The Use of Surface-Modified Nanocrystalline Cellulose Integrated Membranes to Remove Drugs from Waste Water and as Polymers to Clean Oil Sands Tailings Ponds
Abstract
:1. Introduction
2. Materials
3. Methods
3.1. Dioctenyldimethyl Succinic Anhydride-Nanocrystalline Cellulose (DDSA-CNC) Synthesis
3.2. Polydiallyl Dimethyl Ammonium Chloride (PDMC)-CNC Synthesis
3.3. Preparation of Cationic CNC (CTAC-CNC) or Hydrophobic CNC (CTAB-CNC) (Both Non-Covalent Methods)
3.4. Drug or Contaminant Analysis
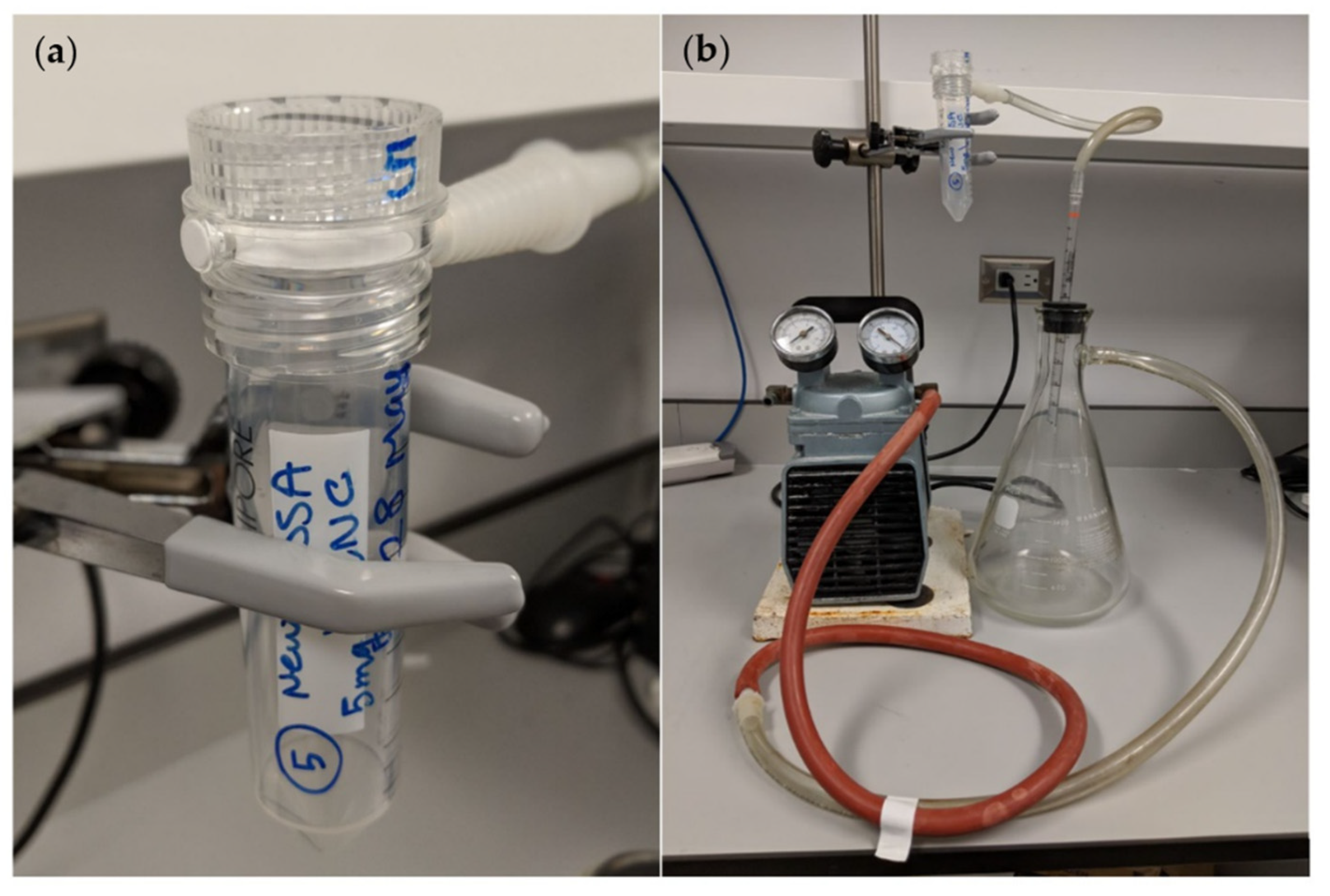
3.5. Filtration
3.6. Loading of CNC on to Small Scale Filters
3.7. Zeta Potential Measurments
3.8. Drug Binding Studies
3.9. Clay Flocculation Experiments
3.10. Naphthenic Acid Binding to CNC
4. Results
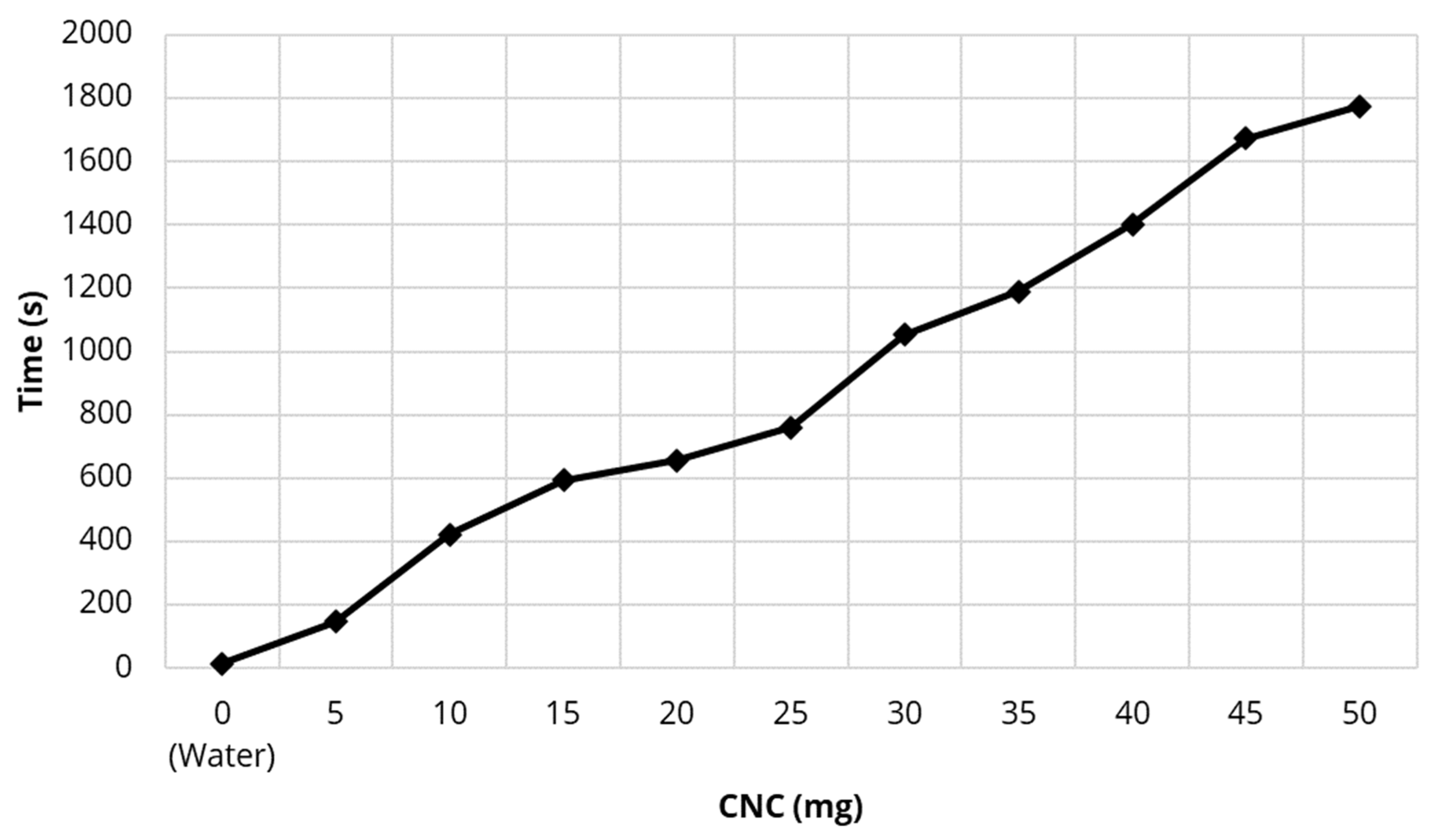
4.1. Filtration Methods
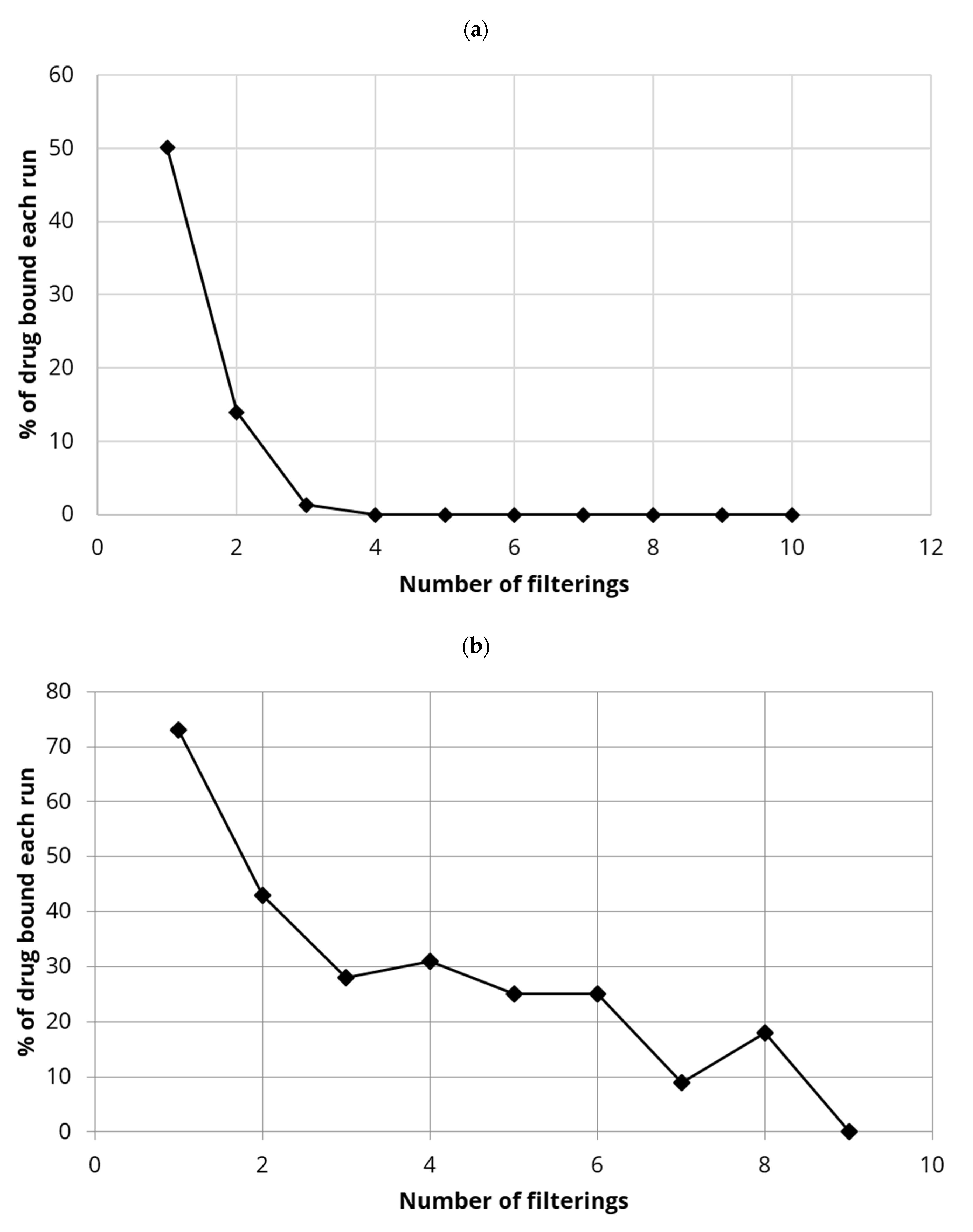
4.2. Binding of Estradiol to CTAB-CNC Integrated Small-Scale Membranes
4.3. Zeta Potential of Covalently Modified CNC
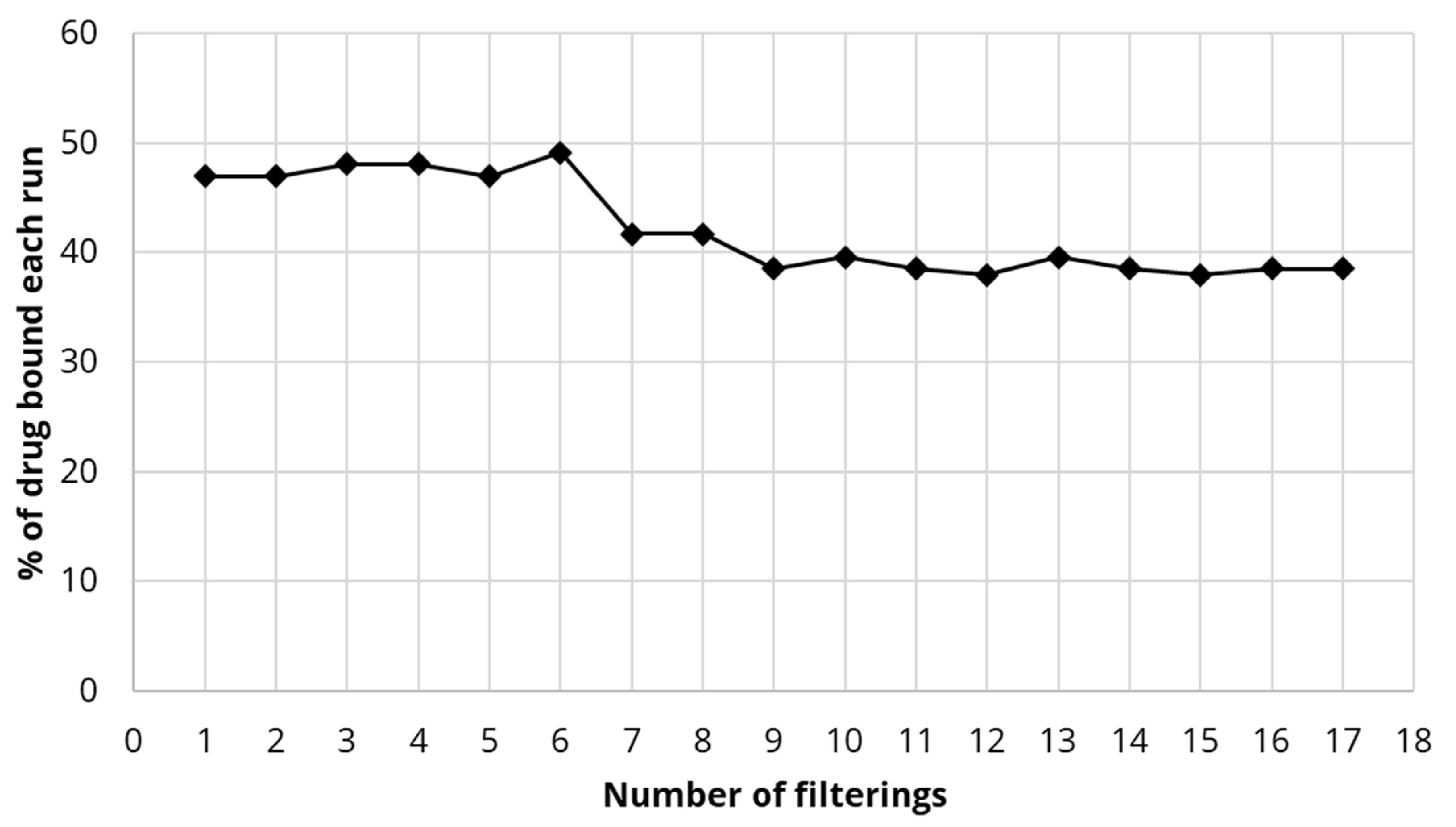
4.4. Binding of Estradiol to DDSA-CNC Integrated Small Scale Membranes
4.5. Binding of Estradiol to DDSA-CNC Integrated Large Commercial Filter Membranes
4.6. Binding of Diclofenac to PDMC Integrated Small Scale Membranes
4.7. Binding of Diclofenac to PDMC Integrated Large Commercial Filter Membranes
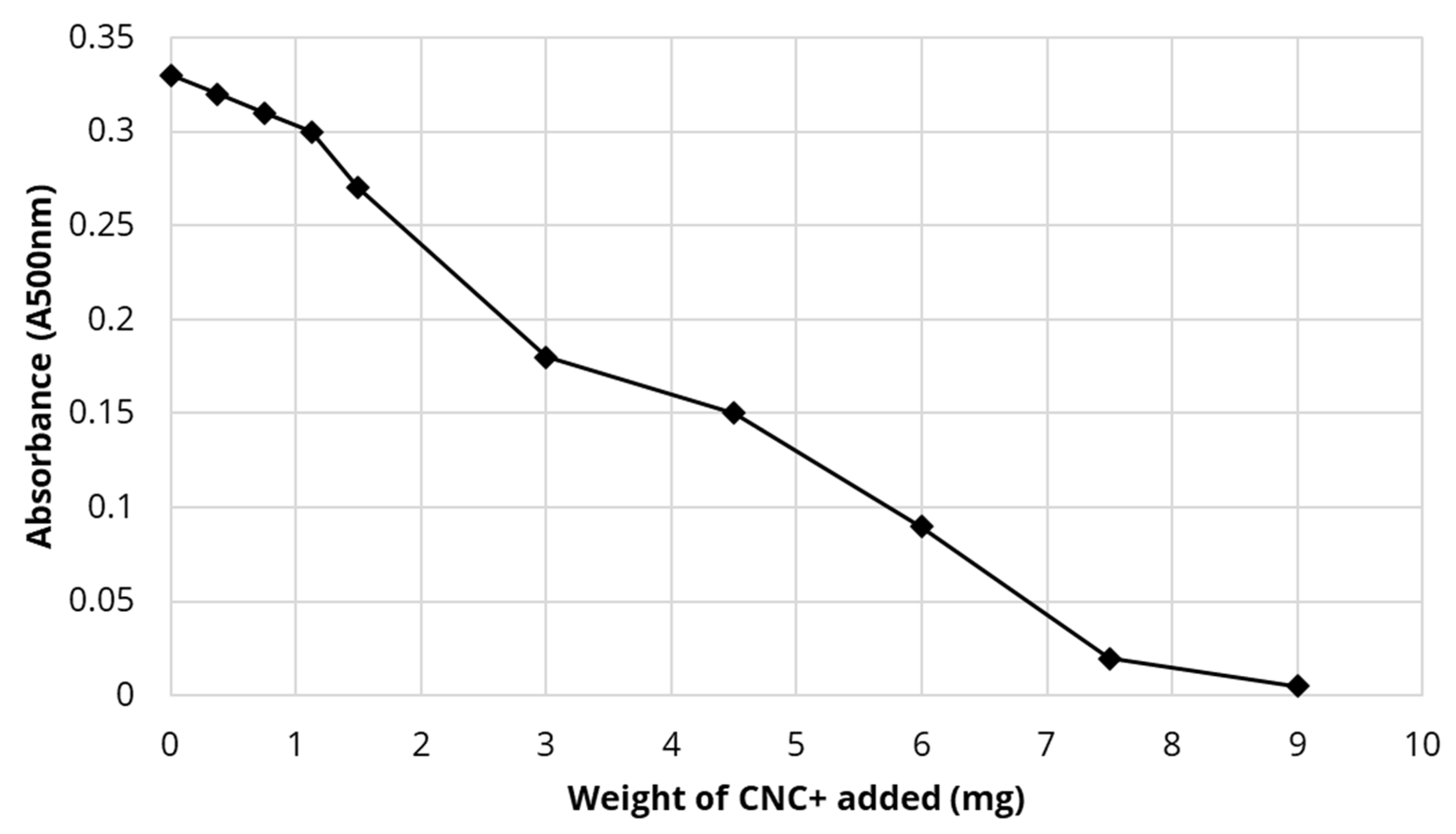
4.8. Oil Sands Treatments and Clay Flocculation
4.9. Binding of Naphthenic Acids to either CTAC-CNC or CTAB-Modified CNC
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Pharmaceuticals in Drinking-Water; WHO: Geneva, Switzerland, 2012; ISBN 978-92-4-150208-5. [Google Scholar]
- World Health Organization. Pharmaceuticals in Drinking-Water (WHO/HSE/WSH/11.05); WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Chauhan, A.; Sillu, D.; Agnihotri, S. Removal of pharmaceutical contaminants in wastewater using nanomaterials: A comprehensive review. Curr. Drug Drug Metab. 2019, 20, 483–505. [Google Scholar] [CrossRef] [Green Version]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in situ remediation: A review of the benefits and potential risks. Environ. Health Perspect. 2009, 117, 1813–1831. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Rodriguez, K.; Renneckar, S.; Vikesland, P.J. Environmental science and engineering applications of nanocellulose-based nanocomposites. Environ. Sci. Nano 2014, 1, 302–316. [Google Scholar] [CrossRef] [Green Version]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Shen, Z. Nanocellulose Based Filtration Membrane in Industrial Waste Water Treatment: A Review. Materials 2021, 14, 5398. [Google Scholar] [CrossRef] [PubMed]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose-based materials for water purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Nanocellulose-Enabled Membranes for Water Purification: Perspectives. Adv. Sustain. Syst. 2020, 4, 1900114. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Jackson, J.K.; Letchford, K.; Wasserman, B.Z.; Ye, L.; Hamad, W.Y.; Burt, H.M. The use of nanocrystalline cellulose for the binding and controlled release of drugs. Int. J. Nanomed. 2011, 6, 321. [Google Scholar]
- Karimian, A.; Parsian, H.; Majidinia, M.; Rahimi, M.; Mir, S.M.; Kafil, H.S.; Shafiei-Irannejad, V.; Kheyrollah, M.; Ostadi, H.; Yousefi, B. Nanocrystalline cellulose: Preparation, physicochemical properties, and applications in drug delivery systems. Int. J. Biol. Macromol. 2019, 133, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Nas, B.; Dolu, T.; Argun, M.E.; Yel, E.; Ateş, H.; Koyuncu, S. Comparison of advanced biological treatment and nature-based solutions for the treatment of pharmaceutically active compounds (PhACs): A comprehensive study for wastewater and sewage sludge. Sci. Total Environ. 2021, 779, 146344. [Google Scholar] [CrossRef]
- Vieno, N.; Sillanpää, M. Fate of diclofenac in municipal wastewater treatment plant—A review. Environ. Int. 2014, 69, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.O.; Moreira, N.F.; Ribeiro, A.R.; Pereira, M.F.; Silva, A.M. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef]
- Nguyen, P.; Carvalho, G.; Reis, M.A.; Oehmen, A. A review of the biotransformations of priority pharmaceuticals in biological wastewater treatment processes. Water Res. 2020, 188, 116446. [Google Scholar] [CrossRef] [PubMed]
- Fair, A. Oil Sands tailings: A historical perspective. In Proceedings of the Fourth International Oil Sands Tailings Conference (IOSTC), Lake Louis, AB, Canada, 7–10 December 2014. [Google Scholar]
- Botha, L.; Soares, J.B. The influence of tailings composition on flocculation. Can. J. Chem. Eng. 2015, 93, 1514–1523. [Google Scholar] [CrossRef]
- Vedoy, D.R.; Soares, J.B. Water-soluble polymers for oil sands tailing treatment: A Review. Can. J. Chem. Eng. 2015, 93, 888–904. [Google Scholar] [CrossRef]
- Wang, X.; Feng, X.; Xu, Z.; Masliyah, J.H. Polymer aids for settling and filtration of oil sands tailings. Can. J. Chem. Eng. 2010, 88, 403–410. [Google Scholar] [CrossRef]
- Sworska, A.; Laskowski, J.; Cymerman, G. Flocculation of the Syncrude fine tailings: Part I. Effect of pH, polymer dosage and Mg2+ and Ca2+ cations. Int. J. Miner. Process. 2000, 60, 143–152. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Jin, X.; Liu, L.; Zhang, Y.; Ni, Q.; Yao, J. Synthesis of hydrophobically modified cellulose-based flocculant and its application in treatments of kaolin suspension and machining wastewater. Cellulose 2017, 24, 5639–5647. [Google Scholar] [CrossRef]
- Jin, L.; Wei, Y.; Xu, Q.; Yao, W.; Cheng, Z. Cellulose nanofibers prepared from TEMPO-oxidation of kraft pulp and its flocculation effect on kaolin clay. J. Appl. Polym. Sci. 2014, 131, 40450. [Google Scholar] [CrossRef]
- Quagraine, E.; Peterson, H.; Headley, J. In situ bioremediation of naphthenic acids contaminated tailing pond waters in the Athabasca oil sands region—Demonstrated field studies and plausible options: A review. J. Environ. Sci. Health 2005, 40, 685–722. [Google Scholar] [CrossRef]
- Scott, A.C.; Mackinnon, M.D.; Fedorak, P.M. Naphthenic acids in Athabasca oil sands tailings waters are less biodegradable than commercial naphthenic acids. Environ. Sci. Technol. 2005, 39, 8388–8394. [Google Scholar] [CrossRef]
- Quinlan, P.J.; Tam, K.C. Water treatment technologies for the remediation of naphthenic acids in oil sands process-affected water. Chem. Eng. J. 2015, 279, 696–714. [Google Scholar] [CrossRef]
- Frank, R.A.; Kavanagh, R.; Burnison, B.K.; Headley, J.V.; Peru, K.M.; Van Der Kraak, G.; Solomon, K.R. Diethylaminoethyl-cellulose clean-up of a large volume naphthenic acid extract. Chemosphere 2006, 64, 1346–1352. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V.; Peru, K.M. Novel materials for environmental remediation of tailing pond waters containing naphthenic acids. Process. Saf. Environ. Prot. 2008, 86, 237–243. [Google Scholar] [CrossRef]
- Wu, C.; De Visscher, A.; Gates, I.D. On naphthenic acids removal from crude oil and oil sands process-affected water. Fuel 2019, 253, 1229–1246. [Google Scholar] [CrossRef]
- Marentette, J.R.; Frank, R.A.; Bartlett, A.J.; Gillis, P.L.; Hewitt, L.M.; Peru, K.M.; Headley, J.V.; Brunswick, P.; Shang, D.; Parrott, J.L. Toxicity of naphthenic acid fraction components extracted from fresh and aged oil sands process-affected waters, and commercial naphthenic acid mixtures, to fathead minnow (Pimephales promelas) embryos. Aquat. Toxicol. 2015, 164, 108–117. [Google Scholar] [CrossRef]
- Toor, N.S.; Franz, E.D.; Fedorak, P.M.; MacKinnon, M.D.; Liber, K. Degradation and aquatic toxicity of naphthenic acids in oil sands process-affected waters using simulated wetlands. Chemosphere 2013, 90, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Leszczyńska, A.; Radzik, P.; Szefer, E.; Mičušík, M.; Omastová, M.; Pielichowski, K. Surface modification of cellulose nanocrystals with succinic anhydride. Polymers 2019, 11, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.; Wang, C.; Huang, Y.; Wu, M. Surface Modification of Nanocellulose. In Nanocellulose: Synthesis, Structure, Properties and Applications; World Scientific: Hadensack, NJ, USA, 2021; pp. 65–92. [Google Scholar]
- Russell, J.A.; Malcolm, R.K.; Campbell, K.; Woolfson, A.D. High-performance liquid chromatographic determination of 17β-estradiol and 17β-estradiol-3-acetate solubilities and diffusion coefficients in silicone elastomeric intravaginal rings. J. Chromatogr. B Biomed. Sci. Appl. 2000, 744, 157–163. [Google Scholar] [CrossRef]
- Wang, D. A critical review of cellulose-based nanomaterials for water purification in industrial processes. Cellulose 2019, 26, 687–701. [Google Scholar] [CrossRef]
- Heydarifard, S.; Nazhad, M.M.; Xiao, H.; Shipin, O.; Olson, J. Water-resistant cellulosic filter for aerosol entrapment and water purification, Part I: Production of water-resistant cellulosic filter. Environ. Technol. 2016, 37, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; de Lannoy, C.-F.; Wiesner, M.R. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef]
- Comber, S.; Gardner, M.; Sörme, P.; Ellor, B. The removal of pharmaceuticals during wastewater treatment: Can it be predicted accurately? Sci. Total Environ. 2019, 676, 222–230. [Google Scholar] [CrossRef]
- Schröder, P.; Helmreich, B.; Škrbić, B.; Carballa, M.; Papa, M.; Pastore, C.; Emre, Z.; Oehmen, A.; Langenhoff, A.; Molinos, M. Status of hormones and painkillers in wastewater effluents across several European states—Considerations for the EU watch list concerning estradiols and diclofenac. Environ. Sci. Pollut. Res. 2016, 23, 12835–12866. [Google Scholar] [CrossRef]
- Boleda, M.R.; Galceran, M.T.; Ventura, F. Behavior of pharmaceuticals and drugs of abuse in a drinking water treatment plant (DWTP) using combined conventional and ultrafiltration and reverse osmosis (UF/RO) treatments. Environ. Pollut. 2011, 159, 1584–1591. [Google Scholar] [CrossRef]
- Sbardella, L.; Comas, J.; Fenu, A.; Rodriguez-Roda, I.; Weemaes, M. Advanced biological activated carbon filter for removing pharmaceutically active compounds from treated wastewater. Sci. Total Environ. 2018, 636, 519–529. [Google Scholar] [CrossRef]
- Kharel, S.; Stapf, M.; Miehe, U.; Ekblad, M.; Cimbritz, M.; Falås, P.; Nilsson, J.; Sehlén, R.; Bregendahl, J.; Bester, K. Removal of pharmaceutical metabolites in wastewater ozonation including their fate in different post-treatments. Sci. Total Environ. 2021, 759, 143989. [Google Scholar] [CrossRef]
- Elk, M.; Baresel, C.; Magnér, J.; Bergström, R.; Harding, M. Activated carbon for the removal of pharmaceutical residues from treated wastewater. Water Sci. Technol. 2014, 69, 2372–2380. [Google Scholar]
- Yangali-Quintanilla, V.; Sadmani, A.; McConville, M.; Kennedy, M.; Amy, G. Rejection of pharmaceutically active compounds and endocrine disrupting compounds by clean and fouled nanofiltration membranes. Water Res. 2009, 43, 2349–2362. [Google Scholar] [CrossRef]
- Soffer, Y.; Ben Aim, R.; Adin, A. Membrane fouling and selectivity mechanisms in effluent ultrafiltration coupled with flocculation. Water Sci. Technol. 2005, 51, 123–134. [Google Scholar] [CrossRef]
- Trisaranakul, W.; Chompoosor, A.; Maneeprakorn, W.; Nacapricha, N.; Choengchan. S.; Teerasong. S. A simple and rapid method based on anti-aggregation of silver nanoparticles for detection of Poly(diallyldimethylammonium chloride) in tap water. Anal. Sci. 2016, 32, 769–773. [Google Scholar] [CrossRef] [Green Version]
- Gamarra, J.S., Jr.; Godoi, A.F.L.; de Vasconcelos, E.C.; de Souza, K.M.T.; de Oliveira, C.M.R. Environmental Risk Assessment (ERA) of diclofenac and ibuprofen: A public health perspective. Chemosphere 2015, 120, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Koshani, R.; Tavakolian, M.; van de Ven, T.G. Cellulose-based dispersants and flocculants. J. Mater. Chem. B 2020, 8, 10502–10526. [Google Scholar] [CrossRef]
- Campano, C.; Lopez-Exposito, P.; Blanco, A.; Negro, C.; van de Ven, T.G. Hairy cationic nanocrystalline cellulose as a novel flocculant of clay. J. Colloid Interface Sci. 2019, 545, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Beddow, J.; Johnson, R.J.; Lawson, T.; Breckels, M.N.; Webster, R.J.; Smith, B.E.; Rowland, S.J.; Whitby, C. The effect of oil sands process-affected water and model naphthenic acids on photosynthesis and growth in Emiliania huxleyi and Chlorella vulgaris. Chemosphere 2016, 145, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKew, B.A.; Johnson, R.; Clothier, L.; Skeels, K.; Ross, M.S.; Metodiev, M.; Frenzel, M.; Gieg, L.M.; Martin, J.W.; Hough, M.A. Differential protein expression during growth on model and commercial mixtures of naphthenic acids in Pseudomonas fluorescens Pf-5. MicrobiologyOpen 2021, 10, e1196. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.A.; Sanderson, H.; Kavanagh, R.; Burnison, B.K.; Headley, J.V.; Solomon, K.R. Use of a (quantitative) structure–activity relationship [(Q) Sar] model to predict the toxicity of naphthenic acids. J. Toxicol. Environ. Health Part A 2009, 73, 319–329. [Google Scholar] [CrossRef]
- Headley, J.V.; McMartin, D.W. A review of the occurrence and fate of naphthenic acids in aquatic environments. J. Environ. Sci. Health Part A 2004, 39, 1989–2010. [Google Scholar] [CrossRef]
- Brown, L.D.; Ulrich, A.C. Oil sands naphthenic acids: A review of properties, measurement, and treatment. Chemosphere 2015, 127, 276–290. [Google Scholar] [CrossRef] [PubMed]








| Sample Materials | Zeta Potential (mV) |
|---|---|
| Cloisite clay | −40 mV |
| Nanocrystalline cellulose (CNC) | −50 mV |
| Cationic CNC (CTAC-CNC) | +41 mV |
| CTAB-CNC (15 mM CTAB) | −20 mV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, J.; Moallemi, A.; Chiao, M.; Plackett, D. The Use of Surface-Modified Nanocrystalline Cellulose Integrated Membranes to Remove Drugs from Waste Water and as Polymers to Clean Oil Sands Tailings Ponds. Polymers 2021, 13, 3899. https://doi.org/10.3390/polym13223899
Jackson J, Moallemi A, Chiao M, Plackett D. The Use of Surface-Modified Nanocrystalline Cellulose Integrated Membranes to Remove Drugs from Waste Water and as Polymers to Clean Oil Sands Tailings Ponds. Polymers. 2021; 13(22):3899. https://doi.org/10.3390/polym13223899
Chicago/Turabian StyleJackson, John, Ali Moallemi, Mu Chiao, and David Plackett. 2021. "The Use of Surface-Modified Nanocrystalline Cellulose Integrated Membranes to Remove Drugs from Waste Water and as Polymers to Clean Oil Sands Tailings Ponds" Polymers 13, no. 22: 3899. https://doi.org/10.3390/polym13223899
APA StyleJackson, J., Moallemi, A., Chiao, M., & Plackett, D. (2021). The Use of Surface-Modified Nanocrystalline Cellulose Integrated Membranes to Remove Drugs from Waste Water and as Polymers to Clean Oil Sands Tailings Ponds. Polymers, 13(22), 3899. https://doi.org/10.3390/polym13223899





