Development of Photocurable Polyacrylate-Based PolyHIPEs and the Study of the Kinetics of Photopolymerization, and of Their Thermal, Mechanical and Hydrocarbon Absorption Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
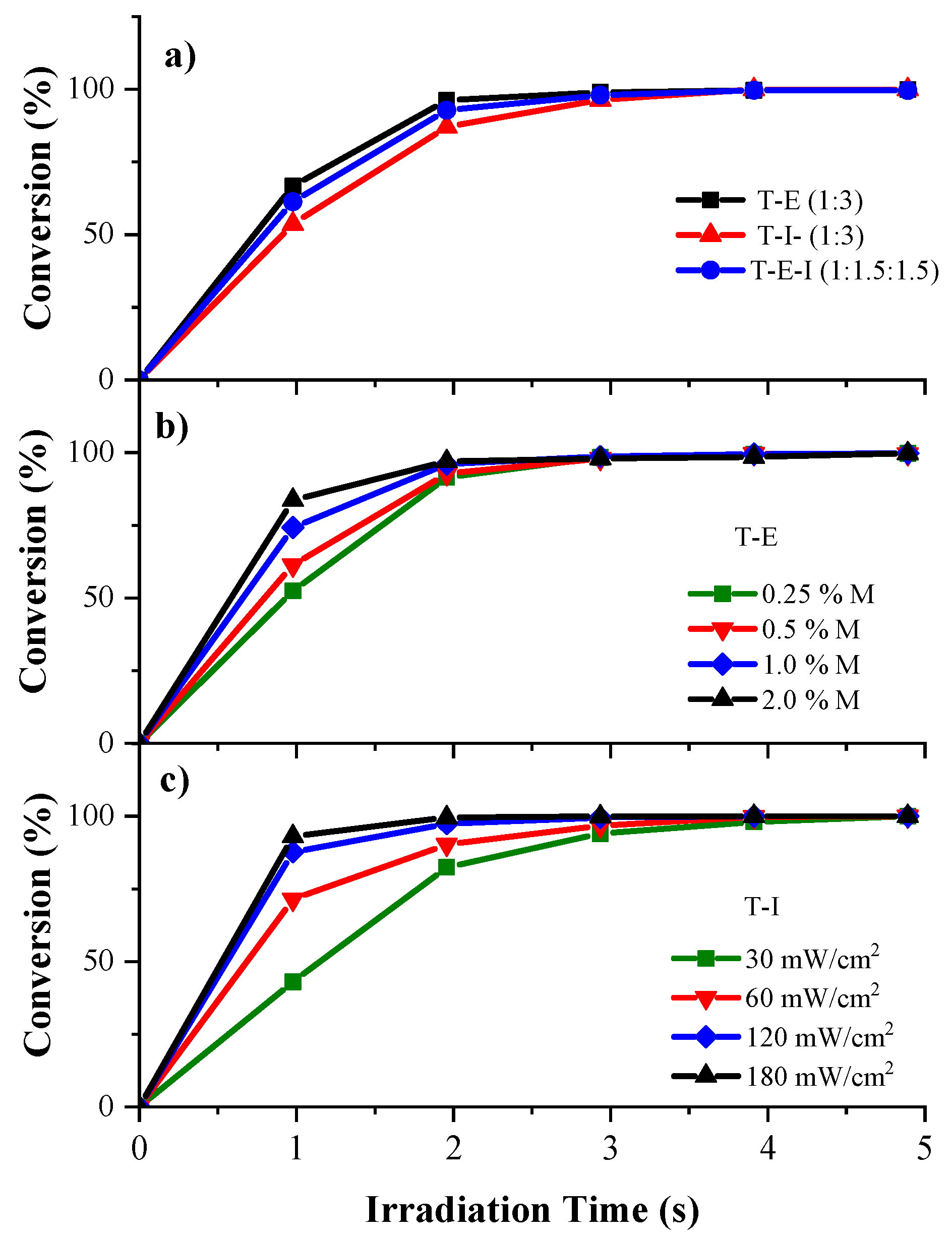
2.2. Determination of Kinetics of Photopolymerization of Pristine Acrylate Monomers by Real-Time Fourier Transforms Infrared Spectroscopy (RT-FTIR)
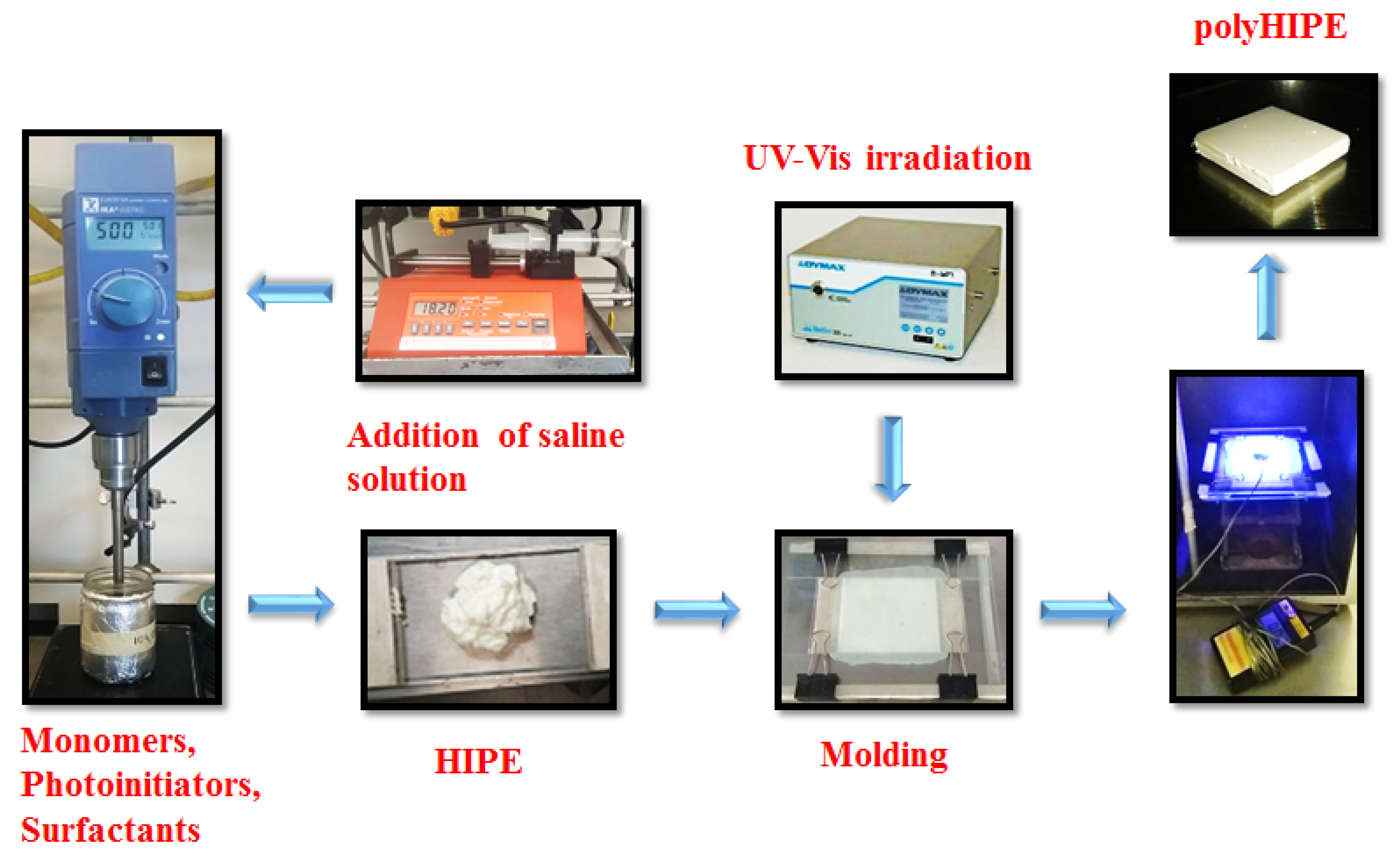
2.3. Preparation of High Internal Phase Emulsions (HIPEs)
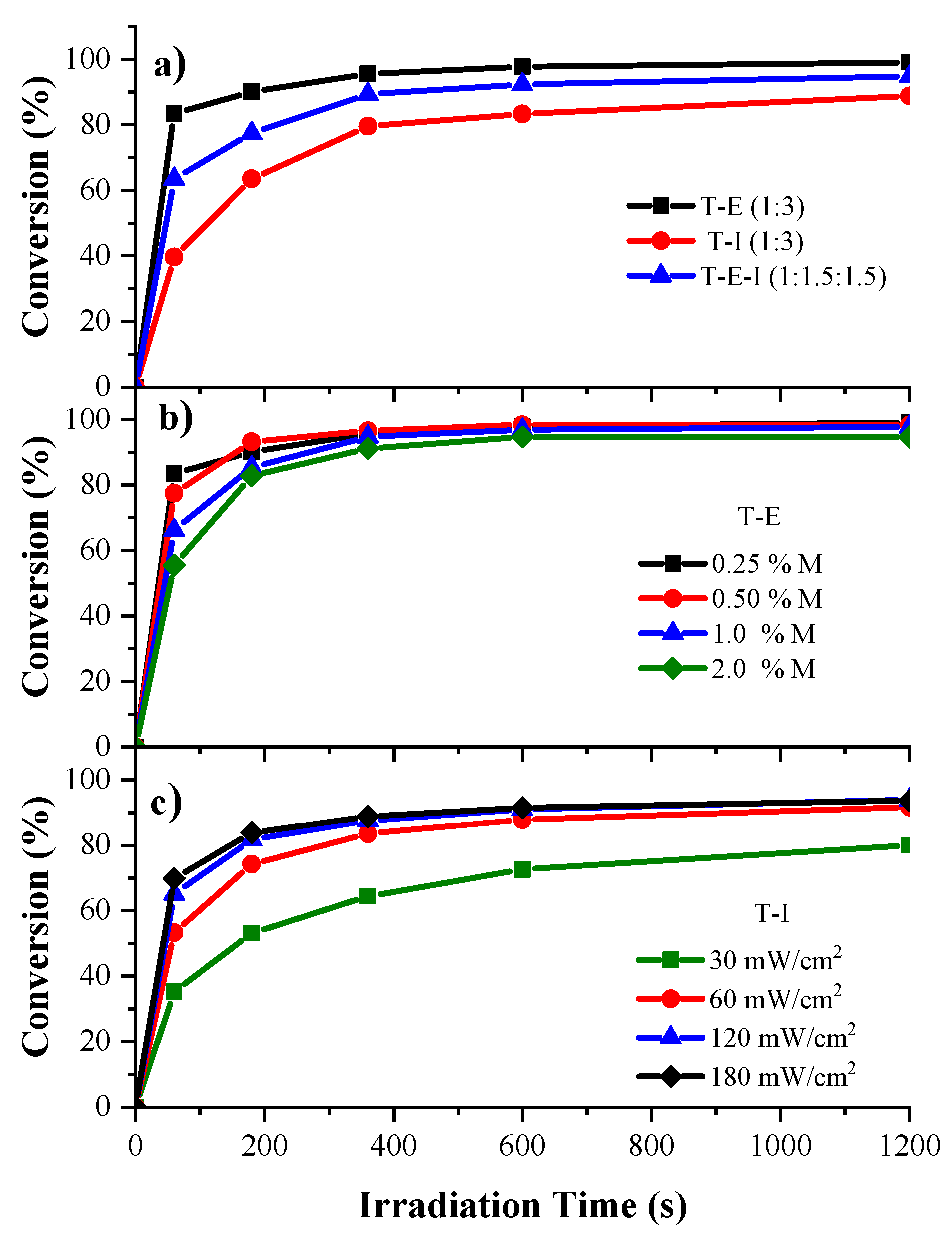
2.4. Determination of Kinetics of Photopolymerization of HIPEs by Gravimetry
2.5. Scanning Electron Microscopy (SEM)
2.6. Analysis of Surface Area and Pore Size Distribution
2.7. Water Contact Angle (WCA)
2.8. Compression Properties
2.9. Thermogravimetric Analysis (TGA)
2.10. Differential Scanning Calorimetry (DSC)
2.11. Determination of Absorption Properties of Hydrocarbons
3. Results and Discussion
3.1. Determination of Kinetics of Photopolymerization of Pristine Monomers
3.2. Preparation of the Photocurable PolyHIPEs
3.3. Kinetics of Photopolymerization of the HIPEs
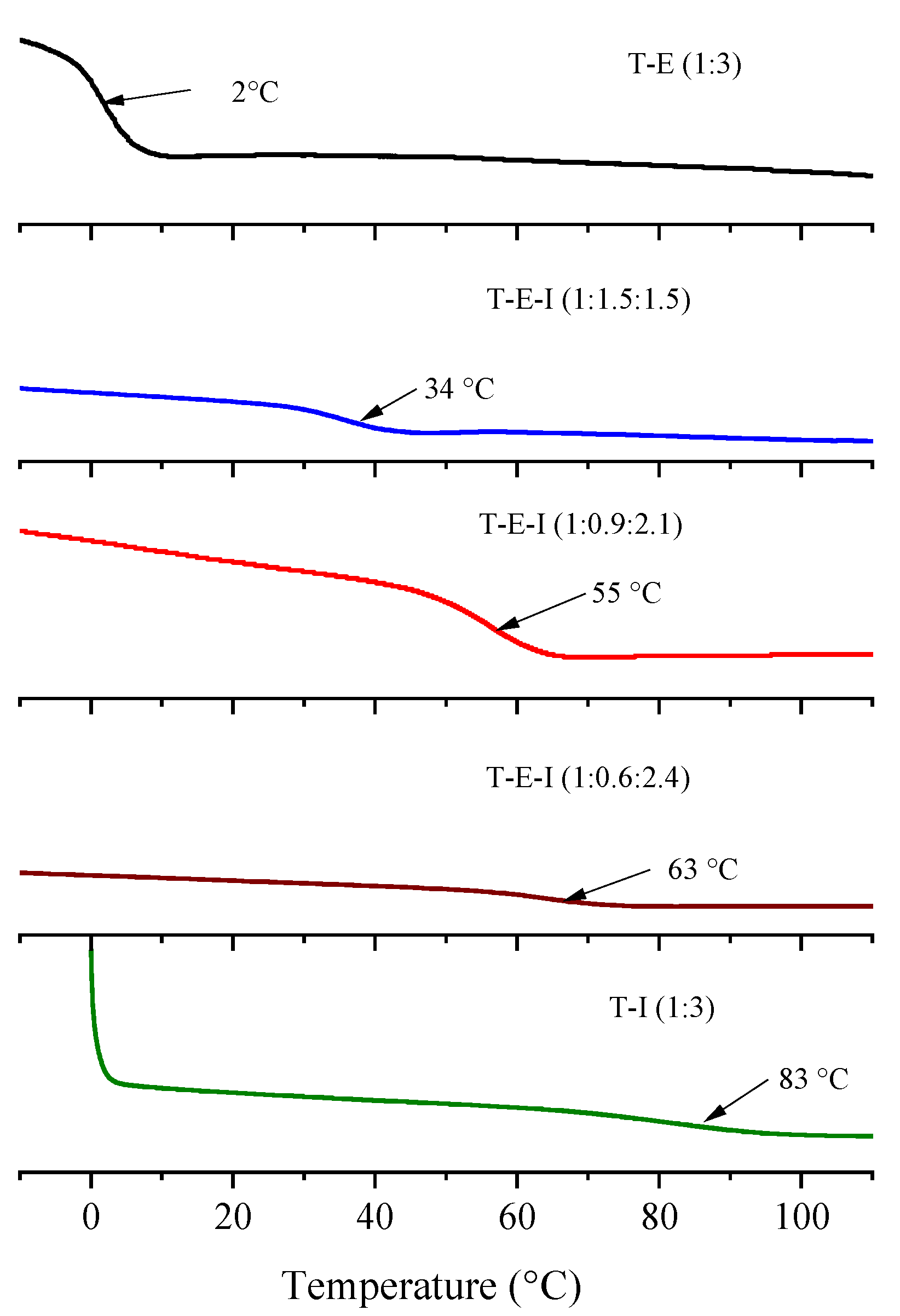
3.4. Thermal Analysis of Monoliths by DSC and TGA
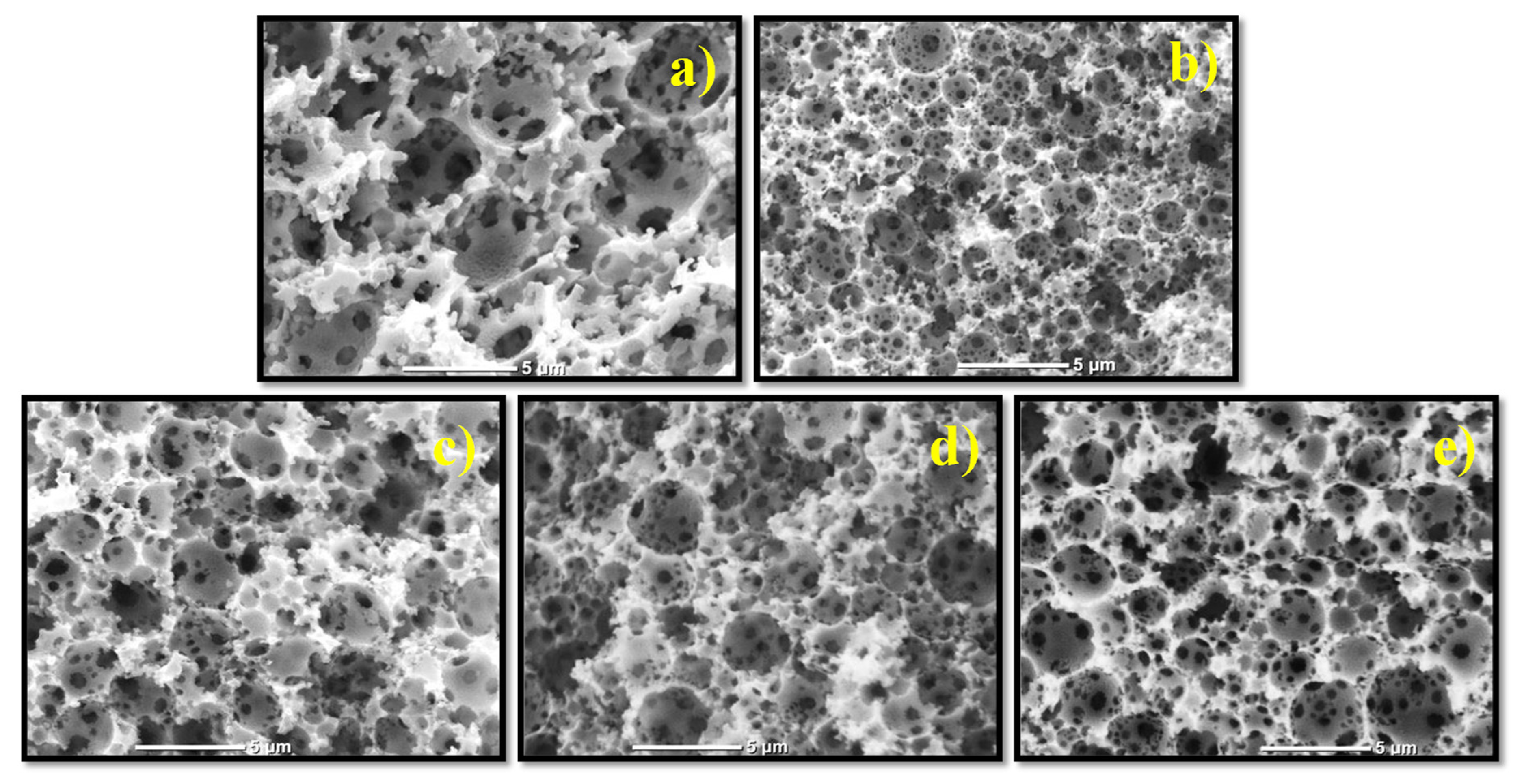
3.5. Morphology of the PolyHIPEs by SEM
3.6. Water Contact Angle (WCA)
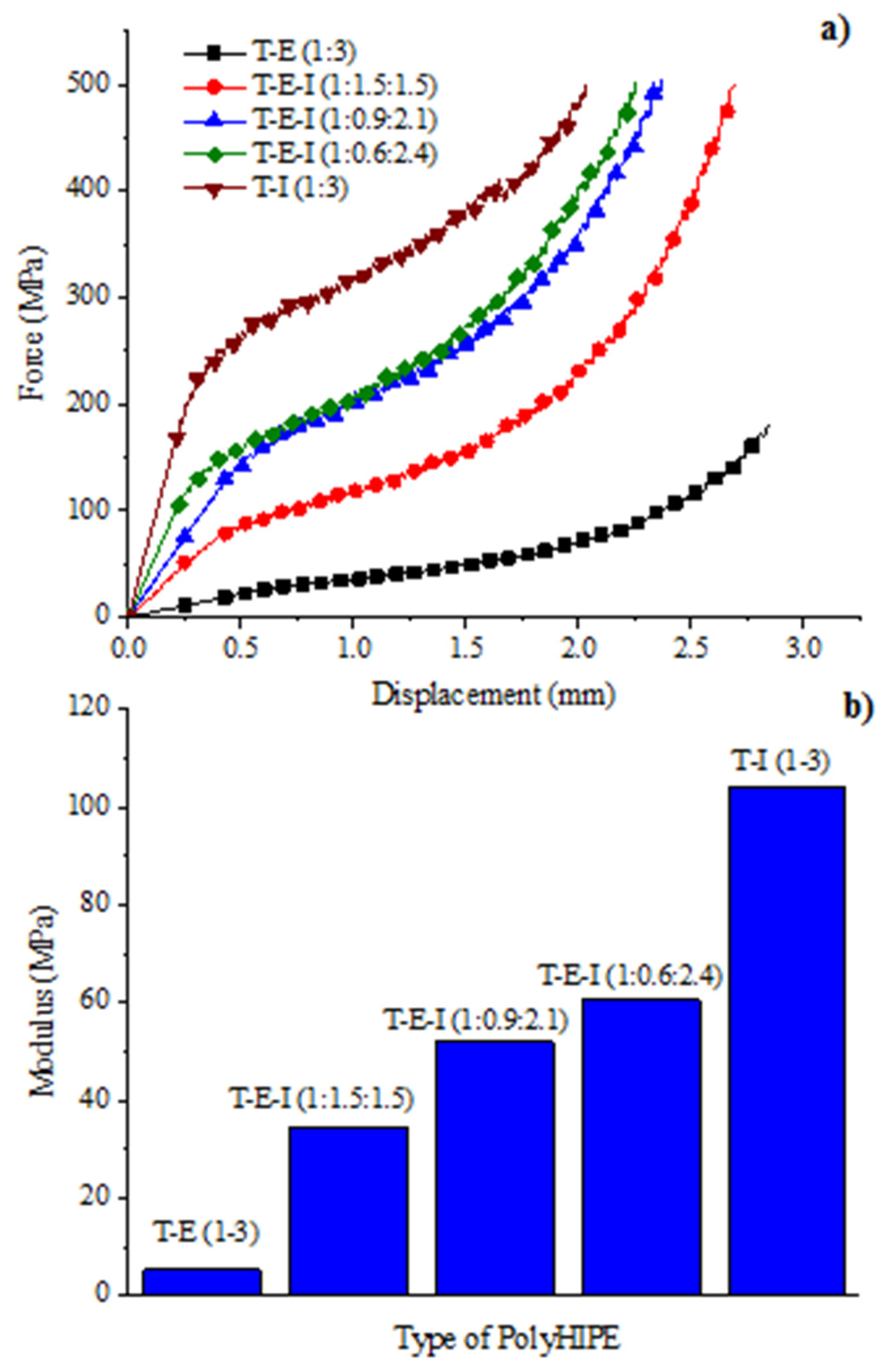
3.7. Mechanical Properties of Compression
3.8. Hydrocarbon Absorption Properties and Study of the Reusability of the PolyHIPEs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiu, S.; Ben, T. Porous Polymers: Design, Synthesis & Applications, 1st ed.; Royal Society: London, UK, 2015; Volume 17. [Google Scholar]
- Kircher, L.; Theato, P.; Cameron, N.R. Functionalyzation of porous polymers from high internal phse emulsions and their applications. In Functional Polymers by Post-Polymerization Modification; Theato, P., Klok, H.A., Eds.; Wiley VCH Verlag GmBH & Co KgaA: Singapore, 2013; pp. 333–352. [Google Scholar]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and Preparation of Porous Polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Ali, A.A.; Hameed, A. Design and synthesis of porous polymeric materials and their applications in gas capture and storage: A review. J. Polym. Res. 2018, 25, 75. [Google Scholar] [CrossRef]
- Wu, J.; Xu, F.; Li, S.; Ma, P.; Zhang, X.; Liu, Q.; Fu, R.; Wu, D. Porous Polymers as Multifunctional Material Platforms toward Task-Specific Applications. Adv. Mater. 2019, 31, 1802922. [Google Scholar] [CrossRef]
- Silverstein, M.S. PolyHIPEs: Recent advances in emulsion-templated porous polymers. Prog. Polym. Sci. 2014, 39, 199–234. [Google Scholar] [CrossRef]
- Zhang, T.; Sanguramath, R.A.; Israel, S.; Silverstein, M.S. Emulsion Templating: Porous Polymers and beyond. Macromolecules 2019, 52, 5445–5479. [Google Scholar]
- Cameron, N.R.; Sherrington, D.C. High Internal Phase Emulsions (HIPEs)—Structure, Properties and Use in Polymer Preparation. Adv. Polym. Sci. 2006, 126, 163–214. [Google Scholar]
- Princen, H.M. Rheology of Foams and Highly Concentrated Emulsions, I. Elastic Properties and Yield Stress of a Cylindrical Model System. J. Colloid Interface Sci. 1983, 91, 160–175. [Google Scholar] [CrossRef]
- Pulko, I.; Krajnc, P. Porous Polymer Monoliths by Emulsion Templating. In Encyclopedia of Polymer Science and Technology; Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 1–28. [Google Scholar] [CrossRef]
- Aronson, M.P.; Petko, M.F. High Internal Phase Emulsions. U.S. Patent 4,606,913, 19 July 1986. [Google Scholar]
- Park, C.I.; Cho, W.-G.; Lee, S.J. Emulsion stability of cosmetic creams based on water-in-oil high internal phase emulsions Emulsion stability of cosmetic creams based on water-in-oil high internal phase emulsions. Korea Australia Rheol. J. 2003, 15, 125–130. [Google Scholar]
- Cameron, N.R. Polymerized High Internal Phase Emulsion Monoliths. In Monolithic Materials: Preparation, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2003; Volume 67, pp. 255–276. [Google Scholar]
- Butler, R.; Hopkinson, I.; Cooper, A.I. Synthesis of Porous Emulsion-Templated Polymers Using High Internal Phase CO2-in-Water Emulsions. J. Am. Chem. Soc. 2003, 125, 14473–14481. [Google Scholar] [CrossRef]
- Livshin, S.; Silverstein, M.S. Crystallinity and Cross-Linking in Porous Polymers Synthesized from Long Side Chain Monomers through Emulsion Templating. Macromolecules 2008, 41, 3930–3938. [Google Scholar] [CrossRef]
- Tan, L.; Wang, K.; Yang, Y.; Li, Q. Organic Porous Polymer Materials: Design, Preparation, and Applications. In Polymer-Engineered Nanostructures for Advanced Energy Applications; Lin, Z., Yang, Z., Zhang, A., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Desforges, A.; Arpontet, M.; Deleuze, H.; Mondain-Monval, O. Synthesis and functionalisation of polyHIPE® beads. React. Funct. Polym. 2002, 53, 183–192. [Google Scholar] [CrossRef]
- Gokmen, M.T.; Van Camp, W.; Colver, P.J.; Bon, S.A.; Du Prez, F.E. Fabrication of porous ‘clickable’ polymer beads and rods through generation of high internal phase emulsion (HIPE) droplets in a simple microfluidic device. Macromolecules 2009, 42, 9289–9294. [Google Scholar] [CrossRef]
- Whitely, M.E.; Robinson, J.L.; Stuebben, M.C.; Pearce, H.A.; McEnery, M.A.; Cosgriff-Hernandez, E. Prevention of Oxygen Inhibition of PolyHIPE Radical Polymerization Using a Thiol-Based Cross-Linker. ACS Biomater. Sci. Eng. 2017, 3, 409–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajnc, P.; Leber, N.; Štefanec, D.; Kontrec, S.; Podgornik, A. Preparation and characterisation of poly(high internal phase emulsion) methacrylate monoliths and their application as separation media. J. Chromatogr. A 2005, 1065, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Thunhorst, K.L.; Gehlsen, M.D.; Wright, R.E.; Nelson, E.W.; Koecher, S.D.; Gold, D. Foams Made By Photopolymerization of Emulsions. U.S. Patent 6573 305, 3 June 2003. [Google Scholar]
- Barby, D.; Haq, Z. Low Density Porous Cross-Linked Polymeric Materials and Their Preparation and Uses as Carriers for Included Liquids. U.S. Patent 4 522 953, 11 June 1985. [Google Scholar]
- Pierre, S.J.; Thies, J.C.; Dureault, A.; Cameron, N.R.; van Hest, J.C.R.; Carette, N.; Michon, T.; Weberskirch, R. Covalent enzyme immobilization onto photopolymerized highly porous monoliths. Adv. Mater. 2006, 18, 1822–1826. [Google Scholar] [CrossRef]
- Cummins, D.; Wyman, P.; Duxbury, C.J.; Thies, J. Synthesis of Functional Photopolymerized Macroporous PolyHIPEs by Atom Transfer Radical Polymerization Surface Grafting. Chem. Mater. 2007, 19, 5285–5292. [Google Scholar] [CrossRef]
- Cummins, D.; Duxbury, C.J.; Quaedflieg, P.J.L.M.; Magusin, P.C.M.M.; Koning, C.E.; Heise, A. Click chemistry as a means to functionalize macroporous PolyHIPE. Soft Matter. 2009, 5, 804–811. [Google Scholar] [CrossRef]
- Gong, X.; Wen, W.; Sheng, P. Microfluidic Fabrication of Porous Polymer Microspheres: Dual Reactions in Single Droplets. Langmuir 2009, 25, 7072–7077. [Google Scholar] [CrossRef]
- Lovelady, E.; Kimmins, S.D.; Wu, J.; Cameron, N.R. Preparation of emulsion-templated porous polymers using thiol-ene and thiol-yne chemistry. Polym. Chem. 2011, 2, 559–562. [Google Scholar] [CrossRef]
- Caldwell, S.; Johnson, D.W.; Didsbury, M.; Murray, B.A. Degradable emulsion-templated scaffolds for tissue engineering from thiol-ene photopolymerisation. Soft Matter. 2012, 8, 10344–10351. [Google Scholar] [CrossRef] [Green Version]
- Kimmins, S.D.; Wyman, P.; Cameron, N.R. Photopolymerised methacrylate-based emulsion-templated porous polymers. React. Funct. Polym. 2012, 72, 947–954. [Google Scholar] [CrossRef]
- Schüler, F.; Schamel, D.; Salonen, A.; Drenckhan, W.; Gilchrist, M.D.; Stubenrauch, C. Synthesis of Macroporous Polystyrene by the Polymerization of Foamed Emulsions. Polym. Foam. 2012, 51, 2213–2217. [Google Scholar] [CrossRef] [Green Version]
- Gokmen, M.T.; Dereli, B.; De Geest, B.G.; Du Prez, F.E. Complexity from Simplicity: Unique Polymer Capsules, Rods, Monoliths, and Liquid Marbles Prepared via HIPE in Microfluidics. Part. Part. Syst. Charact. 2013, 30, 438–444. [Google Scholar] [CrossRef]
- Susec, M.; Ligon, S.C.; Stampfl, J.; Liska, R.; Krajnc, P. Hierarchically porous materials from layer-by-layer photopolymerization of high internal phase emulsions. Macromol. Rapid Commun. 2013, 34, 938–943. [Google Scholar] [CrossRef]
- Johnson, D.W.; Sherborne, C.; Didsbury, M.P.; Pateman, C.; Cameron, N.R.; Claeyssens, F. Macrostructuring of emulsion-templated porous polymers by 3D laser patterning. Adv. Mater. 2013, 25, 3178–3181. [Google Scholar] [CrossRef]
- Mao, D.; Li, T.; Liu, H.; Li, Z.; Shao, H.; Li, M. Preparation of macroporous polyHIPE foams via radiation-induced polymerization at room temperature. Colloid Polym. Sci. 2013, 291, 1649–1656. [Google Scholar] [CrossRef]
- Kircher, L.; Theato, P.; Cameron, N.R. Reactive thiol-ene emulsion-templated porous polymers incorporating penta fluorophenyl acrylate. Polymer 2013, 54, 1755–1761. [Google Scholar] [CrossRef]
- Kimmins, S.D.; Wyman, P.; Cameron, N.R. Amine-functionalization of glycidyl methacrylate-containing emulsion-templated porous polymers and immobilization of proteinase K for biocatalysis. Polymer 2014, 55, 416–425. [Google Scholar] [CrossRef] [Green Version]
- Moglia, R.; Whitely, M.; Brooks, M.; Robinson, J.; Pishko, M.; Cosgriff-Hernandez, E. Solvent-free fabrication of polyHIPE microspheres for controlled release of growth factors. Macromol. Rapid Commun. 2014, 35, 1301–1305. [Google Scholar] [CrossRef]
- Johnson, D.W.; Langford, C.R.; Didsbury, M.P.; Lipp, B.; Przyborski, S.A.; Cameron, N.R. Fully biodegradable and biocompatible emulsion templated polymer scaffolds by thiol-acrylate polymerization of polycaprolactone macromonomers. Polym. Chem. 2015, 6, 7256–7263. [Google Scholar] [CrossRef] [Green Version]
- Susec, M.; Liska, R.; Russmuller, G.; Kotek, J.; Krajnc, P. Microcellular Open Porous Monoliths for Cell Growth by Thiol-Ene Polymerization of Low-Toxicity Monomers in High Internal Phase Emulsions a. Macromol. Biosci. 2015, 15, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.; Sherborne, C.; Paterson, T.; Green, N.H.; Reilly, G.C.; Claeyssens, F. Emulsion templated scaffolds with tunable mechanical properties for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2016, 54, 159–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitely, M.; Rodriguez-River, G.; Waldron, C.; Mohiuddin, S.; Cereceres, S.; Sears, N.; Ray, N.; Cosgriff-Hernandez, E. Porous PolyHIPE microspheres for protein delivery from an injectable bone graft. Acta Biomater. 2019, 93, 169–179. [Google Scholar] [CrossRef]
- Langford, C.R.; Johnson, D.W.; Cameron, N.R. Chemical functionalization of emulsion-templated porous polymers by thiol-ene “click” chemistry. Polym. Chem. 2014, 5, 6200–6206. [Google Scholar] [CrossRef] [Green Version]
- Sušec, M.; Paljevac, M.; Kotek, J.; Krajnc, P. Microcellular open porous polyester membranes from thiol-ene polymerisations of high internal phase emulsions. Des. Monomers Polym. 2016, 19, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Huš, S.; Kolar, M.; Krajnc, P. Separation of heavy metals from water by functionalized glycidyl methacrylate poly (high internal phase emulsions). J. Chromatogr. A 2016, 1437, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Dikici, B.A.; Dikici, S.; Reilly, G.C.; Macneil, S.; Claeyssens, F. A Novel Bilayer Polycaprolactone Membrane for Guided Bone Regeneration: Combining Electrospinning and Emulsion Templating. Materials 2019, 12, 1–24. [Google Scholar]
- Hughes, J.M.; Budd, P.M.; Tiede, K.; Lewis, J. Polymerized High Internal Phase Emulsion Monoliths for the Chromatographic Separation of Engineered Nanoparticles. J. Appl. Polym. Sci. 2014, 132, 41229. [Google Scholar] [CrossRef]
- Jiang, Q.; Barkan, H.; Menner, A.; Bismarck, A. Micropatterned, macroporous polymer springs for capacitive energy harvesters. Polymer 2017, 126, 419–424. [Google Scholar] [CrossRef]
- Danninger, D.; Hartmann, F.; Paschinger, W.; Pruckner, R.; Schwödiauer, R.; Demchyshyn, S.; Kaltenbrunner, M. Stretchable Polymerized High Internal Phase Emulsion Separators for High Performance Soft Batteries. Adv. Energy Mater. 2020, 10, 2000467. [Google Scholar] [CrossRef]
- Jenjob, R.; Seidi, F.; Crespy, D. Recent advances in polymerizations in dispersed media. Adv. Colloid Interface Sci. 2018, 260, 24–31. [Google Scholar] [CrossRef]
- Debecker, D.P.; Boissiere, C.; Laurent, G.; Huet, S.; Eliaers, P.; Sanchez, C.; Backov, R. First Acidic Macro-Mesocellular Aluminosilicate Monolithic foams ”SiAl(HIPE)” and their Catalytic Properties. Chem. Commun. 2015, 51, 13993–14128. [Google Scholar]
- Pan, J.; Zeng, J.; Cao, Q.; Gao, H.; Gen, Y.; Peng, Y.; Dai, X.; Yan, Y. Hierarchical macro and mesoporous foams synthesized by HIPEs template and interface grafted route for simultaneous removal of λ-cyhalothrin and copper ions. Chem. Eng. J. 2016, 284, 1361–1372. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Amer. Chem. Soc. 1938, 60, 309. [Google Scholar] [CrossRef]
- Decker, C. In situ monitoring of ultrafast photopolymerizations by real-time infrared spectroscopy. Polym. News 2005, 30, 34–38. [Google Scholar] [CrossRef]
- Jancovicova, V.; Kindernay, J.; Jacubikova, Z.; Mrlláková, I. Influence of Photoinitiator and Curing Conditions on Polymerization Kinetics and Gloss of UV-Cured Coatings. Chem. Pap. 2007, 61, 383–390. [Google Scholar] [CrossRef]
- Macarie, L.; Ilia, G. The influence of temperature and photoinitiator concentration on photoinitiated polymerization of diacrylate monomer. Cent. Eur. J. Chem. 2005, 3, 721–730. [Google Scholar]
- Drache, M.; Stehle, M.; Mätzig, J.; Brandl, K.; Jungbluth, M.; Namyslo, J.C.; Schmidt, A.; Beuermann, S. Identification of β-scission products from free radical polymerizations of butyl acrylate at high temperature. Polym. Chem. 2019, 10, 1956–1967. [Google Scholar] [CrossRef] [Green Version]
- Barbetta, A.; Cameron, N.R. Morphology and Surface Area of Emulsion-Derived (PolyHIPE) Solid Foams Prepared with Oil-Phase Soluble Porogenic Solvents: Span 80 as Surfactant. Macromolecules 2004, 37, 3188–3201. [Google Scholar] [CrossRef]
- Swedish Transmission Research Institute–STRI. Guide 92/1 Hydrophobicity Classification Guide; Swedish Transmission Research Institute–STRI: Ludvika, Sweden, 1992. [Google Scholar]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley Interscience: Ann Arbor, MI, USA, 1997. [Google Scholar]
- Hiemenz, P.C.; Rajagopalan, R. Principles of Colloids and Surface Chemistry; Chapter 6; CRC Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 1997. [Google Scholar]
- Thomazinia, D.; Gelfusoa, M.V.; Corrêa Altafim, R.A. Hydrophobicity Classification of Polymeric Materials Based on Fractal Dimension. Mater. Res. 2008, 11, 415–419. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nishikawa, N.; Mayama, H.; Nonomura, Y.; Yokojima, S.; Nakamura, S.; Uchida, K. Theoretical explanation of the Lotus Effect: Superhydrophobic property changes by removal of nanostructures form the surface of a Lotus leaf. Langmuir 2015, 31, 7355–7363. [Google Scholar] [CrossRef] [PubMed]
- Speight, J.G. Fuels for Fuell Cells in Fuel Cells: Technologies for Fuel Proceesing; Shekhawat, D., Spivey, J.J., Berry, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Zhang, T.; Guo, Q. Continuous preparation of polyHIPE monoliths from ionomer-stabilized high internal phase emulsions (HIPEs) for efficient recovery of spilled oils. Chem. Eng. J. 2017, 307, 812–819. [Google Scholar] [CrossRef]
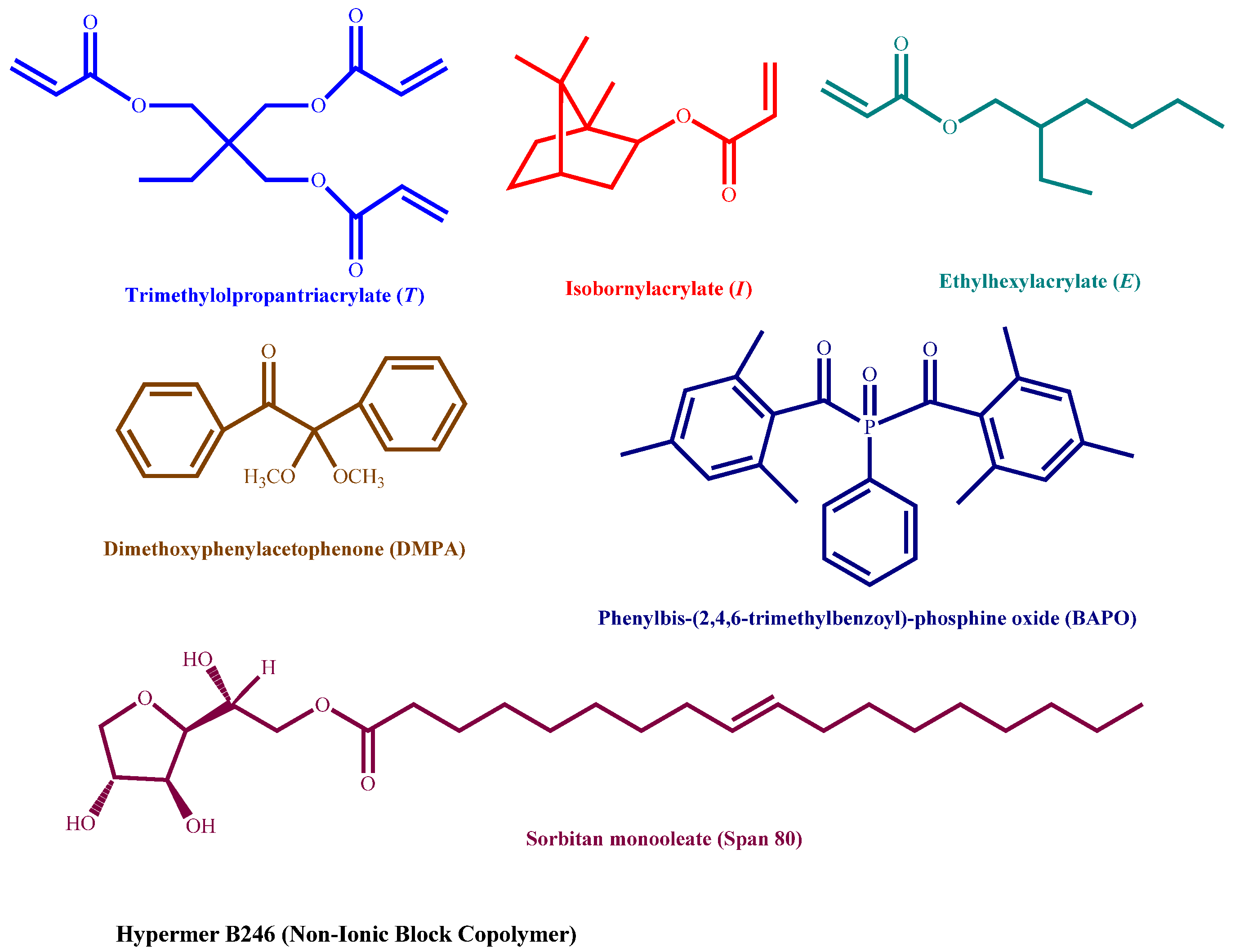


| Phase/Samples | Monomers | Photoinitiators * | |||||
|---|---|---|---|---|---|---|---|
| Nomenclature (Molar Ratios) | E:I %ratio | T (g) (mmol) | E (g) (mmol) | I (g) (mmol) | DMPA (g) (mmol) | BAPO (g) (mmol) | |
| Organic phase | T–E (1:3) | 100:0 | 2.092 | 2.908 | 0 | 0.152 | 0.257 |
| 4.888 | 14.665 | 0 | 0.592 | 0.605 | |||
| T–E–I (1:1.5:1.5) | 50:50 | 2.062 | 1.433 | 1.505 | 0.150 | 0.254 | |
| 4.817 | 7.226 | 7.226 | 0.584 | 0.596 | |||
| T–E–I (1:0.9:2.1) | 30:70 | 2.050 | 0.855 | 2.095 | 0.149 | 0.252 | |
| 4.789 | 4.311 | 10.059 | 0.581 | 0.593 | |||
| T–E–I (1:0.6:2.4) | 20:80 | 2.044 | 0.568 | 2.388 | 0.148 | 0.251 | |
| 4.776 | 2.866 | 11.463 | 0.579 | 0.591 | |||
| T–I (1:3) | 0:100 | 2.032 | 0 | 2.968 | 0.148 | 0.250 | |
| 4.749 | 0 | 14.247 | 0.576 | 0.587 | |||
| Aqueous phase | 18.4 mL (3% w/w CaCl2 solution) | ||||||
| Surfactants | Hypermer B246 = 0.5 g/Span 80 = 0.5 g | ||||||
| Sample | T 5% a | Tmax b |
|---|---|---|
| T–E (1:3) | 257 | 296, 411 |
| T–E–I (1:1.5:1.5) | 289 | 310, 414 |
| T–E–I (1:0.9:2.1) | 296 | 317, 434 |
| T–E–I (1:0.6:2.4) | 302 | 314, 436 |
| T–I (1:3) | 319 | 398 |
| Sample | BET Area (m2/g) | Total Volume of Pores (cm3/g) |
|---|---|---|
| T–E (1:3) | 9.4 | 0.040 |
| T–I (1:3) | 16 | 0.059 |
| T–E–I (1:1.5:1.5) | 11.2 | 0.029 |
| T–E–I (1:0.9:2.1) | 11.5 | 0.028 |
| T–E–I (1:0.6:2.4) | 12.0 | 0.037 |
| Sample | Hexane * | Diesel * | Toluene * | Chloroform * |
|---|---|---|---|---|
| T–E (1:3) | 332 | 265 | 448 | 823 |
| T–I (1:3) | 250 | 158 | 595 | 1570 |
| T–E–I (1:1.5:1.5) | 321 | 253 | 440 | 806 |
| T–E–I (1:0.9:2.1) | 306 | 236 | 378 | 778 |
| T–E–I (1:0.6:2.4) | 294 | 206 | 550 | 1325 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta Ortiz, R.; Reinoza Dávila, J.A.; Guerrero Santos, R. Development of Photocurable Polyacrylate-Based PolyHIPEs and the Study of the Kinetics of Photopolymerization, and of Their Thermal, Mechanical and Hydrocarbon Absorption Properties. Polymers 2021, 13, 3497. https://doi.org/10.3390/polym13203497
Acosta Ortiz R, Reinoza Dávila JA, Guerrero Santos R. Development of Photocurable Polyacrylate-Based PolyHIPEs and the Study of the Kinetics of Photopolymerization, and of Their Thermal, Mechanical and Hydrocarbon Absorption Properties. Polymers. 2021; 13(20):3497. https://doi.org/10.3390/polym13203497
Chicago/Turabian StyleAcosta Ortiz, Ricardo, Jefferson Alberto Reinoza Dávila, and Ramiro Guerrero Santos. 2021. "Development of Photocurable Polyacrylate-Based PolyHIPEs and the Study of the Kinetics of Photopolymerization, and of Their Thermal, Mechanical and Hydrocarbon Absorption Properties" Polymers 13, no. 20: 3497. https://doi.org/10.3390/polym13203497






