Abstract
Although the human eye is an easily accessible sensory organ, it remains a challenge for drug administration due to the presence of several anatomical and physiological barriers which limit the access of drugs to its internal structures. Molecular imprinting technology may be considered the avant-garde approach in advanced drug delivery applications and, in particular, in ocular therapy. In fact, molecularly imprinted polymers hold the promise to compensate for the current shortcomings of the available arsenal of drug delivery systems intended for ocular therapy. The present manuscript aims to review the recent advances, the current challenges and most importantly to raise awareness on the underexplored potential and future perspectives of molecularly imprinted polymer-based drug delivery systems intended for the treatment of eye diseases.
1. Introduction
Vision is responsible for most of the interactions of the human body with the outside world. A function of this importance is performed through complex, yet very fragile peripheral structures, the eyes. In the last decades, the rising life expectancy has favored a demographic transition towards a growing proportion of people over the age of 50, which are particularly at risk of developing non-communicable eye diseases capable of causing vision impairment or even blindness [1]. According to the World Health Organization data from 2019, out of the total population of the world, about 2.2 billion people have visual impairment [2], more than 200 million patients aged 50 and older being diagnosed with moderate and severe visual impairment, making the treatment of eye diseases an important public health problem with significant socio-economic costs [3]. Early detection and adequate medical intervention are necessary to reduce the negative impact of eye diseases on the global health status.
The architecture of the optical system and the eye itself is of the highest complexity. Although an easily accessible sensory organ, it remains a challenge for drug administration due to the presence of anatomical and physiological barriers which may limit the access of drugs to the internal eye structures [4]. Considering the physiological limitations of an efficient and non-invasive drug delivery to distinct segments of the eye, aligned with the binding requirements warranting patient compliance, there is still a pressing need of going beyond state-of-the-art in the field of ocular drug delivery systems (DDS). Research performed in recent years have primarily focused on the optimization of conventional formulations aiming to boost the pharmacological potential of the currently used drug molecules.
Giving new valences to already established polymeric materials coming from various fields of science and technology may represent a fast-track solution to further develop intelligent platforms acting as advanced drug reservoirs and delivery systems. The features of molecularly imprinted polymers (MIPs) demonstrated in separation science, align and compensate for the current shortcomings of the available arsenal of DDS intended for ocular therapy, such as limited loading capacity, suboptimal release time and profile, all converging towards an insufficient bioavailability of the active pharmaceutical ingredient (API).
The present manuscript aims to review the recent advances, the current challenges and most importantly to raise awareness on the underexplored potential and future perspectives of molecularly imprinted polymer-based drug delivery systems intended for the treatment of eye diseases.
2. Eye’s Structure and Therapeutical Approaches for Its Treatment
The eye is one of the few areas of the body with immune privilege. The eye attempts to limit the local immune and inflammatory response to preserve transparency, and in the end, vision. Multiple mechanisms combine to provide this privilege: physical barriers (corneal epithelium, blood-retinal barrier, and retinal pigment epithelium), soluble factors that inhibit the activity of immune-competent cells (including TGF-β and neuropeptides), and anterior chamber immune deviation (ACAID), a unique response to antigens entering the eye, also a mechanism of tolerance to tissue-specific antigens in the healthy eye [5].
The very surface of the eye, the cornea, must carry multiple functions and is the target of many of the DDS applied topically. It must remain transparent to function as a high-power lens (about 44 dioptries), but also must retain integrity as various aggressions constantly bombard it. Corneal surface is designed to be a highly effective barrier, restricting the access to the eye of high-volume molecules, including pathogens. The cornea, together with the lacrimal film, the conjunctiva, and the eyelids, form a functional system that maintain a double homeostasis of the ocular surface–tissular and optical.
Cornea has a thickness of about 550 microns. It consists of three layers with different characteristics: epithelium, stroma, and endothelium, separated by Bowman and Descemet membranes. Epithelial cells have tight junctions (zonula ocludens), especially in the superficial layers, which provide water-tight seal and strong barrier effect. Corneal epithelium is the main obstacle for drug penetration into the eye [6]. Stroma is the thickest part of the cornea and consist of collagen fibrils organized in lamellae, with small number of cells. The endothelial layer, the innermost of the cornea, functions as an ionic pump, dehydrating the stroma to about 60% of water. Hydration of stroma or strong immune response would cause loss of transparency and of vision [7]. Bowman (between epithelium and stroma) and Descemet (between stroma and endothelium) membranes separate the corneal layers and have little importance to permeation. Figure 1 presents a section through cornea and its relationship with conjunctiva and sclera, as viewed In vivo with ocular coherence technology.
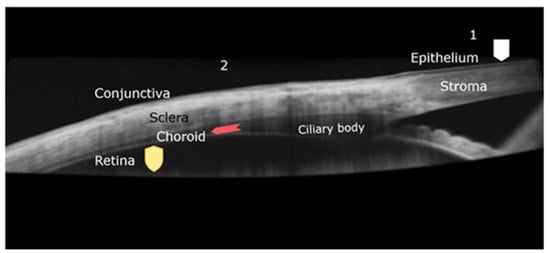
Figure 1.
Ocular coherence technology (OCT) image of the junction between cornea and sclera covered by conjunctiva. Beneath the sclera, the choroid and ciliary body are seen. Barriers that prevent drug permeation are highlighted: 1–corneal pathway, epithelium barrier (squared shield). 2-transscleral pathway, choroid circulation (arrow) and retinal barrier (shield).
The corneal route is the main access for drugs deposited onto ocular surface, into the aqueous humor. Conjunctival surface is much greater, and the permeability is better than in cornea. Sclera lying beneath the conjunctiva (Figure 1) is also porous. The conjunctival and scleral permeability is 15 to 25 times better than cornea, and molecular size has lower importance [8]. However, the choroid and the RPE limit the bioavailability of the drug, as being the case with subconjunctival injection approach.
The corneal epithelium is lipophilic and limit the permeation of hydrophilic molecules. Intercellular pores measure 2.0 ± 0.2 nm in diameter that allow only drugs with a molecular weight of <500 Da/10 Å to penetrate [9]. The stroma, on the other hand, acts as a barrier for lipophilic compounds. The endothelium is more permeable than the other two layers, especially for hydrophilic compounds.
The physiological barriers against the APIs and the need to increase the concentration of drugs in the inner structures of the eye have been permanent challenges for researchers in the attempt to optimize conventional pharmaceutical formulations but also to design new DDSs and to approach alternative routes of administration.
An ideal ocular DDS ought to be fully biocompatible, to provide a controlled release for extended periods of time, to be compliant with non-invasive routes of administration and last but not least, to be in agreement with a patient-friendly posology.
The majority of eye pathologies are addressed by topical administration, most often in the form of eye drops. A topical drug administration to the eye is convenient and well-accepted by patients, lacking the discomfort associated with injectable administrations, and is preferred in the diseases of the anterior segment of the eye like conjunctivitis, diseases of the iris or glaucoma [10]. Eye drops, as molecular (true solutions), mechanical (suspensions, emulsions) or colloidal dispersions are non-invasive, fast action and cost-effective. Nevertheless, significant challenges lay ahead of an efficient ophthalmic treatment for a topically applied drug, since, in general, less than 5% of the substance applied as eye drops is retained on the ocular surface and attempts the entrance through corneal barrier [11]. Drug formulations applied on the corneal surface, designed to reach the anterior segment of the eye are hampered by a series of precorneal factors like blinking reflex, tear turnover and lacrimation which contribute to a low ocular bioavailability [12]. The fast clearance of drugs applied on the cornea also results in a short duration of the pharmacological effect, often requiring a frequent dosing which may reduce in some cases patient acceptance of the administered treatment. Additionally, a variable proportion of the topically administered drug could enter in the systemic circulation through nasolacrimal duct drainage, with possible systemic adverse reactions. Moreover, the multi-layered cornea itself is a barrier against the access of both hydrophilic and lipophilic drugs to the anterior eye segment [13]. Also, the presence of efflux transport systems in the epithelial cells of the cornea can alter the trans-corneal penetration capacity of certain drugs like anti-glaucoma or antiviral agents [14].
Increasing the remanence of the API at conjunctival level ensures an increased absorption and improved tolerance of APIs; this goal can be achieved by using viscosity-modifying agents, the preparation of bioadhesive formulations, or the preparation of gel-forming solutions containing stimuli-responsive excipients [15,16,17].
The use of emulsions as vehicles for water-insoluble drugs has shown increased internalization of the API dissolved in the non-polar phase. The additional use of cationic polymers such as chitosan, able to increase remanence by an interaction with the negatively charged mucin, further improves the bioavailability parameters [16,18]. By gradual dissolution of the dispersed phase, suspensions with optimal particle size ensure high concentrations of sparingly soluble APIs at conjunctival level. The use of excipients that optimize the physical properties of suspensions such as xanthan gum [19] or ion exchange resins [16], which release the API in the presence of tears, is a strategy that improves the API‘s absorption and increases the duration of the therapeutic effect.
Several nanocarriers intended for both the anterior and posterior segment of the eye such as nanomicelles, dendrimers, nanoparticles, nanosuspensions and liposomes showed promising results as means to control drug release and to improve the permeability, the stability, the biocompatibility, and the bioavailability of APIs [17,20]. Liposomes which can efficiently encapsulate both lipophilic, and hydrophilic APIs exhibit structural similarities to biological membranes, good biocompatibility, and were proven to efficiently release APIs in the anterior and posterior segments of the eye [19]. Nanoparticles contain excipients well tolerated by the eye tissues, such as lipids, proteins, natural or synthetic polymers; their surface characteristics and size may favor the internalization kinetics of APIs into the eye [17]. These drug-laden nano-carriers can be used both as fluid colloidal dispersions, or can be incorporated into other pharmaceutical dosage forms, such as semisolid hydrogels or contact lenses.
Contact lenses are hydrogel-based medical devices that are applied directly onto the cornea, initially designed to correct ametropia. Therapeutic contact lenses (CLs) obtained by the inclusion of APIs in the lens matrix are considered an excellent alternative for the treatment of ocular diseases, particularly for chronic ones. Direct contact with the cornea can ensure prolonged and/or controlled drug release, with numerous studies showing a more then 50% increase in bioavailability as compared to eye drops formulations [21]. The critical quality parameters in the development of therapeutic contact lenses are represented by the convenience in use, a visual comfort, and a good tolerance.
CL are composed of polymeric hydrogels or silicone hydrogels. Polyhydroxyethyl methacrylate (pHEMA), was the first monomer used to prepare CLs in the 1960s, and, along with N-vinylpyrrolidone (NVP), it remains the monomer of choice for the manufacture of CLs. Both materials are well tolerated as they confer the lens increased hydrophilicity, adequate mechanical properties, water swelling and oxygen permeability. To optimize these characteristics, other monomers or functionalized compounds can be incorporated into the lens matrix [10,22].
Intraocular injections are usually employed for an efficient drug delivery to the posterior eye segment in diseases like macular degeneration or diabetic retinopathy. Intravitreal injections were developed to bypass the natural barriers of the eye but at greater cost, inconvenience, and risk for the eye: infections, haemorrhages, rise of intraocular pressure, or unwanted perforations of the retina. Moreover, the distribution of the drugs in the vitreous body is variable, subjected to additional pathophysiological influences, and the administration could be painful, affecting patient acceptance [23]. Intravitreal injections are commonly used to deliver anti-VEGF drugs (bevacizumab, ranibizumab, aflibercept) inside the vitreous cavity. Due to their protein structure (being either monoclonal antibodies or soluble receptors) these drugs have long intravitreal half-lives but still require a monthly or bi-monthly administration, sometimes for many years, to control the aggressive form of AMD, the neovascular form [24]. Another example are antibiotics which are rarely effective for intraocular infections (endophthalmitis) upon topical or systemic administration, including intravenous, and must be delivered through intraocular injection.
Implants are devices designed to ensure a prolonged effect of the drugs in the eye, while avoiding frequent intraocular injections, especially in the case of chronic vitreo-retinal diseases. Administration of biodegradable implants (usually PLGA- or polycaprone- based) are preferred due to their high tolerance [17]. Intravitreal administration of a biodegradable PLGA-based implant, for example, was proven to ensure the prolonged release of dexamethasone for 6 months, with beneficial effects in reducing the risk of vision loss in patients with macular edema [17].
Another treatment strategy proposes the use of microneedles technology, that can ensure the controlled release of high concentrations of APIs in the retina/choroid. The approach is promising for eye diseases that threaten vision such as macular degeneration, diabetic retinopathy and posterior uveitis [17].
Alternative periocular routes like retrobulbar, peribulbar, sub-Tenon’s or subconjunctival injections are less invasive, being also capable to deliver drugs to the posterior segment of the eye by transscleral diffusion or through choroidal plexus, but usually in suboptimal concentrations due to the presence of blood-ocular barriers which can limit drug access [25].
Generally, a systemic administration of drugs is rarely used in ophthalmic diseases due to a low intraocular bioavailability of the active substance. Despite the easy access to the eye via the vascular choroid plexus, a systemically administered drug must pass through the anterior blood-aqueous barrier (BAB) or the posterior blood-retinal barrier (BRB) in order to gain access inside different segments of the eye. Particularly, tightly packed retinal pigment epithelial (RPE) cells in the outer blood-retinal barrier can significantly reduce the access of a systemically administered drug to the retina and vitreous body, usually less than 2% of the administered dose being available [26]. Additionally, a possible pre-systemic metabolism of oral drugs or the apparition of significant adverse reactions may furthermore limit the systemic administration to a few clinical emergencies such as the acute angle-closure glaucoma [27].
3. Drug Loading Strategies in Polymeric Matrices for Extended Drug Delivery
Biocompatible polymeric matrices are convenient scaffold materials for the development of drug reservoirs intended for the prevention and treatment of various eye conditions. To increase the API’s bioavailability and the duration of its therapeutic effect, several approaches for drug loading in polymeric matrices may be used: soaking (including supercritical fluid technology), chemical functionalization of the polymeric matrix aiming to induce guest-host interactions (e.g., cyclodextrins), polymerization in the presence of ionic compounds to promote electrostatic interactions with the API, carrier-mediated release (the incorporation of API in nanoparticulate colloidal systems, such as liposomes, micelles, microemulsions and polymer-based nanoparticles, followed by the loading of these systems into the polymeric scaffold), and molecular imprinting [20,22].
3.1. Hydrogels
Hydrogels, mainly designed for topical application (i.e. conjunctival sac), represent networks of natural or synthetic monomers, crosslinked with multifunctional linkers, forming flexible structures upon free-radical polymerization initiated thermally or photochemically. These polymers are insoluble in water but must exhibit a swelling behavior in aqueous solutions while preserving the imprinted sites. Matrix swelling followed by the API‘s release at a certain rate represent fundamental properties of hydrogels and are influenced by the nature of the hydrophilic functional groups and by the degree of crosslinking. The crosslinking of the polymer can be achieved physically or chemically.
Hydrogels can be specifically designed to obtain predictable characteristics (SMART hydrogels) by the appropriate choice of crosslinkers and by the changing of the crosslinking degree. Thus, it is possible to modulate the release rate of the API and the duration of the therapeutic effect by modeling the physical and mechanical characteristics of the hydrogel (such as viscosity, porosity, swelling, mechanical strength, and erosion rates, etc.). Moreover, the addition of functional crosslinking agents enables the gel to react to external stimuli, with major implications on the designed formulation’s pharmacokinetics. Consequently, in situ gel formation at body temperature may occur, or the API‘s release may be triggered by changes in pH or photo-stimulation.
Natural monomers used in the design of hydrogels include agar, alginic acid, collagen, chitosan, gelatin, fibrin, hyaluronic acid (HA), pectin, etc. The resulting polymers are biocompatible and biodegradable as they share a similar structure to ocular tissues (vitreous humor), and have been intensively studied for tissue engineering. Synthetic polymers, such as polyvinylpyrrolidone (PVP), poly(acrylic acid) (PAA), polyacrylamide (PAM), poly(vinyl alcohol) (PVA), poly(hydroxyethyl methacrylate) (PHEMA), poly(ethylene oxide) (PEO), and cellulose derivatives have lower biocompatibility; however, their manufacturing characteristics are more reproducible.
Hydrogel forming polymers are currently used in practice for the design of a wide variety of pharmaceutical formulations in ocular drug delivery such as gel-forming solutions, intravitreal hydrogels, topical hydrogels, and contact lenses.
3.2. Molecularly Imprinted Polymer (MIP)-Based as Drug Reservoirs
Similar to hydrogel synthesis but exploiting a more tailored interaction between the API and the selected functional monomers, both chemically and sterically complementary binding sites (drug-specific cavities) towards the API molecule may be formed in a highly crosslinked polymeric network, through the process of molecular imprinting [28]. The formation of such specific binding sites within the polymeric network enables a significantly higher drug loading, as well as a considerably extended and more controlled drug release in comparison to the aforementioned hydrogels. The beneficial consequences envisaged upon their clinical application lay in the increase of therapeutic efficiency while maintaining non-invasive routes of administration, in an improved patient compliance especially in chronic eye conditions, and last but not least, in a notable cutback of the amount of drug needed to successfully treat ocular diseases.
MIPs can be rationally designed to obtain predictable characteristics by the appropriate choice of functional monomers and crosslinkers, and their molar ratio with respect to the API. Since their inception, MIPs were successfully developed and applied in analytical techniques, therefore the vast majority of MIP-based DDSs later developed were made from the same acrylic monomers and employing the non-covalent imprinting approach. The most adopted functional monomer is meth(acrylic) acid since its carboxyl group can form weak electrostatic attractions and/or functions as a hydrogen donor for H-bond acceptor drugs; most often it is coupled with EGDMA, as crosslinker. Furthermore, in ocular therapy, the traditional CLs were also based on acrylic monomers (e.g., HEMA), thus the main adaptation of the polymerization mixture formulation was the decrease in the crosslinker concentration. Even though these polymers are already acknowledged as being biocompatible, the development of MIP-DDS for drug delivery applications is still in its infancy.
In advanced ocular therapy, contact lenses acting as drug reservoirs are able to provide a sustained or controlled release over the wear duration. As formerly mentioned, there are two strategies for the incorporation of the API into the hydrogel: (i) post-synthesis, via the traditional approach of soaking the premade lens in a drug solution or (ii) during hydrogel polymerization. The first approach offers the advantage of simplicity and ease of fabrication, but it provides a drug release profile characterized by an initial burst release with potentially serious consequences for the patient, followed by subtherapeutic concentrations. In the latter case, drug incorporation occurs simultaneously with the lens synthesis process. Here a distinction needs to be made between the entrapment of drug during polymerization and the molecular imprinting approach. Not every polymer made in the presence of a drug molecule will result in a MIP, because the imprinting effect is not determined by the mere presence of a template in the polymerization mixture. The success of the imprinting depends upon the effectiveness in achieving imprinted cavities in a crosslinked polymer matrix, characterized by (i) shape complementarity to the template molecule (i.e. targeted drug molecule, API) and (ii) specific spatial distribution of the functional groups able to promote multiple, mostly chemical interactions, with the template. This can only be achieved by optimizing the imprinting parameters for each API, since the template and its functionalities determines the choice of the functional monomer(s). As a matter of fact, a complex combination of factors including the nature, concentration and the ratio of the functional and crosslinking monomers, the functional monomer-template ratio, polymerization conditions (temperature, UV, duration, etc.) strongly affect the process of molecular imprinting [28]. Following this optimization, the stability, and the number of the imprinted cavities, as well as the MIP’s affinity towards the template can be adjusted, which in turn dictates the amount of drug loaded and the control over its release profile. In principle, the efficiency of the molecular imprinting process is assessed in comparison to a reference, non-imprinted polymer (NIP).
On the other hand, in cross-linked polymer-based contact lenses in which the API is added during the polymerization process, its molecule is retained in the hydrogel’s polymeric network mainly by physical entrapment. Even though a so called “functional monomer” (i.e. substance capable of interacting with the drug) is used for CL production, they are not able to generate imprinted cavities due to the lack of sufficient and spatially-arranged functionalities that can provide multiple interaction points with the drug. Furthermore, if a subsequent drug washing step is performed, no “drug-specific” cavities will remain in the polymeric matrix, as the lack of effective crosslinking will cause their collapse. For all these reasons, the drug-entrapped contact lenses usually demonstrate low drug loading and a burst release of the API with poor control over the release behavior in comparison with the molecularly imprinted polymers.
4. MIP-Based Systems Intended for Ocular Drug Delivery
There is a long and sinuous road from the early days of these functional polymers towards the MIP-based drug delivery systems intended for ocular therapy, with several noteworthy milestones (Figure 2). In developing ocular drug delivery systems various aspects of the conventional molecular imprinting process need to be carefully adapted for the intended application field and preferred therapeutic strategy, but also aligned to the pharmaceutical requirements and regulations.

Figure 2.
Milestones in the development of MIP-based ocular drug delivery.
MIPs have been initially developed for and employed mainly in analytical applications, as stationary phases/selective sorbents in separations sciences [29] and more recently as interfaces in the development of (bio)chemical sensors. The main feature which differentiated them from the traditional stationary phases/interfaces was their predetermined ligand selectivity, induced during the synthesis process. The standard MIP fabrication procedure involves the dissolution in a proper and inert solvent, also called porogen, of the following four components: template (target) molecule, functional and crosslinking monomers and an initiator. After the polymerization step, the template is washed out, leaving behind permanent cavities generated into the three-dimensional polymer matrix, complementary in shape, size, and functionality to the template molecules. The obtained polymers were used for their abilities to selectively rebind the template molecules or its structurally similar analogues.
However, in the case of MIP-based drug delivery systems, by the nature of their intended use as drug reservoirs with prolonged and, ideally, controlled release, the template removal step seems to be redundant, as long as any potentially unreacted component of the polymerization mixture holds no toxicity for the human tissues. If, however, a thorough wash needs to be carried out (e.g., when monomers with measurable cellular toxicity need to be used), a subsequent drug loading procedure is performed [28]. Obviously, this scenario is objectionable because it will greatly prolong the DDS processing step, may pose additional or unexpected toxicity issues, and it will substantially increase the total amount of drug used.
In ocular drug delivery, the template-functional monomer interactions are based mainly on weak, non-covalent associations responsible for faster binding and release properties during the analyte-polymer interactions, compared to the covalent imprinting approach. Most of the conventional MIPs intended for analytical applications are synthesized in organic aprotic solvents in order to stabilize the template-functional monomer complex during polymerization, but also to confer a macroporous polymeric structure enabling thus an easier diffusion of the template molecule from and into the imprinted cavities. Nevertheless, the presence of residual organic solvents is undesirable in drug delivery applications and especially in the case of ocular DDSs. Moreover, the use of organic solvents in CLs fabrication is often conflicting and thus liquid monomers need to be exploited for template dissolution.
As in the case of MIPs intended for analytical applications, the performances of the imprinted hydrogels developed for drug delivery purposes must be compared with the corresponding NIPs, which are prepared analogously as the imprinted ones, but without the addition of the template drug. In analytical applications (separation and sensing), one of the main features of the obtained functional polymers is selectivity, the supreme goal of an analyst being the specific binding of the target analyte. In drug delivery, however, other fundamental features are primarily sought after, such as: drug loading and releasing properties. Although, seemingly aiming for different objectives based on the field of their application, in fact these features are somewhat interconnected, as higher binding affinity of the template to the imprinted cavities generally implies improvements in drug loading capacity and may hold the premises for prolonged/controlled drug release. As being outlined in the previous section, MIP-based DDS may alleviate shortcomings related to the low bioavailability of various eye drops, non-imprinted polymeric hydrogels and ointments intended for non-invasive, topical, and especially long-term administration, stemming from their high physiological clearance, limited drug-loading, or excessively fast drug release rates, thus holding the promise of a fascinating area of research. Judging by the number of published research in the last two decades (Table 1), the topic of molecularly imprinted DDS for ocular therapy seems to be severely underexplored, for grounds that the following sections will attempt to unveil.

Table 1.
Most relevant studies of the last two decades focusing on the development of MIP-based hydrogels intended for ocular treatment.
4.1. Advantages and Limitations of Molecular Imprinting in Ocular DDS Development
4.1.1. Advantages
Maybe the most promising direction in the current field of topical ophthalmic drug delivery is the molecular imprinting approach as it can offer real solutions to the problems exhibited by the conventional ocular drug delivery formulations (e.g., ophthalmic solutions, ointments, non-imprinted hydrogels, CLs) such as low bioavailability, exceedingly fast release profile, or insufficient loading capacity. Therefore, by creating imprinted binding sites in the polymer network in which numerous functional groups are concentrated and organized in such a manner to attain a strong interaction with the API, a better control over the release profile can be achieved. In fact, using MIPs as drug reservoirs, a sustained, zero-order drug release may be obtained for a long period of time, when compared to conventional drug delivery [28]. The residence time of the drug within the polymer can be adjusted by controlling the number and the strength of interactions between the template and the polymer. This is achieved by opting for the proper imprinting approach (covalent or non-covalent) followed by the empirical or computational modeling-based selection of suitable, but biocompatible, functional monomer(s) available from a large library.
Furthermore, the tailor-made imprinted pockets of the MIPs may help in protecting the active ingredient upon storage or therapeutic use from enzymatic, hydrolytic or photo-degradation, maintaining the drug for a longer period in its bioactive form, thus increasing its bioavailability.
The molecular imprinting technique can be applied to any kind of template, ranging from ions to relatively small pharmaceutical compounds and even large (bio)molecules, like proteins. However, the increase in the size of the template, may hamper its efficient release from the cross-linked polymer, risking of being permanently stuck inside.
From a pharmacological point of view, a very wide variety of APIs were used as templates in the process of imprinted-hydrogel synthesis, such as: β-blockers (timolol as antiglaucoma medication [30,31,32,59]), α2 adrenergic agonist (brimonidine as antiglaucoma medication [52]), antihistamines (ketotifen [36]), non-steroidal anti-inflammatory agents (ibuprofen [49], diclofenac [42]), corticosteroids (prednisolone [48], fluorometholone [50]), antibiotics (ciprofloxacin [37], moxifloxacin [42], minocycline [43], polymyxin B [44], vancomycin [44]), antifungal drugs (voriconazole [45]), antiviral drugs (acyclovir and valacyclovir [46]), carbonic anhydrase inhibitors (dorzolamide [55], acetazolamide [53,54] used as antiglaucoma medication), statins (atorvastatin [56]), bioprotectants (trehalose [49]), hydroxypropyl methylcellulose used to relieve dryness and irritation caused by reduced tear flow [58], hyaluronic acid used in the treatment of dry eye and other conditions [57].
In most cases, various controlled drug release platforms, MIPs included, have focused on the incorporation and the release of a single active molecule, either a comfort molecule or a pharmaceutical agent. However, MIPs, as drug reservoirs, may be adapted for the simultaneous and controlled delivery of multiple active molecules [49].
In conventional drug delivery, the API is loaded into the gel after synthesis by equilibrium partitioning which sometimes requires even days, in contrast to the imprinting approach where the gel is synthesized in the presence of the drug and the obtained imprinted polymer is already fully loaded.
4.1.2. Challenges
Despite its great potential in drug delivery applications, molecular imprinting bears several concerns with respect to ocular therapy. The most important one is finding the right balance between a rigid cross-linked network offering high-fidelity imprints and an elastic hydrogel displaying good optical and pharmacokinetic properties. The presence of a template molecule in the polymerization mixture may lead to a polymer with different morphology and swelling properties [51,58]. Therefore, in principle, for each API an optimization of the imprinting parameters is required to achieve a biocompatible hydrogel with the proper elasticity, optical and swelling properties. For example, imprinting CLs with bimatoprost showed increased drug loads and better release profiles, but with a negative impact on the morphology and the behavior of the MIP from a drug delivery point-of-view [51].
The concentration of the functional monomer, especially if it is endowed with hydrophilic functional groups, influence the swelling and the water content of the resulting hydrogels. By using more functional monomer, which is also beneficial in terms of drug loading capacity, the polymer’s water content will be increased [48]. Several studies showed however that there was no significant difference in the water content between the imprinted and non-imprinted lenses and the molecular imprinting method did not influence the viscoelastic, swelling and transparency properties of the contact lenses [37].
4.2. Balancing between Flexibility of CLs and Imprinting Efficiency
In the MIP synthesis protocol, one compulsory component of the polymerization mixture is the crosslinker whose role is to provide a reticulated three-dimensional polymer matrix and to preserve the structural integrity of the imprinted cavities. The conventional imprinted polymers designed for analytical applications (i.e. chromatographic adsorbents) are relying on a relatively high degree of cross-linking (50–90% cross-linker agent [60]) in order to achieve imprinted sites with maximum fidelity and a porous polymer matrix ensuring high linear flow velocities of the mobile phase and a rapid mass transfer of the targeted analyte (template).
The imprinted hydrogels, and especially the CLs, need to be flexible enough to ensure good biocompatibility and swelling behavior (water content). A high degree of crosslinking also affects the optical clarity and transparency of CLs [61]. Therefore, from a drug delivery perspective, the composition of CLs should contain low concentrations of crosslinker(s). A maximum of 5–10 mol% crosslinker is generally recommended [61,62]. The most employed crosslinker in the development of imprinted CLs is by far EGDMA, presenting two vinyl groups. Up to now, the emphasis in the published studies was on the cross-linker’s concentration and less on its nature.
Achieving the right balance between high-fidelity imprinted sites and elasticity of the CLs it is not an easy and straightforward process and sometimes requires laborious optimization procedure for each targeted API. One of the most important considerations in developing imprinted CLs for drug delivery purposes is to preserve their biocompatibility and swelling behavior, as the latter is affecting the oxygen dissolution and diffusion into the surface of cornea [63]. Therefore, it is not recommended to sacrifice these features for a modest improvement in the imprinting efficiency.
The nature of the functional monomer is another essential factor affecting the imprinting efficiency. The ability of functional monomers to interact with drug templates during polymerization is different. Thus, the selection of co-monomers significantly influences the performance of the final MIP hydrogel, such as release rate, optical clarity, swelling and mechanical properties [58]. Sometimes, a mixture of functional monomers is applied to obtain an optimized MIP with higher loading capacity and improved release kinetics [35,57].
First studies investigated the influence of the functional monomer:template ratio using the empirical approach, in which the concentration of monomer was kept constant while the concentration of the template was varied [48]. As expected, and in line with the non-covalent imprinting approach, increasing the functional monomer content leads to an increase in the affinity of the polymer and thus in the loading capacity. In return, lower ratios of functional monomer, where efficient template complexation is restricted by the limiting amounts of monomer, favors the release of higher amounts of drug (template) in a given timeframe.
Besides the key role of the functional monomer, functional (charged) additives may also bring unexpected advantages to the efficiency molecular imprinting. Long-chained negatively charged comfort agents, such as hyaluronic acid, commonly used as comfort agent to prevent protein deposition on silicone hydrogels [64], through additional electrostatic interactions with the template (e.g., timolol) may beneficially alter drug loading and its release profile [31,33,59,65].
Alternative molecular imprinting strategies, such as metal-ion mediated imprinting, are able to increase the degree of order throughout the polymerization step by well-defined, spatially-directional coordinate bonds [29]. This kosmotropic effect leads to imprinted cavities of higher fidelity, and even to mimic the active sites of enzymes. As efficient ligands of Zn(II), 4-vinylimidazole and 1-vinyl imidazole, resembling aminoacids of the carbonic anhydrase, were used as functional monomers to achieve high-loading molecularly imprinted drug reservoirs for two inhibitors of the metallo-enzyme and in the meantime antiglaucoma drug representatives, namely acetazolamide and its structural analogue, ethoxzolamide [53,54]. Moreover, by playing with the nature of the metal salt (zinc nitrate instead of zinc methacrylate) cytocompatibility and optical transparency of the resulting CLs could also be improved [54].
4.3. Computer Modeling
The empirical selection of the polymerization mixture’s components is time and resource-consuming and might not even offer the best performing imprinted hydrogels. The more efficient approach to achieve high affinity MIPs with appropriate morphological and physico-chemical properties is the computer-assisted molecular modeling, by helping in better understand, but also to rank the interactions taking place during the molecular imprinting process. Eroglu et al. [43] conducted an integrated computational (molecular dynamics) and experimental study to assess the relationship between the design parameters of MI and the drug (minocycline) uptake and release performance of the imprinted hydrogels.
The implementation of computer-assisted techniques in the development of imprinted hydrogels can reduce the tedious and time-consuming laboratory work as well as the amounts of used chemicals, which is very important especially in the case of expensive template drugs.
4.4. Loading/Release Efficiency
By far the main advantage of MI technology in the design of imprinted hydrogels is overcoming their low affinity for most drugs [62,66]. It was repeatedly demonstrated that generating imprinted sites for a specific drug within the hydrogel, not only gives access to efficiently control the residence time of the drug but can also increase drug-loading capacity.
The imprinting efficiency is determined by the stability of the functional monomer-template complex throughout the polymerization step. Therefore, as expected, the component with the highest impact on the drug-loading capacity, is the nature and the concentration of the functional monomer. Generally, the most favorable monomers for the imprinting process are those with the highest binding affinities to the template [31]. Furthermore, the monomer’s concentration strongly influences drug binding, and up to a critical value being directly correlated with the hydrogel’s loading capacity. Going beyond that level, its beneficial effect may be reversed [31].
Drug loading may depend also on the pH at which the drug-stripped hydrogel is exposed for the re-loading step. For example, HEMA-MAA imprinted hydrogel adsorbs the highest amount of timolol (template), at pH 5.5 and 7.5, at which the carboxylic groups of MAA are ionized and are capable of interacting with the positively charged timolol. At pH values where the non-ionic form of the hydrogel (pH < 5.5) and/or template (pH > 9.2) is predominant, a severe cutback of the drug rebinding occurs [31].
The non-covalent interactions between the functional monomer-template are preferred from a drug release perspective due to the faster binding and release kinetics. The delayed kinetics of drug release and slower diffusion rate of the imprinted hydrogels in comparison with the non-imprinted ones, as prerequisites of drug reservoirs designed for long term treatment, are currently explained by the tumbling effect [49], i.e., the migration of the template molecules from one imprinted cavity to another within the hydrogel matrix.
The most notable differences between the release time, but also released amount of drug of the imprinted vs. non-imprinted CLs were observed when exposed to low concentrations of template during drug-rebinding [37]. However, exposed to higher concentrations of template, due to the concurrent increase of non-specific interactions with the polymeric scaffold, drug-release profiles of imprinted and non-imprinted CLs tend to overlap.
As previously mentioned, by the right choice of functional monomer(s) and monomer:template ratio, depending on the therapeutic needs and on the required duration of wear in case of CLs (up to several days, and even longer [49,58]), the rate and duration of drug and comfort molecules’ release can be rigorously programed, creating the premises of simplified treatment plans and improved patient compliance.
Another factor affecting the release behavior is the swelling property of the lens in aqueous media. Upon the rising of water content within the polymer network, the conformation of specific binding sites changes accordingly, which in turn impacts negatively on the hydrogel’s drug binding capacity. The addition of functional monomers (e.g., NVP, DMAA, MAA) to HEMA significantly increases the water content of the hydrogel [62]. However, in the case of hydrophilic drugs, the loading capacity of MIPs with superior swelling properties, is usually higher than that of MIPs with less water content.
Additionally, to avoid potential pitfalls, care must also be taken during In vitro characterization and optimization of the imprinted drug-reservoirs, as the obtained drug-release kinetics may significantly differ for the same formulation in function of the selected experimental models. For example, using an infinite sink dynamic release of ketotifen fumarate from imprinted CLs showed a concentration dependent (Fickian) behavior, whereas testing the same CL under physiological volumetric flow rates and tear volumes using a PDMS microchip, a slower release rate, with an ideal, concentration independent (zero-order) release profile was recorded [35].
4.5. Contact Lenses as Promising MIP-Based DDS for Ocular Treatment
A quick survey of the publications (Table 1) shows that to date, CLs are the preferred formulations under study for the imprinted hydrogels as DDSs. Even though the currently marketed vision corrective CLs are widely used clinically today (only in US there are 45 million lens wearers [67]) and the first patent of a drug-releasing contact lens has been around for over 50 years, only a few therapeutic contact lenses are commercially available today. However, with the advent of silicone-based hydrogels which allow an extended wear, the research in this field intensified in recent years. Drug-soaked and entrapped contact lenses have proved that they are not viable options in developing CLs for therapeutic purposes. MI technology turned out to be the avant-garde approach in drug delivery applications and especially for ocular therapy.
The critical features of such imprinted polymer backbones used for CL manufacture are biocompatibility and non-immunogenicity. Due to their topical route of administration, biodegradability it is not a decisive feature as after payload release, they are removed from the surface of the eye. Process control during optimization and manufacturing of CLs should involve the following In vitro tests: optical transparency, water content, wettability, tribology, refractive index, and oxygen and ion permeability. Furthermore, In vivo studies should validate the aforementioned characteristics and should demonstrate (pre)clinical efficiency.
The imprinted hydrogel-based CLs rely mainly on the same acrylic functional (e.g., MAA, MMA, acrylamide, acrylic acid, HEMA) and crosslinking monomers (e.g., EGDMA, TEGDMA) as the ones employed in analytical applications. Therefore, the transfer of this technology in CLs development does not require multiple adjustments, besides lowering the degree of crosslinking.
The other material of choice in mass-fabricating vision corrective CLs is silicone, however only a few studies attempted to employ it in developing silicone-based molecularly imprinted hydrogels [33,37,40,49,58]. The main issues of using silicone in CLs are its hydrophobicity and rigidity causing poor wettability and abrasiveness [68]. Creating co-polymers with hydrophilic monomers (e.g., HEMA) up to now have not lead to significant increase of the bulk water content (~15%) [37], therefore, surface modifications with such hydrophilic comonomers remains the efficient way in increasing water permeability required by the on-eye movement of CL [69]. Nevertheless, it possesses one important advantage, namely the high permeability to oxygen which enables such CLs to be worn for a long period of time. However, increasing the CLs’ water content, their oxygen permeability substantially decreases.
Commercially available, silicone hydrogel-based contact lens materials (e.g., Lotrafilcon B-containing a mixture of the proprietary silicone hydrogel macromer, TRIS, and dimethyl acrylamide), might not always offer high enough imprinting efficiencies. In this respect, the admix of additional functional monomers (i.e. AA) and crosslinkers (PEG200DMA and EGDMA) lead to considerable enhancement of HPMC loading capacity and release rates [58]. Obviously, the performance of other mixtures of contact lens starting material, containing acrylate or vinyl-pyridine functional monomers were [49] and may be further investigated, as long as the optical properties of the resulting CLs are preserved within acceptable limits.
In the design of therapeutical CLs combined drug loading strategies may also be applied, where the simultaneous and sustained delivery of two or more APIs may be achieved by MI and layer-by-layer (LbL) coating. As such, the inner core of the CL may be imprinted by a drug of interest (e.g., antibiotic, anti-inflammatory agent, etc.), whereas by LbL deposition of various polyelectrolytes (e.g., alginate, poly-L-lysine, sodium hyaluronate), besides the fine-tuning of the drug-release through drug-polyelectrolye interactions, additionally biocompatibility, non-antigenicity, anti-fouling and antibacterial properties may be conferred to the CLs surface [40,70].
To conclude, probably the most promising way of formulating drug-imprinted hydrogels as drug reservoirs is through therapeutic CLs, as it combines the features of a robust drug loading platform, with multiple means of controlling drug release profiles of one or several APIs, while ensuring a patient friendly, non-invasive means of treatment for extended periods of time.
5. Challenges and Perspectives of MIPs as Ocular DDS
Even though the ocular bioavailability of drugs applied on the corneal surface through classical ophthalmic preparations (ocular drops and ointments) is very poor (~5%), the use and commercialization of more advanced ocular DDS are very limited, and they seem to have come to a standstill. Therapeutical contact lenses can be a viable alternative as they can enhance ocular bioavailability of drugs, while reducing the side effects and increasing patient compliance. However, the mere soaking and entrapment of APIs into the polymeric matrix of contact lenses do not provide targeted delivery and sufficient control over the release. Despite its early developing stage in the field of drug delivery, molecular imprinting represents the most promising technology to be employed in the development of efficient ocular drug delivery devices. Imprinted contact lenses proved that they have the ability to improve the drug delivery profile, to prolong the release and residence time of the drug and even to favor the drug loading capacity. According to the reviewed studies, good imprinting efficiency can be achieved without sacrificing the critical properties a standard, vision corrective contact lens should possess, such as optical transparency, good mechanical properties, ion and gas permeability, wettability, or refractive index. Nevertheless, occasionally the lack of careful planning of the experimental goals in line with the particularities of ocular therapy, misleading data interpretation may throw off course the development/optimization process (i.e. drug-release kinetics may significantly differ for the same formulation in function of the selected experimental models; immersing a contact lens in the artificial tear solution under sink conditions do not provide realistic results and tend to overrate the release period and behavior).
In spite of the promising results of numerous In vitro studies published in recent years, consistent In vivo data is still missing. Apart from the known limitations brough on by the MI process (non-rational selection of the polymerization mixture’s components, at times, less than ideal optical transparency, gas permeability, etc.), several other possible reasons may be invoked for the scarcity of In vivo reports. In no particular order of importance, one may be the lack of interdisciplinary approach, as the most active research groups dealing with molecular imprinting are still in the field of materials science, with limited access to animal research facilities. Another important reason might be the lack of appropriate animal models (rats, mice) that relevantly translate the observed pharmacokinetic/pharmacodynamic results to human applications. The use of animal models sharing a higher degree of similarity with the human eye (e.g., rabbits, swine, cats, dogs, horses or primates) imply significantly higher costs or more expensive research infrastructure, but most importantly runs into serious ethical issues concerning their inclusion in such studies.
Future studies should focus on expanding the body of knowledge related to the performance of such molecularly imprinted DDSs in order to ensure the fields maturity towards clinical validation which will pave the way for marketing authorization and ultimately improve the patient’s quality of life.
Author Contributions
Conceptualization, A.E.B., B.-C.I., E.D., O.V., O.S. and E.B.; writing—original draft preparation, A.E.B., B.-C.I., E.D., O.V. and O.S.; writing—review and editing, A.E.B., B.-C.I., E.D., O.V., O.S. and E.B.; visualization A.E.B. and B.-C.I.; supervision, E.B.; funding acquisition, E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Romanian Ministry of Education and Research, CCCDI-UEFISCDI, project number PN-III-P2-2.1-PED-2019-1288, within PNCDI III.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Resnikoff, S.; Kocur, I. Non-communicable eye diseases: Facing the future. Community Eye Health J. 2014, 27, 41–43. [Google Scholar]
- WHO World report on vision. World Health Organ. 2019, 214, 1–160.
- Bourne, R.R.A.; Steinmetz, J.D.; Saylan, M.; Mersha, A.M.; Weldemariam, A.H.; Wondmeneh, T.G.; Sreeramareddy, C.T.; Pinheiro, M.; Yaseri, M.; Yu, C.; et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef]
- Zhou, R.; Caspi, R.R. Ocular immune privilege. F1000 Biol. Rep. 2010, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Mantelli, F.; Mauris, J.; Argüeso, P. The ocular surface epithelial barrier and other mechanisms of mucosal protection: From allergy to infectious diseases. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, K.M.; Kananen, K.; Auriola, S.; Kontturi, K.; Urtti, A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Investig. Ophthalmol. Vis. Sci. 1997, 38, 627–634. [Google Scholar] [PubMed]
- Prausnitz, M.R. Permeability of cornea, sciera, and conjunctiva: A literature analysis for drug delivery to the eye. J. Pharm. Sci. 1998, 87, 1479–1488. [Google Scholar] [CrossRef]
- Morrison, P.W.J.; Khutoryanskiy, V.V. Advances in ophthalmic drug delivery. Ther. Deliv. 2014, 5, 1297–1315. [Google Scholar] [CrossRef] [Green Version]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Barar, J.; Javadzadeh, A.R.; Omidi, Y. Ocular novel drug delivery: Impacts of membranes and barriers. Expert Opin. Drug Deliv. 2008, 5, 567–581. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Lobo, J.M.S. In situ gelling systems: A strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov. Today 2014, 19, 400–412. [Google Scholar] [CrossRef]
- Karla, P.K.; Earla, R.; Boddu, S.H.; Johnston, T.P.; Pal, D.; Mitra, A. Molecular expression and functional evidence of a drug efflux pump (BCRP) in human corneal epithelial cells. Curr. Eye Res. 2009, 34, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef]
- Kuno, N.; Fujii, S. Recent Advances in Ocular Drug Delivery Systems. Polymers 2011, 3, 193–221. [Google Scholar] [CrossRef]
- Patel, A. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47. [Google Scholar] [CrossRef] [PubMed]
- Daull, P.; Amrane, M.; Garrigue, J.-S. Novasorb® Cationic Nanoemulsion and Latanoprost: The Ideal Combination for Glaucoma Management? Journal of Eye Diseases and Disorders. J. Eye Dis. Disord 2017, 2, 1. [Google Scholar] [CrossRef]
- Souto, E.B.; Dias-Ferreira, J.; López-Machado, A.; Ettcheto, M.; Cano, A.; Espuny, A.C.; Espina, M.; Garcia, M.L.; Sánchez-López, E. Advanced formulation approaches for ocular drug delivery: State-of-the-art and recent patents. Pharmaceutics 2019, 11, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Hao, L.; Wang, H.; Yang, X.; Zhang, G.; Wang, G.; Zhang, X. Hydrogel contact lens for extended delivery of ophthalmic drugs. Int. J. Polym. Sci. 2011, 2011, 814163. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, X.; Qi, P. Therapeutic contact lenses for ophthalmic drug delivery: Major challenges. J. Biomater. Sci. Polym. Ed. 2020, 31, 549–560. [Google Scholar] [CrossRef]
- Peral, A.; Martinez-Aguila, A.; Pastrana, C.; Huete-Toral, F.; Carpena-Torres, C.; Carracedo, G. Contact Lenses as Drug Delivery System for Glaucoma: A Review. Appl. Sci. 2020, 10, 5151. [Google Scholar] [CrossRef]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Amo, E.M.; Rimpelä, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.R.; Venkatraman, S.S. Drug delivery to the eye: What benefits do nanocarriers offer? Nanomedicine 2017, 12, 683–702. [Google Scholar] [CrossRef] [Green Version]
- Bisht, R.; Mandal, A.; Jaiswal, J.K.; Rupenthal, I.D. Nanocarrier mediated retinal drug delivery: Overcoming ocular barriers to treat posterior eye diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, 1473. [Google Scholar] [CrossRef]
- Saw, S.M.; Gazzard, G.; Friedman, D.S. Interventions for angle-closure glaucoma: An evidence-based update. Ophthalmology 2003, 110, 1869–1879. [Google Scholar] [CrossRef]
- Bodoki, A.E.; Iacob, B.-C.; Bodoki, E. Perspectives of molecularly imprinted polymer-based drug delivery systems in cancer therapy. Polymers 2019, 11, 2085. [Google Scholar] [CrossRef] [Green Version]
- Bodoki, A.E.; Iacob, B.-C.; Gliga, L.E.; Oprean, S.L.; Spivak, D.A.; Gariano, N.A.; Bodoki, E. Improved Enantioselectivity for Atenolol Employing Pivot Based Molecular Imprinting. Molecules 2018, 23, 1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Lorenzo, C.; Hiratani, H.; Gómez-Amoza, J.L.; Martínez-Pacheco, R.; Souto, C.; Concheiro, A. Soft contact lenses capable of sustained delivery of timolol. J. Pharm. Sci. 2002, 91, 2182–2192. [Google Scholar] [CrossRef] [PubMed]
- Hiratani, H.; Alvarez-Lorenzo, C. Timolol uptake and release by imprinted soft contact lenses made of N,N-diethylacrylamide and methacrylic acid. J. Control. Release 2002, 83, 223–230. [Google Scholar] [CrossRef]
- Hiratani, H.; Fujiwara, A.; Tamiya, Y.; Mizutani, Y.; Alvarez-Lorenzo, C. Ocular release of timolol from molecularly imprinted soft contact lenses. Biomaterials 2005, 26, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Guidi, G.; Korogiannaki, M.; Sheardown, H. Modification of Timolol Release From Silicone Hydrogel Model Contact Lens Materials Using Hyaluronic Acid. Eye Contact Lens Sci. Clin. Pract. 2014, 40, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.; Sizemore, S.P.; Byrne, M.E. Biomimetic hydrogels for enhanced loading and extended release of ocular therapeutics. Biomaterials 2007, 28, 717–724. [Google Scholar] [CrossRef]
- Ali, M.; Horikawa, S.; Venkatesh, S.; Saha, J.; Hong, J.W.; Byrne, M.E. Zero-order therapeutic release from imprinted hydrogel contact lenses within in vitro physiological ocular tear flow. J. Control. Release 2007, 124, 154–162. [Google Scholar] [CrossRef]
- Tieppo, A.; White, C.J.; Paine, A.C.; Voyles, M.L.; McBride, M.K.; Byrne, M.E. Sustained in vivo release from imprinted therapeutic contact lenses. J. Control. Release 2012, 157, 391–397. [Google Scholar] [CrossRef]
- Hui, A.; Sheardown, H.; Jones, L. Acetic and Acrylic Acid Molecular Imprinted Model Silicone Hydrogel Materials for Ciprofloxacin-hcl Delivery. Materials 2012, 5, 85–107. [Google Scholar] [CrossRef] [Green Version]
- Hui, A.; Willcox, M.; Jones, L. In vitro and in vivo evaluation of novel ciprofloxacin-releasing silicone hydrogel contact lenses. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4896–4904. [Google Scholar] [CrossRef]
- Kioomars, S.; Heidari, S.; Malaekeh-Nikouei, B.; Shayani Rad, M.; Khameneh, B.; Mohajeri, S.A. Ciprofloxacin-imprinted hydrogels for drug sustained release in aqueous media. Pharm. Dev. Technol. 2017, 22, 122–129. [Google Scholar] [CrossRef]
- Silva, D.; de Sousa, H.C.; Gil, M.H.; Santos, L.F.; Oom, M.S.; Alvarez-Lorenzo, C.; Saramago, B.; Serro, A.P. Moxifloxacin-imprinted silicone-based hydrogels as contact lens materials for extended drug release. Eur. J. Pharm. Sci. 2021, 156, 105591. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; de Sousa, H.C.; Gil, M.H.; Santos, L.F.; Amaral, R.A.; Saraiva, J.A.; Salema-Oom, M.; Alvarez-Lorenzo, C.; Serro, A.P.; Saramago, B. Imprinted hydrogels with LbL coating for dual drug release from soft contact lenses materials. Mater. Sci. Eng. C 2021, 120, 111687. [Google Scholar] [CrossRef] [PubMed]
- Topete, A.; Barahona, I.; Santos, L.F.; Pinto, C.A.; Saraiva, J.A.; Paula Serro, A.; Saramago, B. The effects of addition of functional monomers and molecular imprinting on dual drug release from intraocular lens material. Int. J. Pharm. 2021, 600, 120513. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, B.; Dalgakiran, D.; Inan, T.; Kurkcuoglu, O.; Güner, F.S. A computational and experimental approach to develop minocycline-imprinted hydrogels and determination of their drug delivery performances. J. Polym. Res. 2018, 25, 1–10. [Google Scholar] [CrossRef]
- Malakooti, N.; Alexander, C.; Alvarez-Lorenzo, C. Imprinted Contact Lenses for Sustained Release of Polymyxin B and Related Antimicrobial Peptides. J. Pharm. Sci. 2015, 104, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Abouelatta, S.M.; Sheta, A.I.; Ibrahim, R.R. Optimized molecular imprints in gamma-irradiated collagen shields of an antifungal drug: In vitro characterization, in-vivo bioavailability enhancement. Eur. J. Pharm. Biopharm. 2021, 166, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Varela-Garcia, A.; Gomez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C. Imprinted contact lenses for ocular administration of antiviral drugs. Polymers 2020, 12, 2026. [Google Scholar] [CrossRef] [PubMed]
- Tieppo, A.; Pate, K.M.; Byrne, M.E. In vitro controlled release of an anti-inflammatory from daily disposable therapeutic contact lenses under physiological ocular tear flow. Eur. J. Pharm. Biopharm. 2012, 81, 170–177. [Google Scholar] [CrossRef]
- Malaekeh-Nikouei, B.; Ghaeni, F.A.; Motamedshariaty, V.S.; Mohajeri, S.A. Controlled release of prednisolone acetate from molecularly imprinted hydrogel contact lenses. J. Appl. Polym. Sci. 2012, 126, 387–394. [Google Scholar] [CrossRef]
- White, C.J.; Di Pasquale, S.A.; Byrne, M.E. Controlled release of multiple therapeutics from silicone hydrogel contact lenses. Optom. Vis. Sci. 2016, 93, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Raesian, P.; Rad, M.S.; Khodaverdi, E.; Motamedshariaty, V.S.; Mohajeri, S.A. Preparation and characterization of fluorometholone molecular imprinted soft contact lenses as ocular controlled drug delivery systems. J. Drug Deliv. Sci. Technol. 2021, 64, 102591. [Google Scholar] [CrossRef]
- Yan, F.; Liu, Y.; Han, S.; Zhao, Q.; Liu, N. Bimatoprost Imprinted Silicone Contact Lens to Treat Glaucoma. AAPS PharmSciTech 2020, 21, 63. [Google Scholar] [CrossRef]
- Omranipour, H.; Sajadi Tabassi, S.; Kowsari, R.; Rad, M.; Mohajeri, S. Brimonidine Imprinted Hydrogels and Evaluation of Their Binding and Releasing Properties as New Ocular Drug Delivery Systems. Curr. Drug Deliv. 2015, 12, 717–725. [Google Scholar] [CrossRef]
- Ribeiro, A.; Veiga, F.; Santos, D.; Torres-Labandeira, J.J.; Concheiro, A.; Alvarez-Lorenzo, C. Bioinspired Imprinted PHEMA-Hydrogels for ocular delivery of carbonic anhydrase inhibitor drugs. Biomacromolecules 2011, 12, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Veiga, F.; Santos, D.; Torres-Labandeira, J.J.; Concheiro, A.; Alvarez-Lorenzo, C. Receptor-based biomimetic NVP/DMA contact lenses for loading/eluting carbonic anhydrase inhibitors. J. Memb. Sci. 2011, 383, 60–69. [Google Scholar] [CrossRef]
- Nikouei, B.M.-; Vahabzadeh, S.A.; Mohajeri, S.A. Preparation of a Molecularly Imprinted Soft Contact Lens as a New Ocular Drug Delivery System for Dorzolamide. Curr. Drug Deliv. 2013, 10, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Da-mota, A.F.; Vivero-Lopez, M.; Topete, A.; Serro, A.P.; Concheiro, A.; Alvarez-Lorenzo, C. Atorvastatin-eluting contact lenses: Effects of molecular imprinting and sterilization on drug loading and release. Pharmaceutics 2021, 13, 606. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Byrne, M.E. Controlled Release of High Molecular Weight Hyaluronic Acid from Molecularly Imprinted Hydrogel Contact Lenses. Pharm. Res. 2009, 26, 714–726. [Google Scholar] [CrossRef] [PubMed]
- White, C.J.; McBride, M.K.; Pate, K.M.; Tieppo, A.; Byrne, M.E. Extended release of high molecular weight hydroxypropyl methylcellulose from molecularly imprinted, extended wear silicone hydrogel contact lenses. Biomaterials 2011, 32, 5698–5705. [Google Scholar] [CrossRef] [PubMed]
- Yañez, F.; Chauhan, A.; Concheiro, A.; Alvarez-Lorenzo, C. Timolol-imprinted soft contact lenses: Influence of the template: Functional monomer ratio and the hydrogel thickness. J. Appl. Polym. Sci. 2011, 122, 1333–1340. [Google Scholar] [CrossRef]
- Sibrian-Vazquez, M.; Spivak, D.A. Improving the Strategy and Performance of Molecularly Imprinted Polymers Using Cross-Linking Functional Monomers. J. Org. Chem. 2003, 68, 9604–9611. [Google Scholar] [CrossRef]
- Tashakori-Sabzevar, F.; Mohajeri, S.A. Development of ocular drug delivery systems using molecularly imprinted soft contact lenses. Drug Dev. Ind. Pharm. 2015, 41, 703–713. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Hiratani, H.; Concheiro, A. Contact lenses for drug delivery: Achieving sustained release with novel systems. Am. J. Drug Deliv. 2006, 4, 131–151. [Google Scholar] [CrossRef]
- Byrne, M.E.; Salian, V. Molecular imprinting within hydrogels II: Progress and analysis of the field. Int. J. Pharm. 2008, 364, 188–212. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, M.; Weeks, A.; Jones, L.; Sheardown, H. Immobilized hyaluronic acid containing model silicone hydrogels reduce protein adsorption. J. Biomater. Sci. Polym. Ed. 2008, 19, 1425–1436. [Google Scholar] [CrossRef]
- Hiratani, H.; Alvarez-Lorenzo, C. The nature of backbone monomers determines the performance of imprinted soft contact lenses as timolol drug delivery systems. Biomaterials 2004, 25, 1105–1113. [Google Scholar] [CrossRef]
- Momose, T.; Ito, N.; Kanai, A.; Watanabe, Y.; Shibata, M. Adsorption of levocabastine eye drops by soft contact lenses and its effects in rabbit eyes. CLAO J. 1997, 23, 96–99. [Google Scholar]
- Cope, J.R.; Collier, S.A.; Rao, M.M.; Chalmers, R.; Mitchell, G.L.; Richdale, K.; Wagner, H.; Kinoshita, B.T.; Lam, D.Y.; Sorbara, L.; et al. Contact Lens Wearer Demographics and Risk Behaviors for Contact Lens-Related Eye Infections–United States, 2014. MMWR. Morb. Mortal. Wkly. Rep. 2015, 64, 865–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [Green Version]
- Donshik, P.C. Extended wear contact lenses. Ophthalmol. Clin. 2003, 16, 305–309. [Google Scholar] [CrossRef]
- Silva, D.; Sousa, H.C.d.; Gil, M.H.; Santos, L.F.; Moutinho, G.M.; Serro, A.P.; Saramago, B. Antibacterial layer-by-layer coatings to control drug release from soft contact lenses material. Int. J. Pharm. 2018, 553, 186–200. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).