Phase Transitions of Polarised PVDF Films in a Standard Curing Process for Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Phase Analysis
2.2.1. DSC
2.2.2. FTIR
2.2.3. XRD
2.3. Autoclave Process
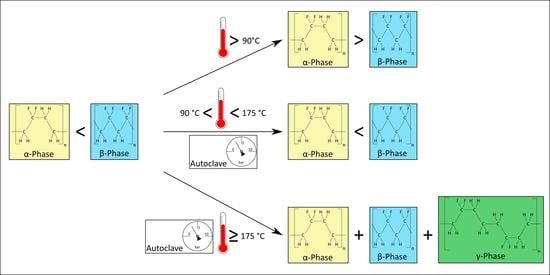
- 90
- 130The adhesive joint is cured at this temperature in an autoclave process as described above.
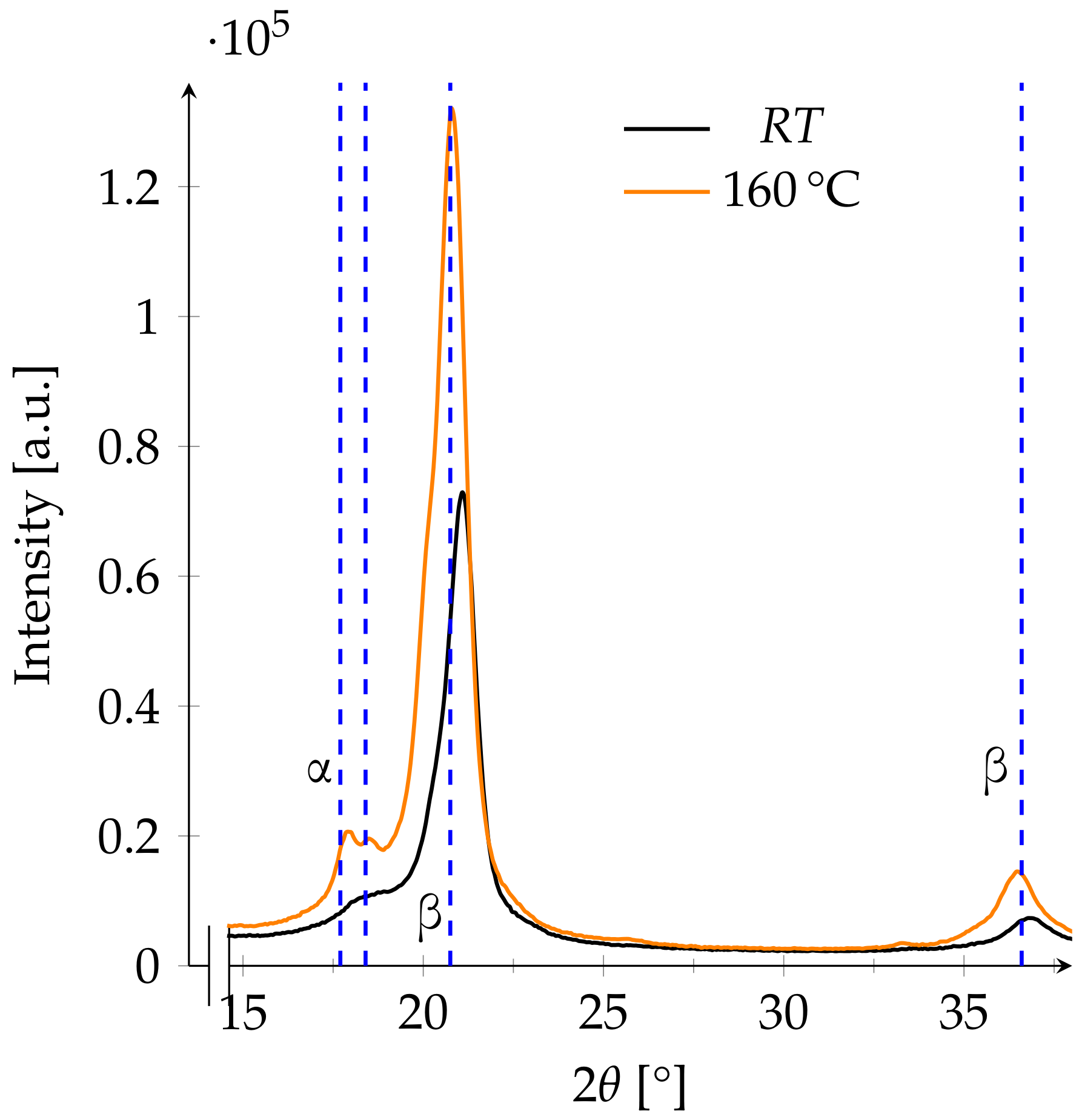
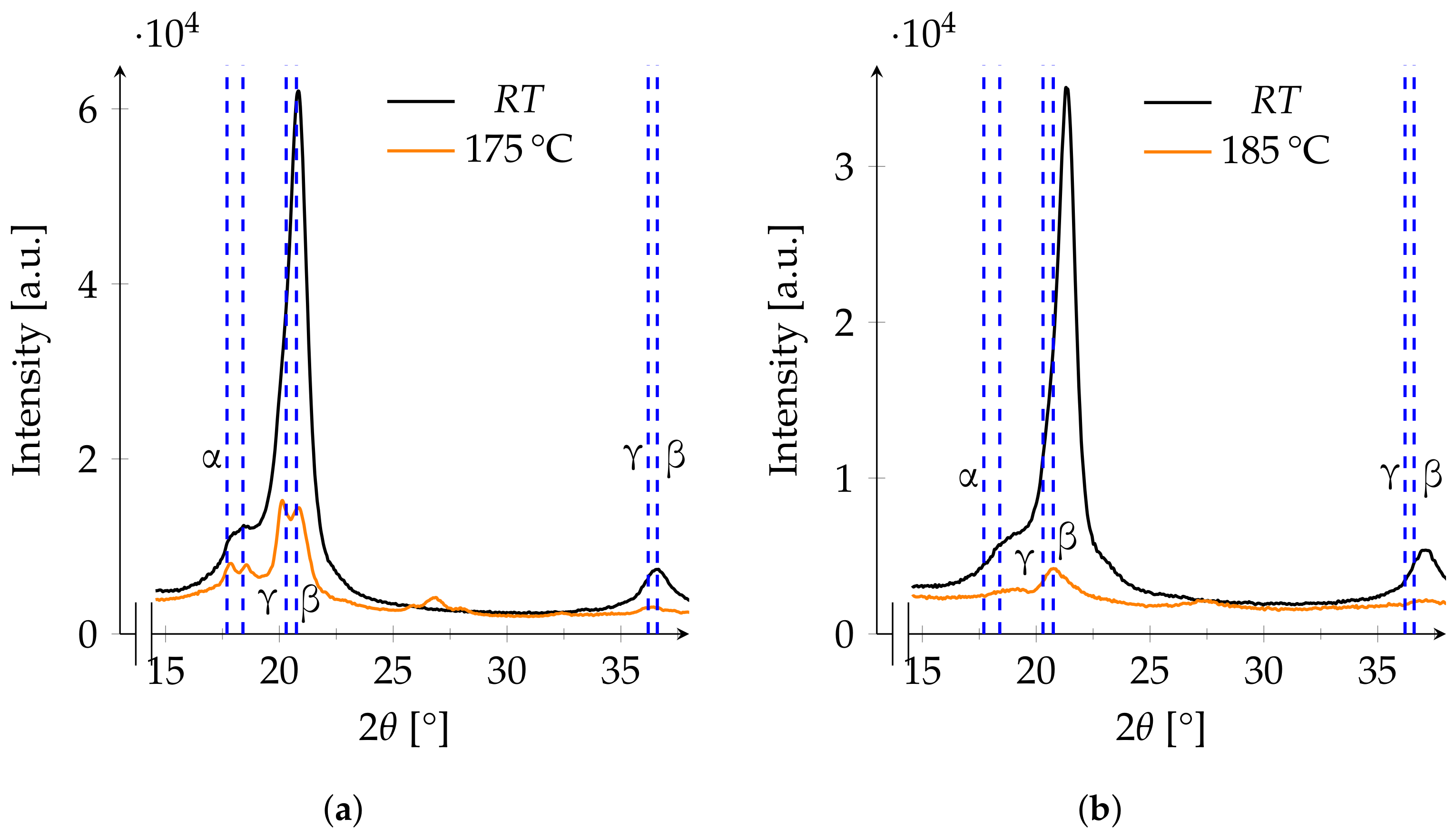
- 160 to 175The melting range of α- and β-PVDF is from 167 to 172 [5]. As the melting points of both phases are not clearly separable, a wide space around the melting range is observed.
- 180 and 185CFRP structures are often cured in these temperature ranges.
3. Results
3.1. DSC
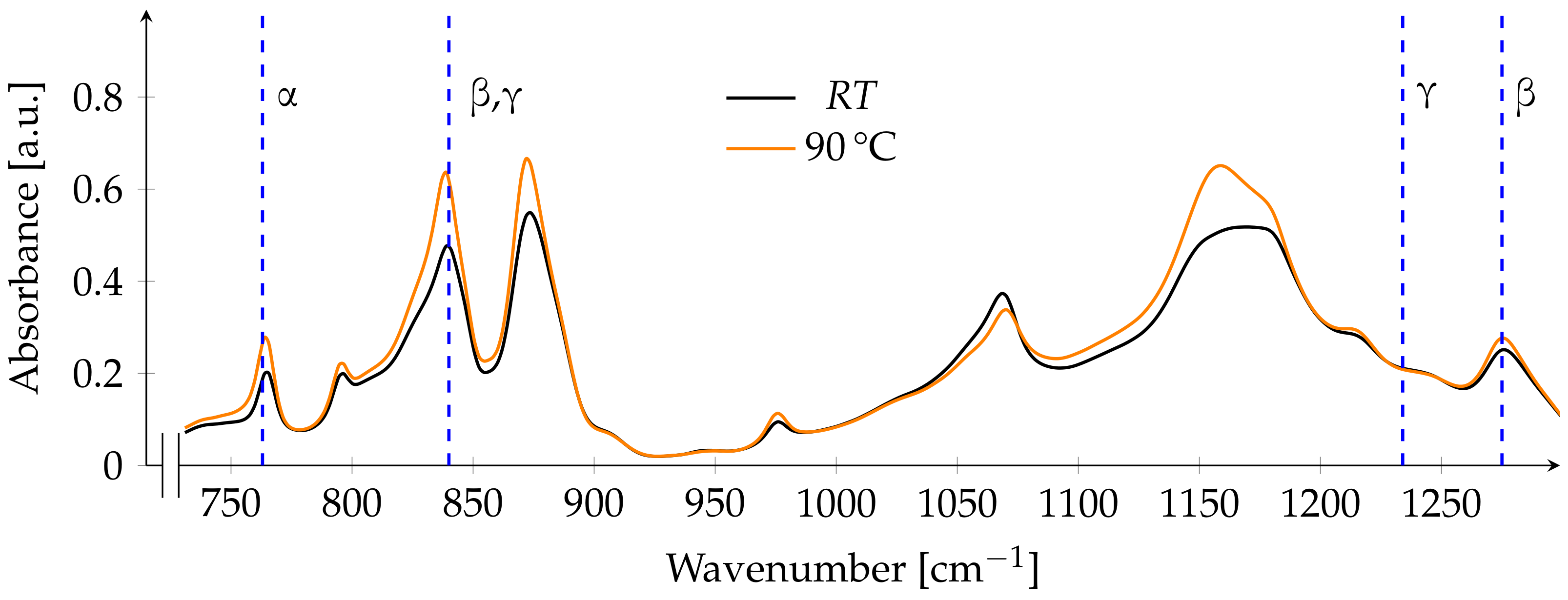
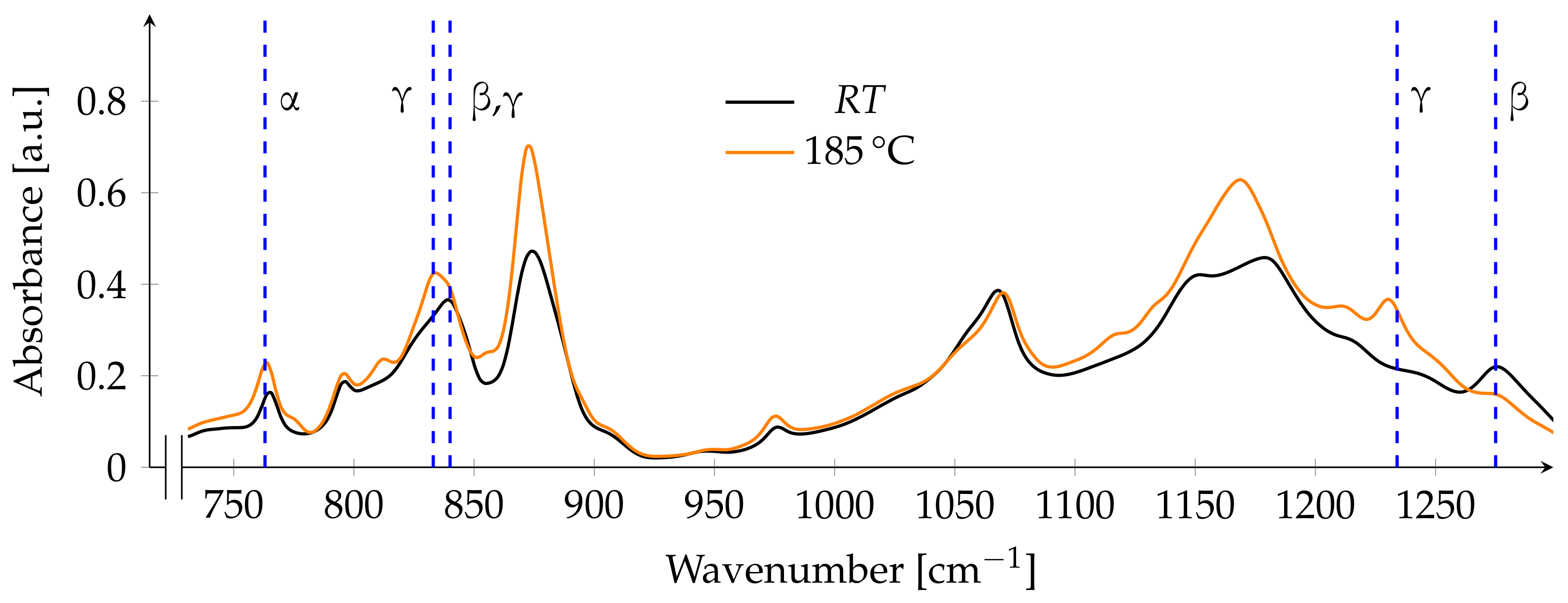
3.2. FTIR
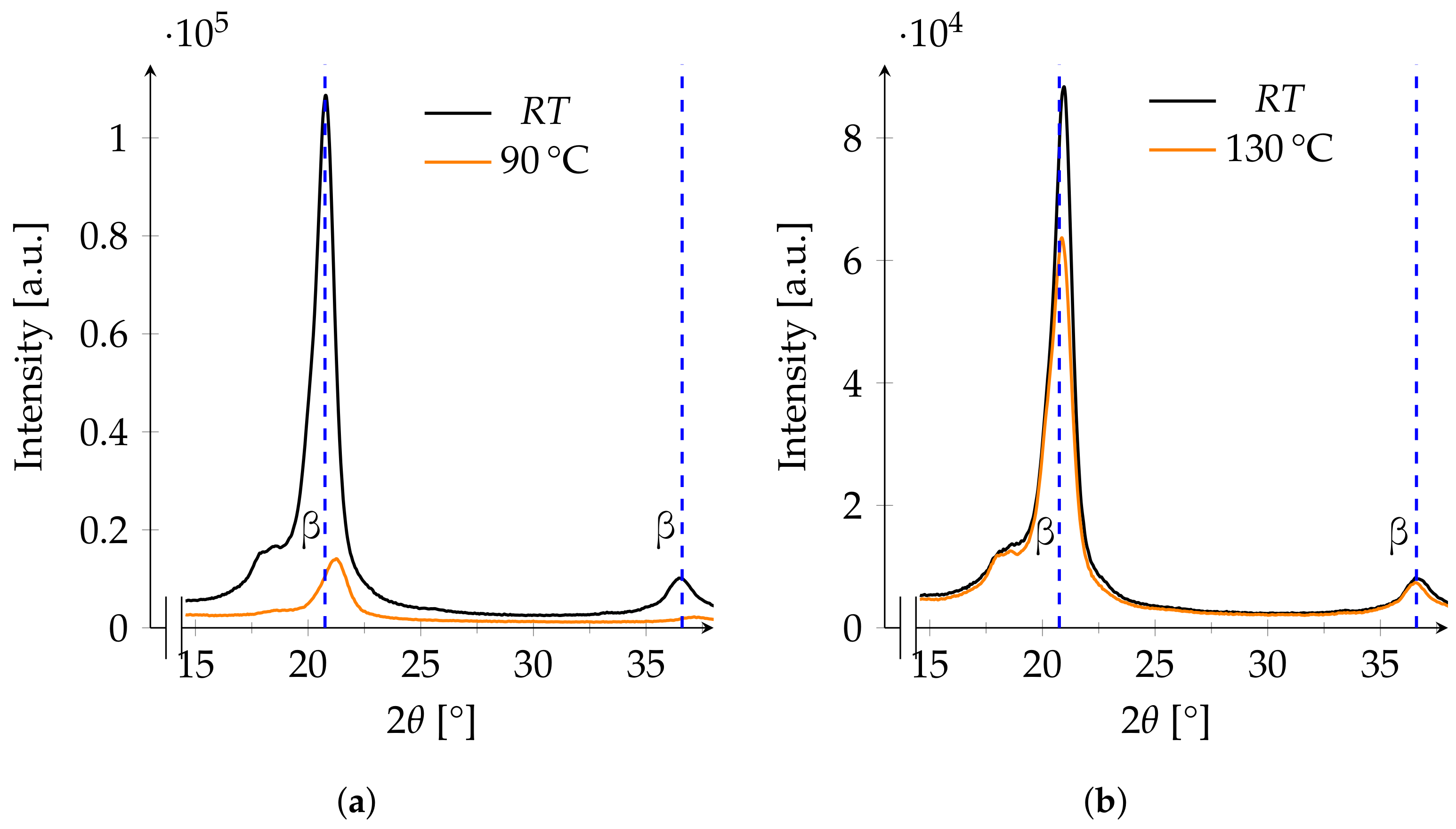
3.3. XRD
3.4. Phase Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATR | attenuated total reflectance |
| CFRP | carbon fibre reinforced plastic |
| DSC | differential scanning calorimetry |
| FTIR | Fourier transformed infrared spectroscopy |
| PVDF | poly(vinylidene fluoride) |
| RT | room temperature |
| SHM | structural health monitoring |
| XRD | X-ray diffraction |
References
- Sinapius, M. Adaptronics—Smart Structures and Materials, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Lovinger, A. Ferroelectric polymers. Science 1983, 220, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Sessler, G. Piezoelectricity in polyvinylidenefluoride. J. Acoust. Soc. Am. 1981, 70, 1596–1608. [Google Scholar] [CrossRef]
- Ueberschlag, P. PVDF piezoelectric polymer. Sens. Rev. 2001, 21, 118–126. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.; Lanceros-Mendez, S. Electroactive phases of poly (vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Steinmetz, J.; Löbel, T.; Völkerink, O.; Hühne, C.; Sinapius, M.; von der Heide, C.; Dietzel, A. The Working Principles of a Multifunctional Bondline with Disbond Stopping and Health Monitoring Features for Composite Structures. J. Compos. Sci. 2021, 5, 51. [Google Scholar] [CrossRef]
- Gregorio, R.; Cestari, M. Effect of crystallization temperature on the crystalline phase content and morphology of poly(vinylidene fluoride). J. Polym. Sci. Part B Polym. Phys. 1994, 32, 859–870. [Google Scholar] [CrossRef]
- Eberle, G.; Eisenmenger, W. Thermal Depolarization of PVDF. Anomaly at 180 °C. IEEE Trans. Electr. Insul. 1992, 27, 768–772. [Google Scholar] [CrossRef]
- Nakamura, K.; Sawai, D.; Watanabe, Y.; Taguchi, D.; Takahashi, Y.; Furukawa, T.; Kanamoto, T. Effect of annealing on the structure and properties of poly(vinylidene fluoride) β-form films. J. Polym. Sci. Part B Polym. Phys. 2003, 14, 1701–1712. [Google Scholar] [CrossRef]
- Silva, M.; Costa, C.; Sencadas, V.; Paleo, A.; Lanceros-Méndez, S. Degradation of the dielectric and piezoelectric response of β-poly(vinylidene fluoride) after temperature annealing. J. Polym. Res. 2011, 18, 1451–1457. [Google Scholar] [CrossRef] [Green Version]
- Bur, A.; Barnes, J.; Wahlstrand, K. A study of thermal depolarization of polyvinylidene fluoride using X-ray pole-figure observations. J. Appl. Phys. 1986, 59, 2345–2354. [Google Scholar] [CrossRef]
- Lopes, A.; Costa, C.; Tavares, C.; Neves, I.; Lanceros-Mendez, S. Nucleation of the Electroactive γ Phase and Enhancement of the Optical Transparency in Low Filler Content Poly(vinylidene)/Clay Nanocomposites. J. Phys. Chem. C 2011, 115, 18076–18082. [Google Scholar] [CrossRef]
- Esterly, D.; Love, B. Phase transformation to β-poly(vinylidene fluoride) by milling. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 91–97. [Google Scholar] [CrossRef]
- Gregorio, R. Determination of the α, β, and γ crystalline phases of poly(vinylidene fluoride) films prepared at different conditions. J. Appl. Polym. Sci. 2006, 100, 3272–3279. [Google Scholar] [CrossRef]
- Gregorio, R.; Ueno, M. Effect of crystalline phase, orientation and temperature on the dielectric properties of poly (vinylidene fluoride) (PVDF). J. Mater. Sci. 1999, 34, 4489–4500. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A critical analysis of the α, β and γ phases in poly(vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef] [Green Version]
- Neese, B. Investigations of Structure-Property Relationships to Enhance the Multifunctional Properties of PVDF-Based Polymers. Ph.D. Thesis, Pennsylvania State University, University Park, PA, USA, 2009. [Google Scholar]
- Bar-Cohen, Y. Electroactive Polymer (EAP) Actuators as Artificial Muscles: Reality, Potential, and Challenges, 1st ed.; SPIE Press: Bellingham, WA, USA, 2001. [Google Scholar]
- Broadhurst, M.; Davis, G. Physical basis for piezoelectricity in PVDF. Ferroelectrics 1984, 60, 3–13. [Google Scholar] [CrossRef]



| Temperature (C) | (%) | Melting Point (%) |
|---|---|---|
| RT | 55.2 ± 2.6 | 171.2 ± 1.0 |
| 90 | 46.6 ± 2.9 | 171.2 ± 1.5 |
| 130 | 52.5 ± 7.3 | 171.4 ± 1.0 |
| 160 | 54.9 ± 6.3 | 172.2 ± 1.4 |
| 165 | 48.4 ± 3.3 | 174.4 ± 1.6 |
| 170 | 48.9 ± 3.5 | 175.8 ± 0.3 |
| 175 | 49.2 ± 3.3 | 177.2 ± 0.7, 183.2 ± 1.3, 190.8 ± 0.3 |
| 180 | 48.3 ± 4.1 | 177.5 ± 0.2, 181.8 ± 0.4, 193.0 ± 0.4 |
| 182 | 49.4 ± 3.5 | 177.7 ± 0.3, 181.4 ± 0.7 |
| 185 | 50.9 ± 3.8 | 177.1 ± 0.2, 180.7 ± 0.2 |
| Temperature (C) | (%) | (%) | Phases | Phases |
|---|---|---|---|---|
| 90 | 63.8 ± 2.0 | 62.5 ± 1.6 | α, β | α, β |
| 130 | 64.6 ± 1.4 | 61.6 ± 1.9 | α, β | α, β |
| 160 | 63.0 ± 2.2 | 65.1 ± 1.8 | α, β | α, β |
| 165 | 65.2 ± 5.3 | 63.0 ± 2.2 | α, β | α, β |
| 170 | 64.2 ± 4.2 | 62.8 ± 3.1 | α, β | α, β |
| 175 | 64.5 ± 1.4 | 35.2 ± 3.0 | α, β | α, β, γ |
| 180 | 64.8 ± 1.9 | 57.0 ± 3.4 | α, β | α, β, γ |
| 182 | 61.7 ± 1.8 | 51.0 ± 9.3 | α, β | α, β, γ |
| 185 | 63.5 ± 2.7 | 59.3 ± 2.4 | α, β | α, β, γ |
| Temperature (C) | (%) | (%) |
|---|---|---|
| 90 | 87.4 ± 1.4 | 90.6 ± 3.2 |
| 130 | 88.2 ± 1.8 | 87.7 ± 4.4 |
| 160 | 87.8 ± 2.1 | 86.6 ± 1.8 |
| 165 | 87.4 ± 2.9 | 87.0 ± 1.0 |
| 170 | 89.0 ± 1.8 | 85.5 ± 0.8 |
| Temperature (C) | (%) | (%) | (%) | (%) |
|---|---|---|---|---|
| RT | 49.6 ± 0.1 | 14.7 ± 2.2 | 5.6 ± 0.3 | 30.1 ± 2.2 |
| 90 | 42.3 ± 2.0 | 20.3 ± 3.6 | 4.4 ± 1.7 | 33.1 ± 2.6 |
| 130 | 46.2 ± 8.0 | 15.5 ± 7.4 | 6.3 ± 2.1 | 32.0 ± 3.3 |
| 160 | 47.6 ± 5.7 | 17.5 ± 4.4 | 7.4 ± 1.2 | 27.6 ± 1.9 |
| 165 | 42.1 ± 2.8 | 21.0 ± 3.4 | 6.3 ± 0.8 | 30.7 ± 2.8 |
| 170 | 41.8 ± 3.1 | 20.9 ± 5.1 | 7.1 ± 0.6 | 30.1 ± 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasic, N.; Steinmetz, J.; Görke, M.; Sinapius, M.; Hühne, C.; Garnweitner, G. Phase Transitions of Polarised PVDF Films in a Standard Curing Process for Composites. Polymers 2021, 13, 3900. https://doi.org/10.3390/polym13223900
Vasic N, Steinmetz J, Görke M, Sinapius M, Hühne C, Garnweitner G. Phase Transitions of Polarised PVDF Films in a Standard Curing Process for Composites. Polymers. 2021; 13(22):3900. https://doi.org/10.3390/polym13223900
Chicago/Turabian StyleVasic, Nils, Julian Steinmetz, Marion Görke, Michael Sinapius, Christian Hühne, and Georg Garnweitner. 2021. "Phase Transitions of Polarised PVDF Films in a Standard Curing Process for Composites" Polymers 13, no. 22: 3900. https://doi.org/10.3390/polym13223900
APA StyleVasic, N., Steinmetz, J., Görke, M., Sinapius, M., Hühne, C., & Garnweitner, G. (2021). Phase Transitions of Polarised PVDF Films in a Standard Curing Process for Composites. Polymers, 13(22), 3900. https://doi.org/10.3390/polym13223900