PLGA Core-Shell Nano/Microparticle Delivery System for Biomedical Application
Abstract
:1. Introduction
2. Drug Delivery
Anti-Cancer Drug Delivery
3. Tissue Regeneration
3.1. Bone Regeneration
3.2. Cartilage Regeneration
3.3. Periodontal Regeneration
4. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sangi, S.; SreeHarsha, N.; Bawadekji, A.; Al Ali, M. Chemotherapeutic drug targeting to lungs by way of microspheres after intravenous administration. Drug Des. Devel. Ther. 2018, 12, 3051–3060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalla, A.; Bhat, M. Chapter Twelve—Smart polymer composites in drug delivery. In Smart Polymer Nanocomposites; Bhawani, S.A., Khan, A., Jawaid, M., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 261–294. [Google Scholar]
- Blasi, P. Poly(lactic acid)/poly(lactic-co-glycolic acid)-based microparticles: An overview. J. Pharm. Investig. 2019, 49, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Ayyanaar, S.; Kesavan, M.P.; Balachandran, C.; Rasala, S.; Rameshkumar, P.; Aoki, S.; Rajesh, J.; Webster, T.J.; Rajagopal, G. Iron oxide nanoparticle core-shell magnetic microspheres: Applications toward targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102134. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Routh, A.F. Benign preparation of aqueous core poly lactic-co-glycolic acid (PLGA) microcapsules. J. Colloid Interface Sci. 2018, 513, 1–9. [Google Scholar] [CrossRef]
- Hayes, R.; Ahmed, A.; Edge, T.; Zhang, H. Core–shell particles: Preparation, fundamentals and applications in high performance liquid chromatography. J. Chromatogr. A 2014, 1357, 36–52. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Shen, L.; Zhu, Z.; Luo, X.; Zhai, Y.; Hua, X.; Zhao, S.; Cen, L.; Zhang, Z. A cell-free therapy for articular cartilage repair based on synergistic delivery of SDF-1 & KGN with HA injectable scaffold. Chem. Eng. J. 2020, 393, 124649. [Google Scholar] [CrossRef]
- Galogahi, F.M.; Zhu, Y.; An, H.; Nguyen, N.-T. Core-shell microparticles: Generation approaches and applications. J. Sci. Adv. Mater. Devices 2020, 5, 417–435. [Google Scholar] [CrossRef]
- Yeh, H.W.; Chen, D.R. In vitro release profiles of PLGA core-shell composite particles loaded with theophylline and budesonide. Int. J. Pharm. 2017, 528, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, N.; Liu, W.; Xie, Z. Polypyrrole coated PLGA core–shell nanoparticles for drug delivery and photothermal therapy. RSC Adv. 2016, 6, 84269–84275. [Google Scholar] [CrossRef]
- He, H.; Markoutsa, E.; Zhan, Y.; Zhang, J.; Xu, P. Mussel-inspired PLGA/polydopamine core-shell nanoparticle for light induced cancer thermochemotherapy. Acta Biomater. 2017, 59, 181–191. [Google Scholar] [CrossRef]
- Shang, T.; Liu, J.; Chen, Y.; Hu, Z.; Deng, L.; Ran, H.; Li, P.; Zheng, Y.; Wang, D.; Wang, Z.; et al. In vivo targeted cancer theranostics by core/shell-structured multifunctional prussian Blue/PLGA “Nanococktails”. Part. Part. Syst. Charact. 2018, 35, 1700306. [Google Scholar] [CrossRef]
- Xu, Q.; Leong, J.; Chua, Q.Y.; Chi, Y.T.; Chow, P.K.; Pack, D.W.; Wang, C.H. Combined modality doxorubicin-based chemotherapy and chitosan-mediated p53 gene therapy using double-walled microspheres for treatment of human hepatocellular carcinoma. Biomaterials 2013, 34, 5149–5162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Xia, Y.; Wang, C.H.; Pack, D.W. Monodisperse double-walled microspheres loaded with chitosan-p53 nanoparticles and doxorubicin for combined gene therapy and chemotherapy. J. Control. Release 2012, 163, 130–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Oller, I.; Padial-Molina, M.; Galindo-Moreno, P.; O’Valle, F.; Jódar-Reyes, A.B.; Peula-García, J.M. Bone regeneration from PLGA micro-nanoparticles. BioMed Res. Int. 2015, 2015, 415289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-García, P.; Reyes, R.; Segredo-Morales, E.; Pérez-Herrero, E.; Delgado, A.; Évora, C. PLGA-BMP-2 and PLA-17β-Estradiol microspheres reinforcing a composite hydrogel for bone regeneration in osteoporosis. Pharmaceutics 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, P.C.; Chung, M.C.; Lei, C.; Chong, L.Y.; Wang, C.H. Biocompatibility of PDGF-simvastatin double-walled PLGA (PDLLA) microspheres for dentoalveolar regeneration: A preliminary study. J. Biomed. Mater. Res. Part A 2012, 100, 2970–2978. [Google Scholar] [CrossRef]
- Chang, P.C.; Chong, L.Y.; Dovban, A.S.; Lim, L.P.; Lim, J.C.; Kuo, M.Y.; Wang, C.H. Sequential platelet-derived growth factor-simvastatin release promotes dentoalveolar regeneration. Tissue Eng. Part A 2014, 20, 356–364. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.C.; Dovban, A.S.; Lim, L.P.; Chong, L.Y.; Kuo, M.Y.; Wang, C.H. Dual delivery of PDGF and simvastatin to accelerate periodontal regeneration in vivo. Biomaterials 2013, 34, 9990–9997. [Google Scholar] [CrossRef]
- Hao, Y.; Tian, R.; Lv, K.; Liu, Z.; Ni, J.; Yuan, P.; Bai, Y.; Chen, X. Stimuli responsive co-delivery of celecoxib and BMP2 from micro-scaffold for periodontal disease treatment. J. Mater. Sci. Technol. 2021, 75, 216–224. [Google Scholar] [CrossRef]
- Patel, R.; Patel, M.; Kwak, J.; Iyer, A.K.; Karpoormath, R.; Desai, S.; Rarh, V. Polymeric microspheres: A delivery system for osteogenic differentiation. Polym. Adv. Technol. 2017, 28, 1595–1609. [Google Scholar] [CrossRef]
- Patel, M.; Jha, A.; Patel, R. Potential application of PLGA microsphere for tissue engineering. J. Polym. Res. 2021, 28, 214. [Google Scholar] [CrossRef]
- Félix Lanao, R.P.; Jonker, A.M.; Wolke, J.G.; Jansen, J.A.; van Hest, J.C.; Leeuwenburgh, S.C. Physicochemical properties and applications of poly(lactic-co-glycolic acid) for use in bone regeneration. Tissue Eng. Part B Rev. 2013, 19, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Bee, S.-L.; Hamid, Z.A.A.; Mariatti, M.; Yahaya, B.H.; Lim, K.; Bee, S.-T.; Sin, L.T. Approaches to improve therapeutic efficacy of biodegradable PLA/PLGA microspheres: A review. Polym. Rev. 2018, 58, 495–536. [Google Scholar] [CrossRef]
- Jenjob, R.; Phakkeeree, T.; Crespy, D. Core–shell particles for drug-delivery, bioimaging, sensing, and tissue engineering. Biomater. Sci. 2020, 8, 2756–2770. [Google Scholar] [CrossRef]
- Yazdian Kashani, S.; Afzalian, A.; Shirinichi, F.; Keshavarz Moraveji, M. Microfluidics for core–shell drug carrier particles—A review. RSC Adv. 2021, 11, 229–249. [Google Scholar] [CrossRef]
- Li, Y.; Yan, D.; Fu, F.; Liu, Y.; Zhang, B.; Wang, J.; Shang, L.; Gu, Z.; Zhao, Y. Composite core-shell microparticles from microfluidics for synergistic drug delivery. Sci. China Mater. 2017, 60, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Yao, Q.; Zhang, Y.; Chen, H.; He, H.; Zhang, Y.; Yin, T.; Tang, X.; Xu, H. Core/shell PLGA microspheres with controllable in vivo release profile via rational core phase design. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1070–1079. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.-C.; Tong, Y.W.; Wang, C.-H. Coaxial electrohydrodynamic atomization toward large scale production of core-shell structured microparticles. AlChE J. 2017, 63, 5303–5319. [Google Scholar] [CrossRef]
- Lim, M.P.A.; Lee, W.L.; Widjaja, E.; Loo, S.C.J. One-step fabrication of core–shell structured alginate–PLGA/PLLA microparticles as a novel drug delivery system for water soluble drugs. Biomater. Sci. 2013, 1, 486–493. [Google Scholar] [CrossRef]
- Qi, P.; Bu, R.; Zhang, H.; Yin, J.; Chen, J.; Zhang, A.; Gou, J.; Yin, T.; Zhang, Y.; He, H.; et al. Goserelin acetate loaded poloxamer hydrogel in PLGA microspheres: Core–shell di-depot intramuscular sustained release delivery system. Mol. Pharm. 2019, 16, 3502–3513. [Google Scholar] [CrossRef]
- Abulateefeh, S.R.; Alkilany, A.M. Synthesis and Characterization of PLGA Shell microcapsules containing aqueous cores prepared by internal phase separation. AAPS Pharm. Sci. Tech. 2016, 17, 891–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abulateefeh, S.R.; Alkawareek, M.Y.; Alkilany, A.M. Tunable sustained release drug delivery system based on mononuclear aqueous core-polymer shell microcapsules. Int. J. Pharm. 2019, 558, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Wang, J.; Wang, C.-H. Double-walled microspheres for the sustained release of a highly water soluble drug: Characterization and irradiation studies. J. Control. Release 2002, 83, 437–452. [Google Scholar] [CrossRef]
- Chen, M.-M.; Cao, H.; Liu, Y.-Y.; Liu, Y.; Song, F.-F.; Chen, J.-D.; Zhang, Q.-Q.; Yang, W.-Z. Sequential delivery of chlorhexidine acetate and bFGF from PLGA-glycol chitosan core-shell microspheres. Colloids Surf. B Biointerfaces 2017, 151, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Liu, Z.; Li, Y.; Zhu, S.; Ma, J.; Li, W.; Gao, G. Development and characterization of cores-shell poly(lactide-co-glycolide)-chitosan microparticles for sustained release of GDNF. Colloids Surf. B Biointerfaces 2017, 159, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kong, T.; Yeung, K.W.K.; Shum, H.C.; Cheung, K.M.C.; Wang, L.; To, M.K.T. Fabrication and characterization of monodisperse PLGA–alginate core–shell microspheres with monodisperse size and homogeneous shells for controlled drug release. Acta Biomater. 2013, 9, 7410–7419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Srivastava, R.S.; Misra, R.D.K. Core−Shell Magnetite Nanoparticles surface encapsulated with smart stimuli-responsive polymer: synthesis, characterization, and LCST of viable drug-targeting delivery system. Langmuir 2007, 23, 6342–6351. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.; Ma, L.; Lin, A.; Gong, Y.; Yuan, G.; Liu, J. A core-shell structure QRu-PLGA-RES-DS NP nanocomposite with photothermal response-induced M2 macrophage polarization for rheumatoid arthritis therapy. Nanoscale 2019, 11, 18209–18223. [Google Scholar] [CrossRef]
- Gajendiran, M.; Gopi, V.; Elangovan, V.; Murali, R.V.; Balasubramanian, S. Isoniazid loaded core shell nanoparticles derived from PLGA-PEG-PLGA tri-block copolymers: In vitro and in vivo drug release. Colloids Surf. B Biointerfaces 2013, 104, 107–115. [Google Scholar] [CrossRef]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.-W.; Langer, R.; Farokhzad, O.C. PLGA–lecithin–PEG core–shell nanoparticles for controlled drug delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef]
- Chitkara, D.; Kumar, N. BSA-PLGA-based core-shell nanoparticles as carrier system for water-soluble drugs. Pharm. Res. 2013, 30, 2396–2409. [Google Scholar] [CrossRef]
- Sadat Akhavi, S.; Moradi Dehaghi, S. Drug delivery of amphotericin B through core-shell composite based on PLGA/Ag/Fe(3)O(4): In vitro test. Appl. Biochem. Biotechnol. 2020, 191, 496–510. [Google Scholar] [CrossRef]
- Varga, N.; Turcsányi, Á.; Hornok, V.; Csapó, E. Vitamin E-loaded PLA- and PLGA-based core-shell nanoparticles: Synthesis, structure optimization and controlled drug release. Pharmaceutics 2019, 11, 357. [Google Scholar] [CrossRef] [Green Version]
- Basu, T.; Pal, B.; Singh, S. Fabrication of core–shell PLGA/PLA–pNIPAM nanocomposites for improved entrapment and release kinetics of antihypertensive drugs. Particuology 2018, 40, 169–176. [Google Scholar] [CrossRef]
- Guan, X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.L.; Guo, W.M.; Ho, V.H.; Saha, A.; Chong, H.C.; Tan, N.S.; Widjaja, E.; Tan, E.Y.; Loo, S.C. Inhibition of 3-D tumor spheroids by timed-released hydrophilic and hydrophobic drugs from multilayered polymeric microparticles. Small 2014, 10, 3986–3996. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 1–12. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, Z.; Qiu, N.; Shen, Y. Rational design of cancer nanomedicine: Nanoproperty integration and synchronization. Adv. Mater. 2017, 29, 1606628. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [Green Version]
- Schöttler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailänder, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly (ethylene glycol)-and poly (phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef]
- Verma, N.K.; Crosbie-Staunton, K.; Satti, A.; Gallagher, S.; Ryan, K.B.; Doody, T.; McAtamney, C.; MacLoughlin, R.; Galvin, P.; Burke, C.S.; et al. Magnetic core-shell nanoparticles for drug delivery by nebulization. J. Nanobiotechnol. 2013, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fang, K.; Song, L.; Gu, Z.; Yang, F.; Zhang, Y.; Gu, N. Magnetic field activated drug release system based on magnetic PLGA microspheres for chemo-thermal therapy. Colloids Surf. B Biointerfaces 2015, 136, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, H.; Liang, C.; Liu, Y.; Li, Z.; Yang, G.; Cheng, L.; Li, Y.; Liu, Z. Iron oxide @ polypyrrole nanoparticles as a multifunctional drug carrier for remotely controlled cancer therapy with synergistic antitumor effect. ACS Nano 2013, 7, 6782–6795. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.L.; Lin, T.T.; Sureshbabu, R.; Chia, W.T.; Hsiao, H.C.; Liu, H.Y.; Yang, C.M.; Sung, H.W. A rapid drug release system with a NIR light-activated molecular switch for dual-modality photothermal/antibiotic treatments of subcutaneous abscesses. J. Control. Release 2015, 199, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Chen, C.-C.; Nguyen, D.; Su, H.-R.; Lin, K.-J.; Chen, H.-L.; Hu, Y.-J.; Lai, P.-L.; Sung, H.-W. Biomimetic engineering of a scavenger-free nitric oxide-generating/delivering system to enhance radiation therapy. Small 2020, 16, 2000655. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.F.; Dias, D.R.; Costa, E.C.; Correia, I.J. Thermo- and pH-responsive nano-in-micro particles for combinatorial drug delivery to cancer cells. Eur. J. Pharm. Sci. 2017, 104, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Schiff, P.B.; Fant, J.; Horwitz, S.B. Promotion of microtubule assembly in vitro by taxol. Nature 1979, 277, 665–667. [Google Scholar] [CrossRef]
- Kuh, H.-J.; Jang, S.H.; Wientjes, M.G.; Weaver, J.R.; Au, J.L.-S. Determinants of paclitaxel penetration and accumulation in human solid tumor. J. Pharm. Exp. Ther. 1999, 290, 871–880. [Google Scholar]
- Dwivedi, P.; Han, S.; Mangrio, F.; Fan, R.; Dwivedi, M.; Zhu, Z.; Huang, F.; Wu, Q.; Khatik, R.; Cohn, D.E.; et al. Engineered multifunctional biodegradable hybrid microparticles for paclitaxel delivery in cancer therapy. Mater. Sci. Eng. C 2019, 102, 113–123. [Google Scholar] [CrossRef]
- Ramasamy, T.; Haidar, Z.S.; Tran, T.H.; Choi, J.Y.; Jeong, J.-H.; Shin, B.S.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Layer-by-layer assembly of liposomal nanoparticles with PEGylated polyelectrolytes enhances systemic delivery of multiple anticancer drugs. Acta Biomater. 2014, 10, 5116–5127. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, Y.; Zhu, Z.; Zhao, H.; Yao, J.; Sun, D. Paclitaxel and etoposide-loaded Poly (lactic-co-glycolic acid) microspheres fabricated by coaxial electrospraying for dual drug delivery. J. Biomater. Sci. Polym. Ed. 2018, 29, 1949–1963. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, F.; Cai, Y.; Chen, Y.; Wei, L.; Liu, Z.; Yuan, W. Local antitumor effects of intratumoral delivery of rlL-2 loaded sustained-release dextran/PLGA-PLA core/shell microspheres. Int. J. Pharm. 2013, 450, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Reardon, P.J.; Parhizkar, M.; Harker, A.H.; Browning, R.J.; Vassileva, V.; Stride, E.; Pedley, R.B.; Edirisinghe, M.; Knowles, J.C. Electrohydrodynamic fabrication of core-shell PLGA nanoparticles with controlled release of cisplatin for enhanced cancer treatment. Int. J. Nanomed. 2017, 12, 3913–3926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liang, Y.; Fei, S.; He, H.; Zhang, Y.; Yin, T.; Tang, X. Formulation and pharmacokinetics of HSA-core and PLGA-shell nanoparticles for delivering gemcitabine. AAPS Pharm Sci. Tech. 2018, 19, 812–819. [Google Scholar] [CrossRef]
- Narayanan, S.; Pavithran, M.; Viswanath, A.; Narayanan, D.; Mohan, C.C.; Manzoor, K.; Menon, D. Sequentially releasing dual-drug-loaded PLGA-casein core/shell nanomedicine: Design, synthesis, biocompatibility and pharmacokinetics. Acta Biomater. 2014, 10, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Mony, U.; Vijaykumar, D.K.; Koyakutty, M.; Paul-Prasanth, B.; Menon, D. Sequential release of epigallocatechin gallate and paclitaxel from PLGA-casein core/shell nanoparticles sensitizes drug-resistant breast cancer cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Gallego, M.R.; Nielsen, L.F.; Jorgensen, L.; Møller, E.H.; Nielsen, H.M. Design and characterization of core-shell mPEG-PLGA composite microparticles for development of cell-scaffold constructs. Eur. J. Pharm. Biopharm. 2013, 85, 87–98. [Google Scholar] [CrossRef]
- Mistry, A.S.; Mikos, A.G. Tissue engineering strategies for bone regeneration. Adv. Biochem. Eng. Biotechnol. 2005, 94, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Y.; Li, R.; Zeng, J.; Li, Z.; Tang, Y.; Sun, D. RhBMP-2-loaded Poly(lactic-co-glycolic acid) microspheres fabricated by coaxial electrospraying for protein delivery. J. Biomater. Sci. Polym. Ed. 2017, 28, 2205–2219. [Google Scholar] [CrossRef]
- Cai, Y.; Tong, S.; Zhang, R.; Zhu, T.; Wang, X. In vitro evaluation of a bone morphogenetic protein-2 nanometer hydroxyapatite collagen scaffold for bone regeneration. Mol. Med. Rep. 2018, 17, 5830–5836. [Google Scholar] [CrossRef]
- Wang, S.; Ju, W.; Shang, P.; Lei, L.; Nie, H. Core–shell microspheres delivering FGF-2 and BMP-2 in different release patterns for bone regeneration. J. Mater. Chem. B 2015, 3, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, Y.; Zhang, X.; Xu, M.; Liu, F.; Ma, Q.; Cai, Q.; Deng, X. PLGA/PDLLA core–shell submicron spheres sequential release system: Preparation, characterization and promotion of bone regeneration in vitro and in vivo. Chem. Eng. J. 2015, 273, 490–501. [Google Scholar] [CrossRef]
- Choi, D.H.; Park, C.H.; Kim, I.H.; Chun, H.J.; Park, K.; Han, D.K. Fabrication of core–shell microcapsules using PLGA and alginate for dual growth factor delivery system. J. Control. Release 2010, 147, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wei, P.; Huang, Y.; Zhang, W.; Chen, F.; Zhang, X.; Mao, J.; Chen, D.; Cai, Q.; Yang, X. Injectable PLGA microspheres with tunable magnesium ion release for promoting bone regeneration. Acta Biomater. 2019, 85, 294–309. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, J.; Qiao, W.; Zhao, Y.; Wong, K.H.M.; Chu, P.K.; Bian, L.; Wu, S.; Zheng, Y.; Cheung, K.M.C.; et al. Precisely controlled delivery of magnesium ions thru sponge-like monodisperse PLGA/nano-MgO-alginate core-shell microsphere device to enable in-situ bone regeneration. Biomaterials 2018, 174, 1–16. [Google Scholar] [CrossRef]
- Vinatier, C.; Bouffi, C.; Merceron, C.; Gordeladze, J.; Brondello, J.-M.; Jorgensen, C.; Weiss, P.; Guicheux, J.; Noël, D. Cartilage tissue engineering: Towards a biomaterial-assisted mesenchymal stem cell therapy. Curr. Stem Cell Res. Ther. 2009, 4, 318–329. [Google Scholar] [CrossRef] [Green Version]
- Atoufi, Z.; Kamrava, S.K.; Davachi, S.M.; Hassanabadi, M.; Saeedi Garakani, S.; Alizadeh, R.; Farhadi, M.; Tavakol, S.; Bagher, Z.; Hashemi Motlagh, G. Injectable PNIPAM/Hyaluronic acid hydrogels containing multipurpose modified particles for cartilage tissue engineering: Synthesis, characterization, drug release and cell culture study. Int. J. Biol. Macromol. 2019, 139, 1168–1181. [Google Scholar] [CrossRef]
- Kaigler, D.; Cirelli, J.A.; Giannobile, W.V. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin. Drug Deliv. 2006, 3, 647–662. [Google Scholar] [CrossRef]
- Chong, L.Y.; Chien, L.Y.; Chung, M.C.; Liang, K.; Lim, J.C.; Fu, J.H.; Wang, C.H.; Chang, P.C. Controlling the proliferation and differentiation stages to initiate periodontal regeneration. Connect. Tissue Res. 2013, 54, 101–107. [Google Scholar] [CrossRef]
- Swider, E.; Koshkina, O.; Tel, J.; Cruz, L.J.; de Vries, I.J.M.; Srinivas, M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018, 73, 38–51. [Google Scholar] [CrossRef] [PubMed]






| Material | Fabrication Technique | Bioactive Molecule | Application | Ref. | |
|---|---|---|---|---|---|
| Core | Shell | ||||
| Aqueous core | PLGA | Modified internal phase separation | Risedronate sodium (hydrophilic) | Osteoporosis | [34] |
| PLGA or PLA | PNIPAM | Single emulsion + aqueous free radical precipitation polymerization | Ramipril | Anti-hypertensive | [47] |
| PLGA | PEG | Nanoprecipitation + self-assembly | Docetaxel | Chemotherapy | [43] |
| PLGA | GC | W/O/W emulsion–solvent evaporation | bFGF/CHA | Chronic wound | [37] |
| QRuNPs | PLGA–DS | Double emulsion | Resveratrol | Rheumatoid arthritis | [41] |
| BSA | PLGA | W/O/W double emulsion | Gemcitabine | Anti-cancer Agent | [44] |
| PLGA | PLGA | W/O/W double emulsion | INH | Mycobacterium tuberculosis | [42] |
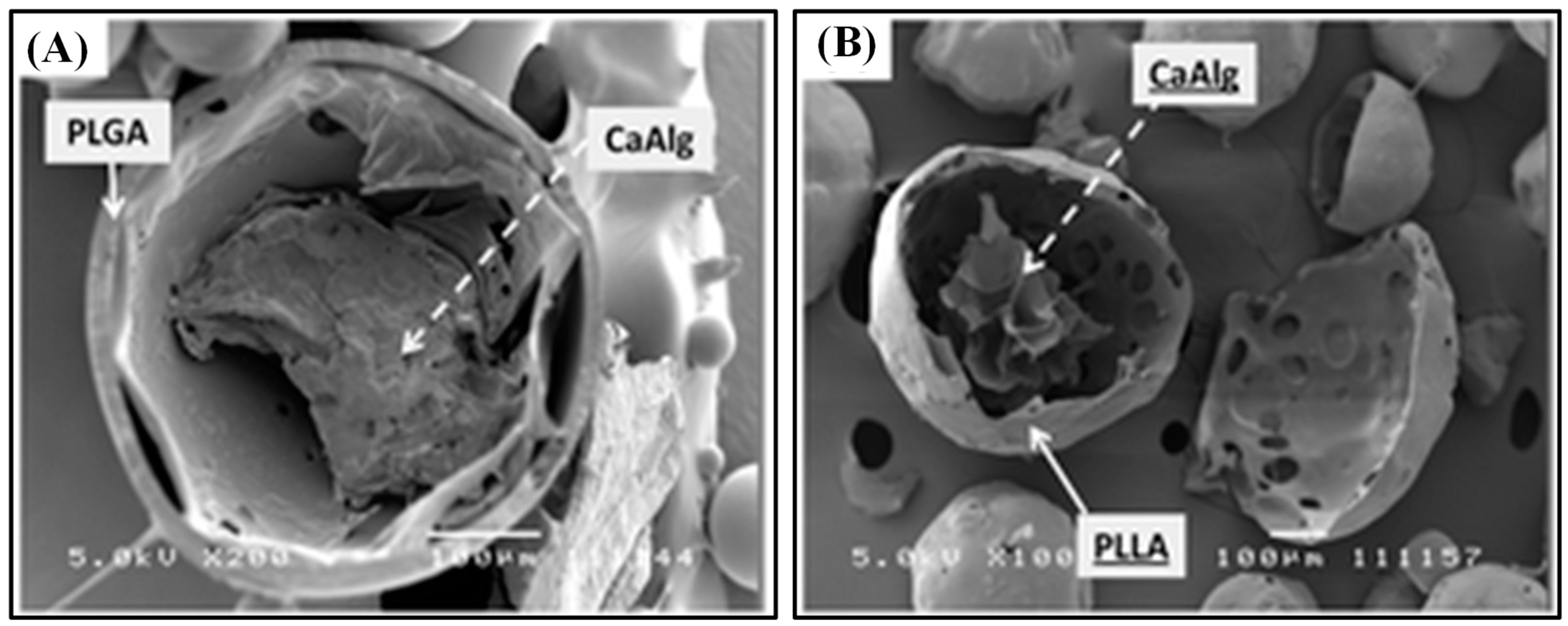
| Ca Alg | PLGA | W/O/W double emulsion | MCA (hydrophilic) | Anti-emetic and gastroprokinetic agent | [32] |
| PLGA | Ppy NPs | Quasi-emulsion diffusion + SPG membrane emulsification | Curcumin (hydrophobic, poor bioavailability) | Anti-cancer | [11] |
| PLGA | Casein | O/W emulsion precipitation | PTX (hydrophobic) EGCG (hydrophilic) | Anti-cancer | [68] |
| PLGA | PLGA | Single emulsion using vegetable oil | Rhodamine B | Hydrophilic model drug | [6] |
| PLGA | PLGA | W/O/W double emulsion | Goserelin acetate | Prostate cancer hormonal therapy | [33] |
| PVP or PLGA | PEG | Emulsion solvent evaporation | Amphotericin B | Fungal infections | [45] |
| PLA or PLGA | Pluronic F127 | Nanoprecipitation | (±)-α-Tocopherol | Vitamin E | [46] |
| HSA | PLGA | Modified W/O/W double emulsification solvent evaporation | Gemcitabine (hydrophilic) | Anti-cancer agent | [67] |
| PLGA | Alginate | Capillary microfluidic method | Rifampicin | Antibiotic | [39] |
| PLGA | PLGA | Dual capillary electrospray | Budesonide (hydrophobic) Theophylline (hydrophilic) | Asthma | [10] |
| LP powder, water, gelatin, or F127 | PLGA | Modified w/o/w double emulsification solvent evaporation | LP (hydrophilic, small) | [30] | |
| Fe3O4 NPs | PLGA/PEG | W/O/W double emulsion | Lecithin curcumin | Reactive oxygen species responsive antioxidant | [5] |
| Aqueous core | PLGA | Capillary fluidic device | Vancomycin, PPy NPs | Photothermal agent | [57] |
| PLGA | Lipid | CEH process | PTX | Ovarian cancer | [62] |
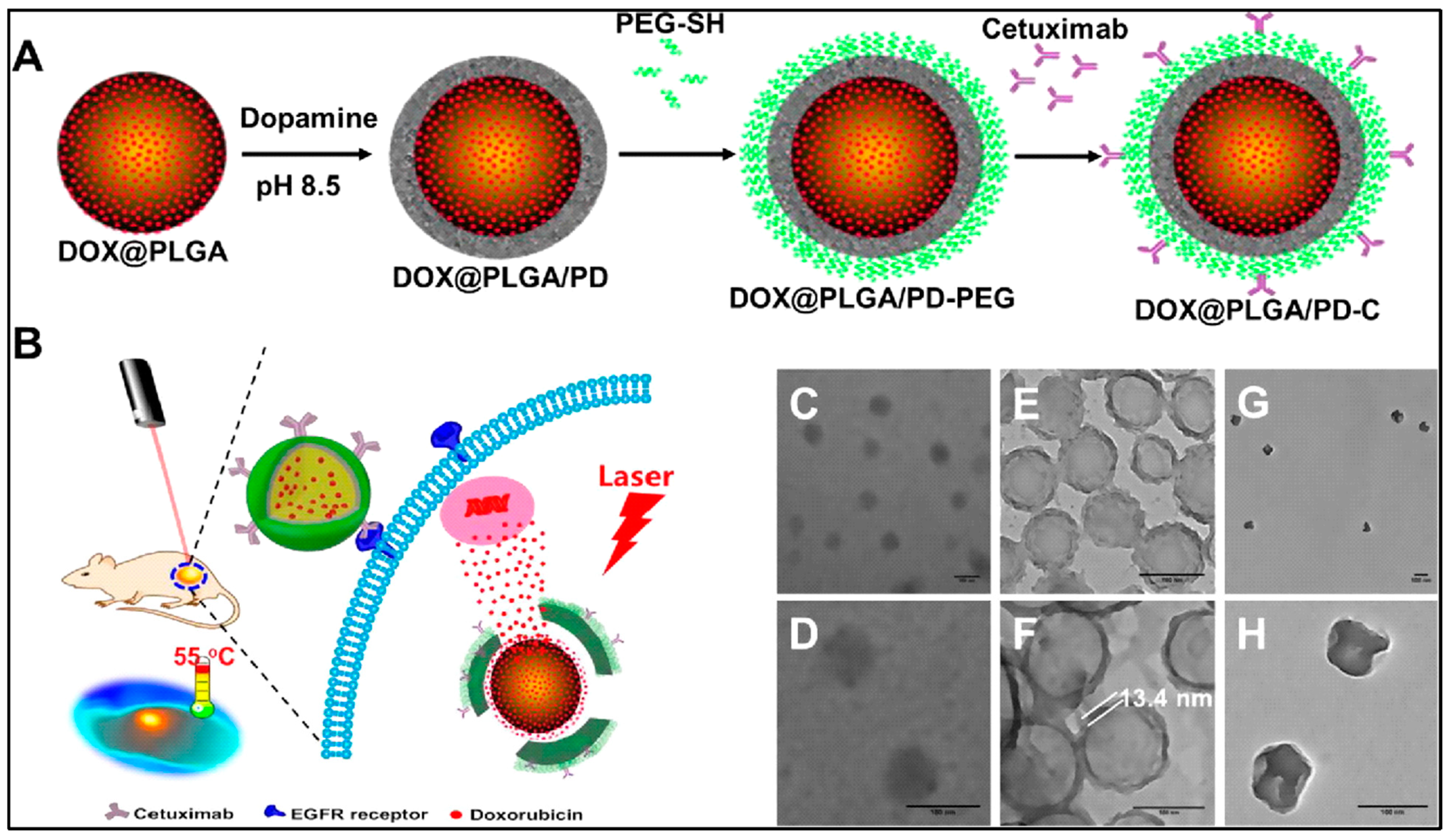
| PLGA | PD | Modified emulsion | DOX/ anti-EGFR antibody | Head and neck cancer photothermal and chemotherapy | [12] |
| PLLA | PLGA | One-step emulsion solvent evaporation | PTX (hydrophobic) DOX HCl (hydrophilic) | Anti-cancer agent | [49] |
| AuMSSs | PLGA | W/O/W double emulsion | DOX/ salicylic acid | pH- and thermo- responsive cervical cancer treatment | [59] |
| PLGA | Casein | Emulsion–precipitation | PTX/EGCG | Targeted breast-cancer therapy | [69] |
| Cisplatin | PLGA | EHDA | CDDP | Cancer chemotherapy | [66] |
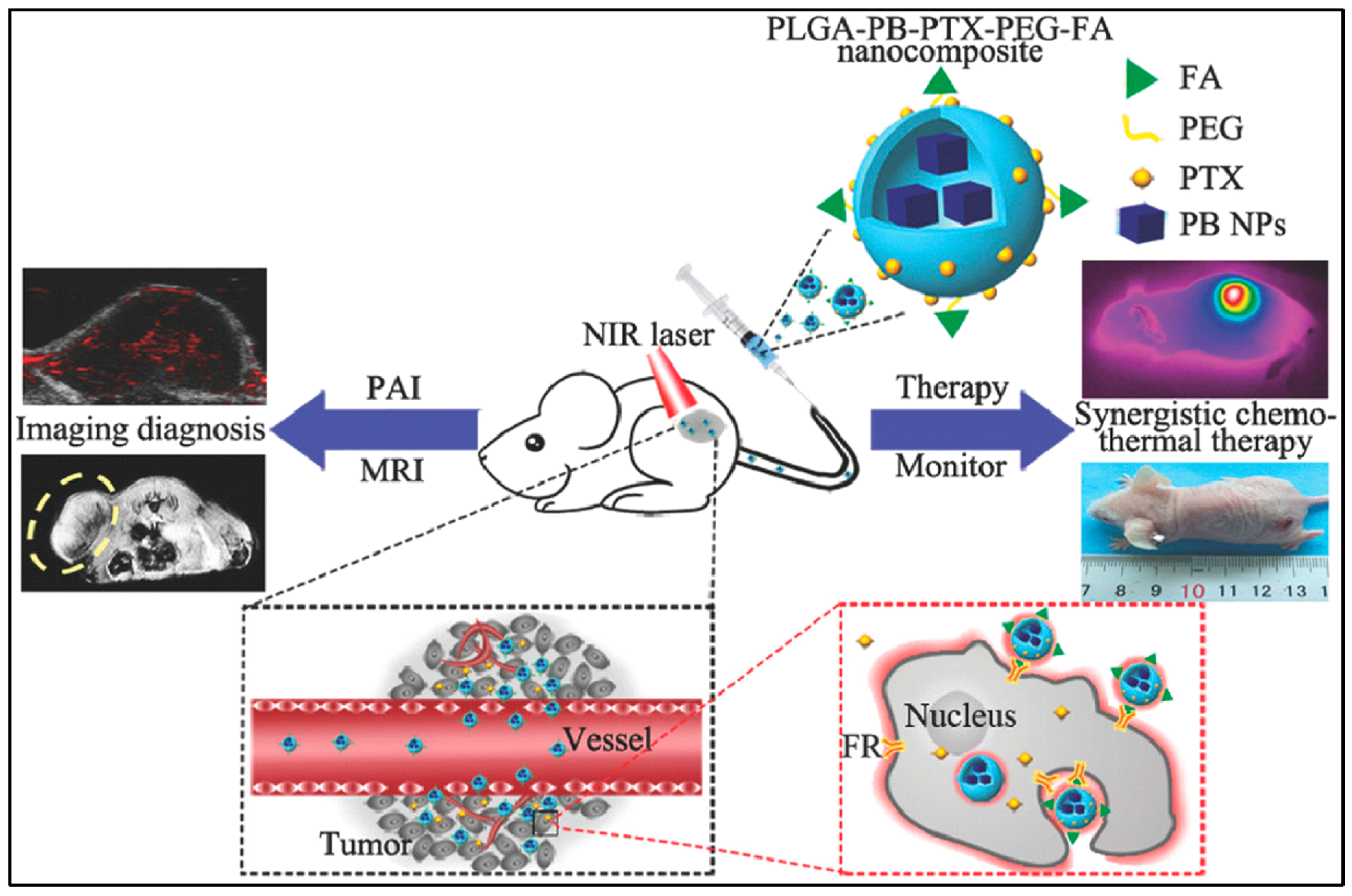
| PB NPs | PLGA/PEG | Modified W/O/W double emulsification solvent evaporation | PTX/FA | Breast-cancer targeted photothermal and chemotherapy | [13] |
| PLGA | PLLA | Precision particle fabrication | DOX/chi-p53 | Hepatocellular carcinoma chemotherapy and gene therapy | [14] |
| PLGA | PLA | Precision particle fabrication | DOX/chi-p53 | Combined gene therapy and chemotherapy | [15] |
| PLGA | PLGA | Coaxial electrospraying | PTX + ETP | Osteosarcoma | [64] |
| PLGA | PLA | Stabilizing aqueous–aqueous emulsion | rIL-2 | Anti-tumor agent | [65] |
| PLGA | PLA | W/O/W double emulsion | BMP2 17β-estradiol | Osteoporosis bone regeneration | [17] |
| PLGA | Alg | Customized microfluidic capillary device | Mg2+ | In situ bone regeneration | [78] |
| PLGA | PLGA | Coaxial electrospraying | rhBMP-2 | Bone regeneration | [72] |
| PLGA | CH-g-AA | Single emulsion solvent evaporation + physical adsorption | Melatonin | Cartilage tissue regeneration | [80] |
| mPEG | PLGA | Coaxial ultrasonic atomization | Alg/CS/DS | Dermatan sulfate deliver system | [70] |
| PLGA | PLGA | W/O/W microfluidic emulsion | SDF-1 KGN | Cartilage tissue regeneration | [8] |
| PDLLA | PLGA | CEHDA | PDGF/simvastatin | Dentoalveolar Regeneration | [19] |
| PDLLA | PLGA | CEHDA | PDGF/simvastatin | Periodontal tissue regeneration | [18] |
| PDLLA | PLGA | CEHDA | PDGF/simvastatin | Periodontal tissue regeneration | [20] |
| MSNs | PLGA | Modified w/o/w double emulsification | Celecoxib BMP-2 | Periodontal disease | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.M.; Patel, M.; Patel, R. PLGA Core-Shell Nano/Microparticle Delivery System for Biomedical Application. Polymers 2021, 13, 3471. https://doi.org/10.3390/polym13203471
Kim SM, Patel M, Patel R. PLGA Core-Shell Nano/Microparticle Delivery System for Biomedical Application. Polymers. 2021; 13(20):3471. https://doi.org/10.3390/polym13203471
Chicago/Turabian StyleKim, Se Min, Madhumita Patel, and Rajkumar Patel. 2021. "PLGA Core-Shell Nano/Microparticle Delivery System for Biomedical Application" Polymers 13, no. 20: 3471. https://doi.org/10.3390/polym13203471
APA StyleKim, S. M., Patel, M., & Patel, R. (2021). PLGA Core-Shell Nano/Microparticle Delivery System for Biomedical Application. Polymers, 13(20), 3471. https://doi.org/10.3390/polym13203471








