Green Chemistry in the Extraction of Natural Dyes from Colored Food Waste, for Dyeing Protein Textile Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Experimental Protocol
- (1)
- Treatment with 10 mL of acetic acid;
- (2)
- Esterification with 10 mL ethanol in an acid medium (pH = 3);
- (3)
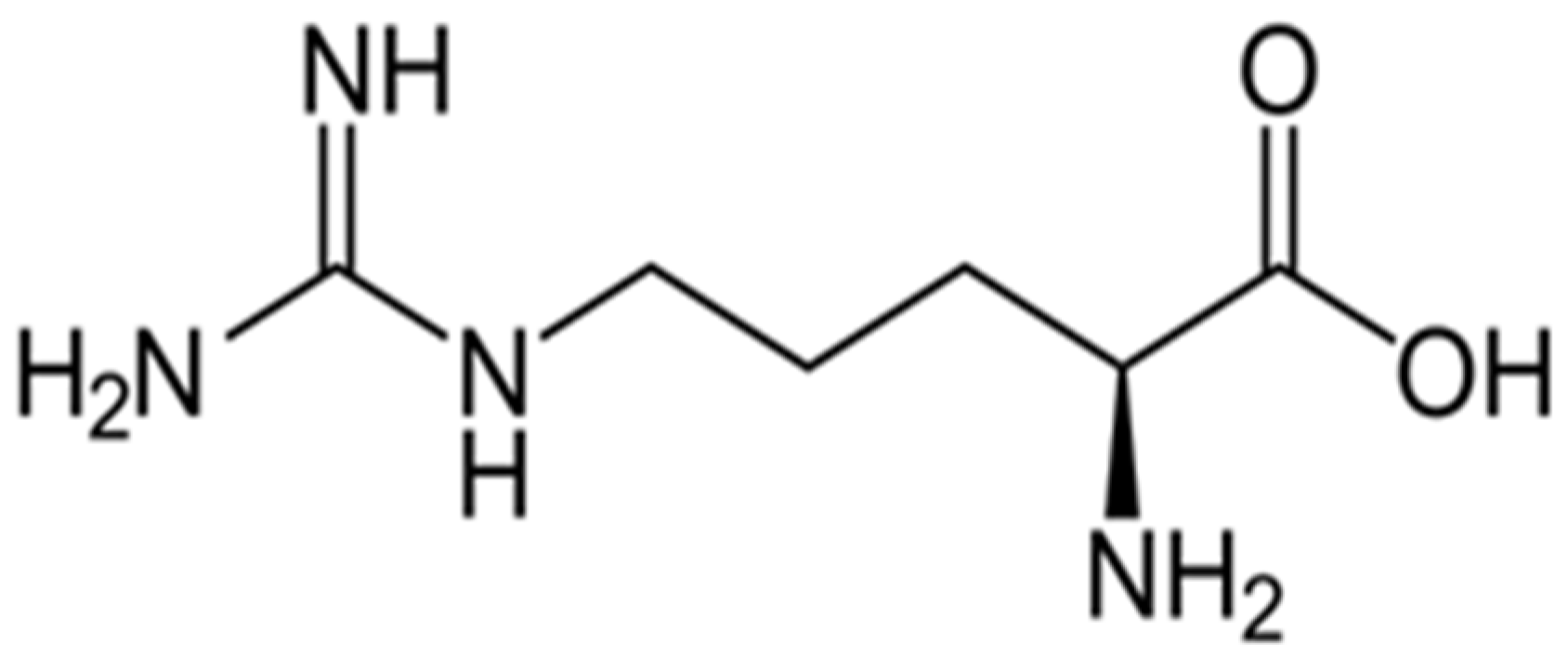
- Amination with 10 mL arginine solution of 50 g/L.
- (4)
- The duration of each functionalization was 30 min, at 25 °C. After the functionalization, the wool samples of 1 g each were dyed, at 40 °C, for 2 h.
- (a)
- When the functionalization was performed on wool: (1) FAAW—functionalization with acid acetic; (2) FEW—functionalization with ethanol; (3) FAW—functionalization with arginine.
- (b)
- When the betalains/extract has been functionalized: (1) FAAD—functionalization with acid acetic; (2) FED—functionalization with ethanol; (3) FAD—functionalization with arginine.
- -
- ISO 105-C10: 2010—Color fastness to washing test;
- -
- ISO 105-X12—Color fastness to rubbing test (also known as the “Crock test”);
- -
- ISO 105-B02: 2013—Color fastness to light test.
2.3. UV-VIS
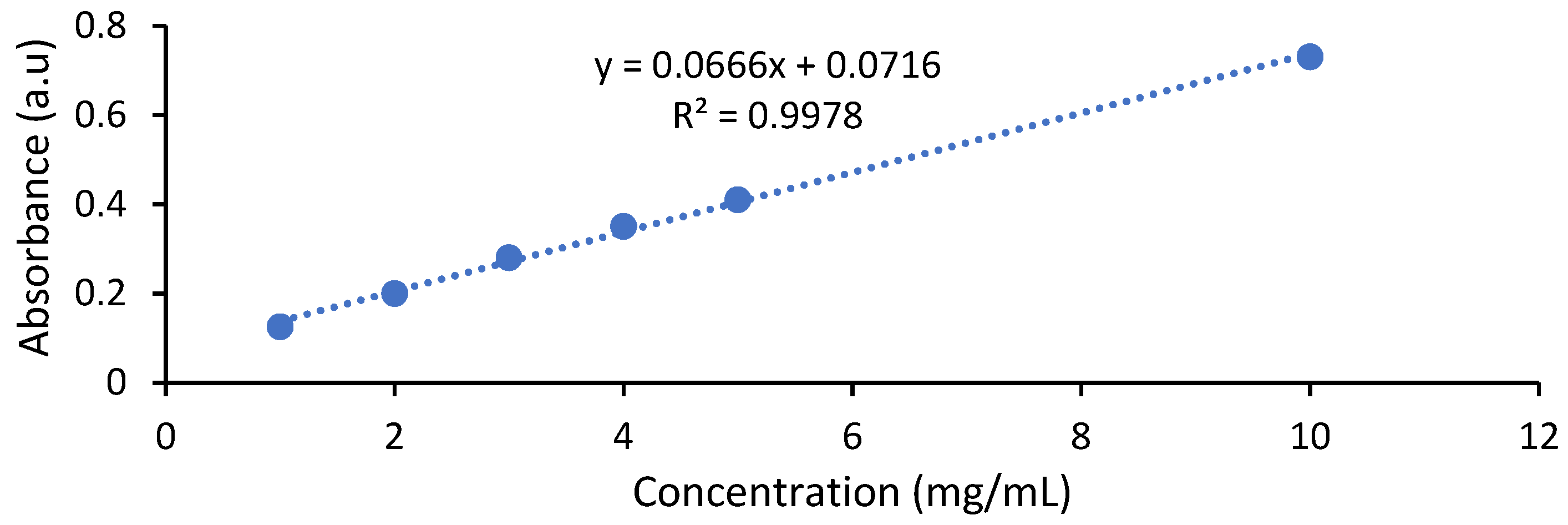
- Betanin content (mg/g fresh weight) = A × MW × Vf × DF/ε × L × Wf;
- Betanin content (mg/L) = [(A × DF × MW × 1000/ε × L)];
- The total monomeric antocyanins (mg/L) = A × DF × MW × 1000/ε × L;
- Color density (control) = (A420 nm − A700 nm) + (Avis max − A700 nm);
- Polymeric density (bisulfite-treated sample) = (A420 nm − A700 nm) + (Avis max − A700 nm);
- Browning index (bisulfite-treated sample) = A420 nm − A700 nm;
- Percent of polymeric color = polymeric color density × 100 %/color density.
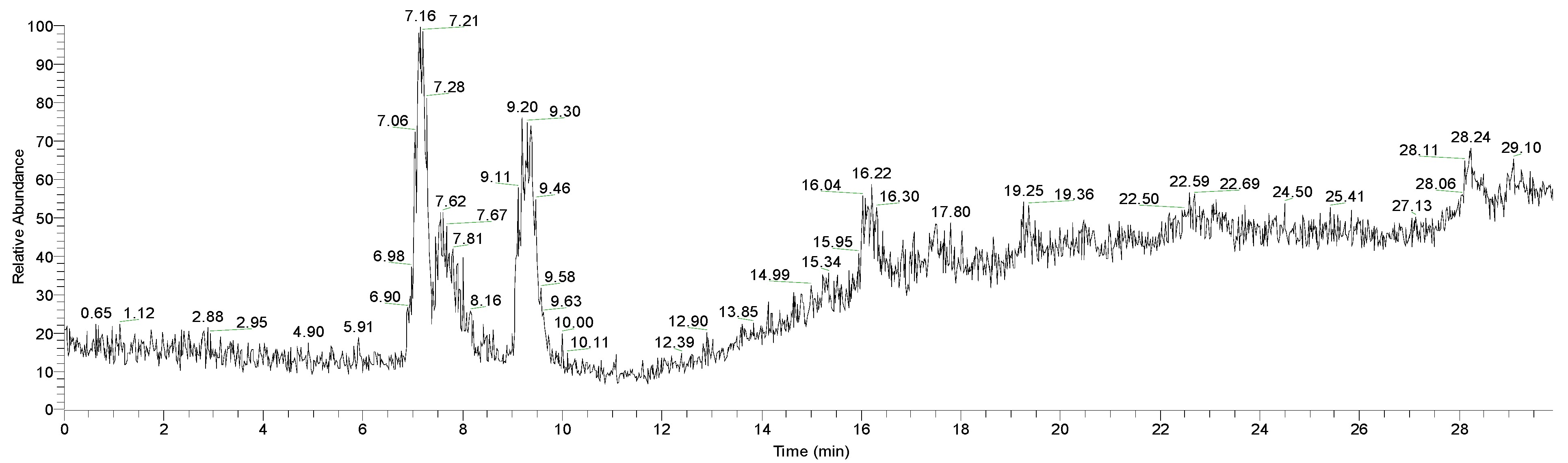
2.4. HPLC Experimental Technique
2.5. FTIR
2.6. CIEL*a*b* Measurements
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Extract
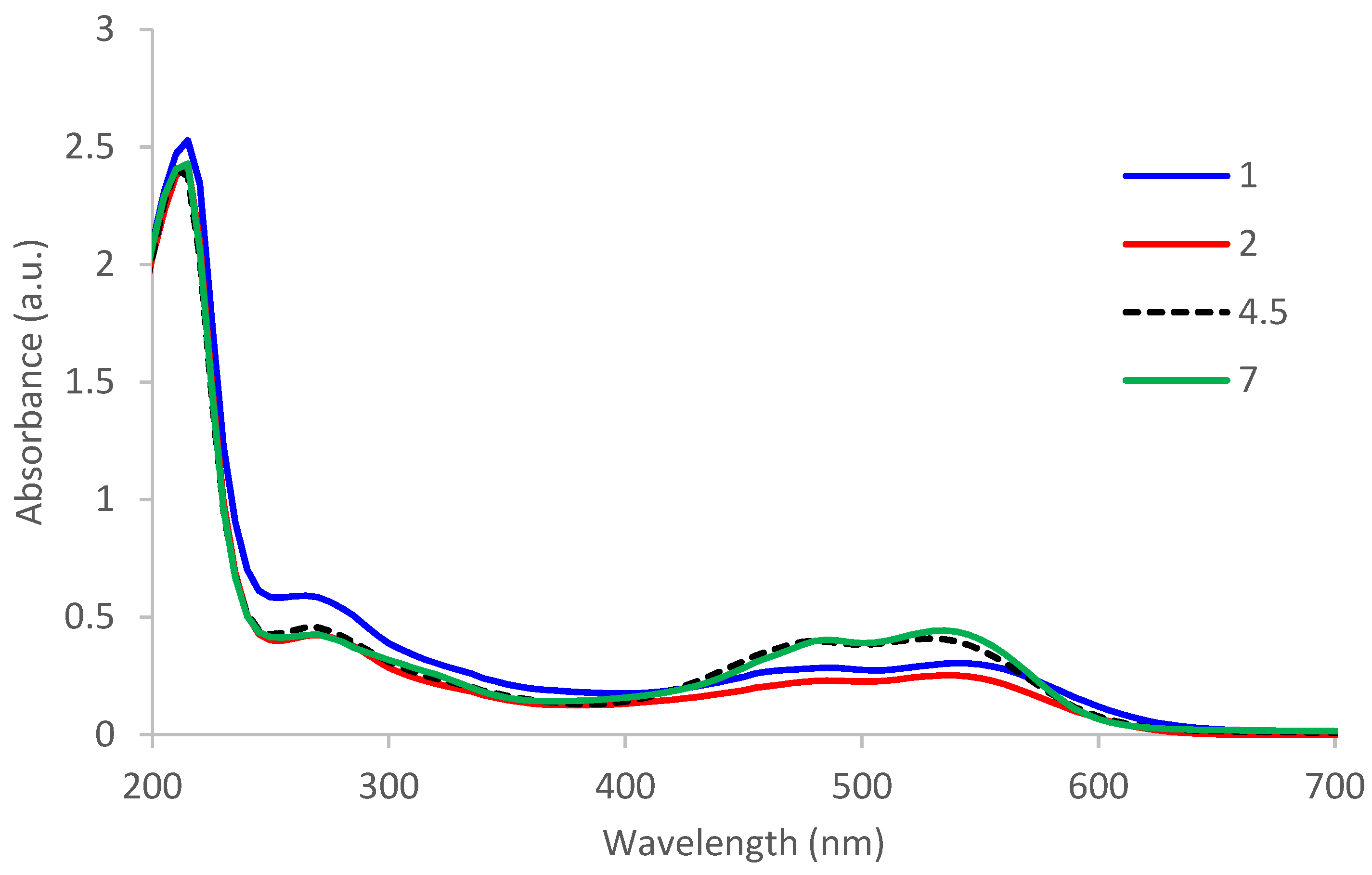
3.1.1. UV-VIS
- (1)
- At zero moment, for the mixture of distilled water with beet peels: L* = 3.04, a* = −0.12, b* = −0.12 and C* = 0.12. The color strength is K/S = 2.16;
- (2)
- After 1 h of continuous stirring, the color became stronger and highlighted by the CIEL*a*b* differences from those at time zero: dL* = −0.88, da* = −0.29, db* = 0.39, dC* = 0.03 and color difference dE* = 1.01. The color strength K/S became 2.66.
3.1.2. HPLC
3.2. Functionalization and Dyeing of Wool with Red Beetroot Peels Extract
- Functionalization/activation of wool and the formation of ionic bonds with COO-groups in betalains;
- Functionalization of the dye, making possible the formation of electrostatic interactions between the functional groups of betalains and those of activated wool.
3.2.1. Functionalization of Wool
3.2.1.1. Functionalization of Wool in Acidic Environment
3.2.1.2. Wool Esterification (FEW) with Ethanol
3.2.1.3. Functionalization of Wool with Arginine
3.2.2. Functionalization of Betalains
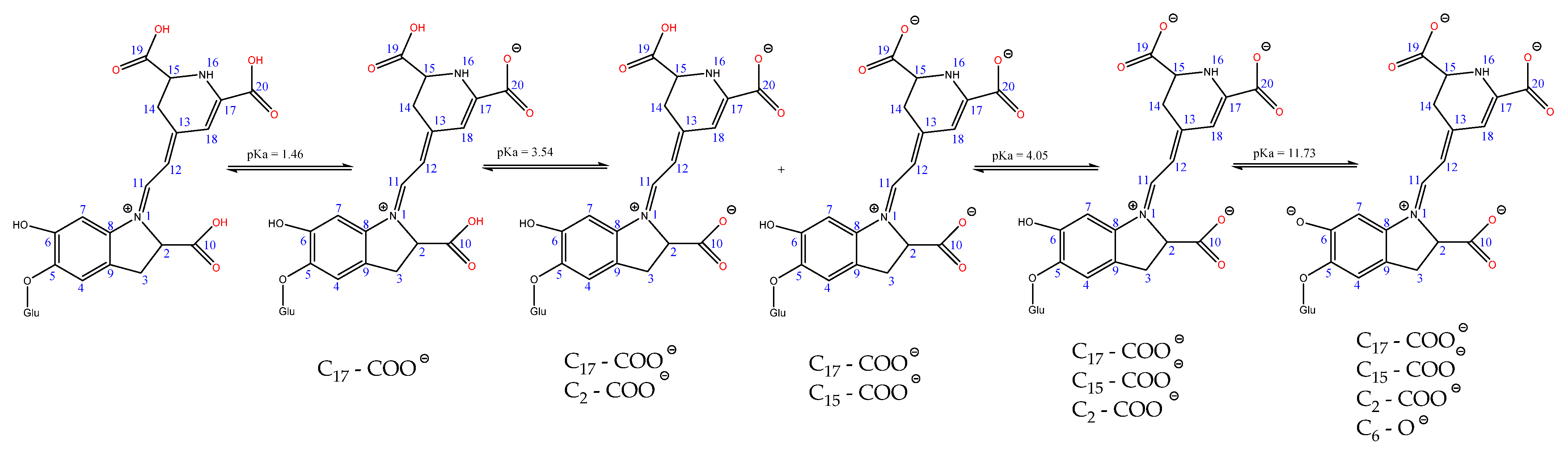
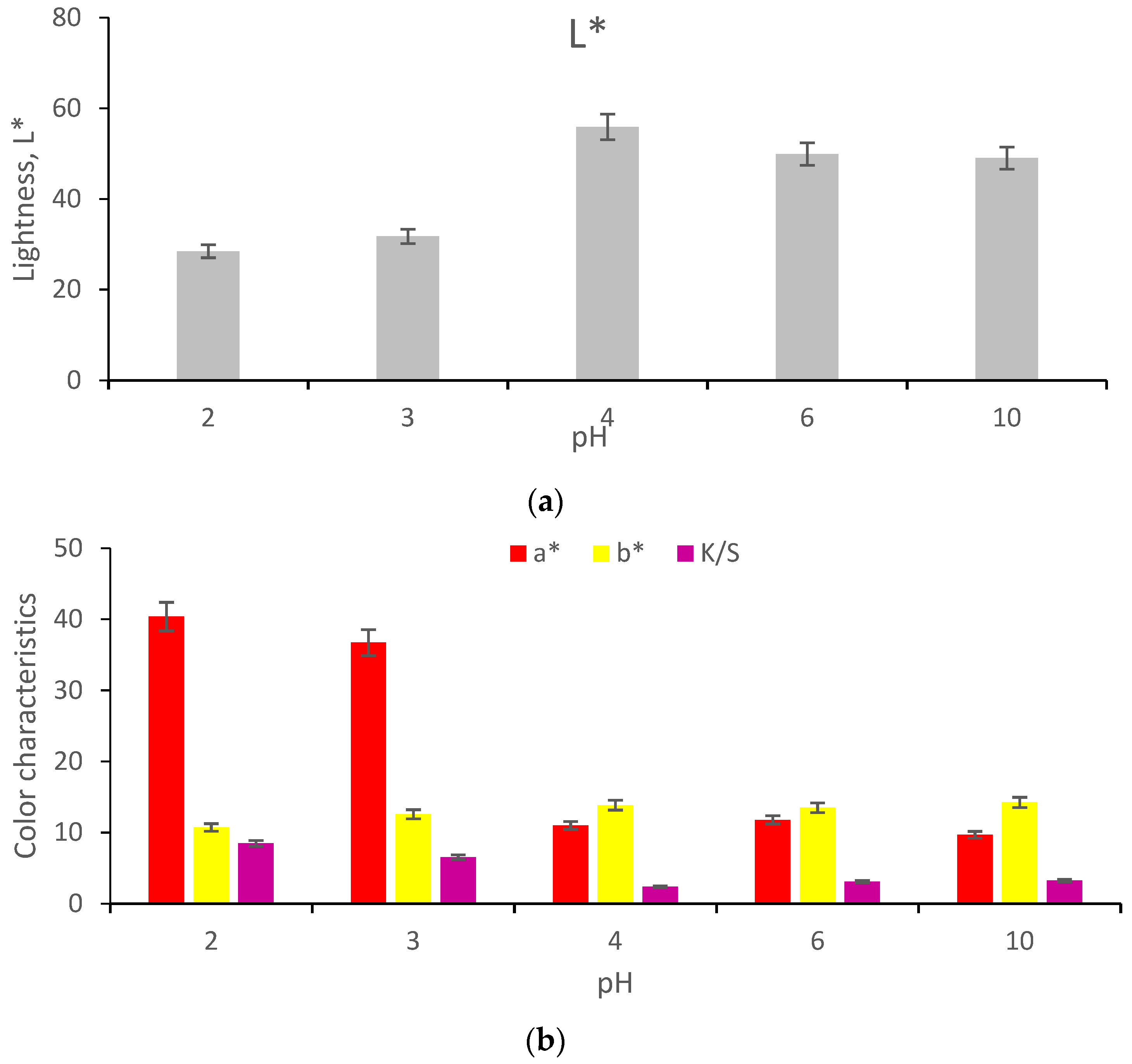
3.2.2.1. Functionalization of Betalains in Acidic Environment
- (a)
- In the mono-deprotonated series:
- (b)
- In the di-deprotonated series:
- (c)
- In the tri-deprotonated series:
- 16N− has DE = 256.5 Kcal/mol;
- C17—COO− has DE = 266.6 Kcal/mol;
- C15—COO− has DE = 270.00 Kcal/mol;
- C2—COO− has DE = 270.6 Kcal/mol;
- C6—O− has DE = 271.9 Kcal/mol.
3.2.2.2. Esterification of Betalains with Ethanol
3.2.2.3. Functionalization of Betalains with Arginine
3.3. The Best Results from Dyeing Wool
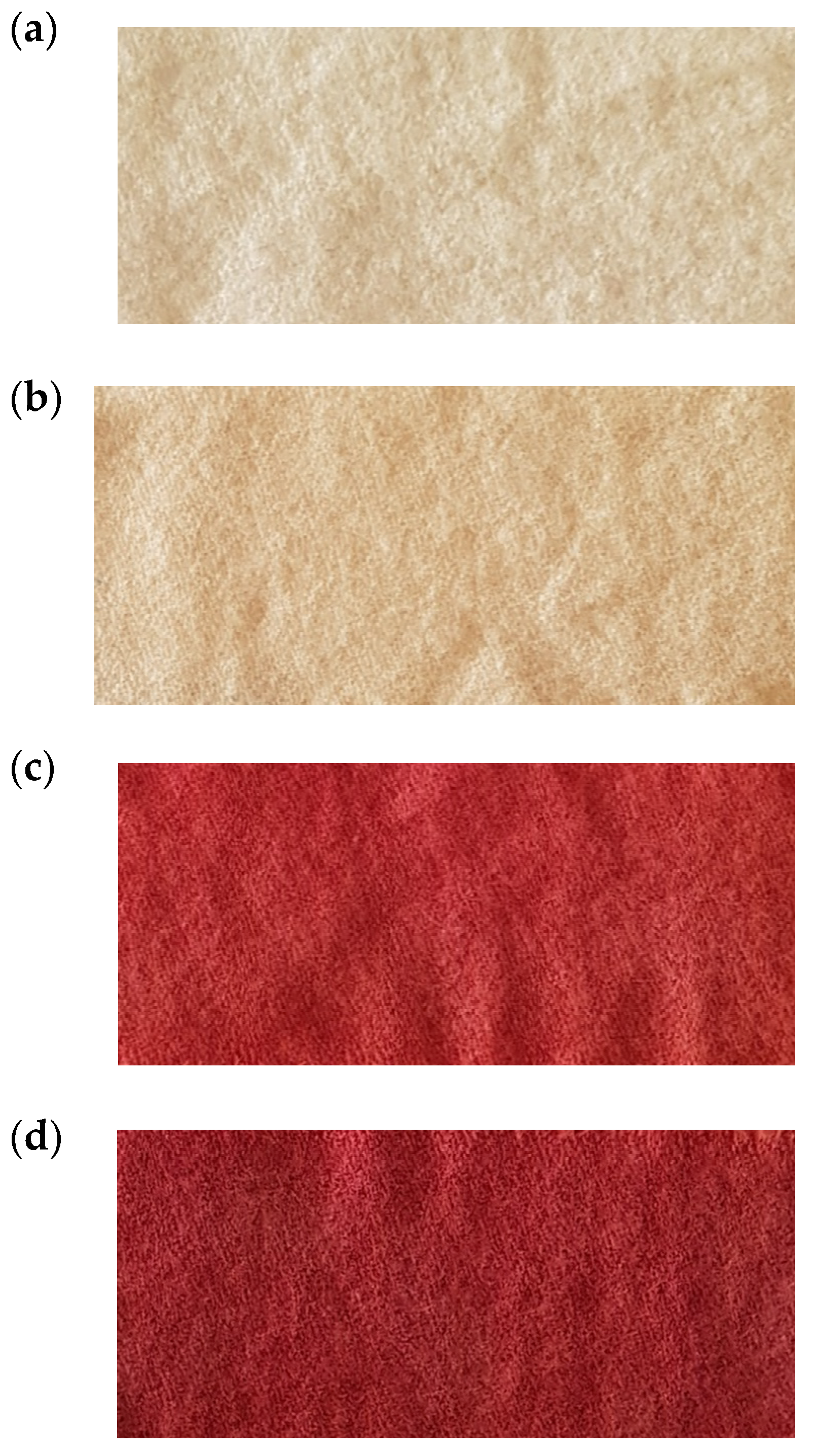
3.3.1. Protonated/Activated Wool Dyeing (FAAW)
3.3.2. Wool Dyeing Results with Acid-Functionalized Dye (FAAD)
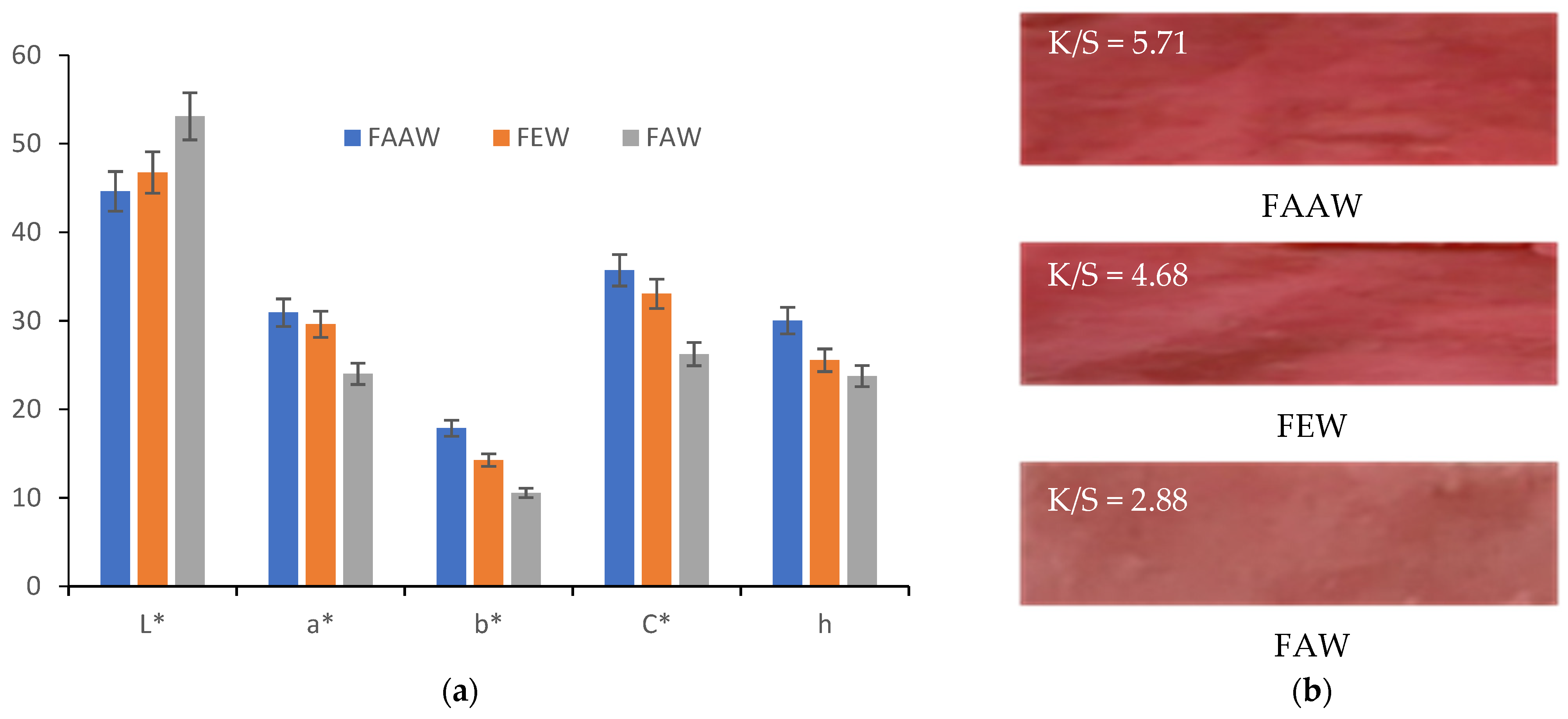
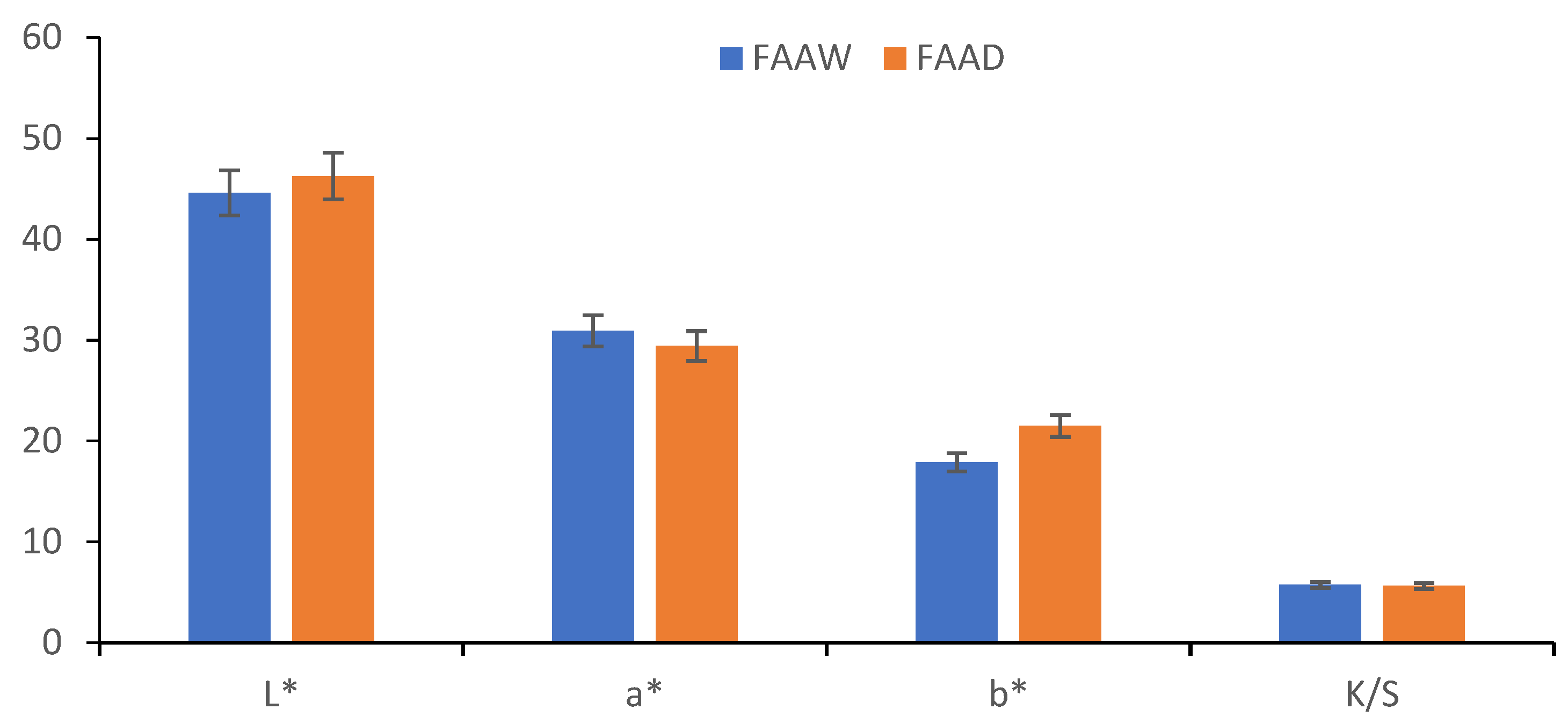
3.3.3. Comparison between FAAW and FAAD Samples
- -
- The dyed FAAW sample was assessed with a grade of 5 on the gray scale for assessing the change in color, after performing the color fastness to washing test;
- -
- The undyed cotton and wool samples that were attached on each side of the dyed sample (FAAW and FAAD, respectively) obtained grades of 5 on the gray scale for staining assessment; this indicates that the ionic bonds established between betalains and wool remained intact throughout the washing, even under the action of the detergent and the impact forces exerted by the steel balls used in the color fastness to washing test.
- In terms of color strength, wool functionalization is better because the FAAW sample has K/S = 5.71 higher than the FAAD sample (K/S = 5.6). The lightness values indicate the same idea: L* = 44.62 in the case of FAAW and L* = 46.27 in the case of FAAD. However, obtaining the FAAW requires increased attention to the handling of the protonated wool, from the functionalization bath to the dyeing bath;
- In terms of shade, the FAAW sample is redder (a* = 30.93) and less yellow (b* = 17.87) than the FAAD sample (a* = 29.42 and b* = 21.48);
- In terms of color-fastness properties, both functionalizations lead to very good results in washing and rubbing and to acceptable results in the light fastness test (Table 4). The values of these tests can be criteria for choosing the type of functionalization, depending on the destination of the dyed fabric;
- In terms of durability for repeated household washes, both functionalizations indicate excellent results.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Communication from the Commission to the European Parliament, The Council the European Economic and Social Committee and the Committee of the Regions, Closing the Loop—An EU Action Plan for the Circular Economy. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A52015DC0614 (accessed on 13 August 2021).
- Standard 100 by OEKO-TEX. Available online: https://www.oeko-tex.com/en/our-standards/standard-100-by-oeko-tex (accessed on 18 August 2021).
- Padma, S.V.; Dhara, S. Newer natural dyes for various textiles. In New Trends in Natural Dyes for Textiles, 1st ed.; Padma, S.V., Dhara, S., Eds.; Woodhead Publishing: Duxford, UK, 2019; pp. 1–69. [Google Scholar]
- Saxena, S.; Raja, A.S.M. Natural dyes: Sources, chemistry, application and sustainability issues. In Roadmap to Sustainable Textiles and Clothing, Textile Science and Clothing Technology; Muthu, S.S., Ed.; Springer Science + Business Media: Singapore, 2014; pp. 1–45. [Google Scholar]
- Yusuf, M.; Shabbir, M.; Mohammad, F. Natural colorants: Historical, processing and sustainable prospects. Nat. Prod. Bioprospecting 2017, 7, 123–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.; Gabr, A.; Kastell, A.; Riedel, H.; Zhenzhen, C.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Janiszeska, E. Microencapsulated beetroot juice as a potential source of betalain. Powder Technol. 2014, 264, 190–196. [Google Scholar] [CrossRef]
- Bhupinder, S.; Bahadur, S.H. Chemical composition, functional properties and processing of Beetroot—A review. Int. J. Sci. Eng. Res. 2014, 5, 679–684. [Google Scholar]
- Chhikaraa, N.; Kushwahaa, K.; Sharmab, P.; Gata, Y.; Panghala, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Knuthsen, P. Investigations on beetroot colours for the purpose of regulation. Z. Für Lebensm.-Unters. Und Forsch. 1981, 172, 195–200. [Google Scholar] [CrossRef]
- Kujala, T.S.; Loponen, J.M.; Klika, K.D.; Pihlaja, K. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: distribution and effect of cold storage on the content of total phenolics and three individual compounds. J. Agric. Food. Chem. 2000, 48, 5338–5342. [Google Scholar] [CrossRef]
- Kujala, T.S.; Loponen, J.M.; Pihlaja, K. Betalains and phenolics in red beetroot (Beta vulgaris) peel extracts: Extraction and characterisation. Z. Naturforsch. C 2001, 56, 343–348. [Google Scholar] [CrossRef]
- Sapers, G.M.; Hornstein, J.S. Varietal differences in colorant properties and stability of red beet pigments. J. Food Sci. 1979, 44, 1245–1248. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Reinhold, C. Betalain stability and degradation-structural and chromatic aspects. J. Food Sci. 2006, 71, 41–50. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Impact of thermal treatment on color and pigment pattern of red beet (Beta vulgaris L.) preparations. J. Food Sci. 2004, 69, 491–498. [Google Scholar] [CrossRef]
- Antigo, J.L.D.; Bergamasco, R.C.; Madrona, G.S. Effect of pH on the stability of red beet extract (Beta vulgaris l.) microcapsules produced by spray drying or freeze drying. Food Sci. Technol. 2018, 38, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Rajabinejad, H.; Bucişcanu, I.I.; Maier, S.S. Current approaches for raw wool waste management and unconventional valorization: A review. Environ. Eng. Manag. J. 2019, 18, 1439–1456. [Google Scholar]
- Popescu, C.; Wortmann, F.-J. Wool–Structure, Mechanical Properties and Technical Products based on Animal Fibres. Structure, Properties and Technical Applications. In Industrial Applications of Natural Fibres, 1st ed.; Müssig, J., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 255–266. [Google Scholar]
- Kumar, V.; Prabha, R. Extraction and analysis of natural dye. J. Nat. Prod. Plant Resour. 2018, 8, 32–38. [Google Scholar]
- Khalida, T.; Hage, M. Isolation of natural dye from beet root and its application on wool and thread with different mordants at different temperatures. Int. J. Adv. Eng. Res. 2016, 3, 52–62. [Google Scholar]
- Yeniocak, M.; Goktas, O.; Ozen, E.; Colak, M.; Ugurlu, M. Determination of leaching features of wood surfaces colored by eco-friendly red beetroot (beta vulgaris) extract. Wood Res. 2018, 63, 443–454. [Google Scholar]
- Yeniocak, M.; Goktas, O.; Ozen, E.; Colak, M.; Ugurlu, M. Natural Coloration of wood material by red beetroot (beta vulgaris) and determination color stability under UV exposure. Maderas. Cienc. Tecnol. 2015, 17, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Yaqub, A.; Chaudhary, N.; Bhatti, R.A.A.; Iqbal, Z.; Habib-ul-Haq, M. Green extraction and dyeing of silk from Beta vulgaris peel dye with ecofriendly acid mordants. Int. J. Biosci. 2018, 13, 308–321. [Google Scholar]
- Stintzing, F.C.; Carle, R. Analysis of betalains. In Food Colorants Chemical and Functional Properties; Socaciu, C., Ed.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Popa, A.; Moldovan, B.; David, L. Betanin from Red Beet (Beta vulgaris L.) Extraction conditions and evaluation of the thermal stability. Rev. Chim. 2015, 66, 413–416. [Google Scholar]
- Neagu, C.; Barbu, V. Principal component analysis of the factors involved in the extraction of beetroot betalains. J. Agroalimenty Process. Technol. 2014, 20, 311–318. [Google Scholar]
- Sturzoiu, A.; Stroescu, M.; Stoica, A.; Dobre, T. Betanine extraction from beta vulgaris—Experimental research and statistical modeling. U.P.B. Sci. Bull. Series B 2011, 73, 145–156. [Google Scholar]
- Xu, W.; Ke, G.; Wu, J.; Wang, X. Modification of wool fiber using steam explosion. Eur. Pol. J. 2006, 42, 2168–2173. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Shi, Z.; Zhao, Q.; Yun, Y. Study on the Structure and Properties of Biofunctional Keratin from Rabbit Hair. Materials 2021, 14, 379. [Google Scholar] [CrossRef] [PubMed]
- Margariti, C. The application of FTIR microspectroscopy in a non-invasive and non-destructive way to the study and conservation of mineralized excavated textiles. Herit. Sci. 2019, 7, 63. [Google Scholar] [CrossRef]
- Devadiga, D.; Ahipa, T.N. Betanin: A Red-Violet Pigment—Chemistry and Applications. In Chemistry and Technology of Natural and Synthetic Dyes and Pigments; Samanta, A.K., Nasser Awwad, N., Algarni, H.M., Eds.; IntechOpen Book Series: London, UK, 2020; pp. 1–19. [Google Scholar]
- Rodriguez, S.A.; Baumgartner, M.T. Betanidin pKa Prediction Using DFT Methods. ACS Omega 2020, 5, 13751–13759. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska-Świglo, A.; Szymusiak, H.; Malinowska, P. Betanin, the main pigment of red beet-molecular origin of its exceptionally high free radical scavenging activity. Food Addit. Contam. 2006, 23, 1079–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumbravă, A.; Enache, I.; Oprea, C.I.; Georgescu, A.; Gîrţu, M.A. Toward a more efficient utilisation of betalains as pigments for Dye-Sensitized solar cells. Digest Dig. J. Nanomater. Biostruct. 2012, 7, 339–351. [Google Scholar]
- Pires Goncalves, L.C.; Martorelli Di Genova, B.; Augusto Dorr, F.; Pinto, E.; Leite Bastos, E. Effect of dielectric microwave heating on the color and antiradical capacity of betanin. J. Food Eng. 2013, 118, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Tuwalska, D.; Starzak, K.; Szot, D.; Wybraniec, S.; Winterhalter, P.; Jerz, G. Semi-synthesis of red beet betacyanin ethyl-esters by esterification. Challenges Mod. Technol. 2014, 5, 27–31. [Google Scholar]
- Belhadj Slimen, I.; Najar, T.; Abderrabba, M. Chemical and Antioxidant Properties of Betalains. J. Agric. Food Chem. 2017, 65, 675–689. [Google Scholar] [CrossRef]
- Sawicki, T.; Bączek, N.; Wiczkowski, W. Betalain profile, content and antioxidant capacity of red beetroot dependent on the genotype and root part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Sawicki, T.; Juśkiewicz, J.; Wiczkowski, W. Using the SPE and Micro-HPLC-MS/MS Method for the Analysis of Betalains in Rat Plasma after Red Beet Administration. Molecules 2017, 22, 2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, A. Contemporary Wool Dyeing and Finishing, Dyeing Methods for Wool, Training Course-Woolwise, 1st ed.; CSIRO Textile and Fibre Technology and Australian Wool Innovation Ltd.: Melbourne, Australia, 2017; pp. 1–146. [Google Scholar]
- Memon, H.; Khatri, A.; Ali, N.; Memon, S. Dyeing Recipe Optimization for Eco-Friendly Dyeing and Mechanical Property Analysis of Eco-Friendly Dyed Cotton Fabric: Better Fixation, Strength, and Color Yield by Biodegradable Salts. J. Nat. Fibers 2016, 13, 749–758. [Google Scholar]
- Dutta, S.; Bansal, P. Cotton Fiber and Yarn Dyeing. In Cotton Science and Processing Technology. Gene, Ginning, Garment and Green Recycling, 1st ed.; Wang, H., Memon, H., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 355–375. [Google Scholar]
- Padma, S.V.; Dhara, S. Dyeing Application of Newer Natural Dyes on Cotton Silk and Wool with Fastness Properties, CIE Lab Values, and Shade Card. In New Trends in Natural Dyes for Textiles, 1st ed.; Padma, S.V., Dhara, S., Eds.; Woodhead Publishing; Elsevier: Sawston, UK, 2019; pp. 159–282. [Google Scholar]
- Padfield, T.; Landi, S. The Light-Fastness of the Natural Dyes Source. Stud. Conserv. 1966, 11, 181–196. [Google Scholar]








| Characteristics | Value | |
|---|---|---|
| 1 | Betanin content (mg/g fresh peels red beetroot) | 1.4235 |
| 2 | Total monomeric antocyanins (betanin content) (mg/L) | 142.35 |
| 3 | Color density | 0.597583 |
| 4 | Polimeric color density (bisulfite) | 0.769485 |
| 5 | Browning index | 0.204574 |
| 6 | Percent of polymeric color (%) | 128.76621 |
| Dye Category | Dye Name | Number of Functional Groups | Dissociation Constant | Water Solubility (g/L) | ||||
|---|---|---|---|---|---|---|---|---|
| COOH | OH | NH2 | SO42− | pKa | pKb | |||
| Betacyanins (Red–violet color) | Betanin | 3 | 5 | - | - | 1.46 | −3.6 | 0.5 |
| Neobetanin | 3 | 5 | - | - | 1.43 | −3.6 | 0.18 | |
| Prebetanin | 3 | 4 | - | 1 | −2.3 | −3.7 | 0.85 | |
| Isobetanin | 3 | 5 | - | - | 1.46 | −3.6 | 0.5 | |
| Betanidin | 3 | 2 | - | - | 1.55 | 0.056 | 0.052 | |
| Isobetanidin | 3 | 2 | - | - | 1.55 | 0.056 | 0.052 | |
| Betaxanthin (Yellow–orange color) | Vulgaxanthin I | 3 | - | 1 | - | 1.64 | 8.59 | 0.26 |
| Vulgaxanthin II | 4 | - | - | - | 1.54 | 8.58 | 0.21 | |
| Indicaxanthin | 3 | - | - | - | 2.12 | 0.082 | 0.21 | |
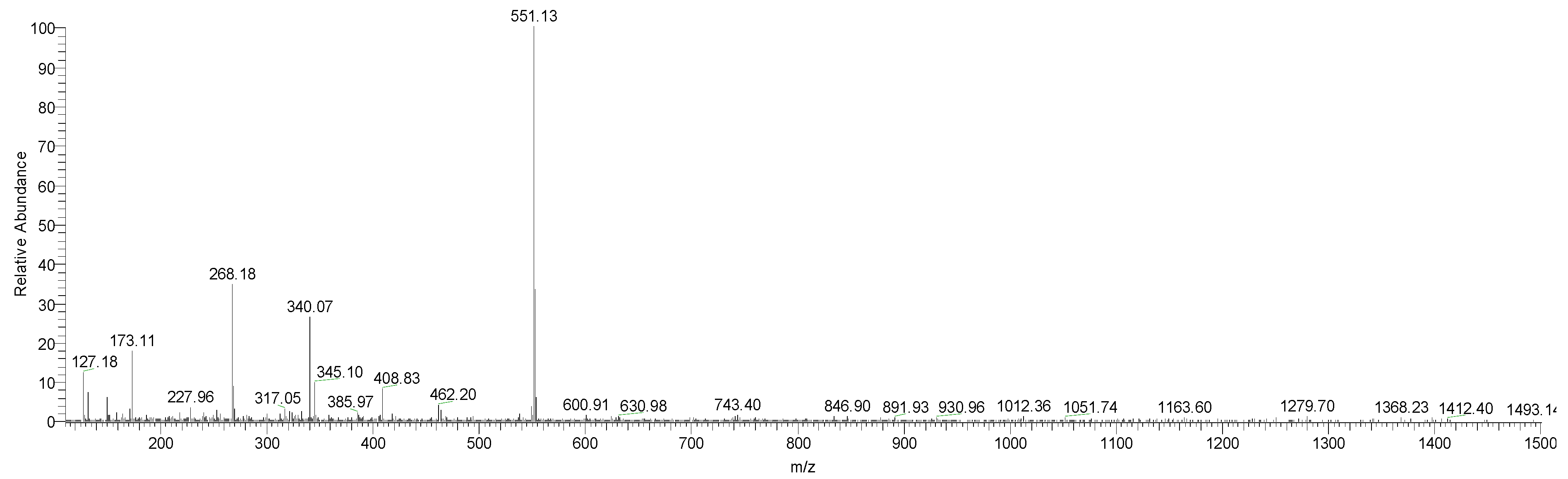
| Sample | Compound | m/z | m/z (Daughter Ions) |
|---|---|---|---|
| Non-functionalized extract (pH = 5.7) RT = 9.27 | Vulgaxanthin I | 340 | 323/277 |
| 17-decarboxy-isobetanidin | 345 | 301.1 | |
| Betanin | 551 | 389/149 | |
| Isobetanin | 551 | 389 | |
| Prebetanin | 631 | 551/389 | |
| Functionalized extract at pH = 2; RT = 4.67 | Portulacaxanthin III | 269 | 225 |
| Vulgaxanthin I | 340 | 323/277 | |
| Vulgaxanthin II | 341 | 325/148 | |
| Betanidin | 389 | 345 | |
| Isobetanidin | 389 | 389/345 |
| Sample Code | Color Fastness to Washing | Color Fastness to Rubbing | Color Fastness to Light | |||
|---|---|---|---|---|---|---|
| Change in Color | Staining | |||||
| Cotton | Wool | Dry | Wet | |||
| FAAW | 5 | 5 | 5 | 5 | 4–5 | 5 |
| FAAD | 4–5 | 5 | 5 | 5 | 4–5 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, V.; Blaga, A.C.; Pruneanu, M.; Cristian, I.N.; Pîslaru, M.; Popescu, A.; Rotaru, V.; Crețescu, I.; Cașcaval, D. Green Chemistry in the Extraction of Natural Dyes from Colored Food Waste, for Dyeing Protein Textile Materials. Polymers 2021, 13, 3867. https://doi.org/10.3390/polym13223867
Popescu V, Blaga AC, Pruneanu M, Cristian IN, Pîslaru M, Popescu A, Rotaru V, Crețescu I, Cașcaval D. Green Chemistry in the Extraction of Natural Dyes from Colored Food Waste, for Dyeing Protein Textile Materials. Polymers. 2021; 13(22):3867. https://doi.org/10.3390/polym13223867
Chicago/Turabian StylePopescu, Vasilica, Alexandra Cristina Blaga, Melinda Pruneanu, Irina Niculina Cristian, Marius Pîslaru, Andrei Popescu, Vlad Rotaru, Igor Crețescu, and Dan Cașcaval. 2021. "Green Chemistry in the Extraction of Natural Dyes from Colored Food Waste, for Dyeing Protein Textile Materials" Polymers 13, no. 22: 3867. https://doi.org/10.3390/polym13223867
APA StylePopescu, V., Blaga, A. C., Pruneanu, M., Cristian, I. N., Pîslaru, M., Popescu, A., Rotaru, V., Crețescu, I., & Cașcaval, D. (2021). Green Chemistry in the Extraction of Natural Dyes from Colored Food Waste, for Dyeing Protein Textile Materials. Polymers, 13(22), 3867. https://doi.org/10.3390/polym13223867









