Improving UV Curing in Organosolv Lignin-Containing Photopolymers for Stereolithography by Reduction and Acylation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
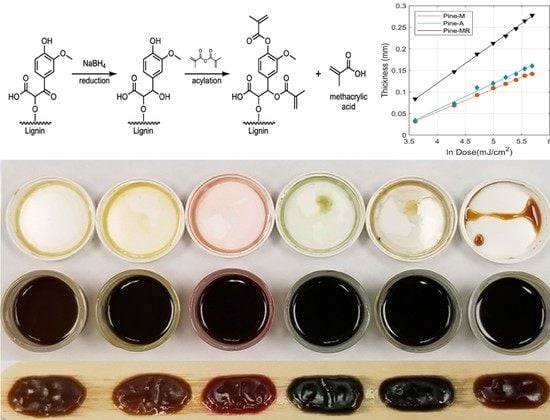
2.2. Lignin Reduction
2.3. Lignin Acylation
2.4. Lignin Characterization
2.5. Resin Formulation
2.6. Cure Scheme and Material Characterization
3. Results and Discussion
3.1. Characterization of Reduced and Acylated Lignin
3.2. Lignin Resins
3.3. Mechanical and Thermal Properties
3.4. 3D Printing with Lignin Resins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, L.; Yu, I.K.M.; Liu, Y.; Ruan, X.; Tsang, D.C.W.; Hunt, A.J.; Ok, Y.S.; Song, H.; Zhang, S. Lignin valorization for the production of renewable chemicals: State-of-the-art review and future prospects. Bioresour. Technol. 2018, 269, 465–475. [Google Scholar] [CrossRef]
- Kalami, S.; Arefmanesh, M.; Master, E.; Nejad, M. Replacing 100% of phenol in phenolic adhesive formulations with lignin. J. Appl. Polym. Sci. 2017, 134, 45124. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, A.; Hamel, J.; Katahira, R.; Zhu, H. Metal-free aqueous flow battery with novel ultrafiltered lignin as electrolyte. ACS Sustain. Chem. Eng. 2018, 6, 5394–5400. [Google Scholar] [CrossRef]
- Morandim-Giannetti, A.A.; Agnelli, J.A.M.; Lanças, B.Z.; Magnabosco, R.; Casarin, S.A.; Bettini, S.H.P. Lignin as additive in polypropylene/coir composites: Thermal, mechanical and morphological properties. Carbohydr. Polym. 2012, 87, 2563–2568. [Google Scholar] [CrossRef]
- Huang, W.; Wu, M.; Liu, W.; Hua, Z.; Wang, Z.; Zhou, L. Value-adding of organosolv lignin: Designing mechanically robust UV-resistant polymeric glass via ARGET ATRP. Appl. Surf. Sci. 2019, 475, 302–311. [Google Scholar] [CrossRef]
- Avelino, F.; de Oliveira, D.R.; Mazzetto, S.E.; Lomonaco, D. Poly(methyl methacrylate) films reinforced with coconut shell lignin fractions to enhance their UV-blocking, antioxidant and thermo-mechanical properties. Int. J. Biol. Macromol. 2019, 125, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Tran, T.M.T.; Choi, J.W.; Won, K. Lignin for white natural sunscreens. Int. J. Biol. Macromol. 2019, 122, 549–554. [Google Scholar] [CrossRef]
- Qian, Y.; Qiu, X.; Zhu, S. Lignin: A nature-inspired sun blocker for broad-spectrum sunscreens. Green Chem. 2015, 17, 320–324. [Google Scholar] [CrossRef]
- Qian, Y.; Qiu, X.; Zhu, S. Sunscreen performance of lignin from different technical resources and their general synergistic effect with synthetic sunscreens. ACS Sustain. Chem. Eng. 2016, 4, 4029–4035. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crops Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Mansouri, N.-E.E.; Salvadó, J. Structural characterization of technical lignins for the production of adhesives: Application to lignosulfonate, kraft, soda-anthraquinone, organosolv and ethanol process lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Kim, Y.; Suhr, J.; Seo, H.-W.; Sun, H.; Kim, S.; Park, I.-K.; Kim, S.-H.; Lee, Y.; Kim, K.-J.; Nam, J.-D. All biomass and UV protective composite composed of compatibilized lignin and poly (lactic-acid). Sci. Rep. 2017, 7, 43596. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Robles, J.; Tamminen, T.; Liitiä, T.; Peresin, M.S.; Rodríguez, A.; Jääskeläinen, A.-S. Aqueous acetone fractionation of kraft, organosolv and soda lignins. Int. J. Biol. Macromol. 2018, 106, 979–987. [Google Scholar] [CrossRef]
- Nitsos, C.; Rova, U.; Christakopoulos, P. Organosolv fractionation of softwood biomass for biofuel and biorefinery applications. Energies 2017, 11, 50. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Saito, T. Solvent fractionation of lignin. In Polymer Precursor-Derived Carbon; American Chemical Society: Washington, DC, USA, 2014; Volume 1173, pp. 153–168. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, J.; Du, X.; Hu, Z.; Chang, H.-m.; Jameel, H. Phenolation to improve lignin reactivity toward thermosets application. ACS Sustain. Chem. Eng. 2018, 6, 5504–5512. [Google Scholar] [CrossRef]
- Kun, D.; Pukánszky, B. Polymer/lignin blends: Interactions, properties, applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef] [Green Version]
- Crivello, J.V.; Reichmanis, E. Photopolymer materials and processes for advanced technologies. Chem. Mater. 2014, 26, 533–548. [Google Scholar] [CrossRef]
- Kafle, A.; Luis, E.; Silwal, R.; Pan, H.M.; Shrestha, P.L.; Bastola, A.K. 3D/4D Printing of polymers: Fused deposition modelling (FDM), selective laser sintering (SLS), and stereolithography (SLA). Polymers 2021, 13, 3101. [Google Scholar] [CrossRef]
- Tan, L.J.; Zhu, W.; Zhou, K. Recent progress on polymer materials for additive manufacturing. Adv. Funct. Mater. 2020, 30, 2003062. [Google Scholar] [CrossRef]
- Zakeri, S.; Vippola, M.; Levänen, E. A comprehensive review of the photopolymerization of ceramic resins used in stereolithography. Addit. Manuf. 2020, 35, 101177. [Google Scholar] [CrossRef]
- Wagner, K.S. Investigate Methods to Increase the Usefulness of Stereolithography 3D Printed Objects by Adding Carbon Nanotubes to Photo-Curable Resins; University of Minnesota: Duluth, MN, USA, 2014; pp. 1–12. Available online: https://hdl.handle.net/11299/187417 (accessed on 8 February 2019).
- Feng, X.; Yang, Z.; Chmely, S.; Wang, Q.; Wang, S.; Xie, Y. Lignin-coated cellulose nanocrystal filled methacrylate composites prepared via 3D stereolithography printing: Mechanical reinforcement and thermal stabilization. Carbohydr. Polym. 2017, 169, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Pansky, A.; Tille, C.; Seitz, H.; Emons, M.; Bens, A.; Roitzheim, B.; Bermes, G.; Tobiasch, E. Non-toxic flexible photopolymers for medical stereolithography technology. Rapid. Prototyp. J. 2007, 13, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Ebers, L.-S.; Arya, A.; Bowland, C.C.; Glasser, W.G.; Chmely, S.C.; Naskar, A.K.; Laborie, M.-P. 3D printing of lignin: Challenges, opportunities and roads onward. Biopolymers 2021, 112, e23431. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Hao, N.; Ragauskas, A.J. Stereolithography 3D printing of lignin-reinforced composites with enhanced mechanical properties. ACS Omega 2019, 4, 20197–20204. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, F.; Mohan, D.; Sajab, M.S.; Bakarudin, S.B.; Kaco, H. Evaluation of the compatibility of organosolv lignin-graphene nanoplatelets with photo-curable polyurethane in stereolithography 3D printing. Polymers 2019, 11, 1544. [Google Scholar] [CrossRef] [Green Version]
- Sutton, J.T.; Rajan, K.; Harper, D.P.; Chmely, S.C. Lignin-containing photoactive resins for 3D printing by stereolithography. ACS Appl. Mater. Interfaces 2018, 10, 36456–36463. [Google Scholar] [CrossRef]
- Langholtz, M.H.; Stokes, B.J.; Eaton, L.M.; Brandt, C.C.; Davis, M.R.; Theiss, T.J.; Turhollow Jr, A.F.; Webb, E.; Coleman, A.; Wigmosta, M. 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy; Oak Ridge National Laboratory (ORNL): Oak Ridge, TN, USA, 2016; Volume 1, pp. 36–137. [Google Scholar] [CrossRef]
- Bozell, J.J.; Black, S.K.; Myers, M.; Cahill, D.; Miller, W.P.; Park, S. Solvent fractionation of renewable woody feedstocks: Organosolv generation of biorefinery process streams for the production of biobased chemicals. Biomass Bioenergy 2011, 35, 4197–4208. [Google Scholar] [CrossRef]
- García-Negrón, V.; Phillip, N.D.; Li, J.; Daniel, C.; Wood, D.; Keffer, D.J.; Rios, O.; Harper, D.P. Processing–structure–property relationships for lignin-based carbonaceous materials used in energy-storage applications. Energy Technol. 2017, 5, 1311–1321. [Google Scholar] [CrossRef]
- Rajan, K.; Mann, J.K.; English, E.; Harper, D.P.; Carrier, D.J.; Rials, T.G.; Labbé, N.; Chmely, S.C. Sustainable hydrogels based on lignin-methacrylate copolymers with enhanced water retention and tunable material properties. Biomacromolecules 2018, 19, 2665–2672. [Google Scholar] [CrossRef]
- Holmberg, A.L.; Nguyen, N.A.; Karavolias, M.G.; Reno, K.H.; Wool, R.P.; Epps, T.H. Softwood lignin-based methacrylate polymers with tunable thermal and viscoelastic properties. Macromolecules 2016, 49, 1286–1295. [Google Scholar] [CrossRef]
- Holzgrabe, U. Quantitative NMR, methods and applications. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Academic Press: Oxford, UK, 2017; pp. 816–823. [Google Scholar] [CrossRef]
- Udagawa, A.; Sakurai, F.; Takahashi, T. In situ study of photopolymerization by Fourier transform infrared spectroscopy. J. Appl. Polym. Sci. 1991, 42, 1861–1867. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Lv, P.; Almeida, G.; Perré, P. TGA-FTIR analysis of torrefaction of lignocellulosic components (cellulose, xylan, lignin) in isothermal conditions over a wide range of time durations. BioRes 2015, 10, 4239–4251. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, P.F. Rapid. Prototyping & Manufacturing: Fundamentals of Stereolithography; Society of Manufacturing Engineers: Dearborn, MI, USA, 1992; pp. 79–105. [Google Scholar] [CrossRef]
- Maines, E.M.; Porwal, M.K.; Ellison, C.J.; Reineke, T.M. Sustainable advances in SLA/DLP 3D printing materials and processes. Green Chem. 2021, 23, 6863–6897. [Google Scholar] [CrossRef]
- Falkehag, S.I.; Marton, J.; Adler, E. Chromophores in kraft lignin. In Lignin Structure and Reactions; American Chemical Society: Washington, DC, USA, 1966; Volume 59, pp. 75–89. [Google Scholar] [CrossRef]
- Jansen, J.F.G.A.; Dias, A.A.; Dorschu, M.; Coussens, B. Fast Monomers: Factors affecting the inherent reactivity of acrylate monomers in photoinitiated acrylate polymerization. Macromolecules 2003, 36, 3861–3873. [Google Scholar] [CrossRef]
- Amen-Chen, C.; Pakdel, H.; Roy, C. Production of monomeric phenols by thermochemical conversion of biomass: A review. Bioresour. Technol. 2001, 79, 277–299. [Google Scholar] [CrossRef]
- Green, W.A. Industrial Photoinitiators: A Technical Guide; CRC Press: Boca Raton, FL, USA, 2010; pp. 75–113. [Google Scholar] [CrossRef]
- Sen, S.; Patil, S.; Argyropoulos, D.S. Thermal properties of lignin in copolymers, blends, and composites: A review. Green Chem. 2015, 17, 4862–4887. [Google Scholar] [CrossRef]
- Britt, P.F.; Buchanan, A.C.; Cooney, M.J.; Martineau, D.R. Flash vacuum pyrolysis of methoxy-substituted lignin model compounds. J. Org. Chem. 2000, 65, 1376–1389. [Google Scholar] [CrossRef]
- Ali, A.H.; Srinivasan, K.S.V. Studies on the thermal degradation of acrylic polymers by simultaneous autostep TG/DTA. J. Polym. Sci. A Polym. Chem. 1997, 34, 235–246. [Google Scholar] [CrossRef]
- Ballistreri, A.; Foti, S.; Maravigna, P.; Montaudo, G.; Scamporrino, E. Mechanism of thermal degradation of polyurethanes investigated by direct pyrolysis in the mass spectrometer. J. Polym. Sci. Polym. Chem. 1980, 18, 1923–1931. [Google Scholar] [CrossRef]
- Chambers, J.; Jiricny, J.; Reese, C.B. The thermal decomposition of polyurethanes and polyisocyanurates. Fire Mater. 1981, 5, 133–141. [Google Scholar] [CrossRef]









| Lignin | Aliphatic –OH | S –OH | G –OH | H –OH | COOH | % Change in Total –OH |
|---|---|---|---|---|---|---|
| Unmodified | 1.65 | 0.97 | 1.75 | 0.24 | 0.27 | - |
| Pine-R | 2.73 | 1.12 | 1.94 | 0.24 | 0.19 | +27.46 |
| Pine-M | 0.24 | 0.29 | 0.23 | 0.04 | 0.32 | −77.05 |
| Pine-A | 0.13 | 0.14 | 0.11 | 0.00 | 0.36 | −84.84 |
| Pine-MR | 0.25 | 0.11 | 0.06 | 0.01 | 0.19 | −87.30 |
| Resin Formulation | Dp (mm) | Ecrit (mJ·cm−2) | Dosage (mJ·cm−2) |
|---|---|---|---|
| Base Resin | 0.201(2) | 23.7(1) | 30(1) |
| Pine-M | 0.054(1) | 26.1(2) | 66(1) |
| Pine-A | 0.061(4) | 24.9(2) | 57(2) |
| Pine-MR | 0.092(3) | 18.6(2) | 32(1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutton, J.T.; Rajan, K.; Harper, D.P.; Chmely, S.C. Improving UV Curing in Organosolv Lignin-Containing Photopolymers for Stereolithography by Reduction and Acylation. Polymers 2021, 13, 3473. https://doi.org/10.3390/polym13203473
Sutton JT, Rajan K, Harper DP, Chmely SC. Improving UV Curing in Organosolv Lignin-Containing Photopolymers for Stereolithography by Reduction and Acylation. Polymers. 2021; 13(20):3473. https://doi.org/10.3390/polym13203473
Chicago/Turabian StyleSutton, Jordan T., Kalavathy Rajan, David P. Harper, and Stephen C. Chmely. 2021. "Improving UV Curing in Organosolv Lignin-Containing Photopolymers for Stereolithography by Reduction and Acylation" Polymers 13, no. 20: 3473. https://doi.org/10.3390/polym13203473
APA StyleSutton, J. T., Rajan, K., Harper, D. P., & Chmely, S. C. (2021). Improving UV Curing in Organosolv Lignin-Containing Photopolymers for Stereolithography by Reduction and Acylation. Polymers, 13(20), 3473. https://doi.org/10.3390/polym13203473