Expanding the Applicability of an Innovative Laccase TTI in Intelligent Packaging by Adding an Enzyme Inhibitor to Change Its Coloration Kinetics
Abstract
:1. Introduction
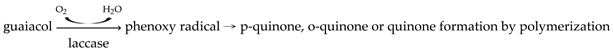
2. Materials and Methods
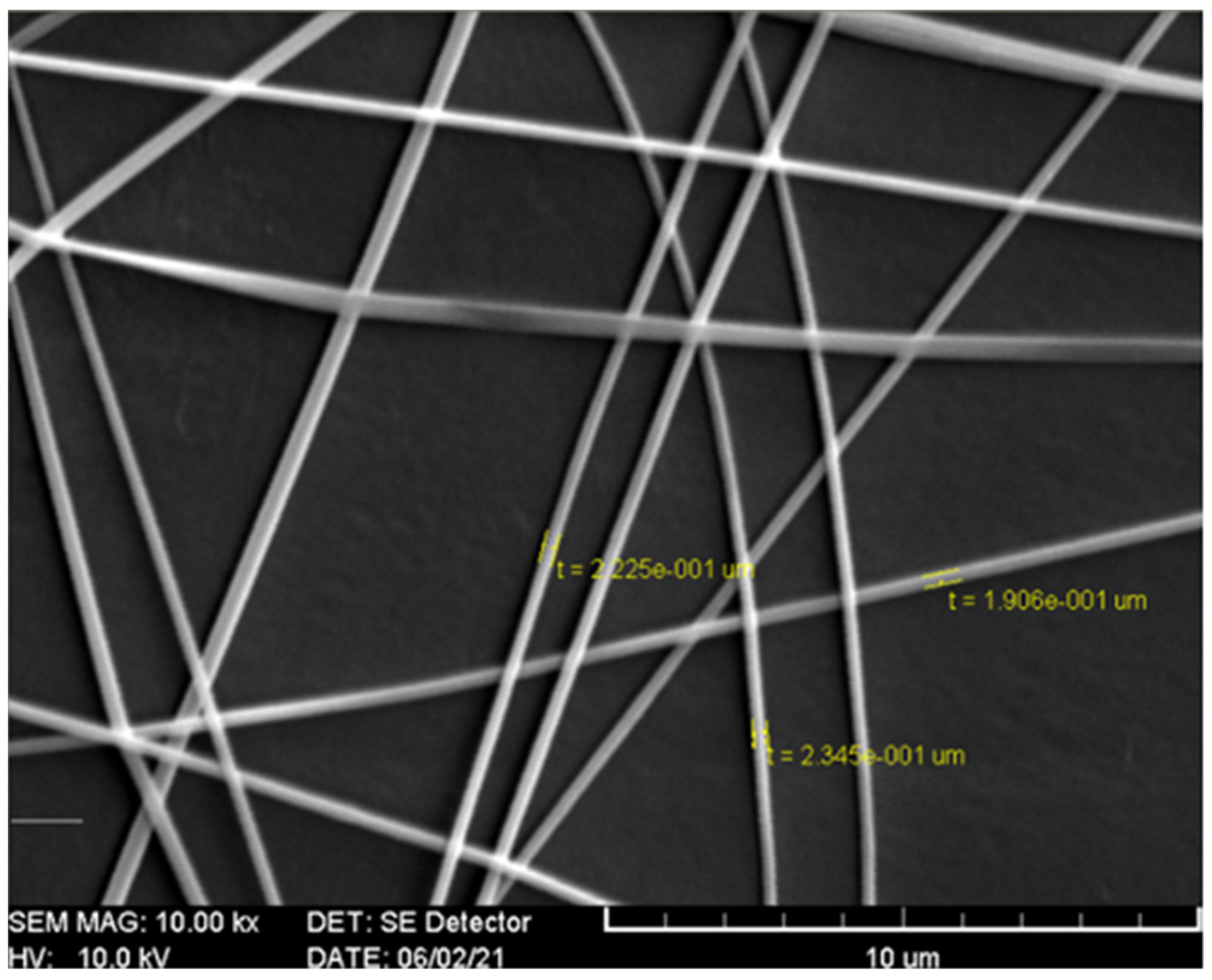
2.1. Immobilizing Laccase on Electrospun Fibers
2.2. Guaiacol Solution Preparation
2.3. Coloration of Laccase TTI Prototype
2.3.1. Color Measurement
2.3.2. Absorbance of Coloration
2.4. Kinetic Analysis of Laccase TTI Prototype Coloration
2.4.1. Enzyme Kinetics
2.4.2. Coloration Rate
2.4.3. Activation Energies
2.4.4. Dynamic Temperature Response Test
2.5. Statistical Analysis
3. Results and Discussion
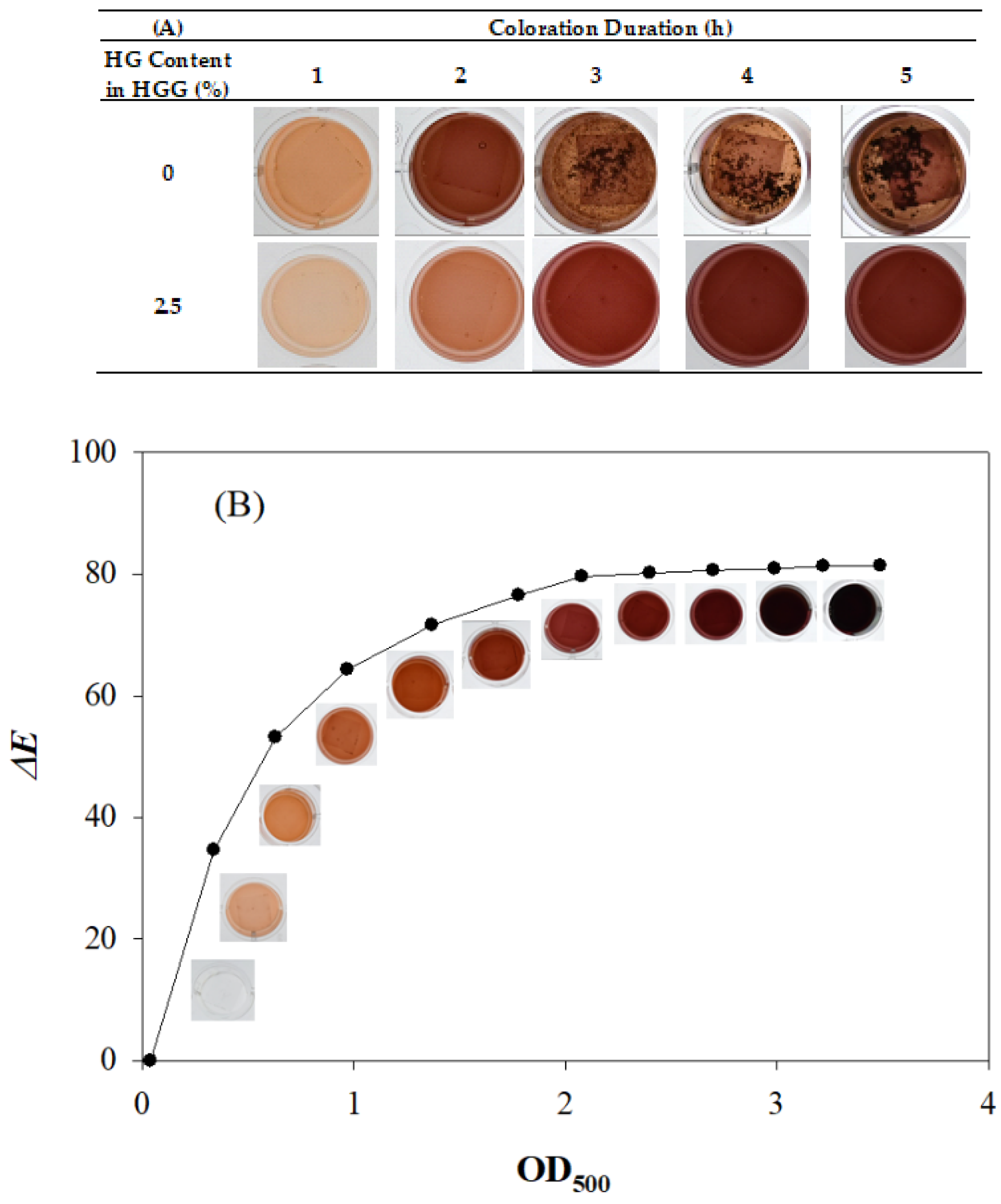
3.1. Coloration of Laccase TTI Prototype
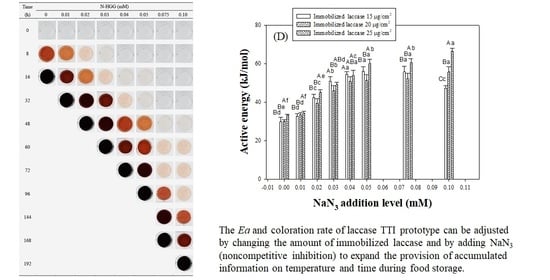
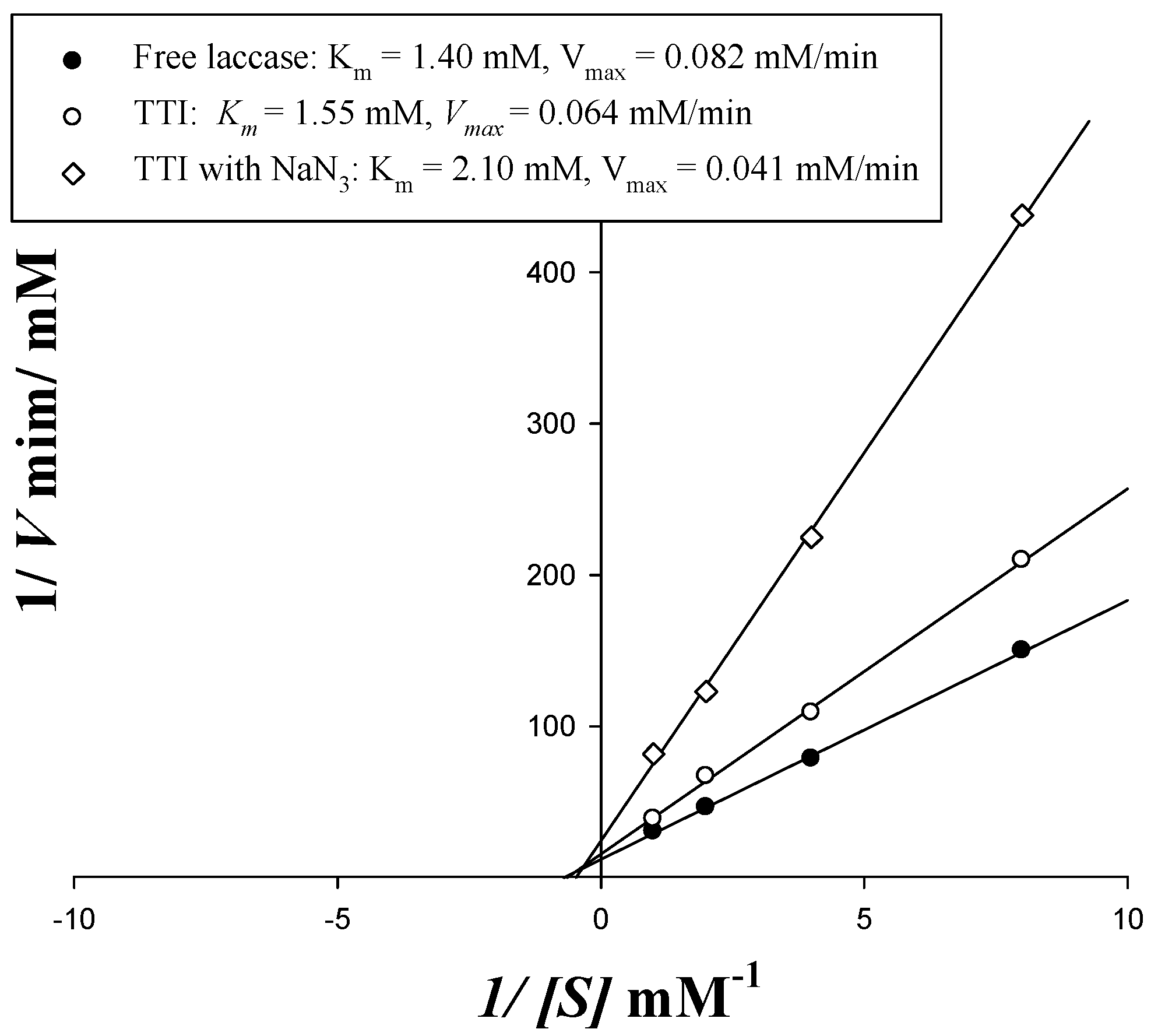
3.2. Coloration of Laccase TTI Prototype with NaN3
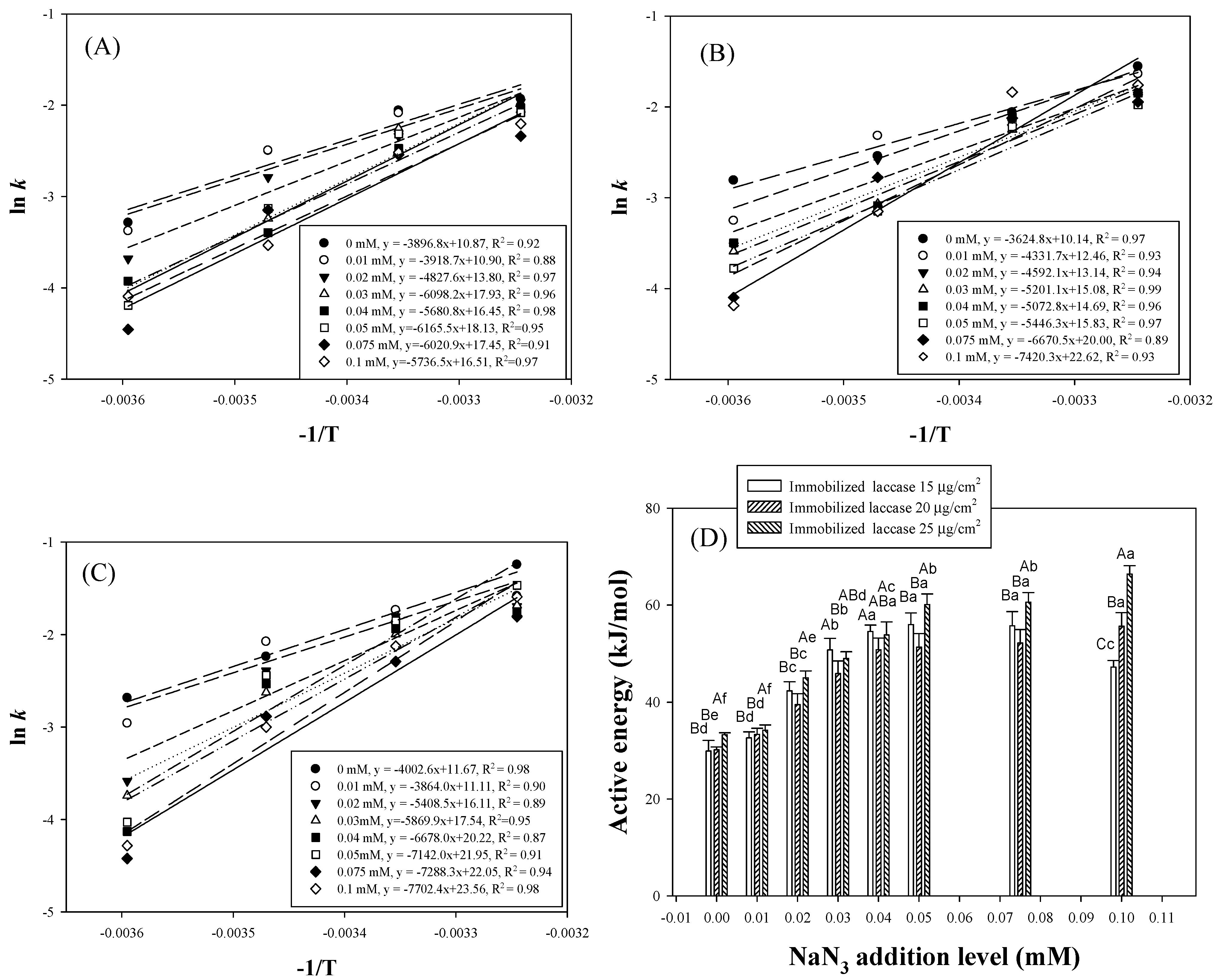
3.3. Coloration Kinetics of Laccase TTI Prototype with Added NaN3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, Y.; Liu, J.; Jiang, L.; Zhu, Z.; He, S.; He, S.; Shao, W. Development of intelligent/active food packaging film based on TEMPO-oxidized bacterial cellulose containing thymol and anthocyanin-rich purple potato extract for shelf life extension of shrimp. Food Packag. Shelf Life 2021, 29, 100709. [Google Scholar] [CrossRef]
- Singh, R.P.; Wells, J.H. Use of time-temperature indicators to monitor quality of frozen hamburger. Food Technol. 1985, 39, 42–50. [Google Scholar]
- Kim, J.U.; Ghafoor, K.; Ahn, J.; Shin, S.; Lee, S.H.; Shahbaz, H.M.; Park, J. Kinetic modeling and characterization of a diffusion-based time-temperature indicator (TTI) for monitoring microbial quality of non-pasteurized angelica juice. LWT 2016, 67, 143–150. [Google Scholar] [CrossRef]
- Tsironi, T.; Ronnow, P.; Giannoglou, M.; Taoukis, P. Developing suitable smart TTI labels to match specific monitoring requirements: The case of Vibrio spp. growth during transportation of oysters. Food Control 2017, 73, 51–56. [Google Scholar] [CrossRef]
- Kim, K.; Kim, E.; Lee, S.J. New enzymatic time-temperature integrator (TTI) that uses laccase. J. Food Eng. 2012, 113, 118–123. [Google Scholar] [CrossRef]
- Park, H.R.; Kim, K.; Lee, S.J. Adjustment of Arrhenius activation energy of laccase-based time-temperature integrator (TTI) using sodium azide. Food Control 2013, 32, 615–620. [Google Scholar] [CrossRef]
- Dwivedi, U.N.; Singh, P.; Pandey, V.P.; Kumar, A. Structure-function relationship among bacterial, fungal and plant laccases. J. Mol. Catal. B Enzym. 2011, 68, 117–128. [Google Scholar] [CrossRef]
- Tonin, F.; Melis, R.; Cordes, A.; Sanchez-Amat, A.; Pollegioni, L.; Rosini, E. Comparison of different microbial laccases as tools for industrial uses. N. Biotechnol. 2016, 33, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Witayakran, S.; Ragauskas, A.J. Synthetic applications of laccase in green chemistry. Adv. Synth. Catal. 2009, 351, 1187–1209. [Google Scholar] [CrossRef]
- Cannatelli, M.D. Exploiting the Oxidizing Capabilities of Laccases Exploiting the Oxidizing Capabilities of Laccases for Sustainable Chemistry. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 28 March 2017. [Google Scholar]
- Wang, S.; Liu, X.; Yang, M.; Zhang, Y.; Xiang, K.; Tang, R. Review of time temperature indicators as quality monitors in food packaging. Packag. Technol. Sci. 2015, 28, 839–867. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Chen, S.H.; Chen, L.C.; Lin, S.B.; Lou, S.N.; Chen, Y.H.; Chen, H.H. Enzymatic time-temperature indicator prototype developed by immobilizing laccase on electrospun fibers to predict lactic acid bacterial growth in milk during storage. Nanomaterials 2021, 11, 1160. [Google Scholar] [CrossRef] [PubMed]
- El-Ghazali, S.; Khatri, M.; Mehdi, M.; Kharaghani, D.; Tamada, Y.; Katagiri, A.; Kobayashi, S.; Kim, I.S. Fabrication of poly(ethylene-glycol 1,4-cyclohexane dimethylene-isosorbide-terephthalate) electrospun nanofiber mats for potential infiltration of fibroblast cells. Polymers 2021, 13, 1245. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Marelli, B. Growing silk fibroin in advanced materials for food security. MRS Commun. 2021, 11, 31–45. [Google Scholar] [CrossRef]
- Sameen, D.E.; Ahmed, S.; Lu, R.; Li, R.; Dai, J.; Qin, W.; Zhang, Q.; Li, S.; Liu, Y. Electrospun nanofibers food packaging: Trends and applications in food systems. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–14. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wan, L.S.; Liu, Z.M.; Huang, X.J.; Xu, Z.K. Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B Enzym. 2009, 56, 189–195. [Google Scholar] [CrossRef]
- Jhuang, J.R.; Lou, S.N.; Lin, S.B.; Chen, S.H.; Chen, L.C.; Chen, H.H. Immobilizing laccase on electrospun chitosan fiber to prepare time-temperature indicator for food quality monitoring. Innov. Food Sci. Emerg. Technol. 2020, 63, 102370. [Google Scholar] [CrossRef]
- Wang, X.; Li, D. A dynamic product quality evaluation based pricing model for perishable food supply chains. Omega 2012, 40, 906–917. [Google Scholar] [CrossRef]
- Johannes, C.; Majcherczyk, A. Laccase activity tests and laccase inhibitors. J. Biotechnol. 2000, 78, 193–199. [Google Scholar] [CrossRef]
- Molina-Guijarro, J.M.; Pérez, J.; Muñoz-Dorado, J.; Guillén, F.; Moya, R.; Hernández, M.; Arias, M.E. Detoxification of azo dyes by a novel pH-versatile, salt-resistant laccase from Streptomyces ipomoea. Int. Microbiol. 2009, 12, 13–21. [Google Scholar]
- Xu, F. Oxidation of phenols, anilines, and benzenethiols by fungal laccases: Correlation between activity and redox potentials as well as halide inhibition. Biochemistry 1996, 35, 7608–7614. [Google Scholar] [CrossRef]
- Zheng, F.; An, Q.; Meng, G.; Wu, X.J.; Dai, Y.C.; Si, J.; Cui, B.K. A novel laccase from white rot fungus Trametes orientalis: Purification, characterization, and application. Int. J. Biol. Macromol. 2017, 102, 758–770. [Google Scholar] [CrossRef]
- Jancikova, S.; Dordevic, D.; Tesikova, K.; Antonic, B.; Tremlova, B. Active edible films fortified with natural extracts: Case study with fresh-cut apple pieces. Membranes 2021, 11, 684. [Google Scholar] [CrossRef]
- Jayalakshmi, T.; Santhakumaran, A. Statistical normalization and back propagation for classification. Int. J. Comput. Theory Eng. 2011, 3, 1793–8201. [Google Scholar]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour measurement and analysis in fresh and processed foods: A review. Food Ioproc. Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Qiu, X.; Qin, J.; Xu, M.; Kang, L.; Hu, Y. Organic-inorganic nanocomposites fabricated via functional ionic liquid as the bridging agent for Laccase immobilization and its application in 2,4-dichlorophenol removal. Colloids Surf. B Biointerfaces 2019, 179, 260–269. [Google Scholar] [CrossRef]
- Minussi, R.C.; Pastore, G.M.; Durán, N. Potential applications of laccase in the food industry. Trends Food Sci. Technol. 2002, 13, 205–216. [Google Scholar] [CrossRef]
- Kang, Y.J.; Kang, J.W.; Choi, J.H.; Park, S.Y.; Rahman, A.M.; Jung, S.W.; Lee, S.J. A feasibility study of application of laccase-based time-temperature indicator to kimchi quality control on fermentation process. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 819–825. [Google Scholar] [CrossRef]
- Shen, Y.C.; Chen, H.H. Adjusting the thermomelting of temperature-sensitive gelatin film using a high-pressure homogenization emulsion. In Proceedings of the 19th IUFoST, Mumbai, India, 23–27 October 2018; p. 89. [Google Scholar]
- Lee, S.B.; Jung, S.W.; Lee, S.J. Air-activation of printed time–temperature integrator: A sandwich package case study. Food Control 2019, 101, 89–96. [Google Scholar] [CrossRef]
- Burke, R.M.; Cairney, J.W.G. Laccases and other polyphenol oxidases in ecto- and ericoid mycorrhizal fungi. Mycorrhiza 2002, 12, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Bobelyn, E.; Hertog, M.L.; Nicolaï, B.M. Applicability of an enzymatic time temperature integrator as a quality indicator for mushrooms in the distribution chain. Postharvest Biol. Technol. 2006, 42, 104–114. [Google Scholar] [CrossRef]
- Aslani, E.; Abri, A.; Pazhang, M. Immobilization of trypsin onto Fe3O4@ SiO2–NH2 and study of its activity and stability. Colloids Surf. B Biointerfaces 2018, 170, 553–562. [Google Scholar] [CrossRef] [PubMed]
- George, R.; Sugunan, S. Kinetic and thermodynamic parameters of immobilized glucoamylase on different mesoporous silica for starch hydrolysis: A comparative study. J. Mol. Catal. B Enzym. 2014, 106, 81–89. [Google Scholar] [CrossRef]
- Zdarta, J.; Jankowska, K.; Wyszowska, M.; Kijeńska-Gawrońska, E.; Zgoła-Grześkowiak, A.; Pinelo, M.; Meyer, A.S.; Moszyński, D.; Jesionowski, T. Robust biodegradation of naproxen and diclofenac by laccase immobilized using electrospun nanofibers with enhanced stability and reusability. Mater. Sci. Eng. C. 2019, 103, 109789. [Google Scholar] [CrossRef]
- Brizio, A.P.D.R.; Prentice, C. Development of a new time temperature indicator for enzymatic validation of pasteurization of meat products. J. Food Sci. 2015, 80, M1271–M1276. [Google Scholar] [CrossRef]
- Wu, D.; Hou, S.; Chen, J.; Sun, Y.; Ye, X.; Liu, D.; Wang, Y. Development and characterization of an enzymatic time-temperature indicator (TTI) based on Aspergillus niger lipase. LWT 2015, 60, 1100–1104. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, H.W.; Lee, S.J. A novel self-powered time-temperature integrator (TTI) using modified biofuel cell for food quality monitoring. Food Control 2016, 70, 167–173. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-X.; Hsu, H.-H.; Chang, Y.-H.; Chen, S.-H.; Lin, S.-B.; Lou, S.-N.; Chen, H.-H. Expanding the Applicability of an Innovative Laccase TTI in Intelligent Packaging by Adding an Enzyme Inhibitor to Change Its Coloration Kinetics. Polymers 2021, 13, 3646. https://doi.org/10.3390/polym13213646
Lin C-X, Hsu H-H, Chang Y-H, Chen S-H, Lin S-B, Lou S-N, Chen H-H. Expanding the Applicability of an Innovative Laccase TTI in Intelligent Packaging by Adding an Enzyme Inhibitor to Change Its Coloration Kinetics. Polymers. 2021; 13(21):3646. https://doi.org/10.3390/polym13213646
Chicago/Turabian StyleLin, Cheng-Xuan, Hao-Hsin Hsu, Yu-Hsuan Chang, Shih-Hsin Chen, Shih-Bin Lin, Shyi-Neng Lou, and Hui-Huang Chen. 2021. "Expanding the Applicability of an Innovative Laccase TTI in Intelligent Packaging by Adding an Enzyme Inhibitor to Change Its Coloration Kinetics" Polymers 13, no. 21: 3646. https://doi.org/10.3390/polym13213646
APA StyleLin, C.-X., Hsu, H.-H., Chang, Y.-H., Chen, S.-H., Lin, S.-B., Lou, S.-N., & Chen, H.-H. (2021). Expanding the Applicability of an Innovative Laccase TTI in Intelligent Packaging by Adding an Enzyme Inhibitor to Change Its Coloration Kinetics. Polymers, 13(21), 3646. https://doi.org/10.3390/polym13213646