Adsorption, Equilibrium Isotherm, and Thermodynamic Studies towards the Removal of Reactive Orange 16 Dye Using Cu(I)-Polyaninile Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Synthesis of Cu(I)-PANI Composite
2.4. Preparation of Dye Solution
2.5. Calibration Curve
2.6. Reusability
3. Results
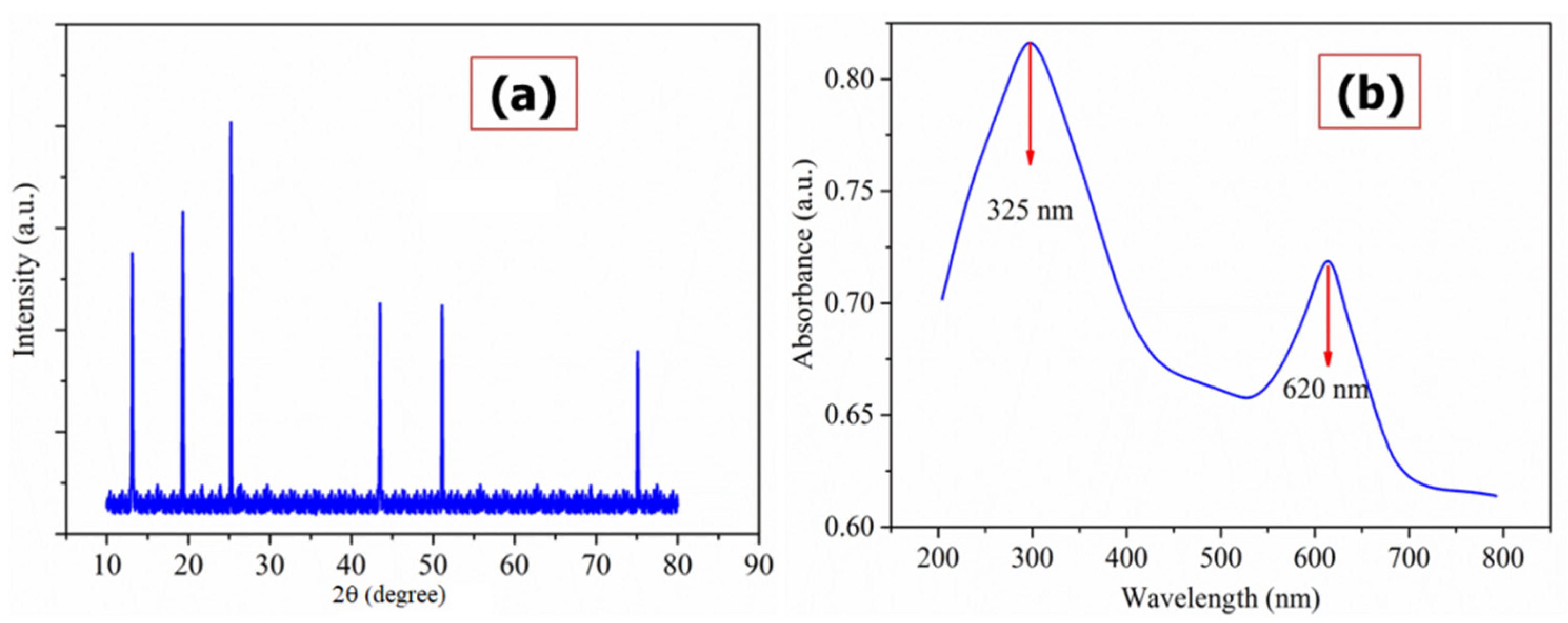
3.1. Physicochemical Studies
3.2. RO16 Adsorption Studies
3.2.1. Effect of pH
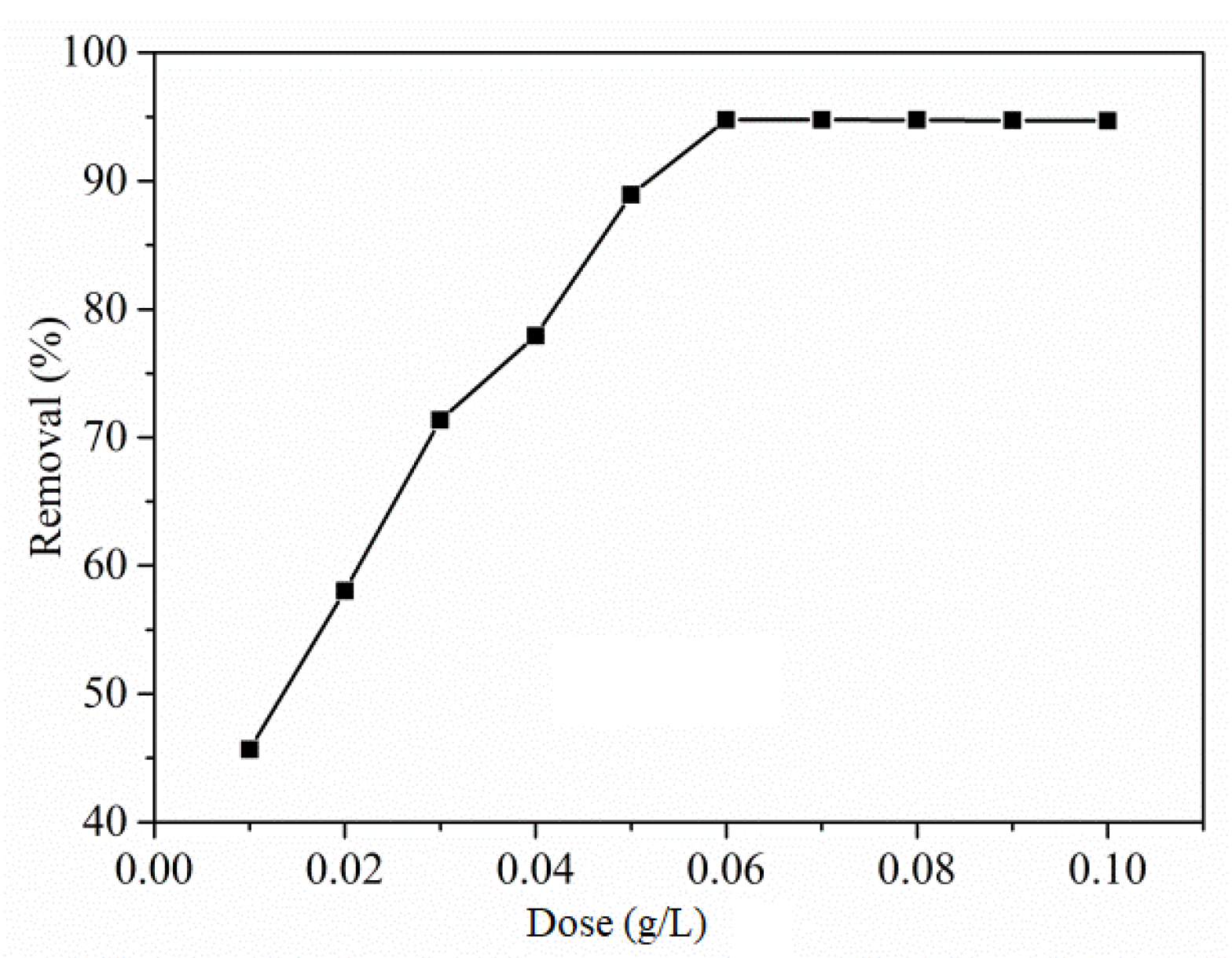
3.2.2. Effect of Dose
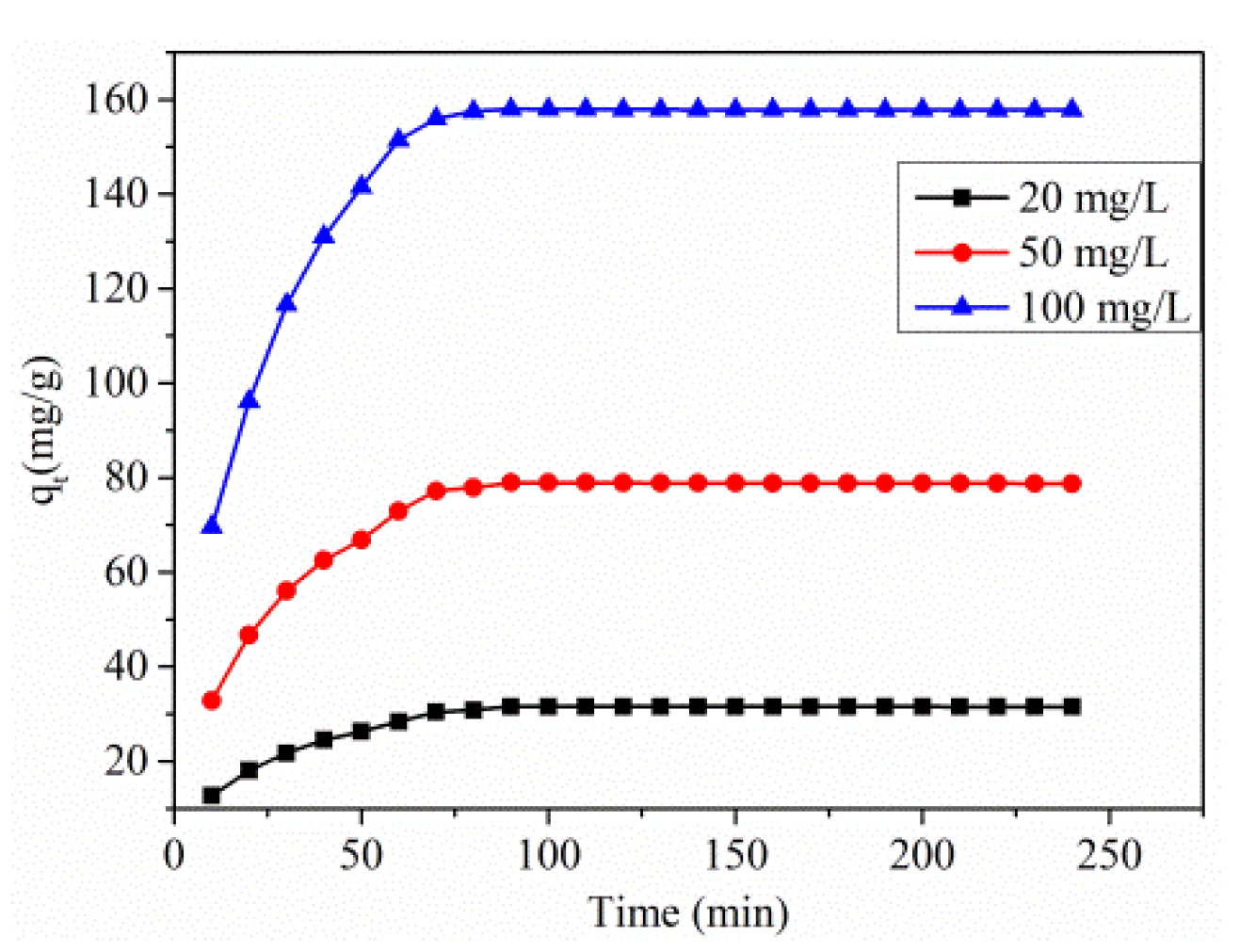
3.2.3. Effect of Initial Concentration and Contact Time
3.2.4. Adsorption Isotherm
3.2.5. Kinetic Studies
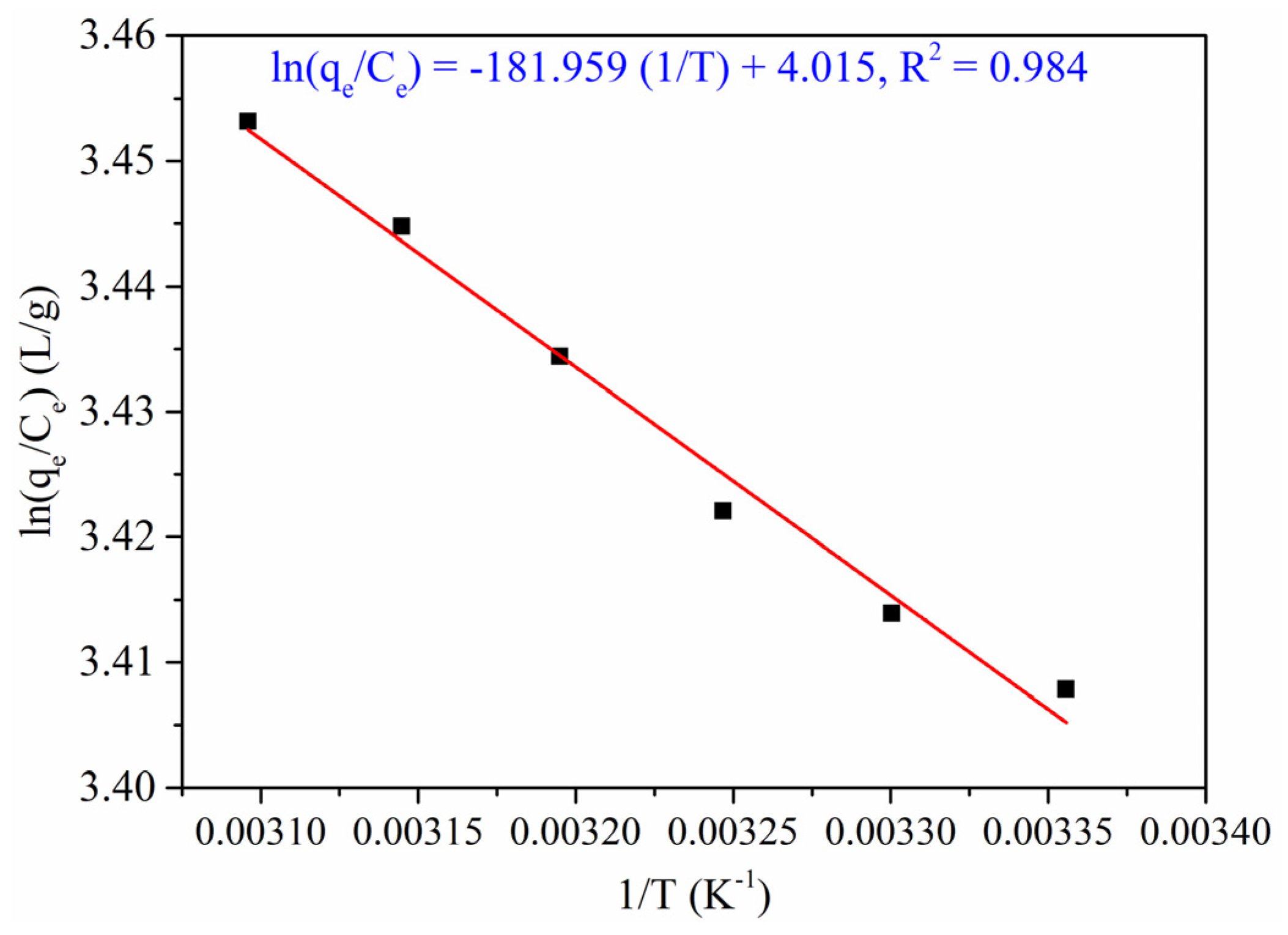
3.2.6. Thermodynamics
3.2.7. Reusability
3.2.8. Comparison with Other Adsorbents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banat, I.M.; Nigam, P.; Singh, D.; Marchant, R. Microbial decolorization of textile-dye containing effluents: A review. Bioresour. Technol. 1996, 58, 217–227. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical treatment review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Janaki, V.; Oh, B.T.; Vijayaraghavan, K.; Kin, J.W.; Kim, S.A.; Ramasamy, A.K.; Kannan, S.K. Application of bacterial extracellular polysaccharides/polyaniline composite for the treatment of remazol effluent. Carbohydr. Polym. 2012, 88, 1002–1008. [Google Scholar] [CrossRef]
- Choi, H.D.; Shin, M.C.; Kim, D.H.; Jeon, C.S.; Baek, K. Removal characteristics of reactive black 5 using surfactant-modified activated carbon. Desalination 2008, 223, 290–298. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Won, S.W.; Yun, Y.S. Treatment of complex remazol dye effluent using sawdust and coal-based activated carbons. J. Hazard. Mater. 2009, 167, 790–796. [Google Scholar] [CrossRef]
- Rezaee, A.; Ghaneian, M.T.; Hashemian, S.J.; Moussavi, G.; Khavanin, A.; Ghanizadeh, G. Decolorization of reactive blue 19 dye from textile wastewater by the UV/H2O2 process. J. Appl. Sci. 2008, 8, 1108–1112. [Google Scholar] [CrossRef] [Green Version]
- Racyte, J.; Rimeika, M.; Bruning, H. pH effect on decolorization of raw textile wastewater polluted with reactive dyes by advanced oxidation with UV/H2O2. Environ. Prot. Eng. 1999, 35, 167–178. [Google Scholar]
- Arfin, T.; Sonawane, K.; Saidankar, P.; Sharma, S. Role of microbes in the bioremediation of toxic dyes. In Integrated Green Chemistry and Sustainable Engineering; ul-Islam, S., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2019; pp. 443–471. [Google Scholar]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Hilal, N.M.; Badawy, N.A.; Mostafa, O.I.; Elrefay, H.M. Synthetic and application of a novel resin from waste foam packaging for adsorption of acid orange 67 from aqueous solution. Bull. Natl. Res. Cent. 2019, 43, 58. [Google Scholar] [CrossRef] [Green Version]
- Arfin, T.; Varshney, N.; Singh, B. Ionic liquid modified activated carbon for the treatment of textile wastewater. In Green Materials for Wastewater Treatment; Naushad, M., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2019; pp. 257–275. [Google Scholar]
- Arfin, T. Reactive and functional polymers. In Advanced Functional Textiles and Polymers: Fabrication, Processing and Applications; ul-Islam, S., Butola, B.S., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 291–308. [Google Scholar]
- Ai, L.; Jiang, J.; Zhang, R. Uniform polyaniline microspheres: A novel adsorbent for dye removal from aqueous solution. Synth. Met. 2010, 160, 762–767. [Google Scholar] [CrossRef]
- Ayad, M.M.; El-Nasr, A.A. Adsorption of cationic dye (methylene blue) from water using polyaniline nanotubes base. J. Phys. Chem. C 2010, 114, 14377–14383. [Google Scholar] [CrossRef]
- Gopal, N.; Asaithambi, M.; Sivakumar, P.; Sivakumar, V. Adsorption studies of a direct dye using polyaniline coated activated carbon prepared prosopis juliflora. J. Water Process Eng. 2014, 2, 87–95. [Google Scholar] [CrossRef]
- McAuley, C.; Du, Y.; Wildgoose, G.G.; Compton, R.G. The use of copper(II) oxide nanorod bundles for the non-enzymatic voltammetric sensing of carbohydrates and hydrogen peroxides. Sens. Actuators B 2008, 135, 230–235. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Tong, S.; Li, X.; Song, W. Three-dimensional network films of electrospun copper oxide nanofibers for glucose determination. Biosens. Bioelectron. 2009, 25, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Mallick, K.; Witcomb, M.J.; Strydom, A.M. Charge transport property of one-dimensional gold-polyaniline composite networks. Phys. Status. Solidi. A 2009, 206, 2245–2248. [Google Scholar] [CrossRef]
- Choudhary, M.; Shukla, S.K.; Taher, A.; Siwal, S.; Mallick, K. Organic-inorganic hybrid supramolecular assembly: An efficient platform for nonenzymatic glucose sensor. ACS Sustain. Chem. Eng. 2014, 2, 2852–2858. [Google Scholar] [CrossRef]
- Calvete, T.; Lima, E.C.; Cardoso, N.F.; Vaghetti, J.C.P.; Dias, S.L.P.; Pavan, F.A. Application of carbon adsorbents prepared from Brazilian-pine fruit shell for the removal of reactive orange 16 from aqueous solution: Kinetic, equilibrium, and thermodynamic studies. J. Environ. Manag. 2010, 91, 1695–1706. [Google Scholar] [CrossRef]
- Ullah, R.; Bilal, S.; Ali, K.; Shah, A.A. Synthesis and characterization of polyaniline doped with Cu(II) chloride by inverse emulsion polymerization. Synth. Met. 2014, 198, 113–117. [Google Scholar] [CrossRef]
- Palaniappan, S.; Narayana, B.H. Temperature effect on conducting polyaniline salts: Thermal and spectral studies. J. Polym. Sci. A Polym. Chem. 1994, 32, 2431–2436. [Google Scholar] [CrossRef]
- Ozkazanc, E.; Zor, S.; Ozkazanc, H.; Guney, H.Y.; Abaci, U. Aynthesis, characterization and dielectric behaviour of (ES)-form polyaniline/cerium(III)-nitrate-hexahydrate composites. Mater. Phys. 2012, 133, 356–362. [Google Scholar] [CrossRef]
- Arfin, T.; Tarannum, A. Rapid determination of lead ions using polyaniline-zirconium (IV) iodate-based ion selective electrode. J. Environ. Chem. Eng. 2019, 7, 102811. [Google Scholar] [CrossRef]
- Liu, A.; Bac, L.H.; Kim, J.-S.; Kim, B.-K.; Kim, J.-C. Synthesis and characterization of conducting polyaniline-copper composites. J. Nanosci. Nanotechnol. 2013, 13, 7728–7733. [Google Scholar] [CrossRef]
- Stejskal, J.; Kratochvil, P.; Radhakrishnan, N. Polyaniline dispersions 2. UV-Vis absorption spectra. Synth. Met. 1993, 61, 225–231. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Aazam, E.S. Preparation and characterization of core–shellpolyaniline/mesoporous Cu2O nanocomposites for the photocatalyticoxidation of thiophene. Appl. Catal. A General 2014, 480, 100–107. [Google Scholar] [CrossRef]
- Elanthamilan, E.; Sathiyan, A.; Rajkumar, S.; Sheryl, E.J.; Merlin, J.P. Polyaniline based charcoal/Ni nanocomposite materil for high performance supercapacitors. Sustain. Energ. Fuels 2018, 2, 811–819. [Google Scholar] [CrossRef]
- Pandimurugan, R.; Thambidurai, S. Seawood-polyaniline nanofiber modified electrode for sensing of uric acid. Anal. Met. 2015, 7, 10422–10432. [Google Scholar] [CrossRef]
- Mohammad, F.; Arfin, T.; Al-Lohedan, H.A. Enhanced biosorption and electrochemical performance of sugarcane bagasse derived a polylactic acid-graphene oxide-CeO2 composite. Mater. Chem. Phys. 2019, 229, 117–123. [Google Scholar] [CrossRef]
- Liu, W.; Yao, C.; Wang, M.; Ji, J.; Ying, L.; Fu, C. Kinetics and thermodynamics characteristics of cationic yellow X-GL adsorption on attapulgite/rice hull-based activated carbon nanocomposutes. Environ. Prog. Sustain. Energy 2013, 32, 655–662. [Google Scholar] [CrossRef]
- Malekbala, M.R.; Hosseini, S.; Yazdi, S.K.; Soltani, S.M.; Malekbala, M.R. The study of the potential capability of sugar beet pulp on the removal efficiency of two cationic dyes. Chem. Eng. Res. Des. 2012, 90, 704–712. [Google Scholar] [CrossRef]
- Ahmad, A.; Rafatullah, M.; Ibrahim, M.H.; Hashim, R. Scavenging behaviour of meranti sawdust in removal of methylene blue from aqueous solution. J. Hazard. Mater. 2009, 170, 357–365. [Google Scholar] [CrossRef]
- Sharma, Y.C. Optimization of parameters for adsorption of methylene blue on a low cost activated carbon. J. Chem. Eng. Data 2010, 55, 435. [Google Scholar] [CrossRef]
- Waghmare, S.; Arfin, T.; Manwar, N.; Lataye, D.; Labhsetwar, N.; Rayalu, S. Preparation and characterization of polyalthia longifolia based adsorbent for removing fluoride from drinking water. Asian J. Adv. Basic Sci. 2015, 4, 12–24. [Google Scholar]
- Satilmis, B.; Budd, P.M. Selective dye adsorption by chemically modified and thermally-treated polymers of intrinsic microporosity. J. Colloid Interf. Sci. 2017, 492, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, S.S.; Arfin, T.; Rayalu, S.; Lataye, D.; Dubey, S.; Tiwari, S. Adsorption behaviour of modified zeolite as novel adsorbents for fluoride removal from drinking water: Surface phenomena, kinetics and thermodynamics studies. Int. J. Sci. Eng. Technol. Res. 2015, 4, 4114–4124. [Google Scholar]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.J.; McKay, G.; Khuder, K.Y.H. Equilibrium adsorption isotherms of basic dyes onto lignite. J. Chem. Technol. Biotechnol. 1989, 45, 291. [Google Scholar] [CrossRef]
- Arfin, T.; Rafiuddin. Transport studies of nickel arsenate membrane. J. Electroanal. Chem. 2009, 636, 113–122. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, B.; Zhang, Y.; He, Y.; Huang, L.; Tan, S.; Cai, X. Simultaneous removal of cationic and anionic dyes from environmental water using montmorillonite-pillared graphene oxide. J. Chem. Eng. Data 2015, 60, 1270–1278. [Google Scholar] [CrossRef]
- Kimura, I.Y.; Laranjeira, M.C.M.; Favere, V.T.; Furlan, L. The interaction between reactive dye containing vinylsulfone group and chitosan micro-spheres. Int. J. Polym. Mater. 2002, 51, 759–768. [Google Scholar] [CrossRef]
- Marrakch, F.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous carbonaceous material from fish scales as low-cost adsorbent for reactive orange 16 adsorption. J. Taiwan Inst. Chem. Eng. 2016, 71, 47–54. [Google Scholar] [CrossRef]
- Won, S.W.; Choi, S.B.; Yun, Y.S. Performance and mechanism in binding of reactive orange 16 to various types of sludge. Biochem. Eng. J. 2006, 28, 208–214. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Optimized and functionalized paper sludge activated with potassium fluoride for single and binary adsorption of reactive dyes. J. Ind. Eng. Chem. 2014, 20, 830–840. [Google Scholar] [CrossRef]
- Marrakchi, F.; Khanday, W.A.; Asif, M.; Hameed, B.H. Cross-linked chitosan/sepiolite composite for the adsorption of methylene blue and reactive orange 16. Int. J. Biol. Macromol. 2016, 93, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Janaki, V.; Vijayaraghavan, K.; Ramasamy, A.K.; Lee, K.-J.; Oh, B.-T.; Kannan, S.K. Competitive adsorption of reactive orange 16 and reactive brilliant blue R on polyaniline/bacterial extracellular polysaccharides composite-a novel eco-friendly polymer. J. Hazards. Mater. 2012, 241-242, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kannusamy, P.; Sivalingam, T. Synthesis of porous chitosan-polyaniline/ZnO hybrid composite and application for removal of reactive orange 16 dye. Colloids Surf. B 2013, 108, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.H.; Islam, M.A.; Hameed, B.H. Cross-linked chitosan thin film coated onto glass plate as an effective adsorbent for adsorption of reactive orange 16. Int. J. Biol. Macromol. 2017, 95, 743–749. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, S.P.; Thiruvenkatachari, R.; Shim, W.G.; Moon, H. Submerged microfiltration membrane coupled with alum coagulation/powdered activated carbon adsorption for complete decolorization of reactive dyes. Water Res. 2006, 40, 435–444. [Google Scholar] [CrossRef] [PubMed]



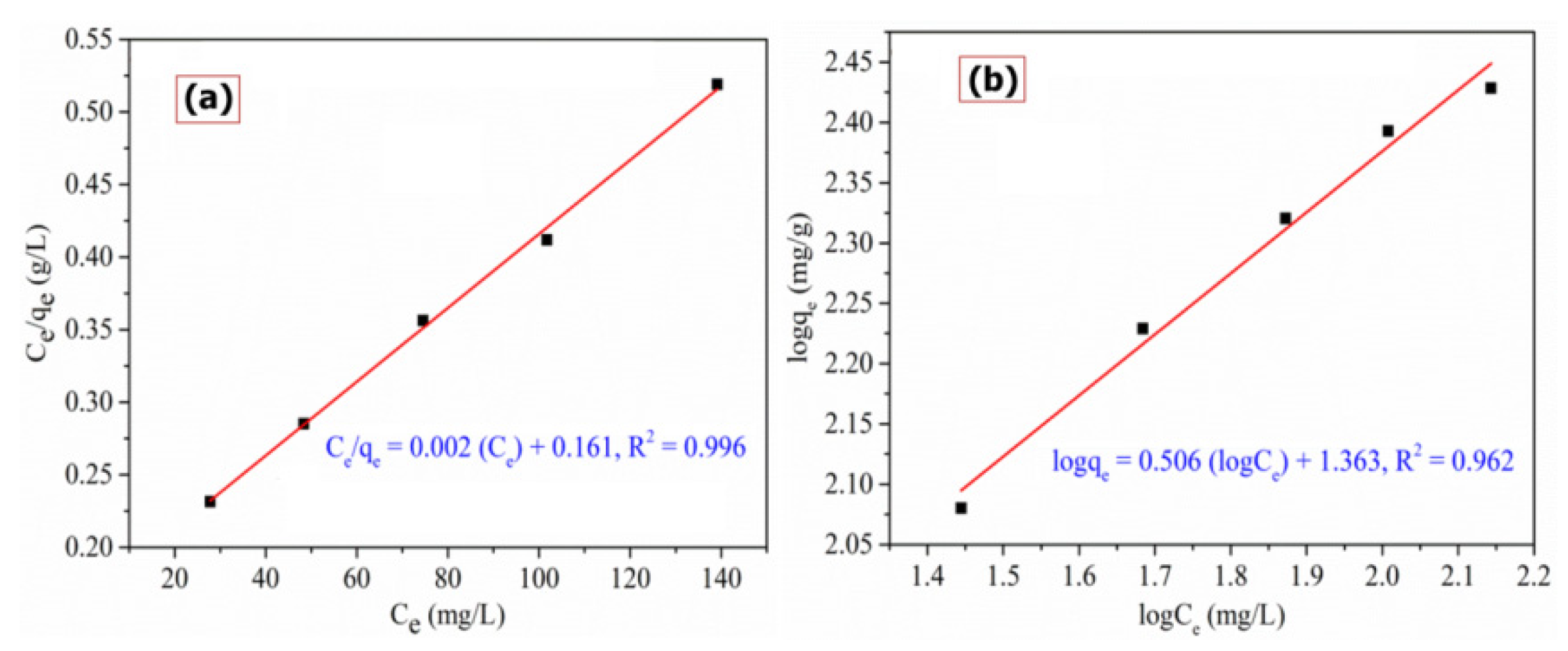


| Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | n | KF (mg/g)(L/mg)1/n | R2 |
| 392.156 | 0.015 | 0.996 | 1.974 | 23.075 | 0.982 |
| Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|
| qm (mg g−1) | k1 (min−1) | R2 | qe (mg g−1) | k2 (g mg−1 min−1) | R2 |
| 16.225 | 0.087 | 0.995 | 190.476 | 2.946 x 10−4 | 0.997 |
| Temp (K) | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (J/mol K) | Removal (%) |
|---|---|---|---|---|
| 298 | −8.437 | 1.512 | 33.387 | 94.77 |
| 303 | −8.604 | 94.80 | ||
| 308 | −8.771 | 94.84 | ||
| 313 | −8.938 | 94.90 | ||
| 318 | −9.105 | 94.95 | ||
| 323 | −9.272 | 94.99 |
| S. No. | Adsorbent | Adsorption Capacity (mg/g) | Isotherm | Dye Concentartion (mg/L) | Kinetic | Ref. |
|---|---|---|---|---|---|---|
| 1. | Chitosan cross-linked (beads) | 30 | Langmuir | 10–70 | PSO | [42] |
| 2. | Carbonized fish scales | 54.940 | Freundlich | 25–400 | PSO | [43] |
| 3. | Digested sludge | 159 | Langmuir | 0–5000 | PSO | [44] |
| 4. | Paper sludge activated carbon | 178 | Langmuir | 50–350 | PSO | [45] |
| 5. | Cross–linked chitosan/sepiolite | 190.965 | Langmuir | 25–400 | PSO | [46] |
| 6. | Polyaniline/bacterial extracellular polysaccharides | 293.2 | Langmuir | - | PSO | [47] |
| 7. | Chitosan-polyaniline | 333.3 | Langmuir | 25–125 | PSO | [48] |
| 8. | Crosslinked chitosan-epichlorohydrine thin film | 356.20 | Langmuir | 25–350 | PSO | [49] |
| 9. | Activated carbon from wood | 367.5 | Langmuir | - | PSO | [50] |
| 10. | Cu(I)-PANI | 392.156 | Langmuir | 100–300 | PSO | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obulapuram, P.K.; Arfin, T.; Mohammad, F.; Khiste, S.K.; Chavali, M.; Albalawi, A.N.; Al-Lohedan, H.A. Adsorption, Equilibrium Isotherm, and Thermodynamic Studies towards the Removal of Reactive Orange 16 Dye Using Cu(I)-Polyaninile Composite. Polymers 2021, 13, 3490. https://doi.org/10.3390/polym13203490
Obulapuram PK, Arfin T, Mohammad F, Khiste SK, Chavali M, Albalawi AN, Al-Lohedan HA. Adsorption, Equilibrium Isotherm, and Thermodynamic Studies towards the Removal of Reactive Orange 16 Dye Using Cu(I)-Polyaninile Composite. Polymers. 2021; 13(20):3490. https://doi.org/10.3390/polym13203490
Chicago/Turabian StyleObulapuram, Prasanna Kumar, Tanvir Arfin, Faruq Mohammad, Sachin K. Khiste, Murthy Chavali, Aisha N. Albalawi, and Hamad A. Al-Lohedan. 2021. "Adsorption, Equilibrium Isotherm, and Thermodynamic Studies towards the Removal of Reactive Orange 16 Dye Using Cu(I)-Polyaninile Composite" Polymers 13, no. 20: 3490. https://doi.org/10.3390/polym13203490
APA StyleObulapuram, P. K., Arfin, T., Mohammad, F., Khiste, S. K., Chavali, M., Albalawi, A. N., & Al-Lohedan, H. A. (2021). Adsorption, Equilibrium Isotherm, and Thermodynamic Studies towards the Removal of Reactive Orange 16 Dye Using Cu(I)-Polyaninile Composite. Polymers, 13(20), 3490. https://doi.org/10.3390/polym13203490






