Conductive Polymers and Their Nanocomposites as Adsorbents in Environmental Applications
Abstract
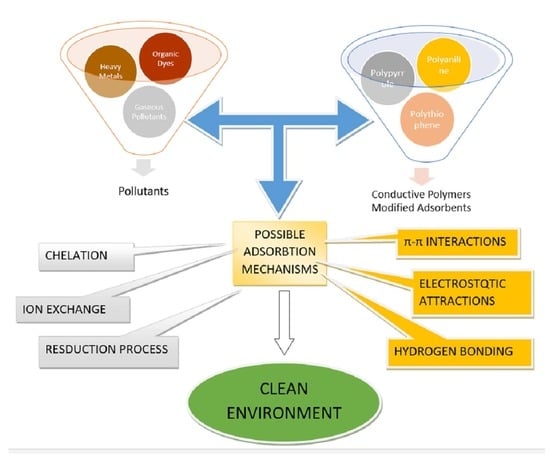
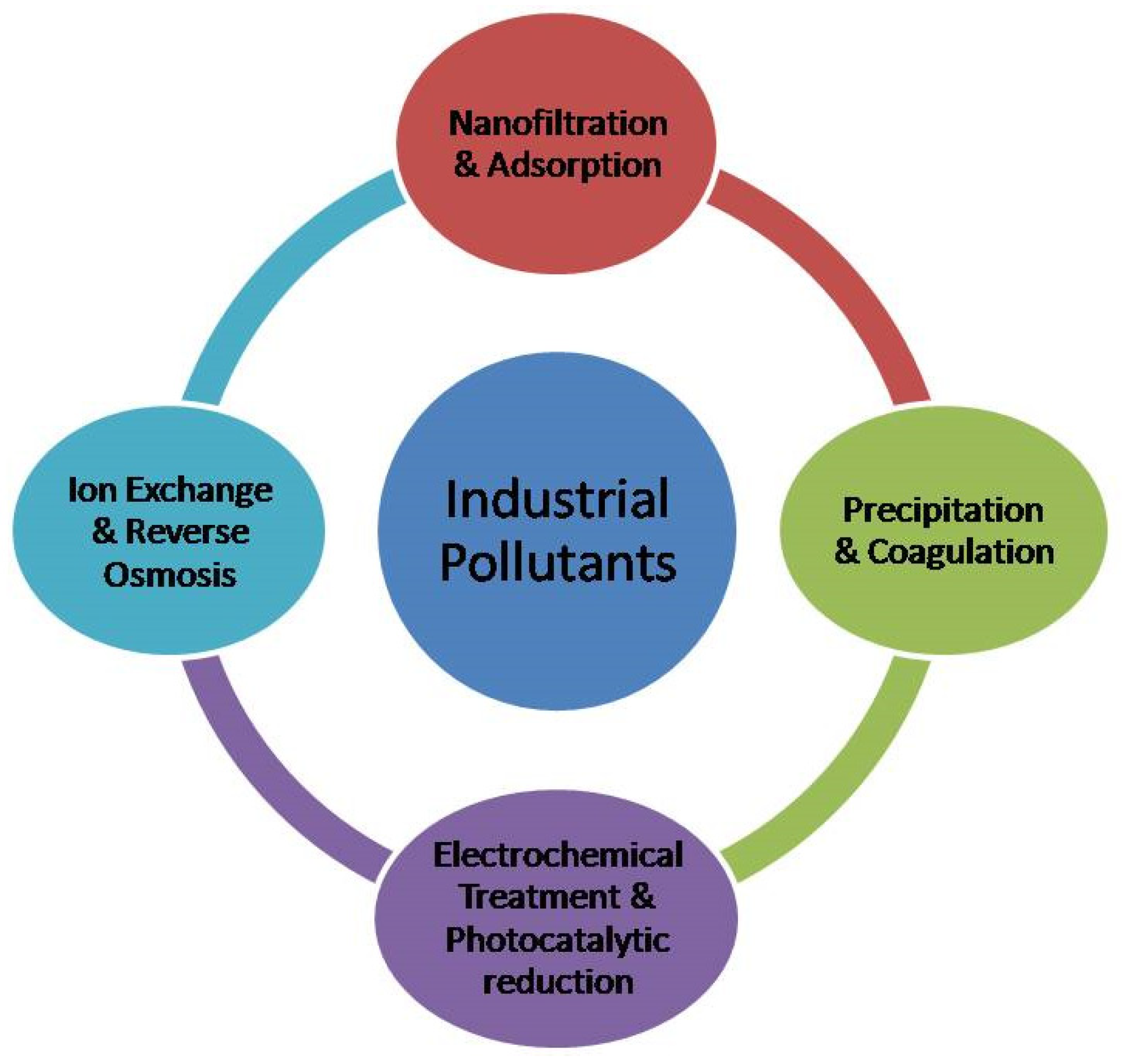
:1. Introduction
2. Conductive Polymers as Adsorbents
2.1. PANI and PANI-Based Composite Adsorbents for the Removal of Heavy Metal Ions (HMIs)
2.2. PANI and PANI-Based Composite Adsorbents for the Removal of Organic Dyes and Other Organic Pollutants from Aqueous Environments
PANI and PANI-Based Composite Adsorbents for the Removal of Gaseous Pollutants
2.3. Polypyrrole-Based Nanocomposites as Adsorbents
2.3.1. HMIs Removal by Ppy and Ppy-Based Composite Adsorbents
2.3.2. Removal of Organic Pollutants and Organic Dyes by Ppy and Ppy-Based Composite Adsorbents
2.3.3. Gaseous Pollutants Removal by Ppy and Ppy-Based Composite Adsorbents
2.4. Polythiophene and Other Conductive Polymer Nanocomposites as Adsorbents
Pollutants Removal by Combined Conductive Polymers
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Derdour, K.; Bouchelta, C.; Naser-Eddine, A.K.; Medjram, M.S.; Magri, P. Removal of Cr (VI) from aqueous solutions by using activated carbon supported iron catalysts as efficient adsorbents. World J. Eng. 2018, 15, 3–13. [Google Scholar] [CrossRef]
- Briggs, D. Environmental pollution and the global burden of disease. Br. Med. Bull. 2003, 68, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Aigbe, U.O.; Osibote, O.A. A review of hexavalent chromium removal from aqueous solutions by sorption technique using nanomaterials. J. Environ. Chem. Eng. 2020, 8, 104503. [Google Scholar] [CrossRef]
- Nasseh, N.; Taghavi, L.; Barikbin, B.; Harifi-Mood, A.R. The removal of Cr (VI) from aqueous solution by almond green hull waste material: Kinetic and equilibrium studies. J. Water Reuse Desalination 2016, 7, 449–460. [Google Scholar] [CrossRef]
- Ali, I.H.; Al Mesfer, M.K.; Khan, M.I.; Danish, M.; Alghamdi, M.M. Exploring Adsorption Process of Lead (II) and Chromium (VI) Ions from Aqueous Solutions on Acid Activated Carbon Prepared from Juniperus procera Leaves. Processes 2019, 7, 217. [Google Scholar] [CrossRef] [Green Version]
- Sorme, L.; Lagerkvist, R. Sources of heavy metals in urban wastewater in Stockholm. Sci. Total Environ. 2002, 298, 131–145. [Google Scholar] [CrossRef]
- Chaari, I.; Medhioub, M.; Jamoussi, F.; Hamzaoui, A.H. Acid-treated clay materials (Southwestern Tunisia) for removing sodium leuco-vat dye: Characterization, adsorption study and activation mechanism. J. Mol. Struct. 2021, 1223, 128944. [Google Scholar] [CrossRef]
- Ameen, S.; Seo, H.-K.; Akhtar, M.S.; Shin, H.S. Novel graphene/polyaniline nanocomposites and its photocatalytic activity toward the degradation of rose Bengal dye. Chem. Eng. J. 2012, 210, 220–228. [Google Scholar] [CrossRef]
- Sani, F.M.; Oyelaran, O.A. Exhaust Gas Treatment in Thermal Power Plants: A Review. Int. J. Adv. Sci. Res. Eng. 2019, 5, 227–233. [Google Scholar] [CrossRef]
- Demirci, S.; Sahiner, N. The Use of Conductive Polymers Embedded Macro Porous Pei and Ionic Liquid Form of Pei Cryogels for Potential Conductometric Sensor Application to CO2. J. Compos. Sci. 2020, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Kausar, A.; Siddiq, M. Conducting Polymer/Graphene Filler-based Hybrids: Energy and Electronic Applications. In Polymer Science: Research Advances, Practical Applications and Educational Aspects; Méndez-Vilas, A., Solano-Martín, A., Eds.; Formatex Research Center: Badajoz, Spain, 2016; pp. 177–187. Available online: https://pdfs.semanticscholar.org/4376/5a93a70d59fa5d4aa8f9c667422c66c6f18a.pdf (accessed on 3 April 2021).
- Kumar, H.; Kumari, N.; Sharma, R. Nanocomposites (conducting polymer and nanoparticles) based electrochemical biosensor for the detection of environment pollutant: Its issues and challenges. Environ. Impact Assess. Rev. 2020, 85, 106438. [Google Scholar] [CrossRef]
- Geetha, S.; Rao, C.R.; Vijayan, M.; Trivedi, D. Biosensing and drug delivery by polypyrrole. Anal. Chim. Acta 2006, 568, 119–125. [Google Scholar] [CrossRef]
- Nicolas-Debarnot, D.; Poncin-Epaillard, F. Polyaniline as a new sensitive layer for gas sensors. Anal. Chim. Acta 2003, 475, 1–15. [Google Scholar] [CrossRef]
- Khan, M.I.; Chaudhry, A.U.; Hashim, S.; Zahoor, M.K.; Iqbal, M.Z. Recent developments in intrinsically conductive polymer coatings for corrosion protection. Chem. Eng. Res. Bull. 2010, 14, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Zarras, P.; Anderson, N.; Webber, C.; Irvin, D.; Irvin, J.; Guenthner, A.; Stenger-Smith, J. Progress in using conductive polymers as corrosion-inhibiting coatings. Radiat. Phys. Chem. 2003, 68, 387–394. [Google Scholar] [CrossRef]
- Bhattacharya, A.; De, A. Conducting composites of polypyrrole and polyaniline a review. In Progress in Solid State Chemistry; Elsevier Ltd.: Amsterdam, The Netherlands, 1996; Volume 24, pp. 141–181. [Google Scholar] [CrossRef]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Oh, W.-K.; Kwon, O.S.; Jang, J. Conducting Polymer Nanomaterials for Biomedical Applications: Cellular Interfacing and Biosensing. Polym. Rev. 2013, 53, 407–442. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincón, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical–Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef]
- Taghizadeh, A.; Taghizadeh, M.; Jouyandeh, M.; Yazdi, M.K.; Zarintaj, P.; Saeb, M.R.; Lima, E.C.; Gupta, V.K. Conductive polymers in water treatment: A review. J. Mol. Liq. 2020, 312, 113447. [Google Scholar] [CrossRef]
- Kang, E.-T. Polyaniline: A polymer with many interesting intrinsic redox states. Prog. Polym. Sci. 1998, 23, 277–324. [Google Scholar] [CrossRef]
- Zare, E.N.; Motahari, A.; Sillanpää, M. Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: A review. Environ. Res. 2018, 162, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Chen, C.; Wang, X. Application of polyaniline and multiwalled carbon nanotube magnetic composites for removal of Pb (II). Chem. Eng. J. 2012, 185–186, 144–150. [Google Scholar] [CrossRef]
- Kumar, P.A.; Chakraborty, S.; Ray, M. Removal and recovery of chromium from wastewater using short chain polyaniline synthesized on jute fiber. Chem. Eng. J. 2008, 141, 130–140. [Google Scholar] [CrossRef]
- Ren, J.; Huang, X.; Wang, N.; Lu, K.; Zhang, X.; Li, W.; Liu, D. Preparation of polyaniline-coated polyacrylonitrile fiber mats and their application to Cr (VI) removal. Synth. Met. 2016, 222, 255–266. [Google Scholar] [CrossRef]
- Qiu, B.; Xu, C.; Sun, D.; Wang, Q.; Gu, H.; Zhang, X.; Weeks, B.L.; Hopper, J.; Ho, T.C.; Guo, Z.; et al. Polyaniline coating with various substrates for hexavalent chromium removal. Appl. Surf. Sci. 2015, 334, 7–14. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Z.; Zeng, G.; Liu, Y.; Shao, B.; Li, Z.; Liu, Y.; Zhang, W.; He, Q. Polyaniline-based adsorbents for removal of hexavalent chromium from aqueous solution: A mini review. Environ. Sci. Pollut. Res. 2018, 25, 6158–6174. [Google Scholar] [CrossRef] [PubMed]
- Karthik, R.; Meenakshi, S. Removal of Cr (VI) ions by adsorption onto sodium alginate-polyaniline nanofibers. Int. J. Biol. Macromol. 2015, 72, 711–717. [Google Scholar] [CrossRef]
- Ghorbani, M.; Lashkenari, M.S.; Eisazadeh, H. Application of polyaniline nanocomposite coated on rice husk ash for removal of Hg (II) from aqueous media. Synth. Met. 2011, 161, 1430–1433. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.; Song, J.; Pan, X.; Liu, J.; Wang, Y.; Tang, L. Synthesis of SBA-15/polyaniline mesoporous composite for removal of resorcinol from aqueous solution. Appl. Surf. Sci. 2014, 290, 260–266. [Google Scholar] [CrossRef]
- Jiang, N.; Xu, Y.; Dai, Y.; Luo, W.; Dai, L. Polyaniline nanofibers assembled on alginate microsphere for Cu2+ and Pb2+ uptake. J. Hazard. Mater. 2012, 215, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Ossman, M.; Farag, H. Removal of Cd (II) ion from waste water by adsorption onto polyaniline coated on sawdust. Desalination 2011, 272, 301–305. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Removal of Pb (II) and Cd (II) ions from aqueous solution using polyaniline grafted chitosan. Chem. Eng. J. 2015, 263, 168–177. [Google Scholar] [CrossRef]
- Soltani, H.; Belmokhtar, A.; Zeggai, F.Z.; Benyoucef, A.; Bousalem, S.; Bachari, K. Copper (II) Removal from Aqueous Solutions by PANI-Clay Hybrid Material: Fabrication, Characterization, Adsorption and Kinetics Study. J. Inorg. Organomet. Polym. Mater. 2019, 29, 841–850. [Google Scholar] [CrossRef]
- Nodeh, M.K.M.; Gabris, M.A.; Nodeh, H.R.; Bidhendi, M.E. Efficient removal of arsenic (III) from aqueous media using magnetic polyaniline-doped strontium–titanium nanocomposite. Environ. Sci. Pollut. Res. 2018, 25, 16864–16874. [Google Scholar] [CrossRef] [PubMed]
- Madi, N.K.; Bhadra, J.; Al-Thani, N.J.; Alashraf, A.; Abdulmalik, D.; Al-Qaradawi, I. Adsorption study of Pb (II) in aqueous medium using polyaniline nanocomposites. J. Vinyl Addit. Technol. 2017, 23, E99–E106. [Google Scholar] [CrossRef]
- Yavuz, A.G.; Dincturk-Atalay, E.; Uygun, A.; Gode, F.; Aslan, E. A comparison study of adsorption of Cr (VI) from aqueous solutions onto alkyl-substituted polyaniline/chitosan composites. Desalination 2011, 279, 325–331. [Google Scholar] [CrossRef]
- Jahan, K.; Kumar, N.; Verma, V. Removal of hexavalent chromium from potable drinking using a polyaniline-coated bacterial cellulose mat. Environ. Sci. Water Res. Technol. 2018, 4, 1589–1603. [Google Scholar] [CrossRef]
- Ben Ali, M.; Wang, F.; Boukherroub, R.; Lei, W.; Xia, M. Phytic acid-doped polyaniline nanofibers-clay mineral for efficient adsorption of copper (II) ions. J. Colloid Interface Sci. 2019, 553, 688–698. [Google Scholar] [CrossRef]
- Dognani, G.; Hadi, P.; Ma, H.; Cabrera, F.C.; Job, A.; Agostini, D.L.; Hsiao, B.S. Effective chromium removal from water by polyaniline-coated electrospun adsorbent membrane. Chem. Eng. J. 2019, 372, 341–351. [Google Scholar] [CrossRef]
- Harijan, D.K.; Chandra, V. Polyaniline functionalized graphene sheets for treatment of toxic hexavalent chromium. J. Environ. Chem. Eng. 2016, 4, 3006–3012. [Google Scholar] [CrossRef]
- Ayad, M.; El-Hefnawy, G.; Zaghlol, S. Facile synthesis of polyaniline nanoparticles; its adsorption behavior. Chem. Eng. J. 2013, 217, 460–465. [Google Scholar] [CrossRef]
- Janaki, V.; Vijayaraghavan, K.; Oh, B.-T.; Lee, K.-J.; Muthuchelian, K.; Ramasamy, A.; Kamala-Kannan, S. Starch/polyaniline nanocomposite for enhanced removal of reactive dyes from synthetic effluent. Carbohydr. Polym. 2012, 90, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.-N.; Jesmeen, S.R.; Hossain, M.M. Removal of dyes from water by conducting polymeric adsorbent. Polym. Adv. Technol. 2004, 15, 633–638. [Google Scholar] [CrossRef]
- Shen, J.; Shahid, S.; Amura, I.; Sarihan, A.; Tian, M.; Emanuelsson, E.A. Enhanced adsorption of cationic and anionic dyes from aqueous solutions by polyacid doped polyaniline. Synth. Met. 2018, 245, 151–159. [Google Scholar] [CrossRef]
- Sharma, V.; Rekha, P.; Mohanty, P. Nanoporous hypercrosslinked polyaniline: An efficient adsorbent for the adsorptive removal of cationic and anionic dyes. J. Mol. Liq. 2016, 222, 1091–1100. [Google Scholar] [CrossRef]
- Khan, N.A.; An, H.J.; Yoo, D.K.; Jhung, S.H. Polyaniline-derived porous carbons: Remarkable adsorbent for removal of various hazardous organics from both aqueous and non-aqueous media. J. Hazard. Mater. 2018, 360, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Pathania, D.; Kothiyal, N.; Sharma, G. Polyaniline zirconium (IV) silicophosphate nanocomposite for remediation of methylene blue dye from waste water. J. Mol. Liq. 2014, 190, 139–145. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, H.; Li, J.; Chen, C. Hierarchical MWCNTs/Fe3O4/PANI magnetic composite as adsorbent for methyl orange removal. J. Colloid Interface Sci. 2015, 450, 189–195. [Google Scholar] [CrossRef]
- Janaki, V.; Oh, B.-T.; Vijayaraghavan, K.; Kim, J.-W.; Kim, S.A.; Ramasamy, A.; Kamala-Kannan, S. Application of bacterial extracellular polysaccharides/polyaniline composite for the treatment of Remazol effluent. Carbohydr. Polym. 2012, 88, 1002–1008. [Google Scholar] [CrossRef]
- Salem, M.A.; Elsharkawy, R.G.; Hablas, M.F. Adsorption of brilliant green dye by polyaniline/silver nanocomposite: Kinetic, equilibrium, and thermodynamic studies. Eur. Polym. J. 2016, 75, 577–590. [Google Scholar] [CrossRef]
- Muhammad, A.; Shah, A.-U.-H.A.; Bilal, S.; Rahman, G. Basic Blue Dye Adsorption from Water Using Polyaniline/Magnetite (Fe3O4) Composites: Kinetic and Thermodynamic Aspects. Materials 2019, 12, 1764. [Google Scholar] [CrossRef] [Green Version]
- Anirudhan, T.S.; Divya, P.L.; Nima, J. Utilization of polypyrrole coated iron-doped titania based hydrogel for the removal of tetracycline hydrochloride from aqueous solutions: Adsorption and photocatalytic degradation studies. Environ. Nanotechnol. Monit. Manag. 2015, 4, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Nasar, A.; Mashkoor, F. Application of polyaniline-based adsorbents for dye removal from water and wastewater—A review. Environ. Sci. Pollut. Res. 2019, 26, 5333–5356. [Google Scholar] [CrossRef]
- Sultana, S.; Rafiuddin; Khan, M.Z.; Umar, K.; Muneer, M. Electrical, Thermal, Photocatalytic and Antibacterial Studies of Metallic Oxide Nanocomposite Doped Polyaniline. J. Mater. Sci. Technol. 2013, 29, 795–800. [Google Scholar] [CrossRef]
- Allahveran, S.; Mehrizad, A. Polyaniline/ZnS nanocomposite as a novel photocatalyst for removal of Rhodamine 6G from aqueous media: Optimization of influential parameters by response surface methodology and kinetic modeling. J. Mol. Liq. 2017, 225, 339–346. [Google Scholar] [CrossRef]
- Eskizeybek, V.; Sari, F.; Gülce, H.; Gülce, A.; Avcı, A. Preparation of the new polyaniline/ZnO nanocomposite and its photocatalytic activity for degradation of methylene blue and malachite green dyes under UV and natural sun lights irradiations. Appl. Catal. B Environ. 2012, 119–120, 197–206. [Google Scholar] [CrossRef]
- Agarwal, S.; Tyagi, I.; Gupta, V.K.; Golbaz, F.; Golikand, A.N.; Moradi, O. Synthesis and characteristics of polyaniline/zirconium oxide conductive nanocomposite for dye adsorption application. J. Mol. Liq. 2016, 218, 494–498. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, G.; Kumar, A.; Kothiyal, N.C. Fabrication of nanocomposite polyaniline zirconium (IV) silicophosphate for photocatalytic and antimicrobial activity. J. Alloys Compd. 2014, 588, 668–675. [Google Scholar] [CrossRef]
- Sun, C.; Xiong, B.; Pan, Y.; Cui, H. Adsorption removal of tannic acid from aqueous solution by polyaniline: Analysis of operating parameters and mechanism. J. Colloid Interface Sci. 2017, 487, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Rashid, M.; Hossain, M.M.; Al Mesfer, M.K.; Arshad, M.; Danish, M.; Lee, M.; El Jery, A.; Kumar, N. Fabrication of polyaniline/activated carbon composite and its testing for methyl orange removal: Optimization, equilibrium, isotherm and kinetic study. Polym. Test. 2019, 77, 105909. [Google Scholar] [CrossRef]
- Gopal, N.; Asaithambi, M.; Sivakumar, P. Adsorption studies of a direct dye using polyaniline coated activated carbon prepared from Prosopis juliflora. J. Water Process. Eng. 2014, 2, 87–95. [Google Scholar] [CrossRef]
- Kanwal, F.; Rehman, R.; Bakhsh, I.Q. Batch wise sorptive amputation of diamond green dye from aqueous medium by novel Polyaniline-Alstonia scholaris leaves composite in ecofriendly way. J. Clean. Prod. 2018, 196, 350–357. [Google Scholar] [CrossRef]
- Janaki, V.; Oh, B.-T.; Shanthi, K.; Lee, K.-J.; Ramasamy, A.; Kamala-Kannan, S. Polyaniline/chitosan composite: An eco-friendly polymer for enhanced removal of dyes from aqueous solution. Synth. Met. 2012, 162, 974–980. [Google Scholar] [CrossRef]
- Mu, B.; Tang, J.; Zhang, L.; Wang, A. Preparation, characterization and application on dye adsorption of a well-defined two-dimensional superparamagnetic clay/polyaniline/Fe3O4 nanocomposite. Appl. Clay Sci. 2016, 132, 7–16. [Google Scholar] [CrossRef]
- Zaremotlagh, S.; Hezarkhani, A. Removal of textile dyes from aqueous solution by conducting polymer modified clinoptilolite. Environ. Earth Sci. 2013, 71, 2999–3006. [Google Scholar] [CrossRef]
- Muhammad, A.; Bilal, S. Comparative Study of the Adsorption of Acid Blue 40 on Polyaniline, Magnetic Oxide and Their Composites: Synthesis, Characterization and Application. Materials 2019, 12, 2854. [Google Scholar] [CrossRef] [Green Version]
- Mahto, T.K.; Chowdhuri, A.R.; Sahu, S.K. Polyaniline-functionalized magnetic nanoparticles for the removal of toxic dye from wastewater. J. Appl. Polym. Sci. 2014, 131, 40840. [Google Scholar] [CrossRef]
- Yan, B.; Chen, Z.; Cai, L.; Chen, Z.; Fu, J.; Xu, Q. Fabrication of polyaniline hydrogel: Synthesis, characterization and adsorption of methylene blue. Appl. Surf. Sci. 2015, 356, 39–47. [Google Scholar] [CrossRef]
- Ballav, N.; Debnath, S.; Pillay, K.; Maity, A. Efficient removal of Reactive Black from aqueous solution using polyaniline coated ligno-cellulose composite as a potential adsorbent. J. Mol. Liq. 2015, 209, 387–396. [Google Scholar] [CrossRef]
- Debnath, S.; Ballav, N.; Maity, A.; Pillay, K. Development of a polyaniline-lignocellulose composite for optimal adsorption of Congo red. Int. J. Biol. Macromol. 2015, 75, 199–209. [Google Scholar] [CrossRef]
- Lyu, W.; Yu, M.; Feng, J.; Yan, W. Highly crystalline polyaniline nanofibers coating with low-cost biomass for easy separation and high efficient removal of anionic dye ARG from aqueous solution. Appl. Surf. Sci. 2018, 458, 413–424. [Google Scholar] [CrossRef]
- Bhaumik, M.; McCrindle, R.I.; Maity, A.; Agarwal, S.; Gupta, V.K. Polyaniline nanofibers as highly effective re-usable adsorbent for removal of reactive black 5 from aqueous solutions. J. Colloid Interface Sci. 2016, 466, 442–451. [Google Scholar] [CrossRef]
- Patil, M.R.; Shrivastava, V.S. Adsorption of malachite green by polyaniline–nickel ferrite magnetic nanocomposite: An isotherm and kinetic study. Appl. Nanosci. 2014, 5, 809–816. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.-D.; He, Y.-J.; Zhang, Y.-H.; Zhu, Q.-Q. Adsorption property of alizarin red S by NiFe2O4/polyaniline magnetic composite. J. Environ. Chem. Eng. 2018, 6, 416–425. [Google Scholar] [CrossRef]
- Zarrini, K.; Rahimi, A.A.; Alihosseini, F.; Fashandi, H. Highly efficient dye adsorbent based on polyaniline-coated nylon-6 nanofibers. J. Clean. Prod. 2017, 142, 3645–3654. [Google Scholar] [CrossRef]
- Rachna, K.; Agarwal, A.; Singh, N. Preparation and characterization of zinc ferrite—Polyaniline nanocomposite for removal of rhodamine B dye from aqueous solution. Environ. Nanotechnol. Monit. Manag. 2018, 9, 154–163. [Google Scholar] [CrossRef]
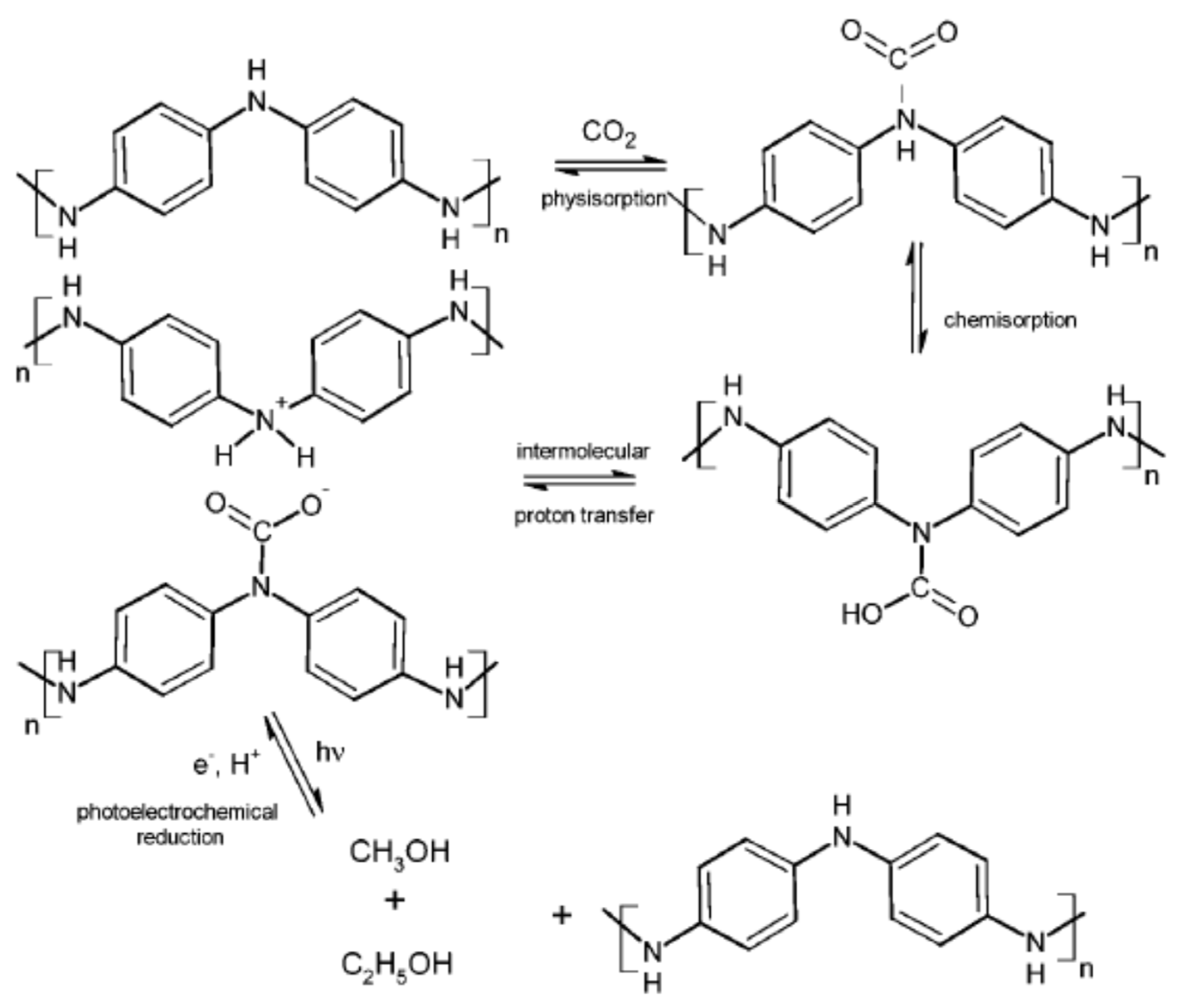
- Li, X.; Sui, Z.Y.; Sun, Y.N.; Xiao, P.W.; Wang, X.Y.; Han, B.H. Polyaniline-derived hierarchically porous nitrogen-doped carbons as gas adsorbents for carbon dioxide uptake. Microporous Mesoporous Mater. 2018, 257, 85–91. [Google Scholar] [CrossRef]
- Yang, S.-B. Removal of ammonia gas via conducting polymer-assisted titania under visible light or UV exposure. Asian J. Chem. 2014, 26, 70–73. [Google Scholar] [CrossRef]
- Hursán, D.; Kormányos, A.; Rajeshwar, K.; Janáky, C. Polyaniline films photoelectrochemically reduce CO2 to alcohols. Chem. Commun. 2016, 52, 8858–8861. [Google Scholar] [CrossRef]
- Della Pina, C.; De Gregorio, M.A.; Clerici, L.; Dellavedova, P.; Falletta, E. Polyaniline (PANI): An innovative support for sampling and removal of VOCs in air matrices. J. Hazard. Mater. 2018, 344, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, L.; Shan, L.; Wang, R.; Min, Y. Comparative Study on the Adsorption of NO2 Using Different Clay/Polyaniline Composites. Ind. Eng. Chem. Res. 2018, 57, 6897–6903. [Google Scholar] [CrossRef]
- Saad, M.; Tahir, H.; Khan, J.; Hameed, U.; Saud, A. Synthesis of polyaniline nanoparticles and their application for the removal of Crystal Violet dye by ultrasonicated adsorption process based on Response Surface Methodology. Ultrason. Sonochem. 2017, 34, 600–608. [Google Scholar] [CrossRef]
- Lyu, W.; Wu, J.; Zhang, W.; Liu, Y.; Yu, M.; Zhao, Y.; Feng, J.; Yan, W. Easy separated 3D hierarchical coral-like magnetic polyaniline adsorbent with enhanced performance in adsorption and reduction of Cr (VI) and immobilization of Cr (III). Chem. Eng. J. 2019, 363, 107–119. [Google Scholar] [CrossRef]
- Aliabadi, R.S.; Mahmoodi, N.O. Synthesis and characterization of polypyrrole, polyaniline nanoparticles and their nanocomposite for removal of azo dyes; sunset yellow and Congo red. J. Clean. Prod. 2018, 179, 235–245. [Google Scholar] [CrossRef]
- Tanzifi, M.; Yaraki, M.T.; Kiadehi, A.D.; Hosseini, S.H.; Olazar, M.; Bharti, A.K.; Agarwal, S.; Gupta, V.K.; Kazemi, A. Adsorption of Amido Black 10B from aqueous solution using polyaniline/SiO2 nanocomposite: Experimental investigation and artificial neural network modeling. J. Colloid Interface Sci. 2018, 510, 246–261. [Google Scholar] [CrossRef]
- Samani, M.R.; Borghei, S.M.; Olad, A.; Chaichi, M.J. Removal of chromium from aqueous solution using polyaniline—Poly ethylene glycol composite. J. Hazard. Mater. 2010, 184, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, T.; Chen, J.; Cai, C. Polyaniline (skin)/polyamide 6 (core) composite fiber: Preparation, characterization and application as a dye adsorbent. Synth. Met. 2013, 175, 163–169. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, K.; Zhao, L. Sono-assisted synthesis of nanostructured polyaniline for adsorption of aqueous Cr (VI): Effect of protonic acids. Chem. Eng. J. 2014, 239, 123–131. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, L.; Wu, W.; Lu, G.; Xu, F.; Tong, Y.; Liu, W.; Du, J. Enhanced adsorption of malachite green onto carbon nanotube/polyaniline composites. J. Appl. Polym. Sci. 2013, 127, 2475–2482. [Google Scholar] [CrossRef]
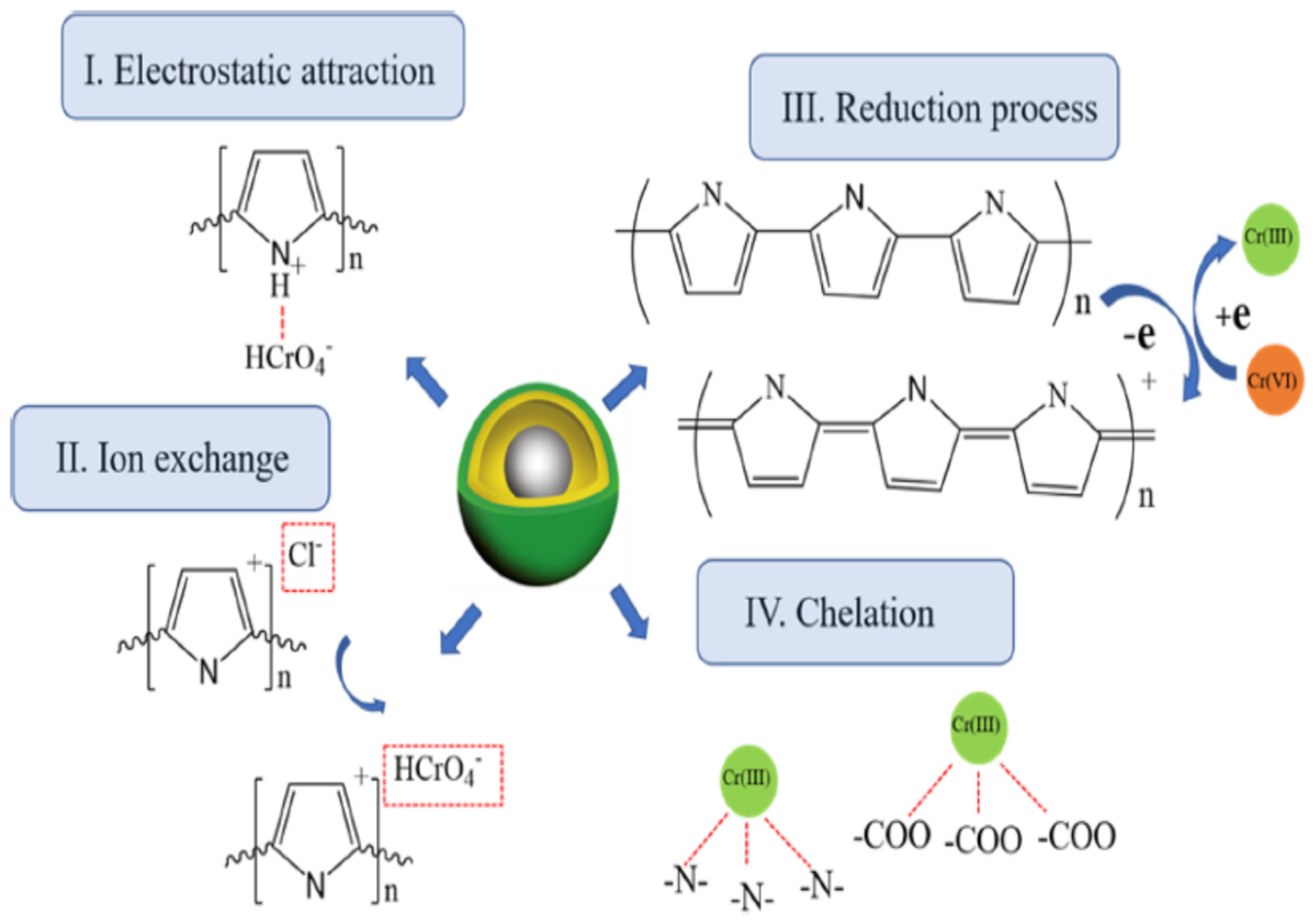
- Setshedi, K.Z.; Bhaumik, M.; Songwane, S.; Onyango, M.S.; Maity, A. Exfoliated polypyrrole-organically modified montmorillonite clay nanocomposite as a potential adsorbent for Cr (VI) removal. Chem. Eng. J. 2013, 222, 186–197. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Synthesis, characterization and Cr (VI) uptake studies of polypyrrole functionalized chitin. Synth. Met. 2014, 198, 181–187. [Google Scholar] [CrossRef]
- Bhaumik, M.; Agarwal, S.; Gupta, V.K.; Maity, A. Enhanced removal of Cr (VI) from aqueous solutions using polypyrrole wrapped oxidized MWCNTs nanocomposites adsorbent. J. Colloid Interface Sci. 2016, 470, 257–267. [Google Scholar] [CrossRef]
- Amalraj, A.; Selvi, M.K.; Rajeswari, A.; Christy, E.J.S.; Pius, A. Efficient removal of toxic hexavalent chromium from aqueous solution using threonine doped polypyrrole nanocomposite. J. Water Process. Eng. 2016, 13, 88–99. [Google Scholar] [CrossRef]
- Kera, N.H.; Bhaumik, M.; Ballav, N.; Pillay, K.; Ray, S.S.; Maity, A. Selective removal of Cr (VI) from aqueous solution by polypyrrole/2,5-diaminobenzene sulfonic acid composite. J. Colloid Interface Sci. 2016, 476, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Ballav, N.; Maity, A.; Mishra, S.B. High efficient removal of chromium (VI) using glycine doped polypyrrole adsorbent from aqueous solution. Chem. Eng. J. 2012, 198–199, 536–546. [Google Scholar] [CrossRef]
- Abdi, S.; Nasiri, M.; Mesbahi, A.; Khani, M. Investigation of uranium (VI) adsorption by polypyrrole. J. Hazard. Mater. 2017, 332, 132–139. [Google Scholar] [CrossRef]
- Hosseini, S.; Mahmud, N.E.; Yahya, R.B.; Ibrahim, F.; Djordjevic, I. Polypyrrole conducting polymer and its application in removal of copper ions from aqueous solution. Mater. Lett. 2015, 149, 77–80. [Google Scholar] [CrossRef]
- Li, S.; Lu, X.; Li, X.; Xue, Y.; Zhang, C.; Lei, J.; Wang, C. Preparation of bamboo-like PPy nanotubes and their application for removal of Cr (VI) ions in aqueous solution. J. Colloid Interface Sci. 2012, 378, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Gao, P.; Meng, Y.; Liu, Y.; Le, S.; Yu, C. Highly Efficient Removal of Cr (VI) from Aqueous Solutions by Polypyrrole/Monodisperse Latex Spheres. ACS Omega 2020, 5, 6651–6660. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Chen, J.; Chen, R.; Yu, P.; Guo, S.; Wang, X. Adsorption and reduction of chromium (VI) from aqueous solution using polypyrrole/calcium rectorite composite adsorbent. Water Res. 2019, 160, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Guajardo, A.E.; Medina-Llamas, J.C.; Maqueira, L.; Andrade, C.A.; Alves, K.; de Melo, C.P. Efficient removal of Cr (VI) and Cu (II) ions from aqueous media by use of polypyrrole/maghemite and polyaniline/maghemite magnetic nanocomposites. Chem. Eng. J. 2015, 281, 826–836. [Google Scholar] [CrossRef]
- Zhao, Y.; Xia, K.; Zhang, Z.; Zhu, Z.; Guo, Y.; Qu, Z. Facile Synthesis of Polypyrrole-Functionalized CoFe2O4@SiO2 for Removal for Hg(II). Nanomaterials 2019, 9, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alawa, B.; Srivstava, J.K.; Srivastava, A.; Palsania, J. Adsorption of Heavy Metal Pb (II) from Synthetic Waste Water by Polypyrrole composites. Int. J. Chem. Stud. 2015, 3, 4–8. [Google Scholar]
- Falahian, Z.; Torki, F.; Faghihian, H. Synthesis and Application of Polypyrrole/Fe3O4 Nanosize Magnetic Adsorbent for Efficient Separation of Hg2+ from Aqueous Solution. Glob. Chall. 2018, 2, 1700078. [Google Scholar] [CrossRef] [Green Version]
- Tanzifi, M.; Nezhad, M.K.; Karimipour, K. Kinetic and Isotherm Studies of Cadmium Adsorption on Polypyrrole/Titanium dioxide Nanocomposite. J. Water Environ. Nanotechnol. 2017, 2, 265–277. [Google Scholar]
- Maity, S.; Dubey, A.; Chakraborty, S. A review on polypyrrole-coated bio-composites for the removal of heavy metal traces from waste water. J. Ind. Text. 2021, 51, 152–173. [Google Scholar] [CrossRef]
- Sathe, S.; Chakraborty, I.; Dubey, B.; Ghangrekar, M. Microbial fuel cell coupled Fenton oxidation for the cathodic degradation of emerging contaminants from wastewater: Applications and challenges. Environ. Res. 2022, 204, 112135. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, M.A.; Khandaker, M.U.; Amin, Y.M.; Mahmud, H.N.M.E. Cadmium-109 Radioisotope Adsorption onto Polypyrrole Coated Sawdust of Dryobalanops aromatic: Kinetics and Adsorption Isotherms Modelling. PLoS ONE 2016, 11, e0164119. [Google Scholar] [CrossRef] [Green Version]
- Eisazadeh, H.; Engineering, C.; Box, P.O. Removal of Arsenic in Water Using Polypyrrole and its Composites. Methods 2008, 3, 10–13. [Google Scholar]
- Zhou, T.; Liang, Q.; Zhou, X.; Luo, H.; Chen, W. Enhanced removal of toxic hexavalent chromium from aqueous solution by magnetic Zr-MOF@polypyrrole: Performance and mechanism. Environ. Sci. Pollut. Res. 2021, 28, 13084–13096. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, M.; Maity, A.; Srinivasu, V.; Onyango, M.S. Removal of hexavalent chromium from aqueous solution using polypyrrole-polyaniline nanofibers. Chem. Eng. J. 2012, 181–182, 323–333. [Google Scholar] [CrossRef]
- Islam, M.; Mishra, S.; Swain, S.K.; Patel, R.; Dey, R.K.; Naushad, M. Evaluation of Phosphate Removal Efficiency from Aqueous Solution by Polypyrrole/BOF Slag Nanocomposite. Sep. Sci. Technol. 2014, 49, 2668–2680. [Google Scholar] [CrossRef]
- Fang, W.; Jiang, X.; Luo, H.; Geng, J. Synthesis of graphene/SiO2@polypyrrole nanocomposites and their application for Cr (VI) removal in aqueous solution. Chemosphere 2018, 197, 594–602. [Google Scholar] [CrossRef]
- Fan, L.; Wei, C.; Xu, Q.; Xu, J. Polypyrrole-coated cotton fabrics used for removal of methylene blue from aqueous solution. J. Text. Inst. 2017, 108, 1847–1852. [Google Scholar] [CrossRef]
- Li, J.; Feng, J.; Yan, W. Excellent adsorption and desorption characteristics of polypyrrole/TiO2 composite for Methylene Blue. Appl. Surf. Sci. 2013, 279, 400–408. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Z.; Hao, R.; Xu, H.; Huang, C. Rapid adsorption and reductive degradation of Naphthol Green B from aqueous solution by Polypyrrole/Attapulgite composites supported nanoscale zero-valent iron. J. Hazard. Mater. 2019, 371, 8–17. [Google Scholar] [CrossRef]
- Zhou, J.; Lü, Q.F.; Luo, J.J. Efficient removal of organic dyes from aqueous solution by rapid adsorption onto polypyrrole–based composites. J. Clean. Prod. 2017, 167, 739–748. [Google Scholar] [CrossRef]
- Boukoussa, B.; Hakiki, A.; Moulai, S.; Chikh, K.; Kherroub, D.E.; Bouhadjar, L.; Guedal, D.; Messaoudi, K.; Mokhtar, F.; Hamacha, R. Adsorption behaviors of cationic and anionic dyes from aqueous solution on nanocomposite polypyrrole/SBA-15. J. Mater. Sci. 2018, 53, 7372–7386. [Google Scholar] [CrossRef]
- Yang, B.Y.; Cao, Y.; Qi, F.F.; Li, X.Q.; Xu, Q. Atrazine adsorption removal with nylon6/polypyrrole core-shell nanofibers mat: Possible mechanism and characteristics. Nanoscale Res. Lett. 2015, 10, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palanisamy, P.N.; Agalya, A.; Sivakumar, P. Polypyrrole Composite—A Potential Material for the Removal of Acid Dyes. Asian J. Chem. 2013, 25, 5891–5896. [Google Scholar] [CrossRef]
- Feng, J.; Li, J.; Lv, W.; Xu, H.; Yang, H.; Yan, W. Synthesis of polypyrrole nano-fibers with hierarchical structure and its adsorption property of Acid Red G from aqueous solution. Synth. Met. 2014, 191, 66–73. [Google Scholar] [CrossRef]
- Pires, B.C.; Nascimento, T.A.D.; Dutra, F.V.A.; Borges, K.B. Removal of a non-steroidal anti-inflammatory by adsorption on polypyrrole/multiwalled carbon nanotube composite—Study of kinetics and equilibrium in aqueous medium. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123583. [Google Scholar] [CrossRef]
- Duan, W.; Li, M.; Xiao, W.; Wang, N.; Niu, B.; Zhou, L.; Zheng, Y. Enhanced adsorption of three fluoroquinolone antibiotics using polypyrrole functionalized Calotropis gigantea fiber. Colloids Surf. A Physicochem. Eng. Asp. 2019, 574, 178–187. [Google Scholar] [CrossRef]
- Yağmur, H.K. Synthesis and characterization of conducting polypyrrole/bentonite nanocomposites and in-situ oxidative polymerization of pyrrole: Adsorption of 4-nitrophenol by polypyrrole/bentonite nanocomposite. Chem. Eng. Commun. 2020, 207, 1171–1183. [Google Scholar] [CrossRef]
- Laabd, M.; El Jaouhari, A.; Haki, M.A.; Eljazouli, H.; Bazzaoui, M.; Kabli, H.; Albourine, A. Simultaneous removal of benzene polycarboxylic acids from water by polypyrrole composite filled with a cellulosic agricultural waste. J. Environ. Chem. Eng. 2016, 4, 1869–1879. [Google Scholar] [CrossRef]
- Stejskal, J. Interaction of conducting polymers, polyaniline and polypyrrole, with organic dyes: Polymer morphology control, dye adsorption and photocatalytic decomposition. Chem. Pap. 2019, 74, 1–54. [Google Scholar] [CrossRef]
- Bhaumik, M.; McCrindle, R.; Maity, A. Efficient removal of Congo red from aqueous solutions by adsorption onto interconnected polypyrrole–polyaniline nanofibres. Chem. Eng. J. 2013, 228, 506–515. [Google Scholar] [CrossRef]
- Ovando-Medina, V.M.; Vizcaíno-Mercado, J.; Gonzalez-Ortega, O.; De La Garza, J.A.R.; Martínez-Gutiérrez, H. Synthesis of α-cellulose/polypyrrole composite for the removal of reactive red dye from aqueous solution: Kinetics and equilibrium modeling. Polym. Compos. 2014, 36, 312–321. [Google Scholar] [CrossRef]
- Ayad, M.; Salahuddin, N.; Fayed, A.; Bastakoti, B.P.; Suzuki, N.; Yamauchi, Y. Chemical design of a smart chitosan–polypyrrole–magnetite nanocomposite toward efficient water treatment. Phys. Chem. Chem. Phys. 2014, 16, 21812–21819. [Google Scholar] [CrossRef]
- Ayad, M.M.; Amer, W.; Zaghlol, S.; Minisy, I.; Bober, P.; Stejskal, J. Polypyrrole-coated cotton textile as adsorbent of methylene blue dye. Chem. Pap. 2018, 72, 1605–1618. [Google Scholar] [CrossRef]
- Ansari, R.R.; Mosayebzadeh, Z. Removal of basic dye methylene blue from aqueous solutions using sawdust and sawdust coated with polypyrrole. J. Iran. Chem. Soc. 2010, 7, 339–350. [Google Scholar] [CrossRef]
- Adhikari, A.; De, S.; Halder, A.; Pattanayak, S.; Dutta, K.; Mondal, D.; Rana, D.; Ghosh, R.; Bera, N.K.; Chattopadhyay, S.; et al. Biosurfactant tailored synthesis of porous polypyrrole nanostructures: A facile approach towards CO2 adsorption and dopamine sensing. Synth. Met. 2018, 245, 209–222. [Google Scholar] [CrossRef]
- Hussein, M.A. Eco-Friendly Polythiophene(keto-amine)s Based on Cyclopentanone Moiety for Environmental Remediation. J. Polym. Environ. 2018, 26, 1194–1205. [Google Scholar] [CrossRef]
- Mishra, A.K.; Agrawal, N.R.; Das, I. Synthesis of water dispersible dendritic amino acid modified polythiophenes as highly effective adsorbent for removal of methylene blue. J. Environ. Chem. Eng. 2017, 5, 4923–4936. [Google Scholar] [CrossRef]
- Kharazi, P.; Rahimi, R.; Rabbani, M. Study on porphyrin/ZnFe2O4@polythiophene nanocomposite as a novel adsorbent and visible light driven photocatalyst for the removal of methylene blue and methyl orange. Mater. Res. Bull. 2018, 103, 133–141. [Google Scholar] [CrossRef]
- Faisal, M.; Harraz, F.A.; Ismail, A.A.; El-Toni, A.M.; Al-Sayari, S.; Al-Hajry, A.; Al-Assiri, M.S. Polythiophene/mesoporous SrTiO 3 nanocomposites with enhanced photocatalytic activity under visible light. Sep. Purif. Technol. 2018, 190, 33–44. [Google Scholar] [CrossRef]
- Ansari, R.; Feizy, J.; Delavar, A.F. Removal of Arsenic Ions from Aqueous Solutions Using Conducting Polymers. Eur. J. Adv. Chem. Res. 2008, 5, 853–863. [Google Scholar] [CrossRef]
- Zoleikani, L.; Issazadeh, H.; ZareNezhad, B. Preparation of new conductive polymer nanocomposites for cadmium removal from industrial wastewaters. J. Chem. Technol. Metall. 2015, 50, 71–80. [Google Scholar]
- Din, M.I.; Ata, S.; Mohsin, I.U.; Rasool, A.; Aziz, A.A. Evaluation of conductive polymers as an adsorbent for eradication of As (III) from aqueous solution using inductively coupled plasma optical emission spectroscopy (ICP-OES). Int. J. Sci. Eng. 2014, 6, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Javadian, H. Application of kinetic, isotherm and thermodynamic models for the adsorption of Co (II) ions on polyaniline/polypyrrole copolymer nanofibers from aqueous solution. J. Ind. Eng. Chem. 2014, 20, 4233–4241. [Google Scholar] [CrossRef]
- Haki, M.A.; Laabd, M.; Chafai, H.; Kabli, H.; Ez-Zahery, M.; Bazzaoui, M.; Lakhmiri, R.; Albourine, A. Comparative adsorption of nitrate ions on the polypyrrole and polyaniline from aqueous solution. J. Dispers. Sci. Technol. 2016, 38, 598–603. [Google Scholar] [CrossRef]
- Chafai, H.; Laabd, M.; Elbariji, S.; Bazzaoui, M.; Albourine, A. Study of congo red adsorption on the polyaniline and polypyrrole. J. Dispers. Sci. Technol. 2016, 38, 832–836. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Kumar, V.B.; Luong, J.H.T.; Gedanken, A. Kinetics, Isotherm, and Thermodynamic Studies of Methylene Blue Adsorption on Polyaniline and Polypyrrole Macro-Nanoparticles Synthesized by C-Dot-Initiated Polymerization. ACS Omega 2018, 3, 7196–7203. [Google Scholar] [CrossRef]
- Chigondo, M.; Paumo, H.K.; Bhaumik, M.; Pillay, K.; Maity, A. Magnetic arginine-functionalized polypyrrole with improved and selective chromium (VI) ions removal from water. J. Mol. Liq. 2019, 275, 778–791. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Chen, X.; Leng, L.; Wang, H.; Li, H.; Zeng, G. Facile synthesis of polypyrrole decorated reduced graphene oxide–Fe3O4 magnetic composites and its application for the Cr (VI) removal. Chem. Eng. J. 2015, 262, 597–606. [Google Scholar] [CrossRef]
- Shao, Y.; Fan, Z.; Zhong, M.; Xu, W.; He, C.; Zhang, Z. Polypyrrole/bacterial cellulose nanofiber composites for hexavalent chromium removal. Cellulose 2021, 28, 2229–2240. [Google Scholar] [CrossRef]
- Liu, W.; Yang, L.; Xu, S.; Chen, Y.; Liu, B.; Li, Z.; Jiang, C. Efficient removal of hexavalent chromium from water by an adsorption–reduction mechanism with sandwiched nanocomposites. RSC Adv. 2018, 8, 15087–15093. [Google Scholar] [CrossRef] [Green Version]
- Deivanayaki, S.; Ponnuswamy, V.; Mariappan, R.; Jayamurugan, P. Synthesis and characterization of polypyrrole/TiO2 composites by chemical oxidative method. Optik (Stuttg) 2013, 124, 1089–1091. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, N.; Feng, C.; Li, M.; Gao, Y. Chromium removal using a magnetic corncob biochar/polypyrrole composite by adsorption combined with reduction: Reaction pathway and contribution degree. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 201–209. [Google Scholar] [CrossRef]
- Feng, J.; Sun, N.; Wu, D.; Yang, H.; Xu, H.; Yan, W. Preparation of Fe3O4/TiO2/Polypyrrole Ternary Magnetic Composite and Using as Adsorbent for the Removal of Acid Red G. J. Polym. Environ. 2016, 25, 781–791. [Google Scholar] [CrossRef]




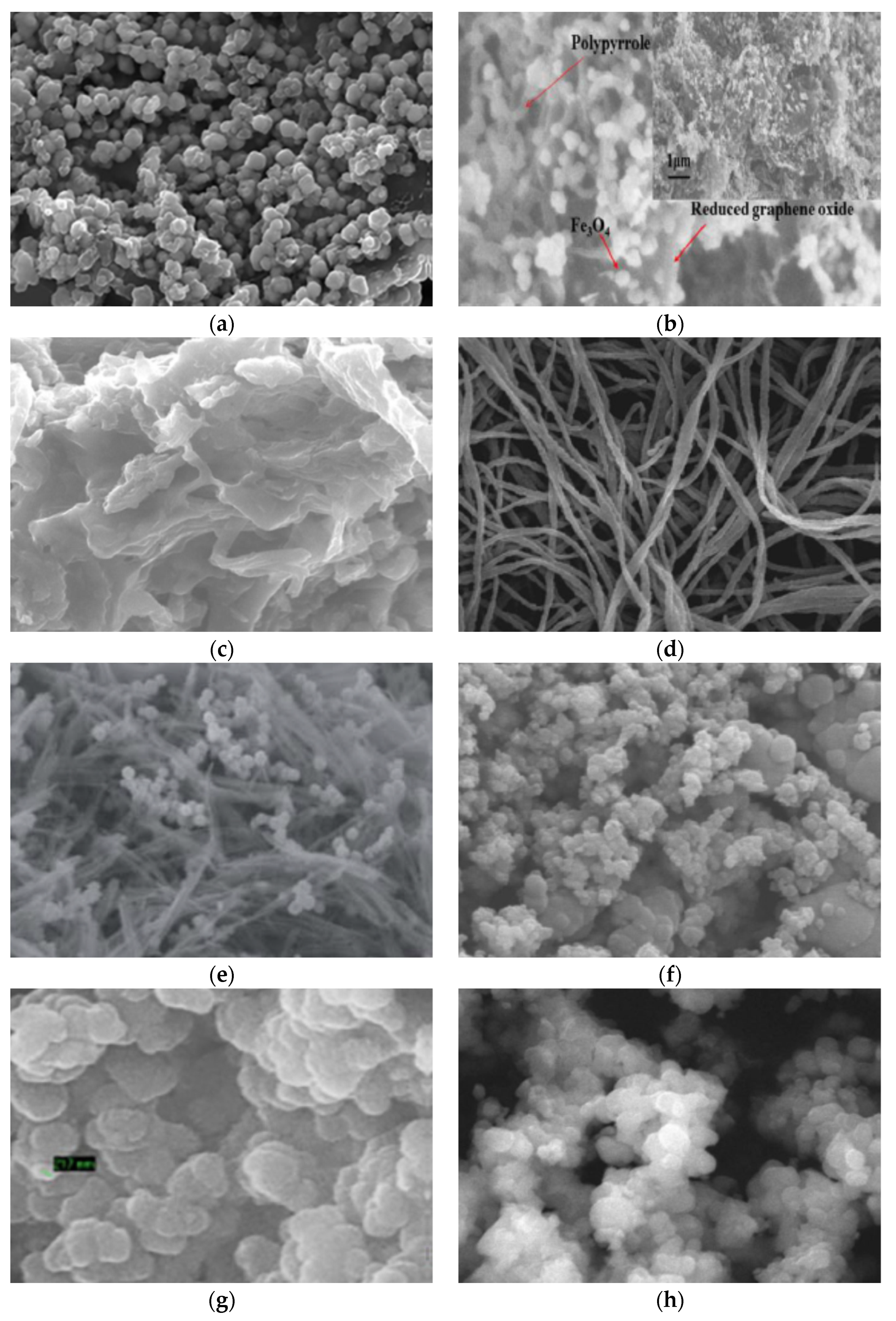
| Polymer | Abbreviation | Structure |
|---|---|---|
| Polythiophene | PTh | 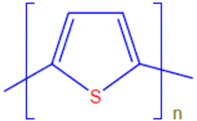 |
| Polypyrrole | Ppy | 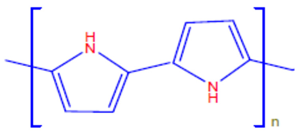 |
| Polyaniline | PANI |  |
| Polyacetylene | PA | 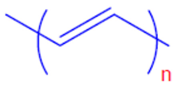 |
| Functionalized Polyacetylene | f-PA |  |
| Poly(phylene vinylene) | PPv | 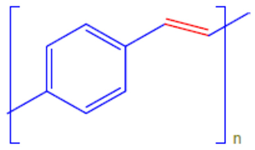 |
| Poly(p-phenylene) | PP |  |
| Adsorbent | Adsorbate | pH | Temperature (°C) | qmax (mg/g) | Ref. |
|---|---|---|---|---|---|
| PANI-JF | Cr (VI) | 3 | 40 | 62.89 | [27] |
| PANI/CA composite | Cu+2, Pb+2 | 6 | NA | 67.95 251.44 | [34] |
| PANI-PGC | Pb+2 Cd+2 | 6 | 30 | 16.07 14.33 | [36] |
| PANI/Clay | Cu+2 | 6 | 25 | 22.77 | [37] |
| PANI-PS | Pb+2 | 2-6 | NA | NA | [39] |
| PANI-Chi composite | Cr (VI) | 3 | 45 | 1.01 | [40] |
| PANI-BC mat | Cr (VI) | 1-2 | NA | NA | [41] |
| P-PANi-MMT | Cu+2 | 5 | NA | 87 | [42] |
| PANI-PVDF-HFP nanofibrous | Cr (VI) | 4.5 | NA | 15.08 | [43] |
| PANI and PANI-G10 | Cr (VI) | 6.5 | 30 | 136, 192 | [44] |
| Adsorbent | Adsorbate | pH | Temperature (°C) | qmax (mg/g) | Ref. |
|---|---|---|---|---|---|
| PANI-SBA-15 | resorcinol | 3 | 25 | 128 | [33] |
| HCPANI | MO, CV | 3, 11 | 27 | 220, 245 | [49] |
| PANI-ZSP | MB | 1 | 85 | 12 | [51] |
| PANI-MWCNTs-Fe3O4 magnetic composite | MO CR | 4 | Room temp. | 446.25, 417.38 | [52] |
| Fe3O4 and PANI-Fe3O4 | BB-3 | 8.5, 12, 10 | 30 | 8.5, 6, 9 | [55] |
| PANI-AC | MO | 6.5 | 25 | 285 | [64] |
| PANI-AC | Direct Red 23 | 3.0 | 45 | 109.89 | [65] |
| PANI and PANI/AL | DG | 1 | 20 | 0.911, 8.13 | [66] |
| PANI-Chi | CR, CBB, RBBR | 3 | 26 | 322.58, 357.14, 303.03 | [67] |
| PANI-MMT-Fe3O4 | MB | 6.3 | Room temp. | 184.48 | [68] |
| PANI/CPL | MO | 4 | Room temp. | 333.33 | [69] |
| PANI, Fe3O4, and PANI-Fe3O4 | AB-40 | 3, 6, 6 | 30 | 130.5, 264.9, 216.9 | [70] |
| PANI-Fe3O4 | MG | 7 | 25 | 4.82 | [71] |
| PANI-HGL | MB | 6.5 | 45 | 71.2 | [72] |
| PANI-LC | RB-5 | 2.0 | Room Temp. | 312 | [73] |
| PANI-LC | CR | 4.29 | 45 | 1672.5 | [74] |
| PANI-NFs/SD | ARG | 2.0 | 35 | 212.97 | [75] |
| PANI-FeCl3 | RB-5 | 6 | 45 | 434.7 | [76] |
| PANI-NiFe2O4 | MG | 7 | N/A | 4.09 | [77] |
| PANI-NiFe2O4 | ARS | 4 8.6 | 30 | 186 | [78] |
| PANI-Ny-6 | MO | 1 | N/A | 370 | [79] |
| PANI-ZnFe2O4 | RH-B | 2 | Room tem. | 229 | [80] |
| Adsorbent | Adsorbate | pH | Temperature (°C) | qmax (mg/g) | Ref. |
|---|---|---|---|---|---|
| Ppy-PANI | Cr (VI) | 2 | 25 | 227 | [115] |
| Ppy-oMMT NC | Cr (VI) | 2 | 25 | 209.6 | [94] |
| Ppy-Chitin | Cr (VI) | 2 | 50 | 35.22 | [95] |
| Ppy/Fe3O4 and Ppy/oMWCNTs NC | Cr (VI) | 2 | 45, 25 | 243.9, 294 | [96] |
| Ppy/DABSA | Cr (VI) | 2 | 25 | 303 | [98] |
| Ppy-gly | Cr (VI) | 2 | 45 | 232.55 | [99] |
| Ppy/MLS | Cr (VI) | 2 | 25 | 343.64 | [103] |
| Ppy/Ca-REC | Cr (VI) | 1.5 | 45 | 833.33 | [104] |
| Ppy-Fe3O4 | Hg2+ | 2.5 | 55 | 173.16 | [108] |
| Ppy–BOFS NC | phosphate | 2 | 45 | 9.13 | [116] |
| Ppy-GSi NC | Cr (VI) | 2 | 25 | 429.2 | [117] |
| Adsorbent | Adsorbate | pH | Temperature (°C) | qmax (mg/g) | Ref. |
|---|---|---|---|---|---|
| Ppy-PANI NF | CR | 4 | 35 | 270.27 | [131] |
| Ppy NF | ARG | 2 | 25 | 121.95 | [125] |
| Ppy-CF | MB | 12 | Room Temp. | 6.0 | [118] |
| Ppy/TiO2 | MB | 13 | 35 | 298.50 | [119] |
| Ppy-Attapulgite-ZVI | NG-B | 2 | 45 | 253.9 | [120] |
| Ppy-Chi-LS | CR | 2 | 50 | 30.12 | [121] |
| Ppy-SBA-15 NC | MB MO | 4.5, 6.5 | 20 | 58.82, 41.66 | [122] |
| Ppy-PA6 NFM | atrazine | 7 | 70 | 14.8 | [123] |
| Ppy-SD | AO-10 | 3 | 45 | 256.41 | [124] |
| Ppy-BNT NC | 4-nitrophenol | N/A | 25 | 96.15 | [128] |
| Ppy-α Cellulose | RR-120 | 2 | 25 | 96.1 | [132] |
| Ppy-Chi-Fe3O4 | AG-25 | N/A | Room temp. | 32.754 | [133] |
| Ppy-CF | MB | 10 | 25 | 3.30 | [134] |
| Ppy/SD | MB | 2 | Room temp. | 34.36 | [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.I.; Almesfer, M.K.; Elkhaleefa, A.; Shigidi, I.; Shamim, M.Z.; Ali, I.H.; Rehan, M. Conductive Polymers and Their Nanocomposites as Adsorbents in Environmental Applications. Polymers 2021, 13, 3810. https://doi.org/10.3390/polym13213810
Khan MI, Almesfer MK, Elkhaleefa A, Shigidi I, Shamim MZ, Ali IH, Rehan M. Conductive Polymers and Their Nanocomposites as Adsorbents in Environmental Applications. Polymers. 2021; 13(21):3810. https://doi.org/10.3390/polym13213810
Chicago/Turabian StyleKhan, Mohammad Ilyas, Mohammed Khaloufa Almesfer, Abubakr Elkhaleefa, Ihab Shigidi, Mohammed Zubair Shamim, Ismat H. Ali, and Mohammad Rehan. 2021. "Conductive Polymers and Their Nanocomposites as Adsorbents in Environmental Applications" Polymers 13, no. 21: 3810. https://doi.org/10.3390/polym13213810
APA StyleKhan, M. I., Almesfer, M. K., Elkhaleefa, A., Shigidi, I., Shamim, M. Z., Ali, I. H., & Rehan, M. (2021). Conductive Polymers and Their Nanocomposites as Adsorbents in Environmental Applications. Polymers, 13(21), 3810. https://doi.org/10.3390/polym13213810