organic frameworks for an environmentally sustainable future. Curr. Opin. Green Sustain. Chem. 2018, 12, 47–56. [Google Scholar] [CrossRef]
- Yu, B.; Zou, B.; Hu, C.-W. Recent applications of polyoxometalates in CO2 capture and transformation. J. CO2 Util. 2018, 26, 314–322. [Google Scholar] [CrossRef]
- Chen, F.; Dong, T.; Chi, Y.; Xu, Y.; Hu, C. Transition-Metal-Substituted Keggin-Type Germanotungstates for Catalytic Conversion of Carbon Dioxide to Cyclic Carbonate. Catal. Lett. 2010, 139, 38–41. [Google Scholar] [CrossRef]
- Ugale, B.; Dhankhar, S.S.; Nagaraja, C.M. Exceptionally Stable and 20-Connected Lanthanide Metal–Organic Frameworks for Selective CO2 Capture and Conversion at Atmospheric Pressure. Cryst. Growth Des. 2018, 18, 2432–2440. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, J.; Sun, X.; Li, G.; Liu, Y.; Verma, G.; Ma, S. Indium-Organic Frameworks Based on Dual Secondary Building Units Featuring Halogen-Decorated Channels for Highly Effective CO2 Fixation. Chem. Mater. 2019, 31, 1084–1091. [Google Scholar] [CrossRef]
- Nguyen, P.T.K.; Nguyen, H.N.; Trickett, C.A.; Ton, Q.T.; Gutiérrez-Puebla, E.; Monge, M.A.; Cordova, K.E.; Gándara, F. New Metal–Organic Frameworks for Chemical Fixation of CO2. ACS Appl. Mater. Interfaces 2017, 10, 733–744. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Yang, X.; Zhao, L.; Wang, G. Functionalized IRMOF-3 as efficient heterogeneous catalyst for the synthesis of cyclic carbonates. J. Mol. Catal. A Chem. 2012, 361–362, 12–16. [Google Scholar] [CrossRef]
- Kiatkittipong, K.; Shukri, M.A.A.M.; Kiatkittipong, W.; Lim, J.W.; Show, P.L.; Lam, M.K.; Assabumrungrat, S. Green Pathway in Utilizing CO2 via Cycloaddition Reaction with Epoxide—A Mini Review. Processes 2020, 8, 548. [Google Scholar] [CrossRef]
- Calabrese, C.; Giacalone, F.; Aprile, C. Hybrid Catalysts for CO2 Conversion into Cyclic Carbonates. Catalysts 2019, 9, 325. [Google Scholar] [CrossRef] [Green Version]
- Di Credico, B.; Redaelli, M.; Bellardita, M.; Calamante, M.; Cepek, C.; Cobani, E.; D’Arienzo, M.; Evangelisti, C.; Marelli, M.; Moret, M.; et al. Step-by-Step Growth of HKUST-1 on Functionalized TiO2 Surface: An Efficient Material for CO2 Capture and Solar Photoreduction. Catalysts 2018, 8, 353. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Huang, Y.-B.; Cao, R. Metal–organic frameworks and porous organic polymers for sustainable fixation of carbon dioxide into cyclic carbonates. Coord. Chem. Rev. 2019, 378, 32–65. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Effects of Functionalization, Catenation, and Variation of the Metal Oxide and Organic Linking Units on the Low-Pressure Hydrogen Adsorption Properties of Metal−Organic Frameworks. J. Am. Chem. Soc. 2006, 128, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, X.; Feng, X.; Li, J.; Huang, Y.; Zhao, J.; Guo, Y.; Dong, X.; Han, R.; Qi, P.; et al. Facile fabrication of magnetically recyclable metal–organic framework nanocomposites for highly efficient and selective catalytic oxidation of benzylic C–H bonds. Chem. Commun. 2014, 50, 8374–8377. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yao, H.-F.; Xi, F.-G.; Gao, E.-Q. Amino-functionalized Zr(IV) metal–organic framework as bifunctional acid–base catalyst for Knoevenagel condensation. J. Mol. Catal. A Chem. 2014, 390, 198–205. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Wasson, M.C.; Buru, C.T.; Chen, Z.; Islamoglu, T.; Farha, O.K. Metal–organic frameworks A tunable platform to access single-site heterogeneous catalysts. Appl. Catal. A Gen. 2019, 586, 117214. [Google Scholar] [CrossRef]
- Du, P.D.; Thanh, H.T.M.; To, T.C.; Thang, H.S.; Tinh, M.X.; Tuyen, T.N.; Hoa, T.T.; Khieu, D.Q. Metal-Organic Framework MIL-101: Synthesis and Photocatalytic Degradation of Remazol Black B Dye. J. Nanomater. 2019, 2019, 6061275. [Google Scholar] [CrossRef]
- Uba, Z.Z.; Hana, N.; Abu, H.; Soraya, N.; Jumbri, K.; Ain, N.; Abdullah, F.; Abdul, E.; Saad, B. Adsorption of Chrysene in Aqueous Solution onto MIL-88(Fe) and NH2-MIL-88(Fe) Metal-Organic Frameworks: Kinetics, Isotherms, Thermodynamics and Docking Simulation Studies. J. Environ. Chem. Eng. 2019, 88, 103544. [Google Scholar] [CrossRef]
- Zhuo, N.; Lan, Y.; Yang, W.; Yang, Z.; Li, X.; Zhou, X.; Liu, Y.; Shen, J.; Zhang, X. Adsorption of three selected pharmaceuticals and personal care products (PPCPs) onto MIL-101(Cr)/natural polymer composite beads. Sep. Purif. Technol. 2017, 177, 272–280. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, Y.; Zhao, H.; Gao, Z.; Zhang, Y.; Zhi, L.; Wang, Y.; Huang, H. Functionalized metal-organic frameworks for effective removal of rocephin in aqueous solutions. J. Colloid Interface Sci. 2018, 514, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.W.; Khan, N.A.; Jhung, S.H. Removal of nitroimidazole antibiotics from water by adsorption over metal–organic frameworks modified with urea or melamine. Chem. Eng. J. 2017, 315, 92–100. [Google Scholar] [CrossRef]
- Hasan, Z.; Khan, N.A.; Jhung, S.H. Adsorptive removal of diclofenac sodium from water with Zr-based metal–organic frameworks. Chem. Eng. J. 2016, 284, 1406–1413. [Google Scholar] [CrossRef]
- Zango, Z.U.; Sambudi, N.S.; Jumbri, K.; Abu Bakar, N.H.H.; Abdullah, N.A.F.; Negim, E.-S.M.; Saad, B. Experimental and molecular docking model studies for the adsorption of polycyclic aromatic hydrocarbons onto UiO-66(Zr) and NH2-UiO-66(Zr) metal-organic frameworks. Chem. Eng. Sci. 2020, 220, 115608. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, N.; Gan, C.; Liu, Y.; Chen, L.; Zhang, C.; Fang, Y. Synthesis of In2S3/UiO-66 hybrid with enhanced photocatalytic activity towards methyl orange and tetracycline hydrochloride degradation under visible-light irradiation. Mater. Sci. Semicond. Process. 2019, 91, 212–221. [Google Scholar] [CrossRef]
- Wu, T.; Yan, T.; Zhang, X.; Feng, Y.; Wei, D.; Sun, M.; Du, B.; Wei, Q. A competitive photoelectrochemical immunosensor for the detection of diethylstilbestrol based on an Au/UiO-66(NH2)/CdS matrix and a direct Z-scheme Melem/CdTe heterojunction as labels. Biosens. Bioelectron. 2018, 117, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, T.; Kukkar, D.; Kim, K.-H.; Sohn, J.R.; Deep, A. Cyclodextrin-metal–organic framework (CD-MOF): From synthesis to applications. J. Ind. Eng. Chem. 2019, 72, 50–66. [Google Scholar] [CrossRef]
- Lan, J.; Qu, Y.; Zhang, X.; Ma, H.; Xu, P.; Sun, J. A novel water-stable MOF Zn(Py)(Atz) as heterogeneous catalyst for chemical conversion of CO2 with various epoxides under mild conditions. J. CO2 Util. 2020, 35, 216–224. [Google Scholar] [CrossRef]
- Patel, P.; Parmar, B.; Pillai, R.S.; Ansari, A.; Khan, N.-U.H.; Suresh, E. CO2 fixation by cycloaddition of mono/disubstituted epoxides using acyl amide decorated Co(II) MOF as a synergistic heterogeneous catalyst. Appl. Catal. A Gen. 2020, 590, 117375. [Google Scholar] [CrossRef]
- Gupta, A.K.; Guha, N.; Krishnan, S.; Mathur, P.; Rai, D.K. A Three-Dimensional Cu(II)-MOF with Lewis acid-base dual functional sites for Chemical Fixation of CO2 via Cyclic Carbonate Synthesis. J. CO2 Util. 2020, 39, 101173. [Google Scholar] [CrossRef]
- Gallardo-Fuentes, S.; Contreras, R.; Isaacs, M.; Honores, J.; Quezada, D.; Landaeta, E.; Ormazábal-Toledo, R. On the mechanism of CO2 electro-cycloaddition to propylene oxides. J. CO2 Util. 2016, 16, 114–120. [Google Scholar] [CrossRef]
- Wu, Y.; Song, X.; Zhang, J.; Xu, S.; Xu, N.; Yang, H.; Miao, Y.; Gao, L.; Zhang, J.; Xiao, G. Zn2(C9H3O6)(C4H5N2)(C4H6N2)3 MOF as a highly efficient catalyst for chemical fixation of CO2 into cyclic carbonates and kinetic studies. Chem. Eng. Res. Des. 2018, 140, 273–282. [Google Scholar] [CrossRef]
- Cai, Z.; Qian, J.; Zhao, Y.; Cheng, Y. Microporous MOF with open metal sites for CO2 fixation and protective effect on osteoarthritis by regulating the activation of PI3K/AKT pathway. J. Solid State Chem. 2020, 283, 121169. [Google Scholar] [CrossRef]
- Zanon, A.; Chaemchuen, S.; Mousavi, B.; Verpoort, F. 1 Zn-doped ZIF-67 as catalyst for the CO2 fixation into cyclic carbonates. J. CO2 Util. 2017, 20, 282–291. [Google Scholar] [CrossRef]
- Ciprian, M.; Ruiz, K.H.; Kassymova, M.; Wang, T.; Zhuiykov, S.; Chaemchuen, S.; Tu, R.; Verpoort, F. 3D derived N-doped carbon matrix from 2D ZIF-L as an enhanced stable catalyst for chemical fixation. Microporous Mesoporous Mater. 2019, 285, 80–88. [Google Scholar] [CrossRef]
- Liu, E.; Zhu, J.; Yang, W.; Liu, F.; Huang, C.; Yin, S. PCN-222(Co) Metal–Organic Framework Nanorods Coated with 2D Metal–Organic Layers for the Catalytic Fixation of CO2 to Cyclic Carbonates. ACS Appl. Nano Mater. 2020, 3, 3578–3584. [Google Scholar] [CrossRef]
- Dhankhar, S.S.; Ugale, B.; Nagaraja, C.M. Co-Catalyst-Free Chemical Fixation of CO2 into Cyclic Carbonates by using Metal-Organic Frameworks as Efficient Heterogeneous Catalysts. Chem. Asian J. 2020, 15, 2403–2427. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, F.; Khavasi, H.R. Diversity-Oriented Metal Decoration on UiO-Type Metal–Organic Frameworks: An Efficient Approach to Increase CO2 Uptake and Catalytic Conversion to Cyclic Carbonates. ACS Omega 2019, 4, 19037–19045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jang, M.-S.; Lee, J.; Puthiaraj, P.; Ahn, W.-S. Zeolite-Like Metal Organic Framework (ZMOF) with a rho Topology for a CO2 Cycloaddition to Epoxides. ACS Sustain. Chem. Eng. 2020, 8, 7078–7086. [Google Scholar] [CrossRef]
- Dhankhar, S.S.S.; Nagaraja, C.M. Construction of 3D lanthanide based MOFs with pores decorated with basic imidazole groups for selective capture and chemical fixation of CO2. New J. Chem. 2020, 44, 9090–9096. [Google Scholar] [CrossRef]
- Hwang, G.-Y.; Roshan, R.; Ryu, H.-S.; Jeong, H.-M.; Ravi, S.; Kim, M.-I.; Park, D.-W. A highly efficient zeolitic imidazolate framework catalyst for the co-catalyst and solvent free synthesis of cyclic carbonates from CO2. J. CO2 Util. 2016, 15, 123–130. [Google Scholar] [CrossRef]
- Liu, X.; Tang, B.; Long, J.; Zhang, W.; Liu, X.; Mirza, Z. The development of MOFs-based nanomaterials in heterogeneous organocatalysis. Sci. Bull. 2018, 63, 502–524. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.-D.; Jiang, Y.; Ding, Y.-H. Computational screening of metal-substituted HKUST-1 catalysts for chemical fixation of carbon dioxide into epoxides. J. Mater. Chem. A 2019, 7, 14825–14834. [Google Scholar] [CrossRef]
- Kim, H.S.; Yu, K.; Puthiaraj, P.; Ahn, W.-S. CO2 cycloaddition to epichlorohydrin over an aluminum fumarate metal-organic framework synthesized by a sonochemical route. Microporous Mesoporous Mater. 2020, 306, 110432. [Google Scholar] [CrossRef]
- Feng, C.; Qiao, S.; Guo, Y.; Xie, Y.; Zhang, L.; Akram, N.; Li, S.; Wang, J. Adenine-assisted synthesis of functionalized F-Mn-MOF-74 as an efficient catalyst with enhanced catalytic activity for the cycloaddition of carbon dioxide. Colloids Surf. A Physicochem. Eng. ASP 2020, 597, 124781. [Google Scholar] [CrossRef]
- Guo, F. A novel 2D Cu(II)-MOF as a heterogeneous catalyst for the cycloaddition reaction of epoxides and CO2 into cyclic carbonates. J. Mol. Struct. 2019, 1184, 557–561. [Google Scholar] [CrossRef]
- Chen, D.-M.; Zhang, X.-J. A polyhedron-based metal-organic framework with a rare hexanuclear Co(II) cluster for selective sorption and chemical conversion for CO2. J. Solid State Chem. 2019, 278, 120906. [Google Scholar] [CrossRef]
- Mohammadian, R.; Kamyar, N.; Kaffashian, A.; Amini, M.M.; Shaabani, A. Synthesis of Defect-Engineered Homochiral Metal-Organic Frameworks Using L-Amino Acids: A Comprehensive Study of Chiral Catalyst Performance in CO2 Fixation Reaction. ChemistrySelect 2020, 5, 10346–10354. [Google Scholar] [CrossRef]
- Wu, Y.; Song, X.; Xu, S.; Yu, T.; Zhang, J.; Qi, Q.; Gao, L.; Zhang, J.; Xiao, G. [(CH3)2NH2][M(COOH)3] (M=Mn, Co, Ni, Zn) MOFs as highly efficient catalysts for chemical fixation of CO2 and DFT studies. Mol. Catal. 2019, 475, 110485. [Google Scholar] [CrossRef]
- Wu, Y.; Song, X.; Zhang, J.; Xu, S.; Gao, L.; Zhang, J.; Xiao, G. Mn-based MOFs as efficient catalysts for catalytic conversion of carbon dioxide into cyclic carbonates and DFT studies. Chem. Eng. Sci. 2019, 201, 288–297. [Google Scholar] [CrossRef]
- Huang, X.; Hu, Q.; Gao, L.; Hao, Q.; Wang, P.; Qin, D. Adsorption characteristics of metal–organic framework MIL-101(Cr) towards sulfamethoxazole and its persulfate oxidation regeneration. RSC Adv. 2018, 8, 27623–27630. [Google Scholar] [CrossRef] [Green Version]
- Yin, D.; Li, C.; Ren, H.; Shekhah, O.; Liu, J.; Liang, C. Efficient Pd@MIL-101(Cr) hetero-catalysts for 2-butyne-1,4-diol hydrogenation exhibiting high selectivity. RSC Adv. 2017, 7, 1626–1633. [Google Scholar] [CrossRef] [Green Version]
- Leng, K.; Sun, Y.; Li, X.; Sun, S.; Xu, W. Rapid Synthesis of Metal–Organic Frameworks MIL-101(Cr) Without the Addition of Solvent and Hydrofluoric Acid. Cryst. Growth Des. 2016, 16, 1168–1171. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, S.; Xu, Z.; Ding, L.; Cheng, Y. Metal-organic frameworks of MIL-100(Fe, Cr) and MIL-101(Cr) for Aromatic Amines Adsorption from Aqueous Solutions. Molecules 2019, 24, 3718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafiei, M.; Alivand, M.S.; Rashidi, A.; Samimi, A.; Mohebbi-Kalhori, D. Synthesis and adsorption performance of a modified micro-mesoporous MIL-101(Cr) for VOCs removal at ambient conditions. Chem. Eng. J. 2018, 341, 164–174. [Google Scholar] [CrossRef]
- Zhao, T.; Jeremias, F.; Boldog, I.; Nguyen, B.; Henninger, S.K.; Janiak, C. High-yield, fluoride-free and large-scale synthesis of MIL-101(Cr). Dalton Trans. 2015, 44, 16791–16801. [Google Scholar] [CrossRef] [Green Version]
- Hasan, Z.; Jeon, J.; Jhung, S.H. Adsorptive removal of naproxen and clofibric acid from water using metal-organic frameworks. J. Hazard. Mater. 2012, 209–210, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Hupp, J.T.; Poeppelmeier, K.R. CHEMISTRY: Enhanced: Better Living through Nanopore Chemistry. Science 2005, 309, 2008–2009. [Google Scholar] [CrossRef] [PubMed]
- Ertas, I.E.; Gulcan, M.; Bulut, A.; Yurderi, M.; Zahmakiran, M. Metal-organic framework (MIL-101) stabilized ruthenium nanoparticles: Highly efficient catalytic material in the phenol hydrogenation. Microporous Mesoporous Mater. 2016, 226, 94–103. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, S.; Liang, R.; Li, Z.; Shi, Z.; Chen, G. Graphene-wrapped chromium-MOF(MIL-101)/sulfur composite for performance improvement of high-rate rechargeable Li–S batteries. J. Mater. Chem. A 2014, 2, 13509–13512. [Google Scholar] [CrossRef]
- Sarker, M.; Song, J.Y.; Jhung, S.H. Adsorptive removal of anti-inflammatory drugs from water using graphene oxide/metal-organic framework composites. Chem. Eng. J. 2018, 335, 74–81. [Google Scholar] [CrossRef]
- Gordon, J.; Kazemian, H.; Rohani, S. MIL-53(Fe), MIL-101, and SBA-15 porous materials: Potential platforms for drug delivery. Mater. Sci. Eng. C 2015, 47, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Jhung, S.H. Adsorption of pharmaceuticals and personal care products over metal-organic frameworks functionalized with hydroxyl groups: Quantitative analyses of H-bonding in adsorption. Chem. Eng. J. 2017, 322, 366–374. [Google Scholar] [CrossRef]
- Juan-Alcañiz, J.; Ramos-Fernandez, E.V.; Lafont, U.; Gascon, J.; Kapteijn, F. Building MOF bottles around phosphotungstic acid ships: One-pot synthesis of bi-functional polyoxometalate-MIL-101 catalysts. J. Catal. 2010, 269, 229–241. [Google Scholar] [CrossRef]
- Hu, X.; Lu, Y.; Dai, F.; Liu, C.; Liu, Y. Host–guest synthesis and encapsulation of phosphotungstic acid in MIL-101 via “bottle around ship”: An effective catalyst for oxidative desulfurization. Microporous Mesoporous Mater. 2013, 170, 36–44. [Google Scholar] [CrossRef]
- Akimana, E.; Wang, J.; Likhanova, N.V.; Chaemchuen, S.; Verpoort, F. MIL-101(Cr) for CO2 Conversion into Cyclic Carbonates, Under Solvent and Co-Catalyst Free Mild Reaction Conditions. Catalysts 2020, 10, 453. [Google Scholar] [CrossRef]
- Dai, W.; Mao, P.; Liu, Y.; Zhang, S.; Li, B.; Yang, L.; Luo, X.; Zou, J. Quaternary phosphonium salt-functionalized Cr-MIL-101: A bifunctional and efficient catalyst for CO2 cycloaddition with epoxides. J. CO2 Util. 2020, 36, 295–305. [Google Scholar] [CrossRef]
- Wang, T.; Song, X.; Luo, Q.; Yang, X.; Chong, S.; Zhang, J.; Ji, M. Acid-base bifunctional catalyst: Carboxyl ionic liquid immobilized on MIL-101-NH2 for rapid synthesis of propylene carbonate from CO2 and propylene oxide under facile solvent-free conditions. Microporous Mesoporous Mater. 2018, 267, 84–92. [Google Scholar] [CrossRef]
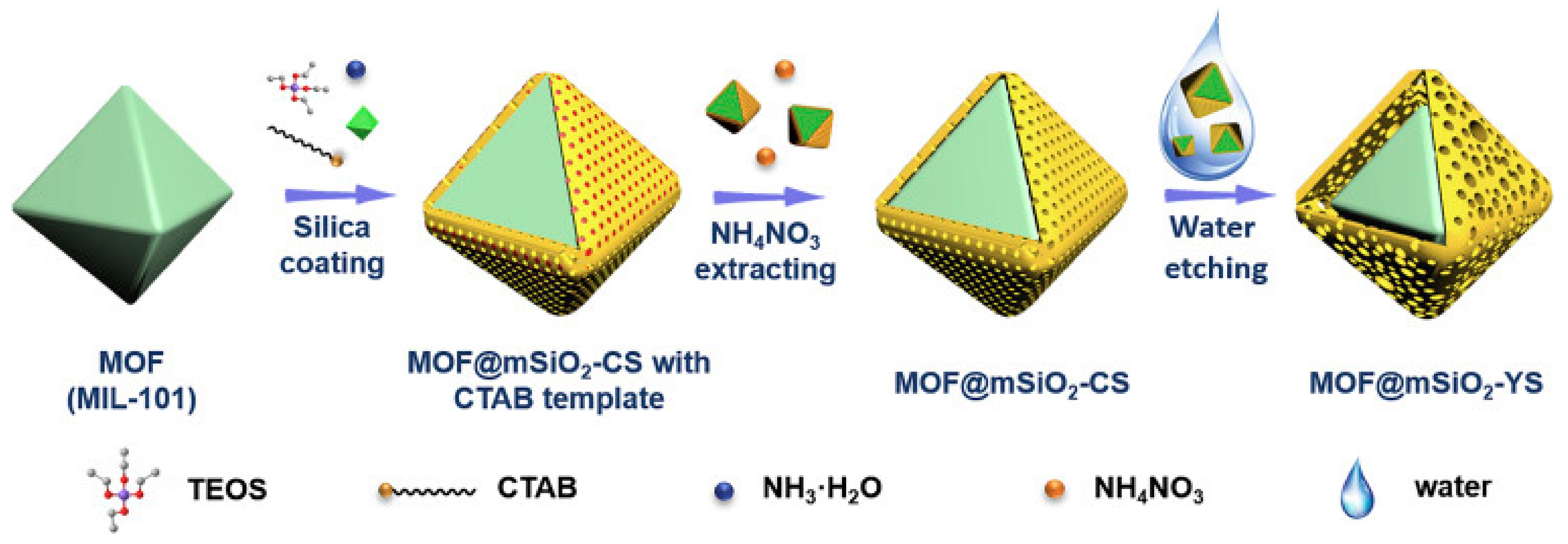
- Bao, S.; Li, J.; Guan, B.; Jia, M.; Terasaki, O.; Yu, J. A Green Selective Water-Etching Approach to MOF@Mesoporous SiO2 Yolk-Shell Nanoreactors with Enhanced Catalytic Stabilities. Matter 2020, 3, 498–508. [Google Scholar] [CrossRef]
- Liu, D.; Li, G.; Liu, H. Functionalized MIL-101 with imidazolium-based ionic liquids for the cycloaddition of CO2 and epoxides under mild condition. Appl. Surf. Sci. 2018, 428, 218–225. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J. Nanoporous metal-organic framework (MOF-199): Synthesis, characterization and photocatalytic degradation of Basic Blue 41. Microchem. J. 2019, 144, 436–442. [Google Scholar] [CrossRef]
- Murray, L.J.; Dinca, M.; Yano, J.; Chavan, S.; Bordiga, S.; Brown, C.M.; Long, J.R. Highly-Selective and Reversible O2 Binding in Cr3(1,3,5-benzenetricarboxylate)2. J. Am. Chem. Soc. 2010, 132, 7856–7857. [Google Scholar] [CrossRef] [PubMed]
- Maniam, P.; Stock, N. Investigation of Porous Ni-Based Metal–Organic Frameworks Containing Paddle-Wheel Type Inorganic Building Units via High-Throughput Methods. Inorg. Chem. 2011, 50, 5085–5097. [Google Scholar] [CrossRef] [PubMed]
- Feldblyum, J.I.; Liu, M.; Gidley, D.W.; Matzger, A.J. Reconciling the Discrepancies between Crystallographic Porosity and Guest Access as Exemplified by Zn-HKUST-1. J. Am. Chem. Soc. 2011, 133, 18257–18263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Wojtas, L.; Eddaoudi, M.; Zaworotko, M.J. Template-Directed Synthesis of Nets Based upon Octahemioctahedral Cages That Encapsulate Catalytically Active Metalloporphyrins. J. Am. Chem. Soc. 2011, 134, 928–933. [Google Scholar] [CrossRef]
- Crane, J.L.; Anderson, K.E.; Conway, S.G. Hydrothermal Synthesis and Characterization of a Metal–Organic Framework by Thermogravimetric Analysis, Powder X-ray Diffraction, and Infrared Spectroscopy: An Integrative Inorganic Chemistry Experiment. J. Chem. Educ. 2014, 92, 373–377. [Google Scholar] [CrossRef]
- Gotthardt, M.A.; Schoch, R.; Wolf, S.; Bauer, M.; Kleist, W. Synthesis and characterization of bimetallic metal–organic framework Cu–Ru-BTC with HKUST-1 structure. Dalton Trans. 2014, 44, 2052–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Mu, X.; Lester, E.; Wu, T. High efficiency synthesis of HKUST-1 under mild conditions with high BET surface area and CO2 uptake capacity. Prog. Nat. Sci. 2018, 28, 584–589. [Google Scholar] [CrossRef]
- Functionalizable, A.C.; Material, N.; Tma, C.; Chui, S.S.; Lo, S.M.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D.; Ho, C.T.M.A.; Chui, S.S.; et al. Published by American Association for the Advancement of Science Linked References Are Available on JSTOR for This Article. Nanoporous Mater. 2016, 283, 1148–1150. [Google Scholar]
- O’Neill, L.D.; Zhang, H.; Bradshaw, D. Macro-/Microporous MOF Composite Beads. J. Mater. Chem. 2010, 20, 5720–5726. [Google Scholar] [CrossRef]
- Xin, C.; Jiao, X.; Yin, Y.; Zhan, H.; Li, H.; Li, L.; Zhao, N.; Xiao, F.; Wei, W. Enhanced CO2 Adsorption Capacity and Hydrothermal Stability of HKUST-1 via Introduction of Siliceous Mesocellular Foams (MCFs). Ind. Eng. Chem. Res. 2016, 55, 7950–7957. [Google Scholar] [CrossRef]
- MacIas, E.E.; Ratnasamy, P.; Carreon, M.A. Catalytic Activity of Metal Organic Framework Cu3(BTC)2 in the Cycloaddition of CO2 to Epichlorohydrin Reaction. Catal. Today 2012, 198, 215–218. [Google Scholar] [CrossRef]

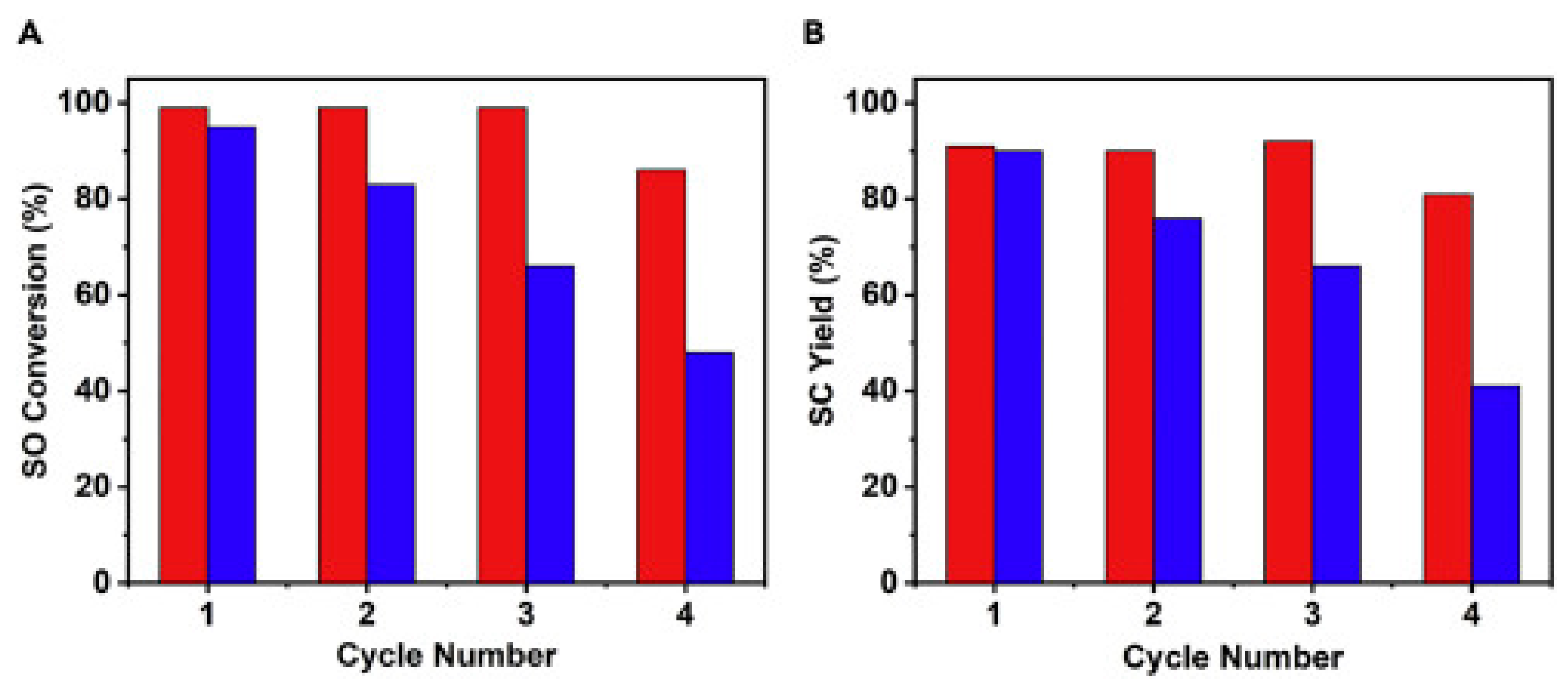
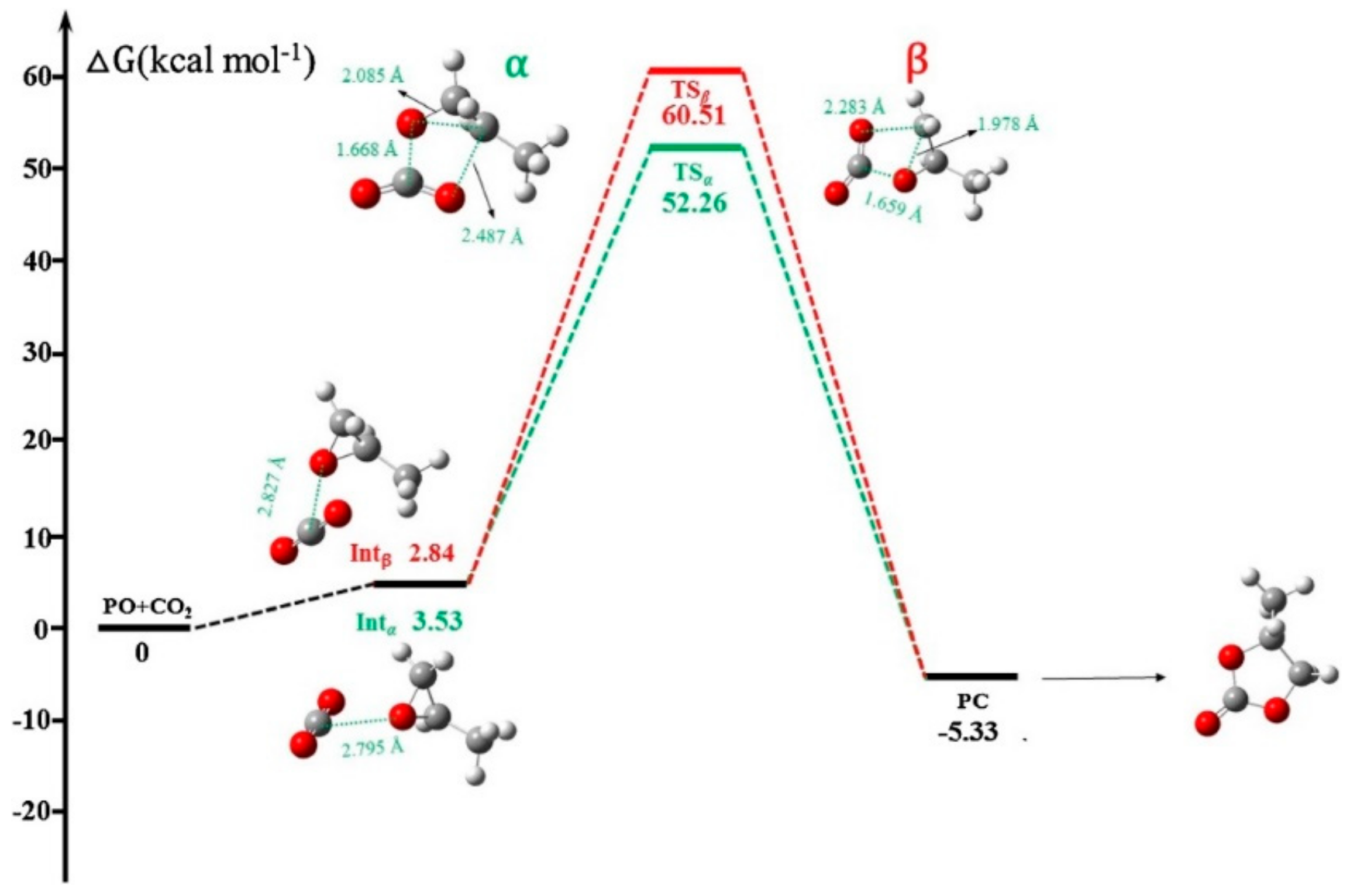
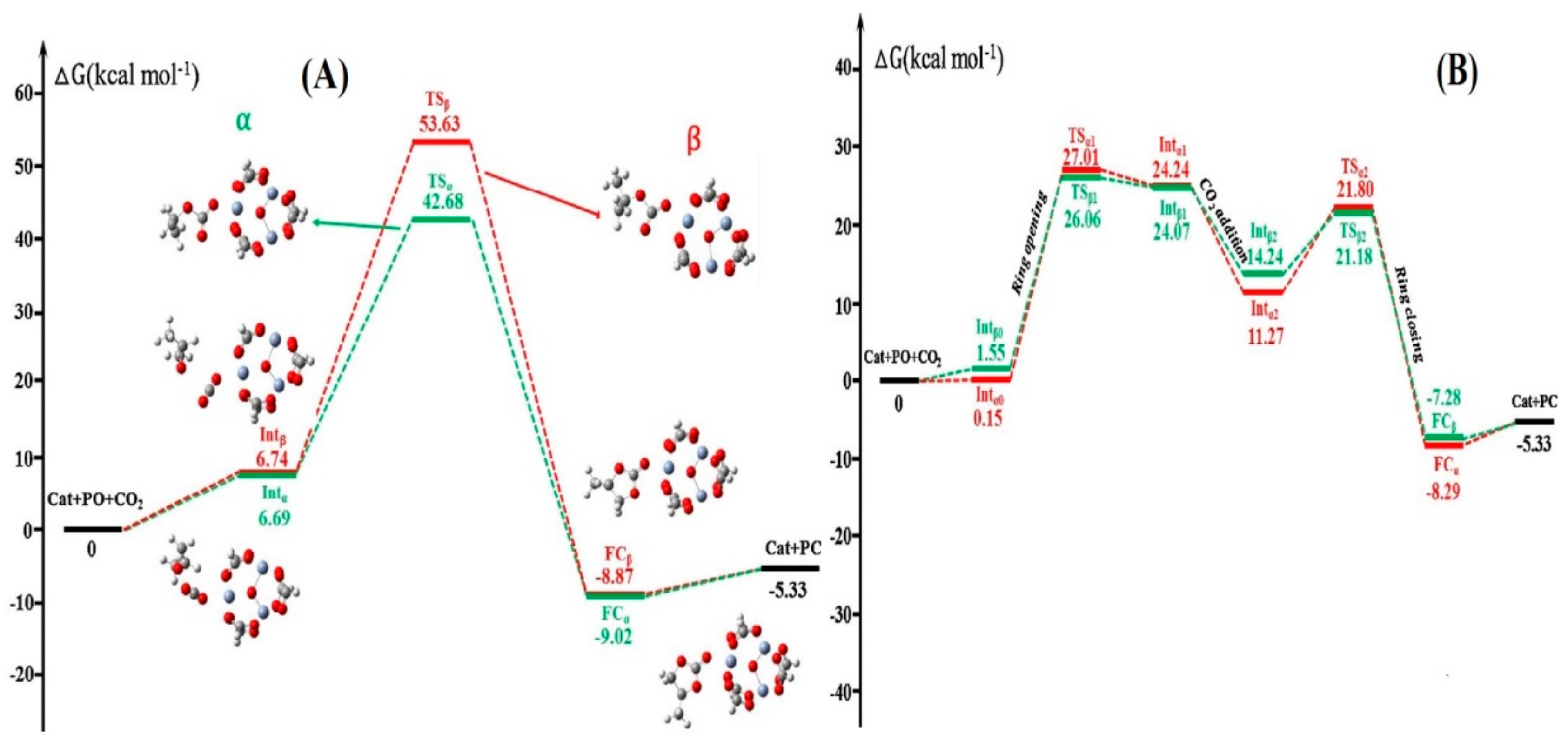
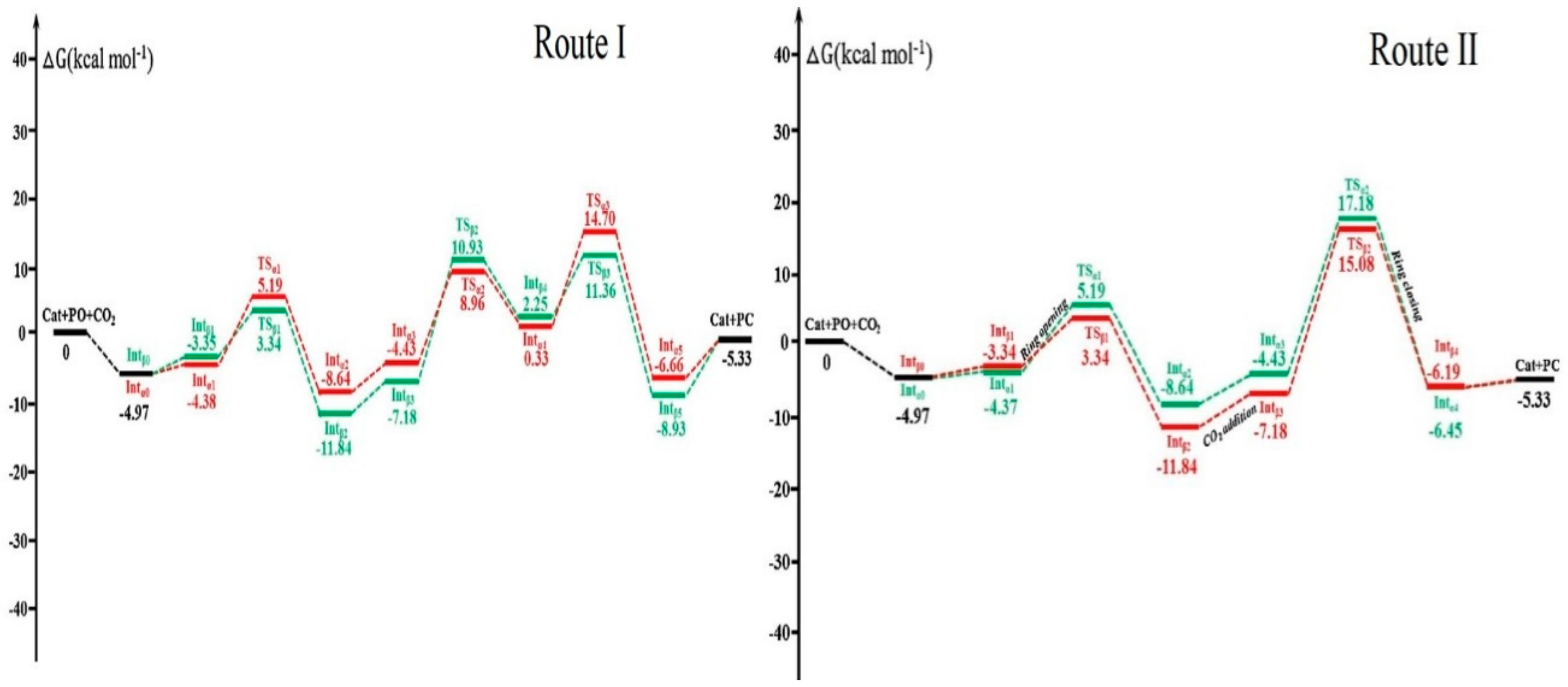

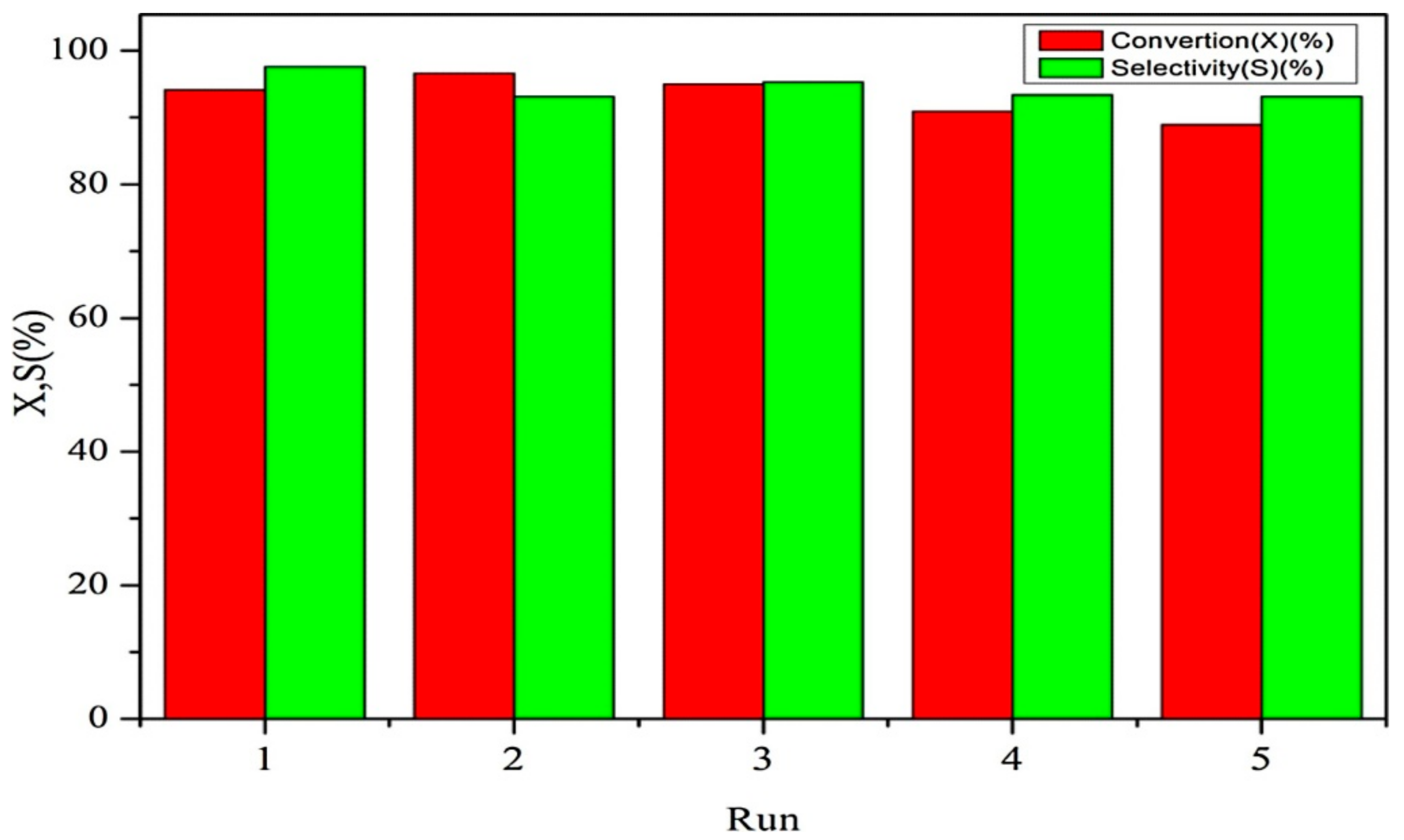
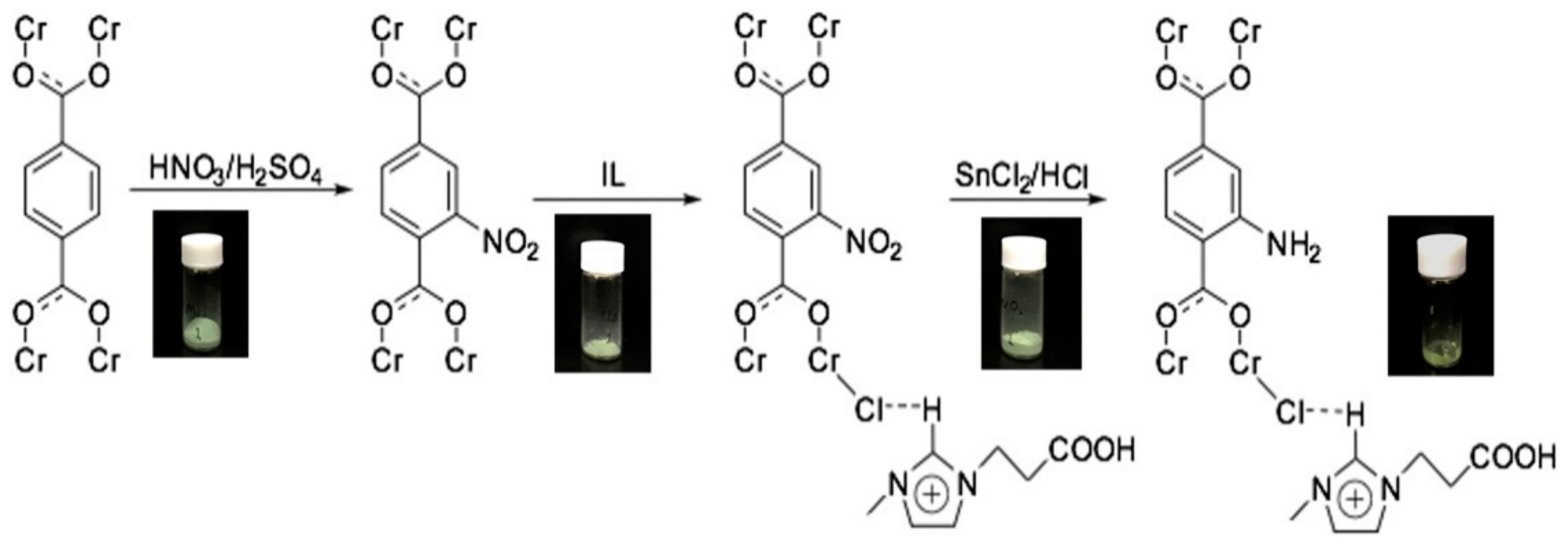



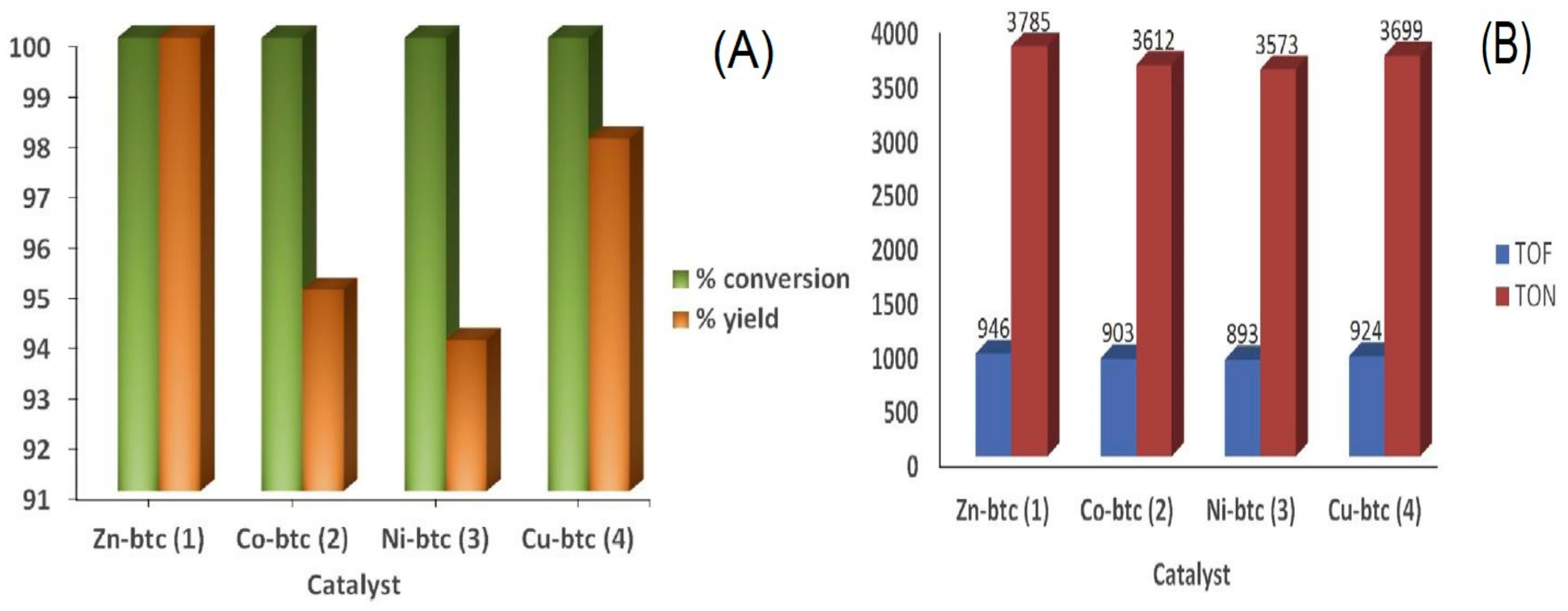

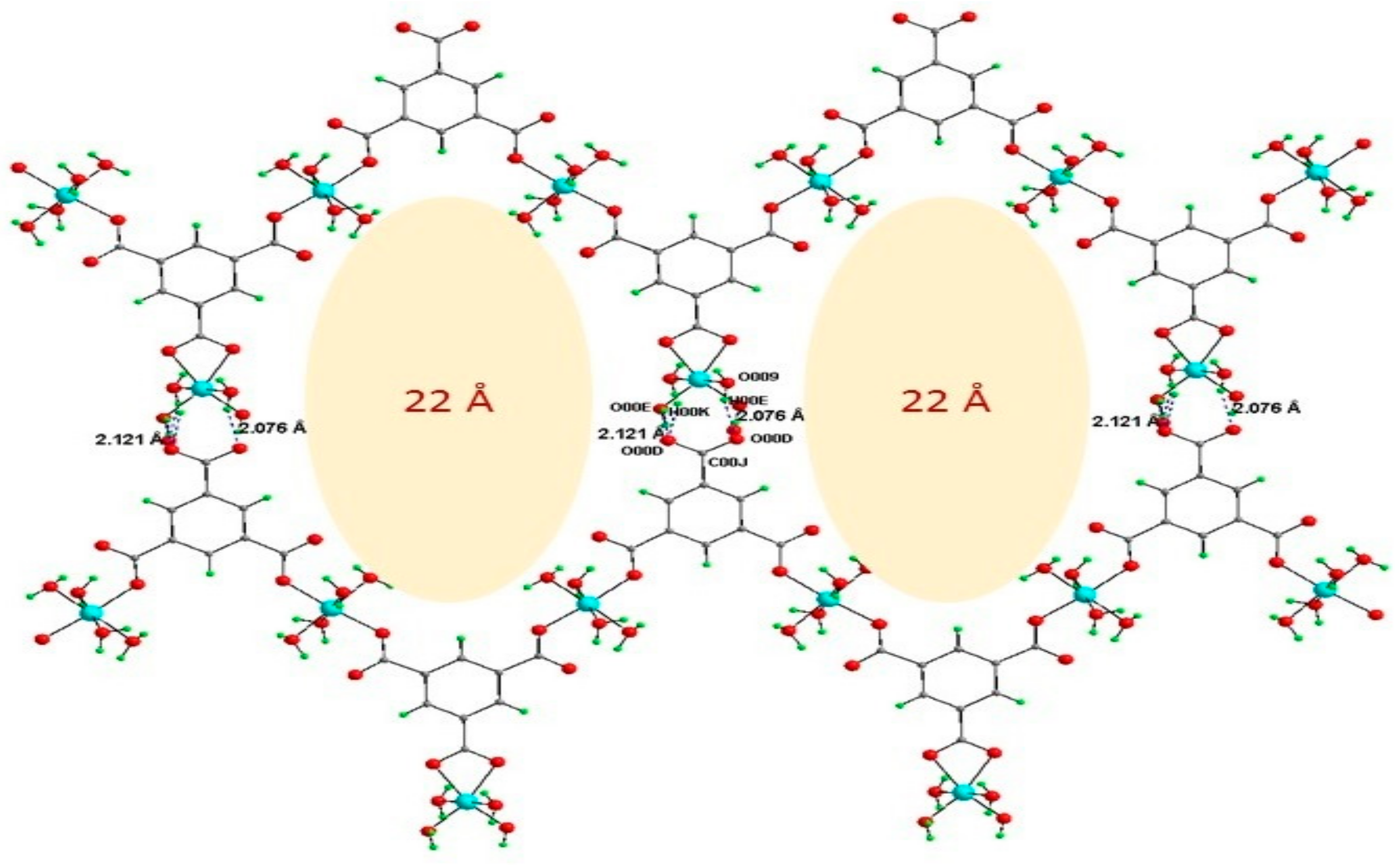


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, S.G.; Aljunid Merican, Z.M.; Akbarzadeh, O. Study on Selected Metal-Organic Framework-Based Catalysts for Cycloaddition Reaction of CO2 with Epoxides: A Highly Economic Solution for Carbon Capture and Utilization. Polymers 2021, 13, 3905. https://doi.org/10.3390/polym13223905
Musa SG, Aljunid Merican ZM, Akbarzadeh O. Study on Selected Metal-Organic Framework-Based Catalysts for Cycloaddition Reaction of CO2 with Epoxides: A Highly Economic Solution for Carbon Capture and Utilization. Polymers. 2021; 13(22):3905. https://doi.org/10.3390/polym13223905
Chicago/Turabian StyleMusa, Suleiman Gani, Zulkifli Merican Aljunid Merican, and Omid Akbarzadeh. 2021. "Study on Selected Metal-Organic Framework-Based Catalysts for Cycloaddition Reaction of CO2 with Epoxides: A Highly Economic Solution for Carbon Capture and Utilization" Polymers 13, no. 22: 3905. https://doi.org/10.3390/polym13223905
APA StyleMusa, S. G., Aljunid Merican, Z. M., & Akbarzadeh, O. (2021). Study on Selected Metal-Organic Framework-Based Catalysts for Cycloaddition Reaction of CO2 with Epoxides: A Highly Economic Solution for Carbon Capture and Utilization. Polymers, 13(22), 3905. https://doi.org/10.3390/polym13223905








