Validating Poly(3,4-ethylene dioxythiophene) Polystyrene Sulfonate-Based Textile Electroencephalography Electrodes by a Textile-Based Head Phantom
Abstract
1. Introduction
2. Experiment
2.1. Conductive Fabric Development
2.2. Textile Electrode Design and Construction
2.3. Head Phantom Construction
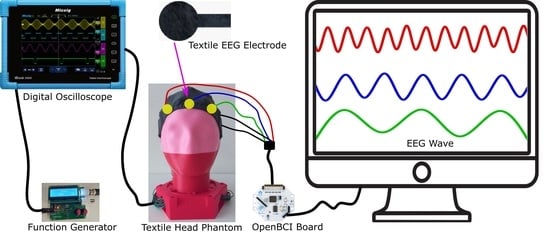
2.4. Synthetic Sine Wave Generation
2.5. Phantom-to-Electrode Impedance Measurement
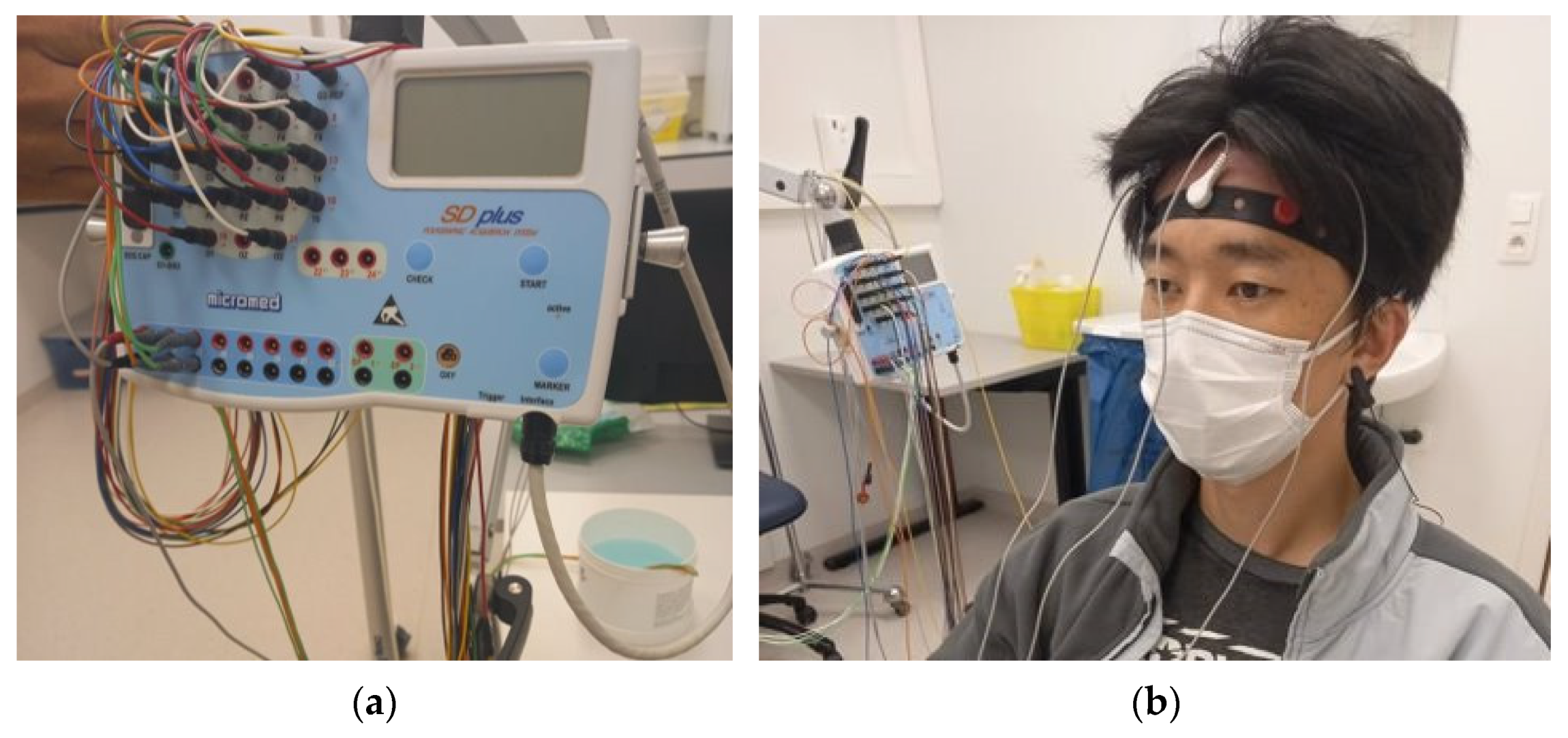
2.6. EEG Measurement and Analysis
2.7. On-Body EEG Measurement
3. Results
3.1. Phantom-to-Electrode Impedance
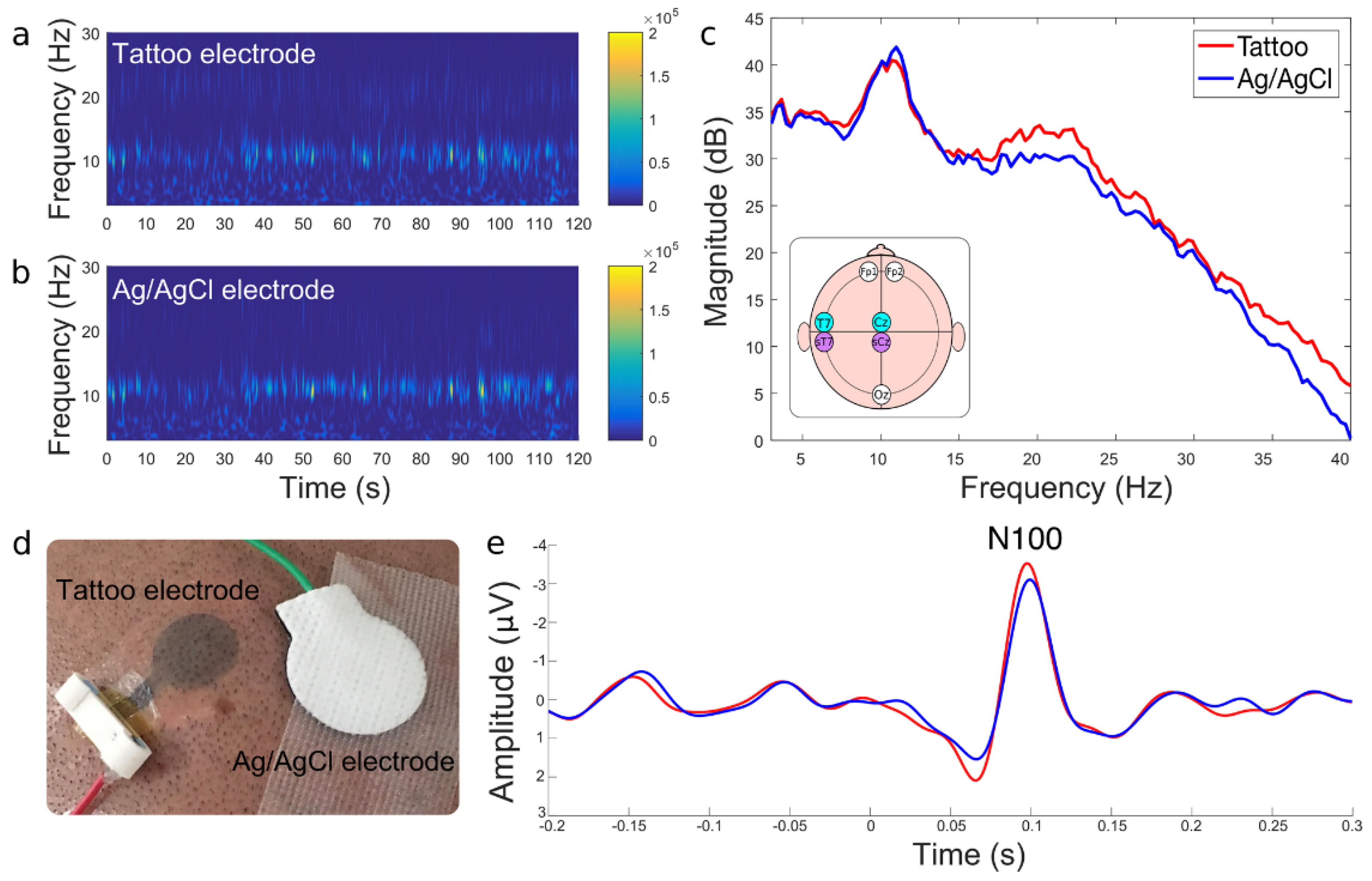
3.2. EEG Signal Analysis
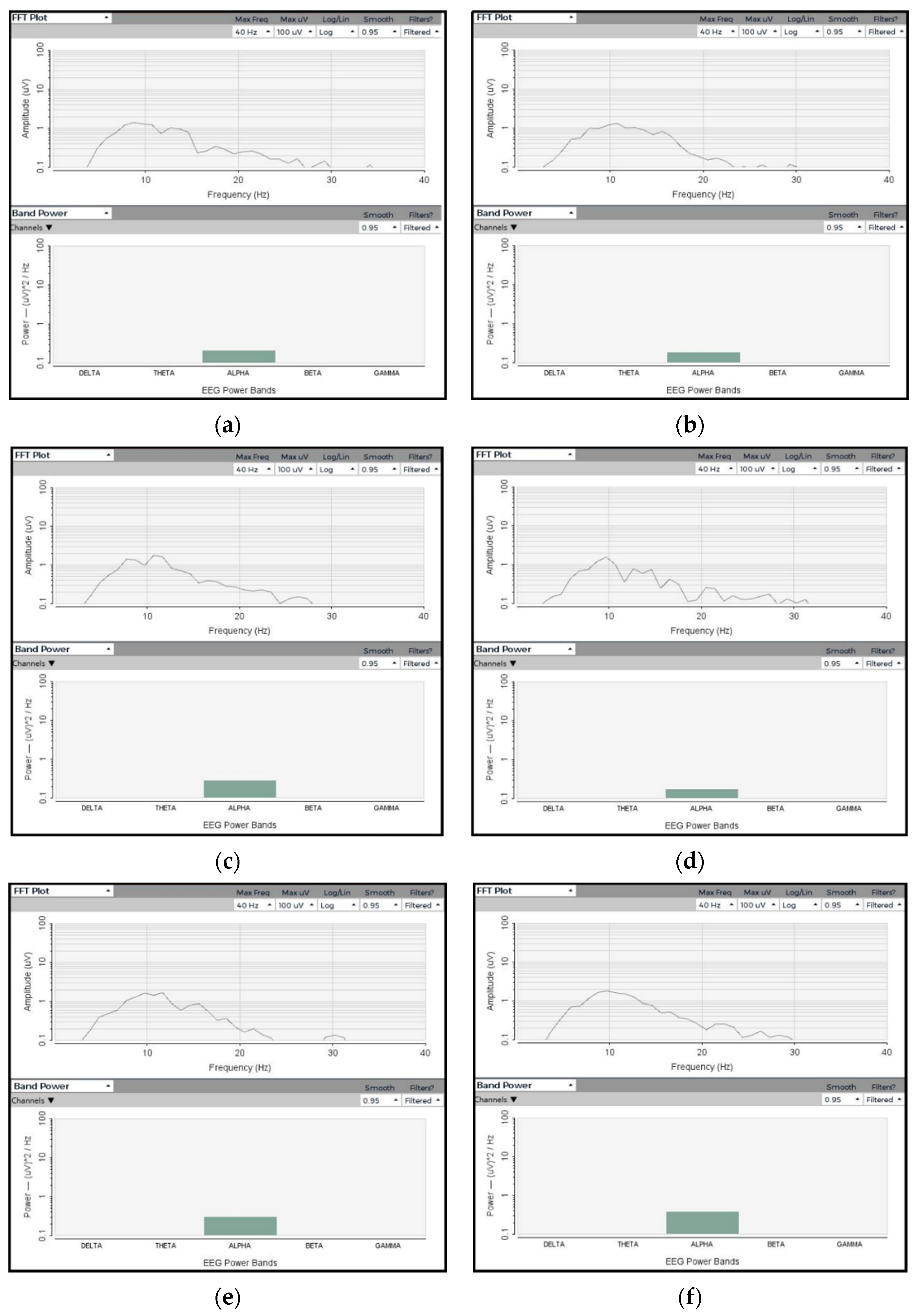
3.2.1. Amplitude and Frequency
3.2.2. SNR Analysis
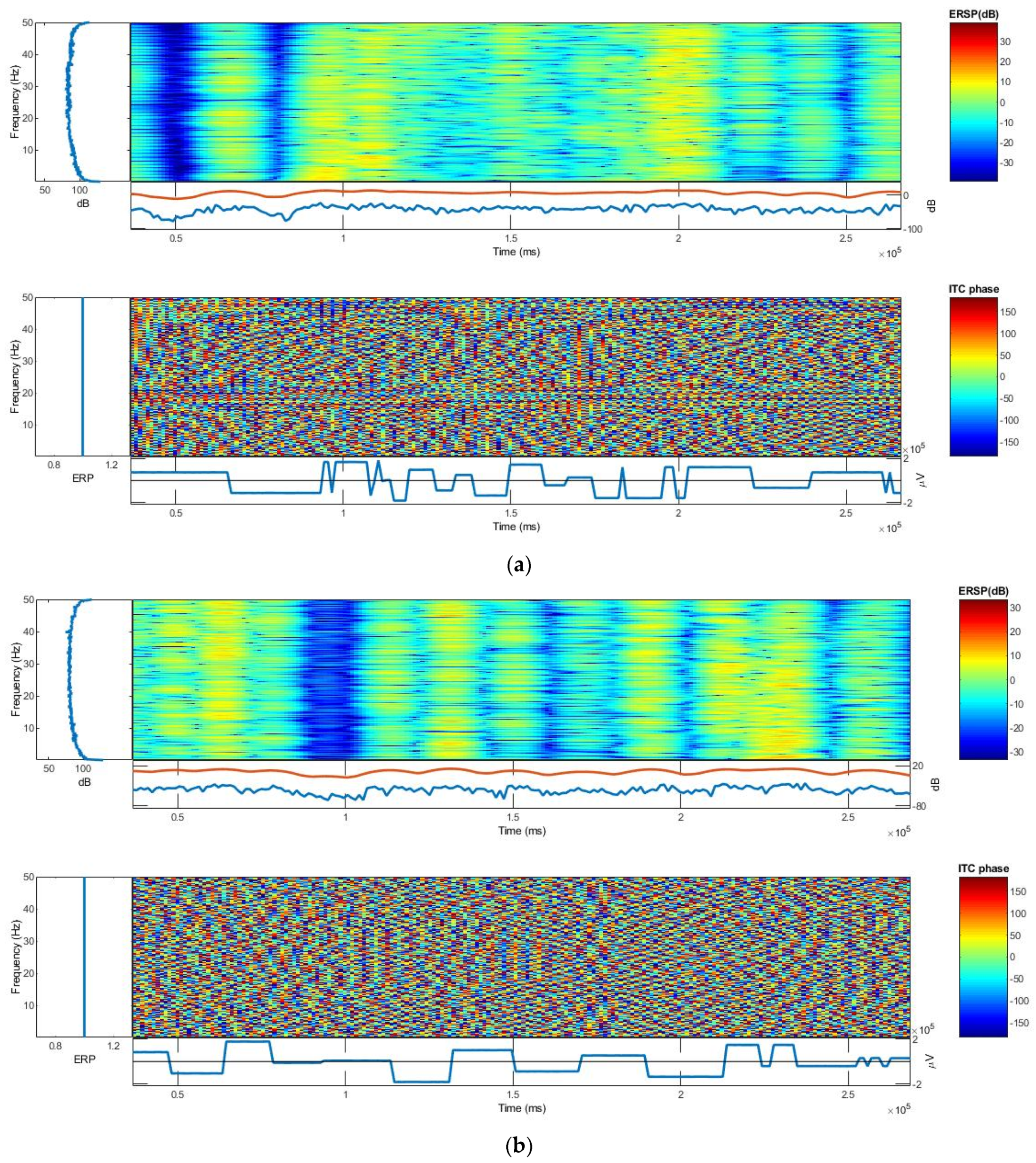
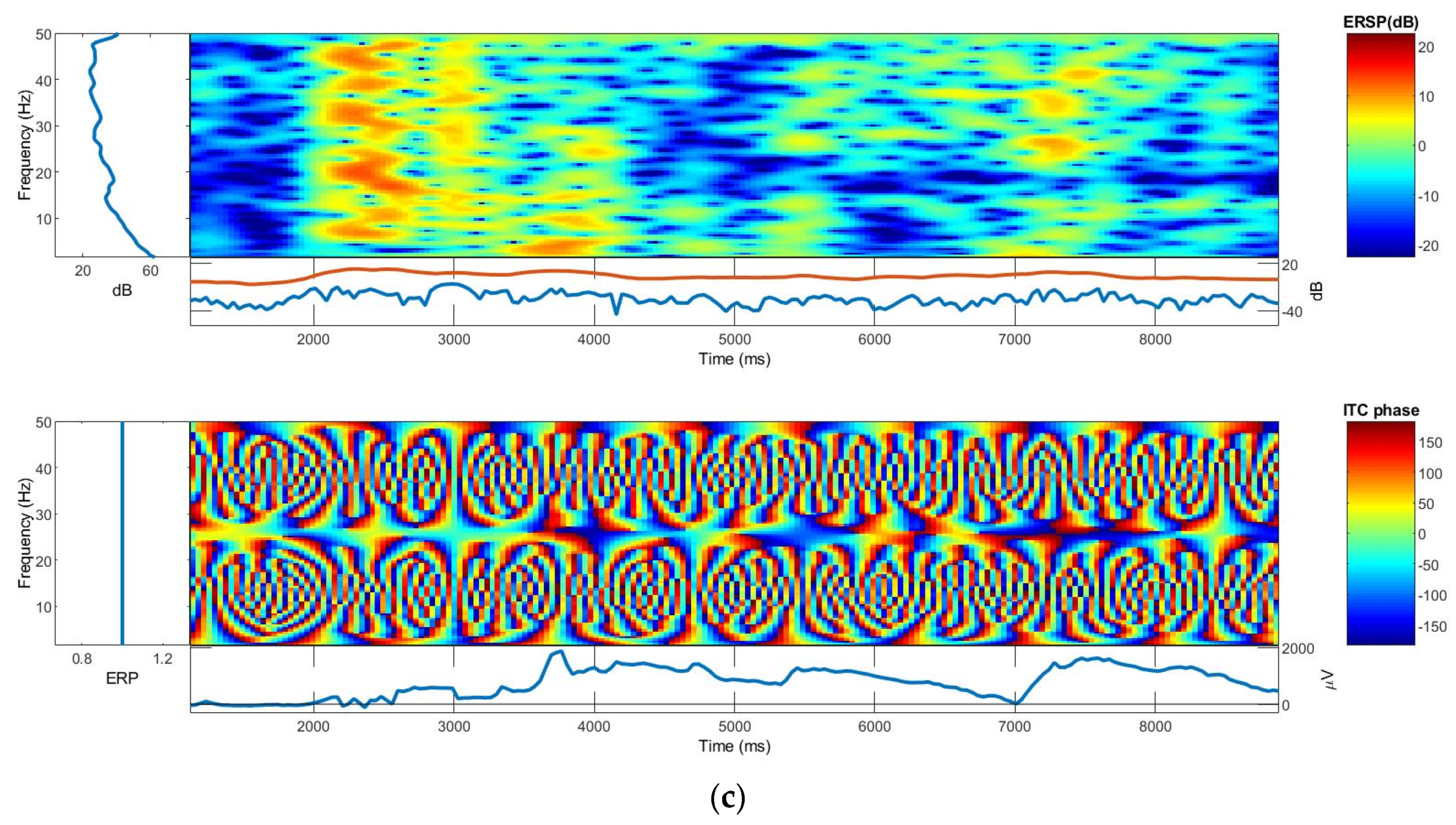
3.2.3. ERSP and ITC
3.3. On-Body EEG Signals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Von Bartheld, C.S.; Bahney, J.; Herculano-Houzel, S. The Search for True Numbers of Neurons and Glial Cells in the Human Brain: A Review of 150 Years of Cell Counting: Quantifying Neurons and Glia in Human Brain. J. Comp. Neurol. 2016, 524, 3865–3895. [Google Scholar] [CrossRef]
- Noback, C.R. (Ed.) The Human Nervous System: Structure and Function, 6th ed.; Humana Press: Totowa, NJ, USA, 2005; ISBN 978-1-59259-730-7. [Google Scholar]
- Liu, J.; Sheng, Y.; Liu, H. Corticomuscular Coherence and Its Applications: A Review. Front. Hum. Neurosci. 2019, 13, 100. [Google Scholar] [CrossRef]
- Berger, H. Uber Das Elektrenkephalogramm Des Menschen. Arch. Psychiatr. 1929, 87, 527–570. [Google Scholar] [CrossRef]
- Alturki, F.A.; AlSharabi, K.; Abdurraqeeb, A.M.; Aljalal, M. EEG Signal Analysis for Diagnosing Neurological Disorders Using Discrete Wavelet Transform and Intelligent Techniques. Sensors 2020, 20, 2505. [Google Scholar] [CrossRef] [PubMed]
- Purdon, P.L.; Sampson, A.; Pavone, K.J.; Brown, E.N. Clinical Electroencephalography for Anesthesiologists. Anesthesiology 2015, 123, 937–960. [Google Scholar] [CrossRef]
- Jacob, J.E.; Nair, G.K.; Iype, T.; Cherian, A. Diagnosis of Encephalopathy Based on Energies of EEG Subbands Using Discrete Wavelet Transform and Support Vector Machine. Neurol. Res. Int. 2018, 2018, 1613456. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cao, J.; Cao, Y.; Zhang, Y.; Gu, F.; Zhu, G.; Hong, Z.; Wang, B.; Cichocki, A. An Empirical EEG Analysis in Brain Death Diagnosis for Adults. Cogn. Neurodyn. 2008, 2, 257–271. [Google Scholar] [CrossRef]
- Lazarou, I.; Nikolopoulos, S.; Petrantonakis, P.C.; Kompatsiaris, I.; Tsolaki, M. EEG-Based Brain-Computer Interfaces for Communication and Rehabilitation of People with Motor Impairment: A Novel Approach of the 21st Century. Front. Hum. Neurosci. 2018, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Peng, S.; Song, A.; Yang, R.; Pan, L. Robot-Aided Upper-Limb Rehabilitation Based on Motor Imagery EEG. Int. J. Adv. Robot. Syst. 2011, 8, 40. [Google Scholar] [CrossRef]
- Namazi, H. Investigating the Brain Development in Newborns by Information-Based Analysis of Electroencephalography (EEG) Signal. Fluct. Noise Lett. 2020, 19, 2050043. [Google Scholar] [CrossRef]
- Tseghai, G.B.; Malengier, B.; Fante, K.A.; Van Langenhove, L. A Long-Lasting Textile-Based Anatomically Realistic Head Phantom for Validation of EEG Electrodes. Sensors 2021, 21, 4658. [Google Scholar] [CrossRef]
- Nigusse, A.B.; Malengier, B.; Mengistie, D.A.; Tseghai, G.B.; Van Langenhove, L. Development of Washable Silver Printed Textile Electrodes for Long-Term ECG Monitoring. Sensors 2020, 20, 6233. [Google Scholar] [CrossRef] [PubMed]
- Tseghai, G.B.; Malengier, B.; Fante, K.A.; Nigusse, A.B.; Etana, B.B.; Van Langenhove, L. PEDOT:PSS/PDMS-Coated Cotton Fabric for ECG Electrode. In Proceedings of the 2020 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Manchester, UK, 16–19 August 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–4. [Google Scholar]
- Kim, S.; Lee, S.; Jeong, W. EMG Measurement with Textile-Based Electrodes in Different Electrode Sizes and Clothing Pressures for Smart Clothing Design Optimization. Polymers 2020, 12, 2406. [Google Scholar] [CrossRef] [PubMed]
- Löfhede, J.; Seoane, F.; Thordstein, M. Textile Electrodes for EEG Recording—A Pilot Study. Sensors 2012, 12, 16907–16919. [Google Scholar] [CrossRef] [PubMed]
- Tseghai, G.B.; Malengier, B.; Fante, K.A.; Van Langenhove, L. A Dry EEG Textrode from a PEDOT:PSS/PDMS-Coated Cotton Fabric for Brain Activity Monitoring. In Proceedings of the 2021 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Manchester, UK, 20–23 June 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–4. [Google Scholar]
- Tseghai, G.B.; Malengier, B.; Fante, K.A.; Nigusse, A.B.; Langenhove, L.V. Integration of Conductive Materials with Textile Structures, an Overview. Sensors 2020, 20, 6910. [Google Scholar] [CrossRef]
- Worfolk, B.J.; Andrews, S.C.; Park, S.; Reinspach, J.; Liu, N.; Toney, M.F.; Mannsfeld, S.C.B.; Bao, Z. Ultrahigh Electrical Conductivity in Solution-Sheared Polymeric Transparent Films. Proc. Natl. Acad. Sci. USA 2015, 112, 14138–14143. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.M.; Ismailov, U.; Badier, J.-M.; Greco, F.; Ismailova, E. Conducting Polymer Tattoo Electrodes in Clinical Electro- and Magneto-Encephalography. NPJ Flex. Electron. 2020, 4, 4. [Google Scholar] [CrossRef]
- Tseghai, G.B.; Malengier, B.; Fante, K.A.; Van Langenhove, L. Dry Electroencephalography Textrode for Brain Activity Monitoring. IEEE Sens. J. 2021, 21, 22077–22085. [Google Scholar] [CrossRef]
- Hallez, H.; Vanrumste, B.; Grech, R.; Muscat, J.; De Clercq, W.; Vergult, A.; D’Asseler, Y.; Camilleri, K.P.; Fabri, S.G.; Van Huffel, S.; et al. Review on Solving the Forward Problem in EEG Source Analysis. J. Neuroeng. Rehabil. 2007, 4, 46. [Google Scholar] [CrossRef]
- Kohli, S.; Casson, A.J. Removal of Gross Artifacts of Transcranial Alternating Current Stimulation in Simultaneous EEG Monitoring. Sensors 2019, 19, 190. [Google Scholar] [CrossRef]
- Richardson, C.; Bernard, S.; Dinh, V.A. A Cost-Effective, Gelatin-Based Phantom Model for Learning Ultrasound-Guided Fine-Needle Aspiration Procedures of the Head and Neck. J. Ultrasound Med. 2015, 34, 1479–1484. [Google Scholar] [CrossRef]
- Md Said, M.S.; Seman, N.; Sulaiman, N.R.; Abd Rahman, T. Modeling of Gelatin-Based Head Phantom Based on Its Electrical Properties for Wideband Microwave Imaging Application. AMM 2015, 781, 608–611. [Google Scholar] [CrossRef]
- Symeonidou, E.-R.; Nordin, A.; Hairston, W.; Ferris, D. Effects of Cable Sway, Electrode Surface Area, and Electrode Mass on Electroencephalography Signal Quality during Motion. Sensors 2018, 18, 1073. [Google Scholar] [CrossRef]
- Owda, A.Y.; Casson, A.J. Electrical Properties, Accuracy, and Multi-Day Performance of Gelatine Phantoms for Electrophysiology. bioRxiv 2020. Available online: https://www.biorxiv.org/content/10.1101/2020.05.30.125070v1.full?__cf_chl_jschl_tk__=pmd_m20SP1lcyLrMlAY3p8bQt9PFWwApfIlCh7buOSlTdMs-1634701758-0-gqNtZGzNAiWjcnBszQhRP (accessed on 25 August 2021).
- Oliveira, A.S.; Schlink, B.R.; Hairston, W.D.; König, P.; Ferris, D.P. Induction and Separation of Motion Artifacts in EEG Data Using a Mobile Phantom Head Device. J. Neural Eng. 2016, 13, 036014. [Google Scholar] [CrossRef] [PubMed]
- Said, M.S.M.; Seman, N. Preservation of Gelatin-Based Phantom Material Using Vinegar and Its Life-Span Study for Application in Microwave Imaging. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 528–534. [Google Scholar] [CrossRef]
- Tseghai, G.B.; Malengier, B.; Fante, K.A.; Nigusse, A.B.; Van Langenhove, L. Development of a Flex and Stretchy Conductive Cotton Fabric Via Flat Screen Printing of PEDOT:PSS/PDMS Conductive Polymer Composite. Sensors 2020, 20, 1742. [Google Scholar] [CrossRef]
- Zeglio, E.; Rutz, A.L.; Winkler, T.E.; Malliaras, G.G.; Herland, A. Conjugated Polymers for Assessing and Controlling Biological Functions. Adv. Mater. 2019, 31, e1806712. [Google Scholar] [CrossRef] [PubMed]
- Bießmann, L.; Saxena, N.; Hohn, N.; Hossain, M.A.; Veinot, J.G.C.; Müller-Buschbaum, P. Highly Conducting, Transparent PEDOT:PSS Polymer Electrodes from Post-Treatment with Weak and Strong Acids. Adv. Electron. Mater. 2019, 5, 1800654. [Google Scholar] [CrossRef]
- Be’langer, M.; Marois, Y. Hemocompatibility, Biocompatibility, Inflammatory and in Vivo Studies of Primary Reference Materials Low-density Polyethylene and Polydimethylsiloxane: A Review. J. Biomed. Mater. Res. 2001, 58, 467–477. [Google Scholar] [CrossRef] [PubMed]
- De Ville, M.; Coquet, P.; Brunet, P.; Boukherroub, R. Simple and Low-Cost Fabrication of PDMS Microfluidic Round Channels by Surface-Wetting Parameters Optimization. Microfluid. Nanofluid. 2012, 12, 953–961. [Google Scholar] [CrossRef]
- Akther, F.; Yakob, S.B.; Nguyen, N.-T.; Ta, H.T. Surface Modification Techniques for Endothelial Cell Seeding in PDMS Microfluidic Devices. Biosensors 2020, 10, 182. [Google Scholar] [CrossRef]
- Karbovnyk, I.; Olenych, I.; Aksimentyeva, O.; Klym, H.; Dzendzelyuk, O.; Olenych, Y.; Hrushetska, O. Effect of Radiation on the Electrical Properties of PEDOT-Based Nanocomposites. Nanoscale Res. Lett. 2016, 11, 84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- George, J.; Sabapathi, S. Cellulose Nanocrystals: Synthesis, Functional Properties, and Applications. NSA 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Musa, A.B.H.; Malengier, B.; Vasile, S.; Van Langenhove, L.; De Raeve, A. Analysis and Comparison of Thickness and Bending Measurements from Fabric Touch Tester (FTT) and Standard Methods. Autex Res. J. 2018, 18, 51–60. [Google Scholar] [CrossRef]
- Jia, W.; Wu, J.; Gao, D.; Sun, M.; Sclabassi, R.J. Characteristics of Skin-Electrode Impedance for a Novel Screw Electrode. In Proceedings of the 2014 40th Annual Northeast Bioengineering Conference (NEBEC), Boston, MA, USA, 25–27 April 2014; pp. 1–2. [Google Scholar]
- Vargas Luna, J.L.; Krenn, M.; Cortés Ramírez, J.A.; Mayr, W. Dynamic Impedance Model of the Skin-Electrode Interface for Transcutaneous Electrical Stimulation. PLoS ONE 2015, 10, e0125609. [Google Scholar] [CrossRef]
- Texas Instruments ADS1299-x Low-Noise, 4-, 6-, 8-Channel, 24-Bit, Analog-to-Digital Converter for EEG and Biopotential Measurements, Texas Instruments. 2017. Available online: www.ti.com/lit/ds/symlink/ads1299.pdf (accessed on 20 June 2021).
- Klem, G.H.; LuÈders, H.O.; Jasper, H.H.; Elger, C. The Ten±twenty Electrode System of the International Federation. Int. Fed. Clin. Neurophysiol. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar]
- Ferree, T.C.; Luu, P.; Russell, G.S.; Tucker, D.M. Scalp Electrode Impedance, Infection Risk, and EEG Data Quality. Clin. Neurophysiol. 2001, 112, 536–544. [Google Scholar] [CrossRef]
- Welvaert, M.; Rosseel, Y. On the Definition of Signal-To-Noise Ratio and Contrast-To-Noise Ratio for FMRI Data. PLoS ONE 2013, 8, e77089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, L. Temporal and Spatial Features of Single-Trial EEG for Brain-Computer Interface. Comput. Intell. Neurosci. 2007, 2007, 037695. [Google Scholar] [CrossRef]
- Nash-Kille, A.; Sharma, A. Inter-Trial Coherence as a Marker of Cortical Phase Synchrony in Children with Sensorineural Hearing Loss and Auditory Neuropathy Spectrum Disorder Fitted with Hearing Aids and Cochlear Implants. Clin. Neurophysiol. 2014, 125, 1459–1470. [Google Scholar] [CrossRef] [PubMed][Green Version]









| Time (s) | Textile-Based Electrode | Dry Ag/AgCl Electrode | ||||
|---|---|---|---|---|---|---|
| Vavg 1 | Zraw 2 | Zact 3 | Vavg | Zraw | Zact | |
| 30 | 41.18 | 6863.33 | 1863.33 | 43.93 | 7321.67 | 2321.67 |
| 60 | 43.81 | 7301.67 | 2301.67 | 43.98 | 7330 | 2330 |
| 90 | 42.66 | 7110 | 2110 | 44.54 | 7423.33 | 2423.33 |
| 120 | 42.92 | 7153.33 | 2153.33 | 43.87 | 7311.67 | 2311.67 |
| 150 | 43.13 | 7188.33 | 2188.33 | 42.49 | 7081.67 | 2081.67 |
| 180 | 42.44 | 7073.33 | 2073.33 | 44.12 | 7353.33 | 2353.33 |
| 210 | 41.33 | 6888.33 | 1888.33 | 43.72 | 7286.67 | 2286.67 |
| 240 | 42.72 | 7120 | 2120 | 43.63 | 7271.67 | 2271.67 |
| Mean | 41.18 | 6863.33 | 1863.33 | 43.79 | 7297.5 | 2297.5 |
| V Max (µV) | V Min (µV) | V Pk-Pk (µV) | SNR (dB) | |
|---|---|---|---|---|
| Synthetic Wave | 168,000.00 | 192,000.00 | 360,000.00 | - |
| Ag/AgCl electrode | 166,830.00 | −185,800.00 | 352,630.00 | 16.8 |
| Textile electrode 1 | 165,616.73 | −187,825.55 | 353,442.27 | 17.32 |
| Textile electrode 2 | 165,689.17 | −187,867.88 | 353,557.04 | 17.39 |
| Textile electrode 3 | 165,596.89 | −187,795.33 | 353,392.21 | 17.28 |
| Textile electrode 4 | 165,614.41 | −187,967.12 | 353,581.53 | 17.41 |
| Textile electrode 5 | 165,689.79 | −188,001.02 | 353,690.81 | 17.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseghai, G.B.; Malengier, B.; Fante, K.A.; Van Langenhove, L. Validating Poly(3,4-ethylene dioxythiophene) Polystyrene Sulfonate-Based Textile Electroencephalography Electrodes by a Textile-Based Head Phantom. Polymers 2021, 13, 3629. https://doi.org/10.3390/polym13213629
Tseghai GB, Malengier B, Fante KA, Van Langenhove L. Validating Poly(3,4-ethylene dioxythiophene) Polystyrene Sulfonate-Based Textile Electroencephalography Electrodes by a Textile-Based Head Phantom. Polymers. 2021; 13(21):3629. https://doi.org/10.3390/polym13213629
Chicago/Turabian StyleTseghai, Granch Berhe, Benny Malengier, Kinde Anlay Fante, and Lieva Van Langenhove. 2021. "Validating Poly(3,4-ethylene dioxythiophene) Polystyrene Sulfonate-Based Textile Electroencephalography Electrodes by a Textile-Based Head Phantom" Polymers 13, no. 21: 3629. https://doi.org/10.3390/polym13213629
APA StyleTseghai, G. B., Malengier, B., Fante, K. A., & Van Langenhove, L. (2021). Validating Poly(3,4-ethylene dioxythiophene) Polystyrene Sulfonate-Based Textile Electroencephalography Electrodes by a Textile-Based Head Phantom. Polymers, 13(21), 3629. https://doi.org/10.3390/polym13213629