Impact of Magnesium Salt on the Mechanical and Thermal Properties of Poly(vinyl alcohol)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Measurements
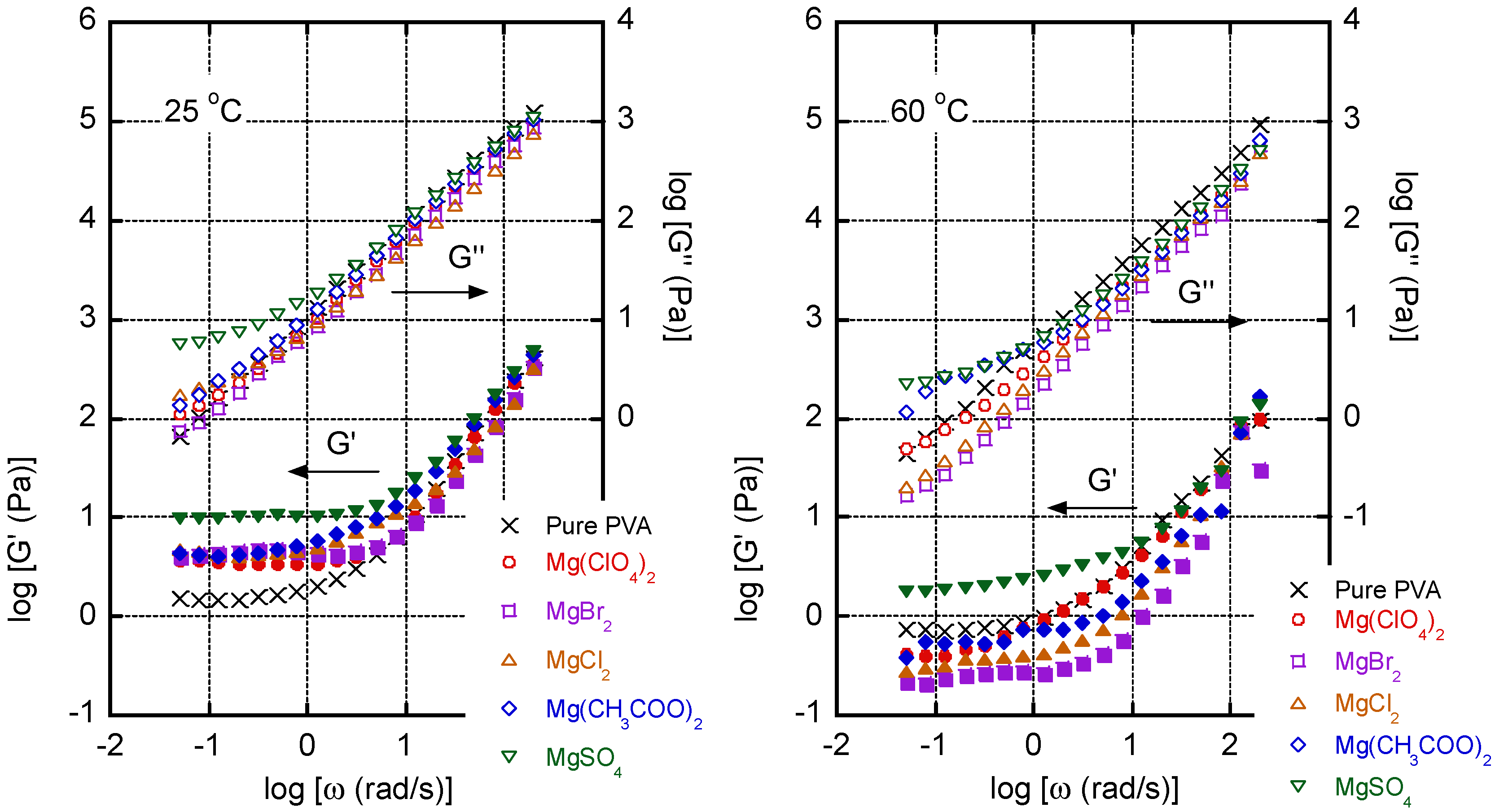
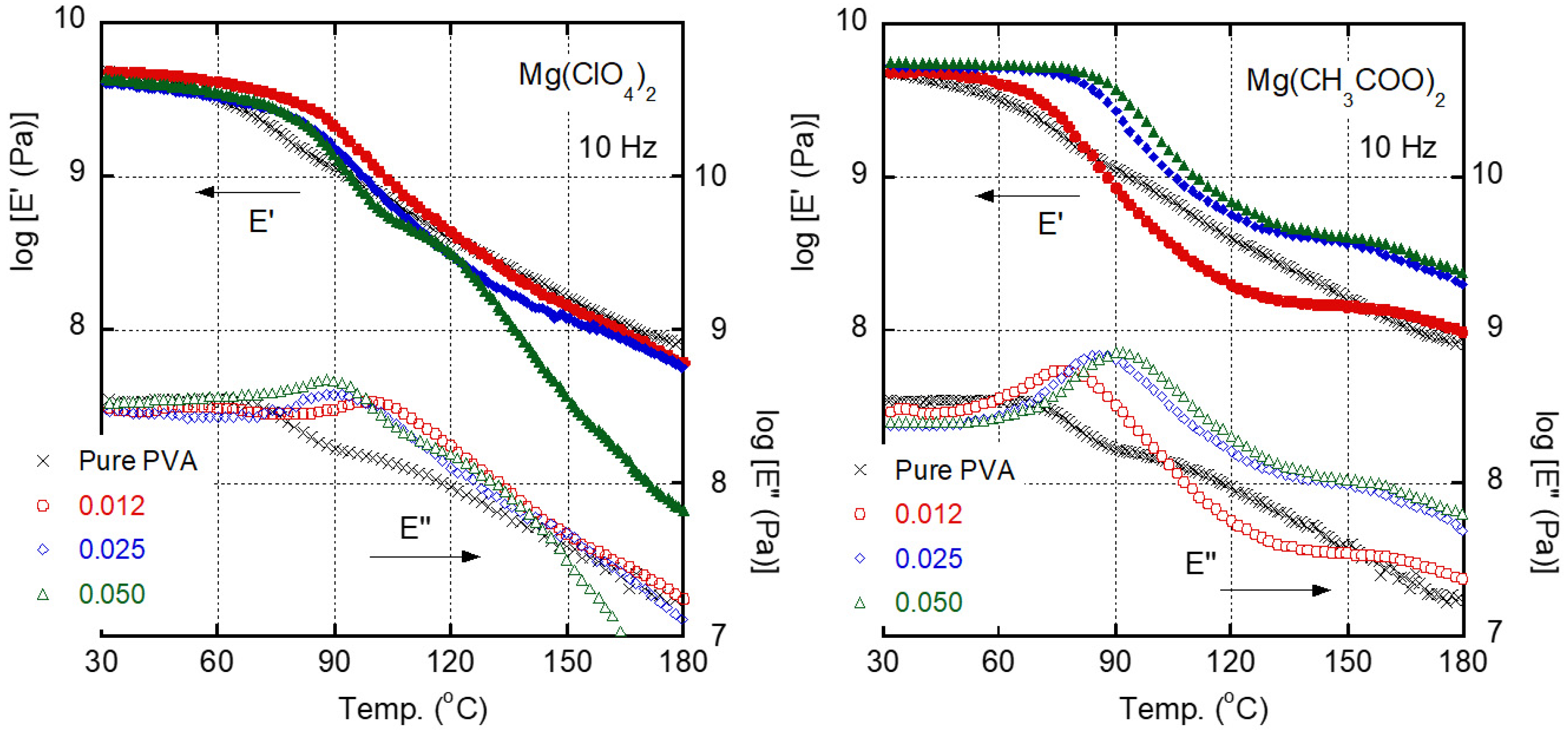
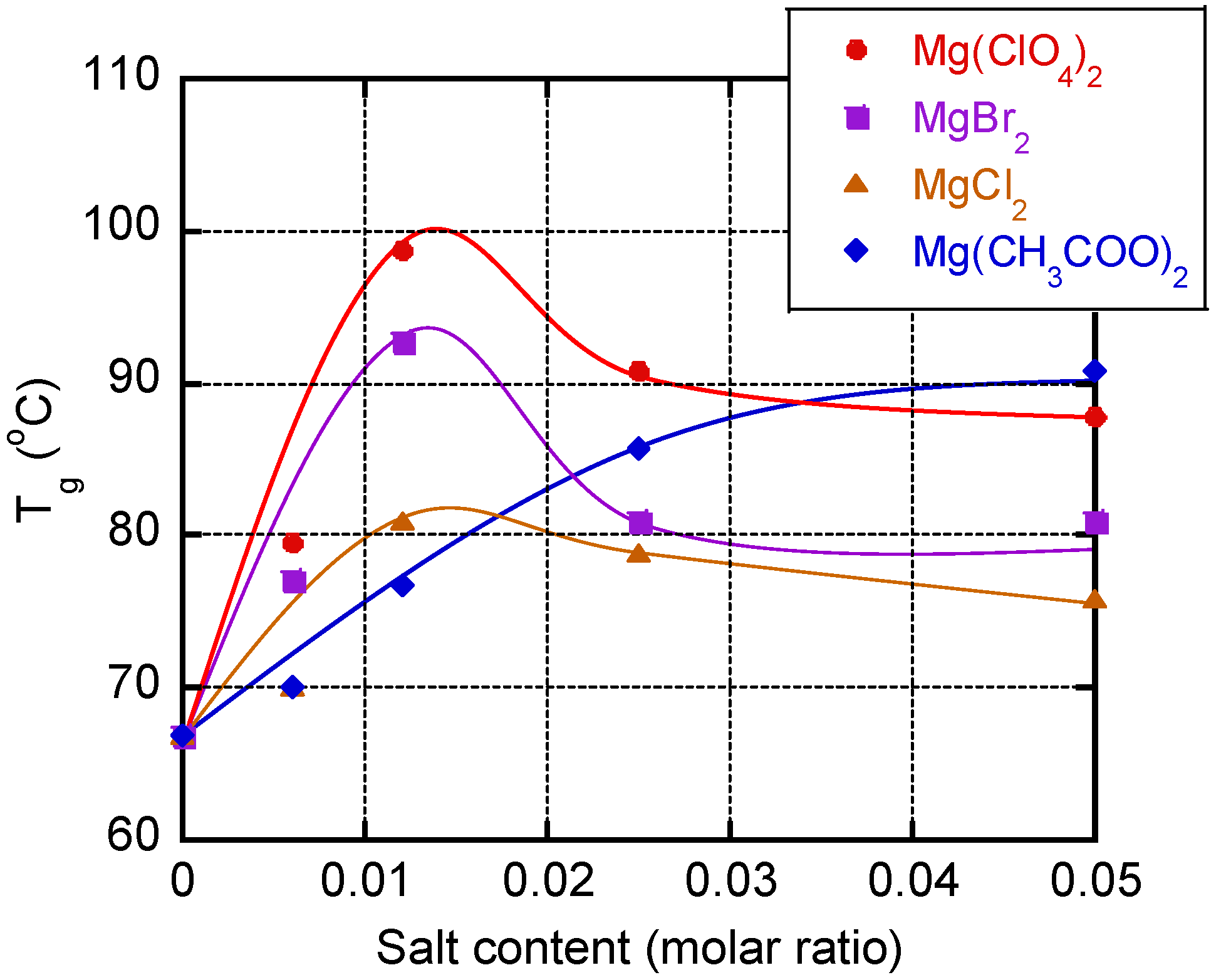
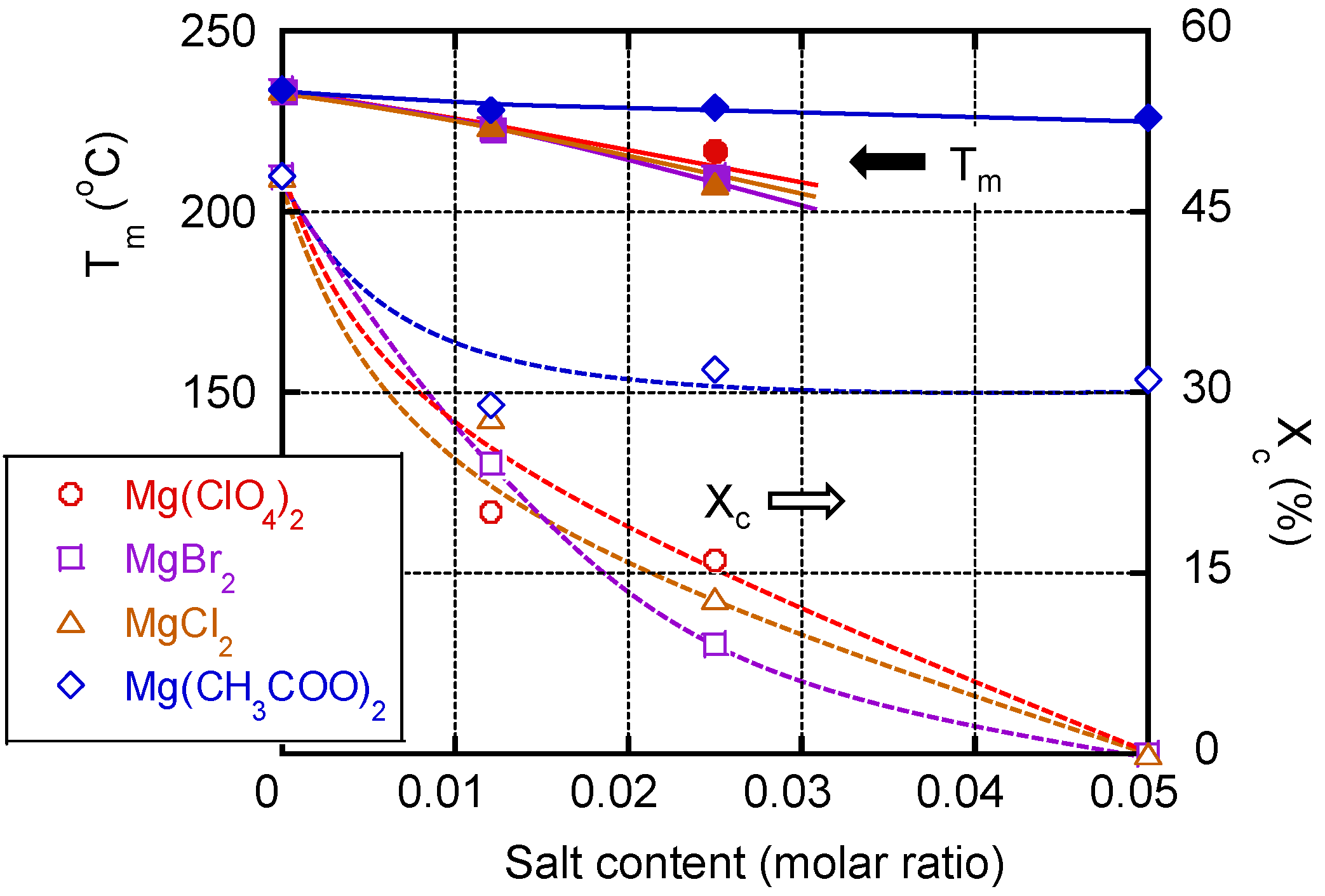
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.-Z.; Wang, Y.-Y.; Wang, C.-L.; Xiang, H. Synthesis and characterization of a PVA/LiCl blend membrane for air dehumidification. J. Membr. Sci. 2008, 308, 198–206. [Google Scholar] [CrossRef]
- Ahad, N.; Saion, E.; Gharibshahi, E. Structural, thermal, and electrical properties of PVA-sodium salicylate solid composite polymer electrolyte. J. Nanomater. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Dai, H.; Zhang, X. Effect of magnesium chloride hexahydrate on thermal and mechanical properties of starch/PVA films. Plast. Rubber Compos. 2015, 44, 299–305. [Google Scholar] [CrossRef]
- Saari, R.A.; Maeno, R.; Marujiwat, W.; Nasri, M.S.; Matsumura, K.; Yamaguchi, M. Modification of poly(vinyl alcohol) fibers with lithium bromide. Polymer 2020, 213, 123193. [Google Scholar] [CrossRef]
- Chen, N.; Li, L.; Wang, Q. New technology for thermal processing of poly(vinyl alcohol). Plast. Rubber Compos. 2007, 36, 283–290. [Google Scholar] [CrossRef]
- Saari, R.A.; Maeno, R.; Tsuyuguchi, R.; Marujiwat, W.; Phulkerd, P.; Yamaguchi, M. Impact of lithium halides on rheological properties of aqueous solution of poly(vinyl alcohol). J. Polym. Res. 2020, 27, 1–8. [Google Scholar] [CrossRef]
- Nishikawa, R.; Aridome, N.; Ojima, N.; Yamaguchi, M. Structure and properties of fiber-reinforced polypropylene prepared by direct incorporation of aqueous solution of poly(vinyl alcohol). Polymer 2020, 199, 122566. [Google Scholar] [CrossRef]
- Song, Q.; Yu, R.; Shui, Z.; Wang, X.; Rao, S.; Lin, Z.; Wang, Z. Key parameters in optimizing fibres orientation and distribution for ultra-high performance fibre reinforced concrete (UHPFRC). Constr. Build. Mater. 2018, 188, 17–27. [Google Scholar] [CrossRef]
- Lai, D.; Wei, Y.; Zou, L.; Xu, Y.; Lu, H. Wet spinning of PVA composite fibers with a large fraction of multi-walled carbon nanotubes. Prog. Nat. Sci. Mater. Int. 2015, 25, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Kubo, J.-I.; Rahman, N.; Takahashi, N.; Kawai, T.; Matsuba, G.; Nishida, K.; Kanaya, T.; Yamamoto, M. Improvement of poly(vinyl alcohol) properties by the addition of magnesium nitrate. J. Appl. Polym. Sci. 2009, 112, 1647–1652. [Google Scholar] [CrossRef]
- Tretinnikov, O.N.; Zagorskaya, S.A. Determination of the degree of crystallinity of poly(vinyl alcohol) by FTIR spectroscopy. J. Appl. Spectrosc. 2012, 79, 521–526. [Google Scholar] [CrossRef]
- Jiang, X.; Jiang, T.; Zhang, X.; Dai, H.; Zhang, X. Melt processing of poly(vinyl alcohol) through adding magnesium chloride hexahydrate and ethylene glycol as a complex plasticizer. Polym. Eng. Sci. 2012, 52, 2245–2252. [Google Scholar] [CrossRef]
- Wang, B.; Lu, C.; Hu, J.; Lu, W. Property improvements of EVOH by enhancing the hydrogen bonding. Plast. Rubber Compos. 2020, 49, 18–24. [Google Scholar] [CrossRef]
- Sako, T.; Miyagawa, A.; Yamaguchi, M. Modulus enhancement of polycarbonate by addition of lithium perchlorate. J. Appl. Polym. Sci. 2017, 134, 44882–44887. [Google Scholar] [CrossRef]
- Tomie, S.; Tsugawa, N.; Yamaguchi, M. Modifying the thermal and mechanical properties of poly(lactic acid) by adding lithium trifluoromethanesulfonate. J. Polym. Res. 2018, 25, 206. [Google Scholar] [CrossRef]
- Sato, Y.; Ito, A.; Maeda, S.; Yamaguchi, M. Structure and optical properties of transparent polyamide 6 containing lithium bromide. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1513–1520. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Takatani, R.; Sato, Y.; Maeda, S. Moisture-sensitive smart hot-melt adhesive from polyamide 6. SN Appl. Sci. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Miyagawa, A.; Ayerdurai, V.; Nobukawa, S.; Yamaguchi, M. Viscoelastic properties of poly(methyl methacrylate) with high glass transition temperature by lithium salt addition. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 2388–2394. [Google Scholar] [CrossRef]
- Ito, A.; Maeno, R.; Yamaguchi, M. Control of optical and mechanical properties of poly(methyl methacrylate) by introducing lithium salt. Opt. Mater. 2018, 83, 152–156. [Google Scholar] [CrossRef]
- Ito, A.; Phulkerd, P.; Ayerdurai, V.; Soga, M.; Courtoux, A.; Miyagawa, A.; Yamaguchi, M. Enhancement of the glass transition temperature of poly(methyl methacrylate) by salt. Polym. J. 2018, 50, 857–863. [Google Scholar] [CrossRef]
- Tsugawa, N.; Ito, A.; Yamaguchi, M. Effect of lithium salt addition on the structure and optical properties of PMMA/PVB blends. Polymer 2018, 146, 242–248. [Google Scholar] [CrossRef]
- Saari, R.A.; Nasri, M.S.; Marujiwat, W.; Maeno, R.; Yamaguchi, M. Application of the Hofmeister series to the structure and properties of poly(vinyl alcohol) films containing metal salts. Polym. J. 2021, 53, 557–564. [Google Scholar] [CrossRef]
- Okazaki, Y.; Ishizuki, K.; Kawauchi, S.; Satoh, M.; Komiyama, J. Ion-Specific Swelling and Deswelling Behaviors of Ampholytic Polymer Gels. Macromolecules 1996, 29, 8391–8397. [Google Scholar] [CrossRef]
- Nakano, T.; Yuasa, H.; Kanaya, Y. Suppression of agglomeration in fluidized bed coating. III. Hofmeister series in suppression of particle agglomeration. Pharm. Res. 1999, 16, 1616–1620. [Google Scholar] [CrossRef]
- Muta, H.; Kawauchi, S.; Satoh, M. Ion-specific swelling behavior of uncharged poly(acrylic acid) gel. Colloid Polym. Sci. 2003, 282, 149–155. [Google Scholar] [CrossRef]
- Mori, M.; Wang, J.; Satoh, M. Anti-Hofmeister series properties found for a polymer having a π electron system and acidic protons. Colloid Polym. Sci. 2009, 287, 123–127. [Google Scholar] [CrossRef]
- Wang, J.; Satoh, M. Novel PVA-based polymers showing an anti-Hofmeister series property. Polymer 2009, 50, 3680–3685. [Google Scholar] [CrossRef]
- Konidari, M.V.; Papadokostaki, K.G.; Sanopoulou, M. Moisture-induced effects on the tensile mechanical properties and glass-transition temperature of poly(vinyl alcohol) films. J. Appl. Polym. Sci. 2011, 120, 3381–3386. [Google Scholar] [CrossRef]
- Saeed, M.A.M.; Abdullah, O.G. Effect of structural features on ionic conductivity and dielectric response of PVA proton conductor-based solid polymer electrolytes. J. Electron. Mater. 2020, 50, 432–442. [Google Scholar] [CrossRef]
- Lee, K.E.; Khan, I.; Morad, N.; Teng, T.T.; Poh, B.T. Thermal behavior and morphological properties of novel magnesium salt-polyacrylamide composite polymers. Polym. Compos. 2011, 32, 1515–1522. [Google Scholar] [CrossRef]
- Arayachukiat, S.; Doan, V.A.; Murakami, T.; Nobukawa, S.; Yamaguchi, M. Autonomic self-healing of poly(vinyl butyral). J. Appl. Polym. Sci. 2015, 132, 42008. [Google Scholar] [CrossRef]
- Qiao, C.; Wang, X.; Zhang, J.; Yao, J. Influence of salts in the Hofmeister series on the physical gelation behavior of gelatin in aqueous solutions. Food Hydrocoll. 2020, 110, 106150. [Google Scholar] [CrossRef]
- Wang, X.; Park, S.Y.; Yoon, K.H.; Lyoo, W.S.; Gil Min, B. The effect of multi-walled carbon nanotubes on the molecular orientation of poly(vinyl alcohol) in drawn composite films. Fibers Polym. 2006, 7, 323–327. [Google Scholar] [CrossRef]
- Jang, J.; Lee, D.K. Plasticizer effect on the melting and crystallization behavior of polyvinyl alcohol. Polymer 2003, 44, 8139–8146. [Google Scholar] [CrossRef]


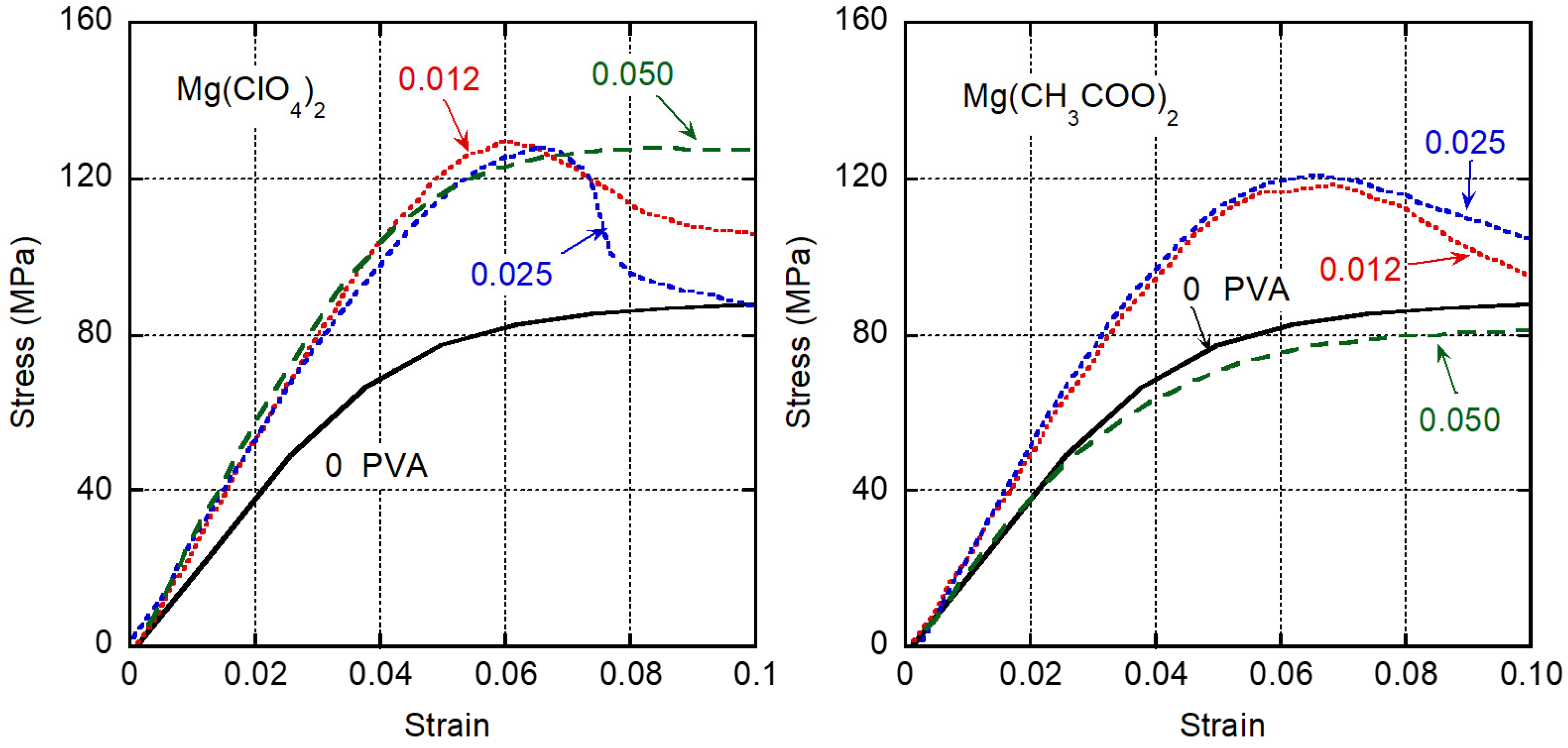

| Salt | Molar Ratio (wt %) | Tensile Modulus GPa | Yield Stress MPa |
|---|---|---|---|
| Pure PVA | - | 2.4 <0.2> | 88 <1> |
| Mg(ClO4)2 | 0.012 (5.7) | 3.3 <0.2> | 130 <1> |
| 0.025 (11.3) | 3.0 <0.3> | 128 <1> | |
| 0.050 (20.2) | 3.1 <0.6> | 129 <3> | |
| Mg(CH3COO)2 | 0.012 (3.7) | 2.8 <0.2> | 119 <1> |
| 0.025 (7.5) | 2.9 <0.3> | 120 <1> | |
| 0.050 (13.9) | 2.5 <0.6> | 86 <1> |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saari, R.A.; Nasri, M.S.; Kida, T.; Yamaguchi, M. Impact of Magnesium Salt on the Mechanical and Thermal Properties of Poly(vinyl alcohol). Polymers 2021, 13, 3760. https://doi.org/10.3390/polym13213760
Saari RA, Nasri MS, Kida T, Yamaguchi M. Impact of Magnesium Salt on the Mechanical and Thermal Properties of Poly(vinyl alcohol). Polymers. 2021; 13(21):3760. https://doi.org/10.3390/polym13213760
Chicago/Turabian StyleSaari, Riza Asmaa, Muhammad Shahrulnizam Nasri, Takumitsu Kida, and Masayuki Yamaguchi. 2021. "Impact of Magnesium Salt on the Mechanical and Thermal Properties of Poly(vinyl alcohol)" Polymers 13, no. 21: 3760. https://doi.org/10.3390/polym13213760
APA StyleSaari, R. A., Nasri, M. S., Kida, T., & Yamaguchi, M. (2021). Impact of Magnesium Salt on the Mechanical and Thermal Properties of Poly(vinyl alcohol). Polymers, 13(21), 3760. https://doi.org/10.3390/polym13213760





