Preparation of a Cage-Type Polyglycolic Acid/Collagen Nanofiber Blend with Improved Surface Wettability and Handling Properties for Potential Biomedical Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
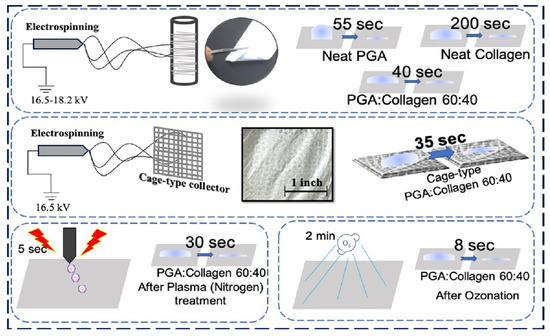
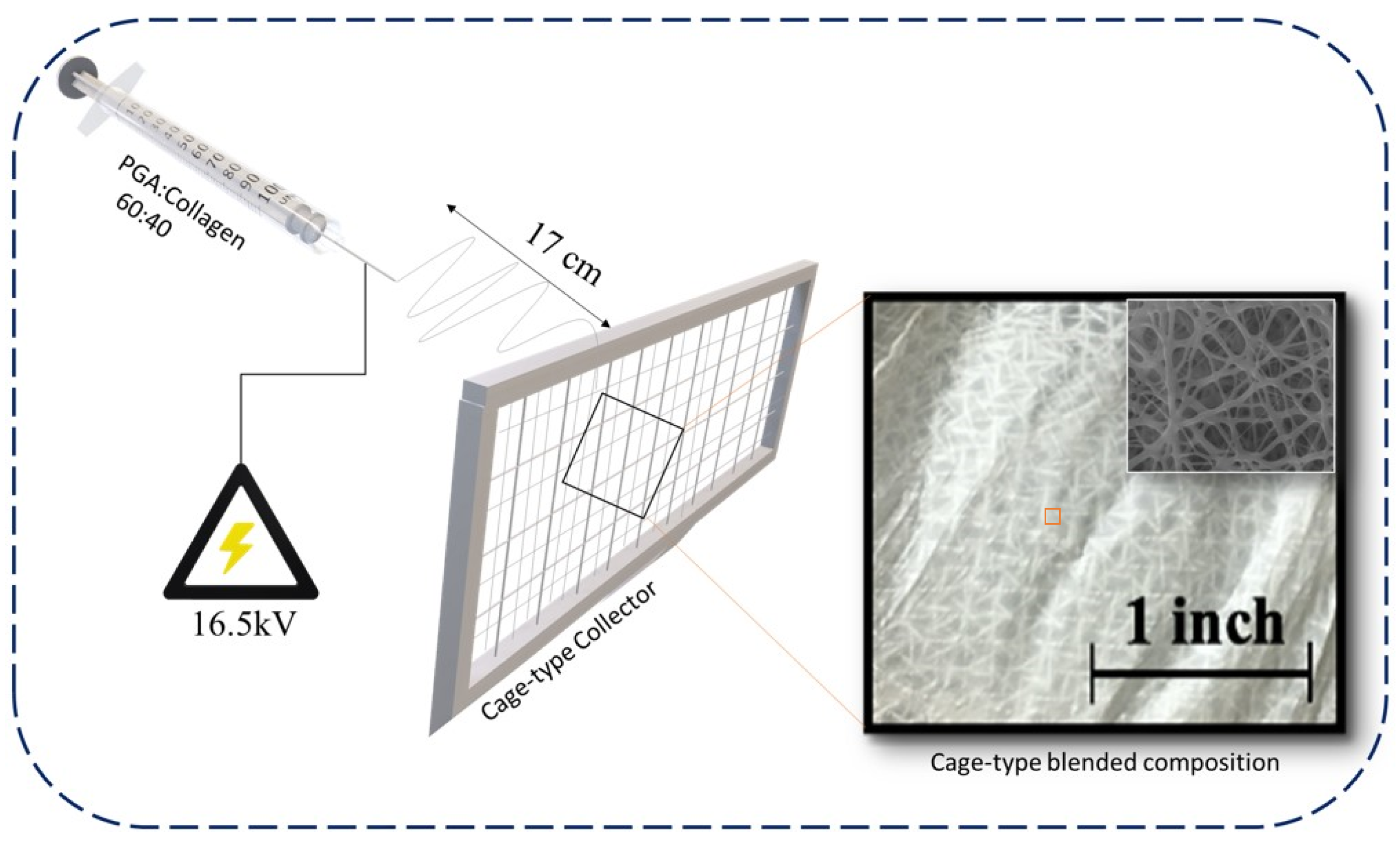
Preparation of PGA and Collagen and Cage-Type Nanofiber Blend via Electrospinning
3. Characterization
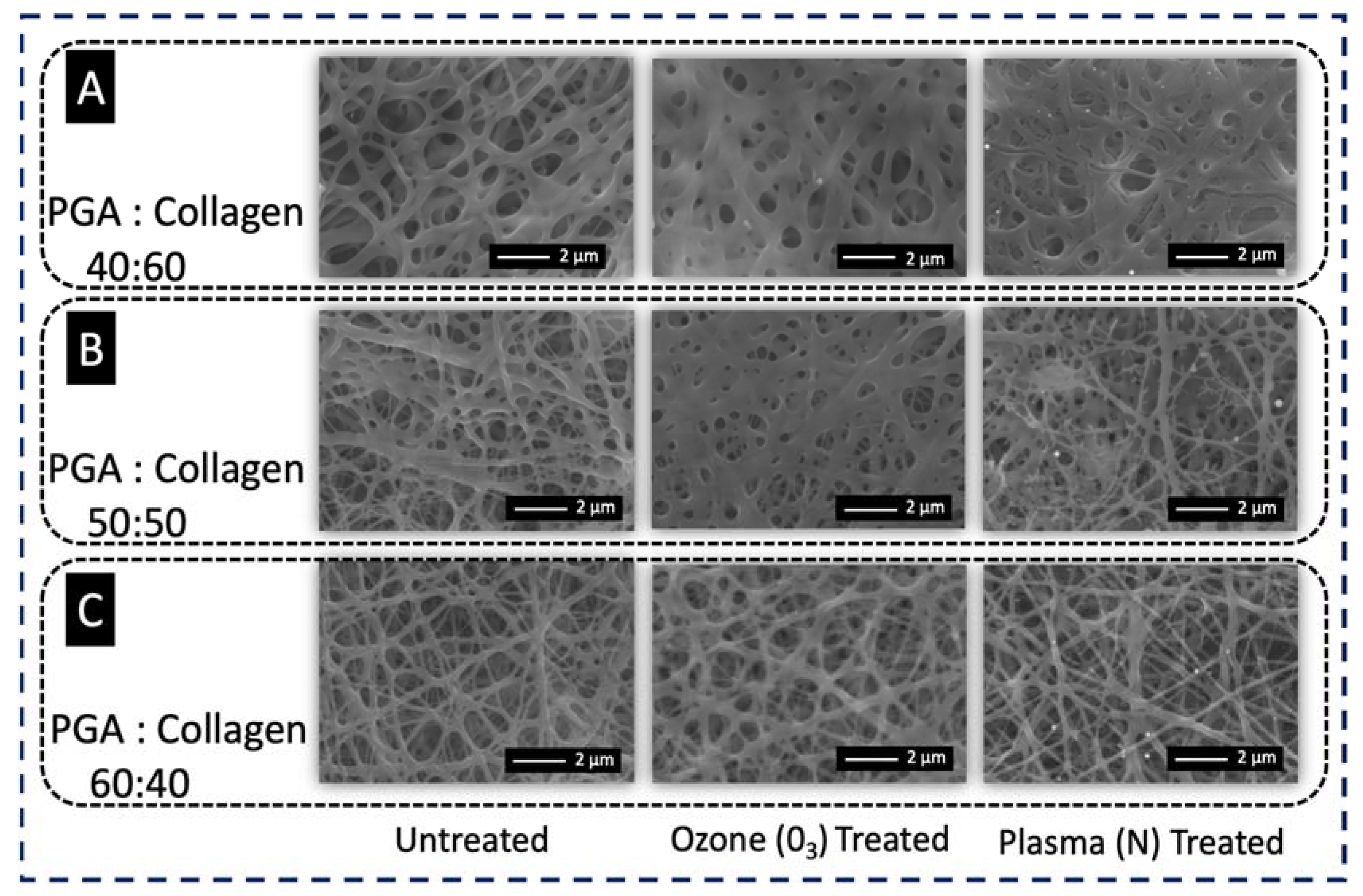
3.1. Scanning Electron Microscopy (SEM)
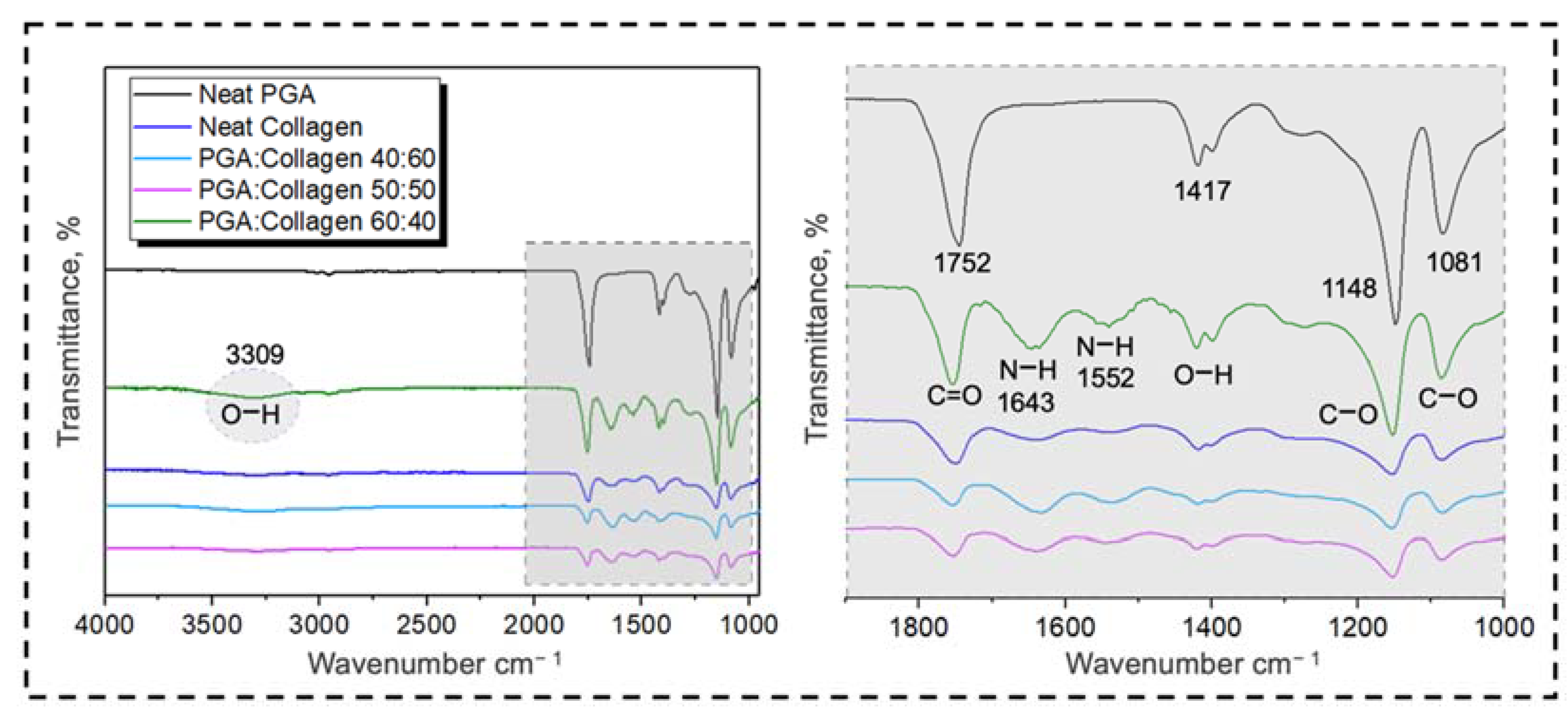
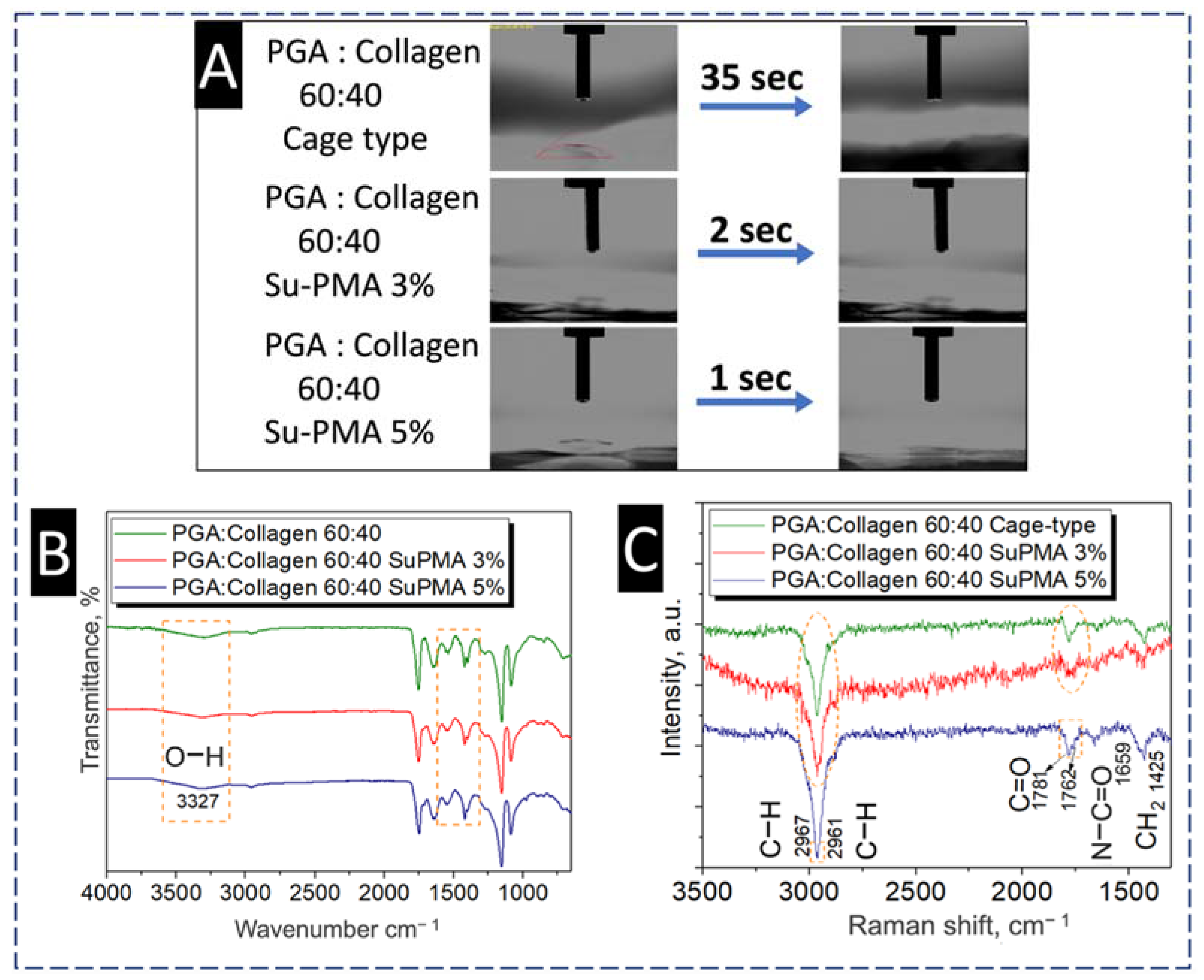
3.2. Fourier Transform Infrared (FTIR) Spectroscopy
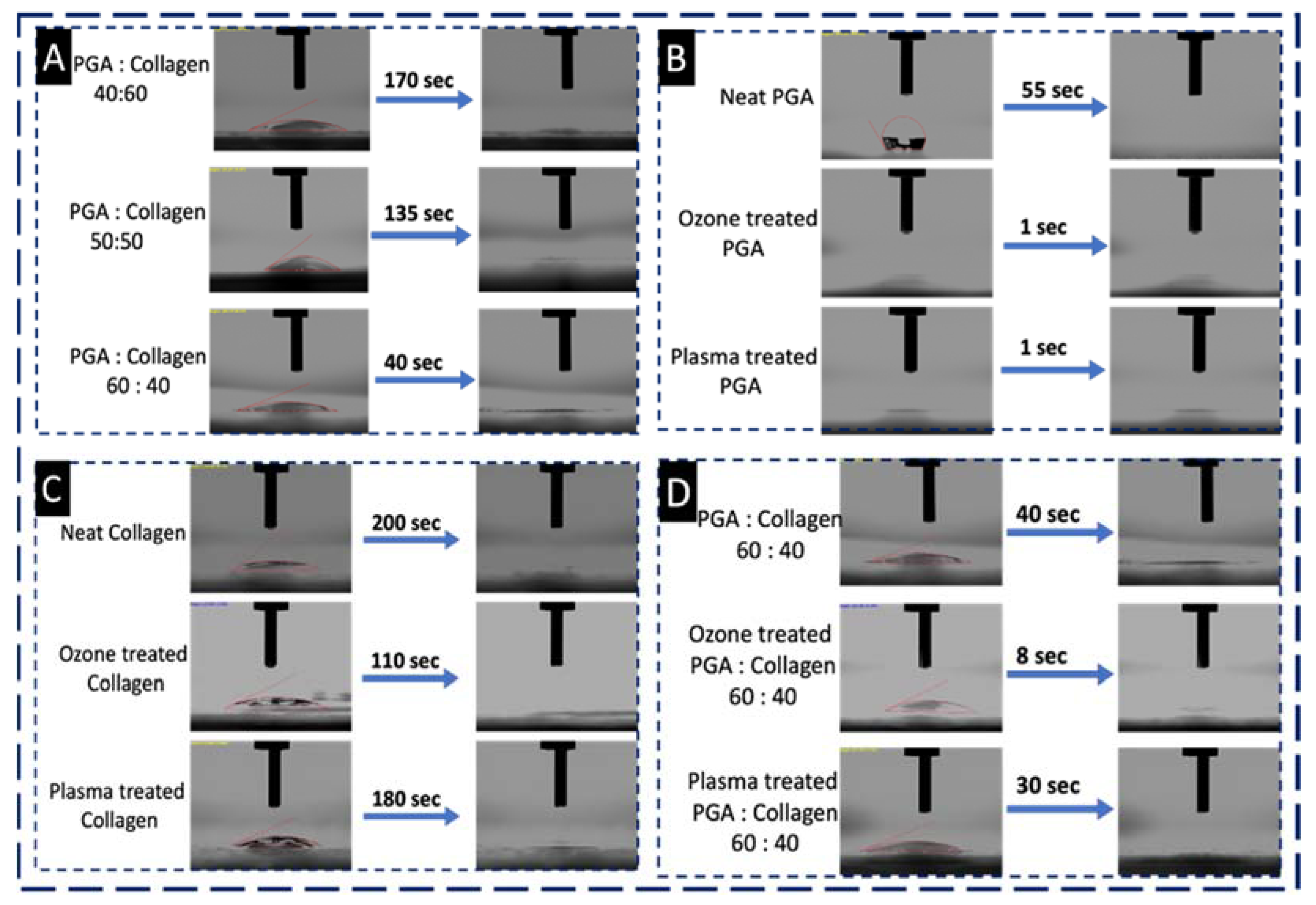
3.3. Water Contact Angle (WCA) Test
3.4. Raman Spectroscopy
4. Results and Discussion
4.1. Morphology of PGA, Collagen, and Their Blend Nanofibers
4.2. Effect of Ozonation and Plasma Treatment on the Morphology of PGA/Collagen Blends
4.3. Physico-Chemical Properties of PGA, Collagen, and Their Blend Nanofibers
4.4. Effect on Wettability of Nanofibers from Neat and Blends of PGA and Collagen
4.4.1. Ozonation and Plasma Treatment Effect on the Wettability of Nanofibers
4.4.2. Effect of SuPMA Incorporation and Cage-type Collector on Wettability of Blend Nanofibers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, H.; Kim, H.S.; Qazi, R.; Kwon, Y.; Jeong, J.; Yeo, W. Advanced soft materials, sensor integrations, and applications of wearable flexible hybrid electronics in healthcare, energy, and environment. Adv. Mater. 2020, 32, 1901924. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Cámara-Torres, M.; Scopece, P.; Falzacappa, E.V.; Patelli, A.; Moroni, L.; Mota, C. A hybrid additive manufacturing platform to create bulk and surface composition gradients on scaffolds for tissue regeneration. Nat. Commun. 2021, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Pushp, P.; Gupta, M.K. Cardiac Tissue Engineering: A Role for Natural Biomaterials. In Bioactive Natural Products for Pharmaceutical Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 617–641. [Google Scholar]
- Ibrahim, H.M.; Klingner, A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020, 90, 106647. [Google Scholar] [CrossRef]
- Deng, X.; Qasim, M.; Ali, A. Engineering and polymeric composition of drug-eluting suture: A review. J. Biomed. Mater. Res. Part A 2021, 109, 2065–2081. [Google Scholar] [CrossRef]
- Daniel, S. Biodegradable Polymeric Materials for Medicinal Applications. In Green Composites; Springer: Berlin/Heidelberg, Germany, 2021; pp. 351–372. [Google Scholar]
- Baldwin, A.; Uy, L.; Booth, B.W. Characterization of collagen type I/tannic acid beads as a cell scaffold. J. Bioact. Compat. Polym. 2021, 36, 124–138. [Google Scholar] [CrossRef]
- Tian, F.; Hosseinkhani, H.; Hosseinkhani, M.; Khademhosseini, A.; Yokoyama, Y.; Estrada, G.G.; Kobayashi, H. Quantitative analysis of cell adhesion on aligned micro- and nanofibers. J. Biomed. Mater. Res. Part A 2008, 84, 291–299. [Google Scholar] [CrossRef]
- Sekiya, N.; Ichioka, S.; Terada, D.; Tsuchiya, S.; Kobayashi, H. Efficacy of a poly glycolic acid (PGA)/collagen composite nanofibre scaffold on cell migration and neovascularisation in vivo skin defect model. J. Plast. Surg. Hand Surg. 2013, 47, 498–502. [Google Scholar]
- Sayanagi, J.; Tanaka, H.; Ebara, M.; Okada, K.; Oka, K.; Murase, T.; Yoshikawa, H. Combination of electrospun nanofiber sheet incorporating methylcobalamin and PGA-collagen tube for treatment of a sciatic nerve defect in a rat model. JBJS 2020, 102, 245–253. [Google Scholar] [CrossRef]
- Kobayashi, H.; Terada, D.; Yokoyama, Y.; Moon, D.W.; Yasuda, Y.; Koyama, H.; Takato, T. Vascular-inducing poly (glycolic acid)-collagen nanocomposite-fiber scaffold. J. Biomed. Nanotechnol. 2013, 9, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Concagh, D.; Core, L.; Kuang, Y.; You, C.; Pham, Q.; Zugates, G.; Busold, R.; Webber, S.; Merlo, J. The development of bioresorbable composite polymeric implants with high mechanical strength. Nat. Mater. 2018, 17, 96–103. [Google Scholar] [CrossRef]
- Park, K.I.; Teng, Y.D.; Snyder, E.Y. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat. Biotechnol. 2002, 20, 1111–1117. [Google Scholar] [CrossRef]
- Sun, A.; He, X.; Li, L.; Li, T.; Liu, Q.; Zhou, X.; Ji, X.; Li, W.; Qian, Z. An injectable photopolymerized hydrogel with antimicrobial and biocompatible properties for infected skin regeneration. NPG Asia Mater. 2020, 12, 25. [Google Scholar] [CrossRef]
- Low, Y.J.; Andriyana, A.; Ang, B.C.; Zainal Abidin, N.I. Bioresorbable and degradable behaviors of PGA: Current state and future prospects. Polym. Eng. Sci. 2020, 60, 2657–2675. [Google Scholar] [CrossRef]
- El-Ghazali, S.; Khatri, M.; Hussain, N.; Khatri, Z.; Yamamoto, T.; Kim, S.H.; Kobayashi, S.; Kim, I.S. Characterization and biocompatibility evaluation of artificial blood vessels prepared from pristine poly (Ethylene-glycol-co-1, 4-cyclohexane dimethylene-co-isosorbide terephthalate), poly (1, 4 cyclohexane di-methylene-co-isosorbide terephthalate) nanofi. Mater. Today Commun. 2021, 26, 102113. [Google Scholar] [CrossRef]
- Langston, C.; Patterson, K.; Dishop, M.K.; Baker, P.; Chou, P.; Cool, C.; Coventry, S.; Cutz, E.; Davis, M.; Deutsch, G. A protocol for the handling of tissue obtained by operative lung biopsy: Recommendations of the child pathology co-operative group. Pediatr. Dev. Pathol. 2006, 9, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Coemert, S.; Roth, R.; Strauss, G.; Schmitz, P.M.; Lueth, T.C. A handheld flexible manipulator system for frontal sinus surgery. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Wen, N.; Zhang, L.; Jiang, D.; Wu, Z.; Li, B.; Sun, C.; Guo, Z. Emerging flexible sensors based on nanomaterials: Recent status and applications. J. Mater. Chem. A 2020, 8, 25499–25527. [Google Scholar] [CrossRef]
- Farmer, Z.-L.; Domínguez-Robles, J.; Mancinelli, C.; Larrañeta, E.; Lamprou, D.A. Urogynecological surgical mesh implants: New trends in materials, manufacturing and therapeutic approaches. Int. J. Pharm. 2020, 585, 119512. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly (glycolic acid)(PGA): A versatile building block expanding high performance and sustainable bioplastic applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Liu, L.; Stephens, B.; Bergman, M.; May, A.; Chiang, T. Role of collagen in airway mechanics. Bioengineering 2021, 8, 13. [Google Scholar] [CrossRef]
- Kanzaki, M.; Takagi, R.; Washio, K.; Kokubo, M.; Mitsuboshi, S.; Isaka, T.; Yamato, M. Bio-artificial pleura using autologous dermal fibroblast sheets to mitigate air leaks during thoracoscopic lung resection. Regen. Med. 2021, 6, 2. [Google Scholar]
- Charlton, N.P.; Swain, J.M.; Brozek, J.L.; Ludwikowska, M.; Singletary, E.; Zideman, D.; Epstein, J.; Darzi, A.; Bak, A.; Karam, S. Control of severe life-threatening external bleeding in the out-of-hospital setting: A systematic review. Prehosp. Emerg. Care 2021, 25, 235–267. [Google Scholar] [CrossRef] [PubMed]
- Madhumanchi, S.; Srichana, T.; Domb, A.J. Polymeric Biomaterials. In Biomedical Materials; Springer: Berlin/Heidelberg, Germany, 2021; pp. 49–100. [Google Scholar]
- Christoff-Tempesta, T.; Cho, Y.; Kim, D.-Y.; Geri, M.; Lamour, G.; Lew, A.J.; Zuo, X.; Lindemann, W.R.; Ortony, J.H. Self-assembly of aramid amphiphiles into ultra-stable nanoribbons aligned nanoribbon threads. Nat. Nanotechnol. 2021, 16, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Akoumeh, R.; Elzein, T.; Martínez-Campos, E.; Reviriego, F.; Rodríguez-Hernández, J. Fabrication of porous films from immiscible polymer blends: Role of the surface structure on the cell adhesion. Polym. Test. 2020, 91, 106797. [Google Scholar] [CrossRef]
- Bilginer, R.; Ozkendir-Inanc, D.; Yildiz, U.H.; Arslan-Yildiz, A. Biocomposite scaffolds for 3D cell culture: Propolis enriched polyvinyl alcohol nanofibers favoring cell adhesion. J. Appl. Polym. Sci. 2021, 138, 50287. [Google Scholar] [CrossRef]
- Balu, R.; Dutta, N.K.; Dutta, A.K.; Choudhury, N.R. Resilin-mimetics as a smart biomaterial platform for biomedical applications. Nat. Commun. 2021, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Daum, R.; Mrsic, I.; Hutterer, J.; Junginger, A.; Hinderer, S.; Meixner, A.J.; Gauglitz, G.; Chassé, T.; Schenke-Layland, K. Fibronectin adsorption on oxygen plasma-treated polyurethane surfaces modulates endothelial cell response. J. Mater. Chem. B 2021, 9, 1647–1660. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.M.; Yang, Y.; Carnes, D.L.; Ong, J.L.; Sylvia, V.L.; Dean, D.D.; Agrawal, C.M.; Reis, R.L. Modulating bone cells response onto starch-based biomaterials by surface plasma treatment and protein adsorption. Biomaterials 2007, 28, 307–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorasani, M.T.; Mirzadeh, H.; Irani, S. Plasma surface modification of poly (l-lactic acid) and poly (lactic-co-glycolic acid) films for improvement of nerve cells adhesion. Radiat. Phys. Chem. 2008, 77, 280–287. [Google Scholar] [CrossRef]
- Poncin-Epaillard, F.; Legeay, G. Surface engineering of biomaterials with plasma techniques. J. Biomater. Sci. Polym. Ed. 2003, 14, 1005–1028. [Google Scholar] [CrossRef]
- Kasai, K.; Kimura, Y.; Miyata, S. Improvement of adhesion and proliferation of mouse embryonic stem cells cultured on ozone/UV surface-modified substrates. Mater. Sci. Eng. C 2017, 78, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Qiu, Y.; Sang, H.; Mei, H.; Zhu, A.; Shen, J.; Lin, S. Various approaches to modify biomaterial surfaces for improving hemocompatibility. Adv. Colloid Interface Sci. 2004, 110, 5–17. [Google Scholar] [CrossRef]
- Khan, M.Q.; Lee, H.; Khatri, Z.; Kharaghani, D.; Khatri, M.; Ishikawa, T.; Im, S.-S.; Kim, I.S. Fabrication and characterization of nanofibers of honey/poly (1, 4-cyclohexane dimethylene isosorbide trephthalate) by electrospinning. Mater. Sci. Eng. C 2017, 81, 247–251. [Google Scholar] [CrossRef]
- Phan, D.-N.; Rebia, R.A.; Saito, Y.; Kharaghani, D.; Khatri, M.; Tanaka, T.; Lee, H.; Kim, I.-S. Zinc oxide nanoparticles attached to polyacrylonitrile nanofibers with hinokitiol as gluing agent for synergistic antibacterial activities and effective dye removal. J. Ind. Eng. Chem. 2020, 85, 258–268. [Google Scholar] [CrossRef]
- Hussain, N.; Ullah, S.; Sarwar, M.N.; Hashmi, M.; Khatri, M.; Yamaguchi, T.; Khatri, Z.; Kim, I.S. Fabrication and Characterization of Novel Antibacterial Ultrafine Nylon-6 Nanofibers Impregnated by Garlic Sour. Fibers Polym. 2020, 21, 2780–2787. [Google Scholar] [CrossRef]
- Sabra, S.; Ragab, D.M.; Agwa, M.M.; Rohani, S. Recent advances in electrospun nanofibers for some biomedical applications. Eur. J. Pharm. Sci. 2020, 144, 105224. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.; Ko, F. Biomedical applications of nanofibers. Polym. Adv. Technol. 2011, 22, 350–365. [Google Scholar] [CrossRef]
- Khatri, M.; Ahmed, F.; Jatoi, A.W.; Mahar, R.B.; Khatri, Z.; Kim, I.S. Ultrasonic dyeing of cellulose nanofibers. Ultrason. Sonochem. 2016, 31, 350–354. [Google Scholar] [CrossRef]
- Qureshi, U.A.; Khatri, Z.; Ahmed, F.; Khatri, M.; Kim, I.-S. Electrospun zein nanofiber as a green and recyclable adsorbent for the removal of reactive black 5 from the aqueous phase. ACS Sustain. Chem. Eng. 2017, 5, 4340–4351. [Google Scholar] [CrossRef]
- Khatri, M.; Khatri, Z.; El-Ghazali, S.; Hussain, N.; Qureshi, U.A.; Kobayashi, S.; Ahmed, F.; Kim, I.S. Zein nanofibers via deep eutectic solvent electrospinning: Tunable morphology with super hydrophilic properties. Sci. Rep. 2020, 10, 15307. [Google Scholar] [CrossRef]
- Ibupoto, A.S.; Qureshi, U.A.; Ahmed, F.; Khatri, Z.; Khatri, M.; Maqsood, M.; Brohi, R.Z.; Kim, I.S. Reusable carbon nanofibers for efficient removal of methylene blue from aqueous solution. Chem. Eng. Res. Des. 2018, 136, 744–752. [Google Scholar] [CrossRef]
- Khatri, M.; Hussain, N.; El-Ghazali, S.; Yamamoto, T.; Kobayashi, S.; Khatri, Z.; Ahmed, F.; Kim, I.S. Ultrasonic-assisted dyeing of silk fibroin nanofibers: An energy-efficient coloration at room temperature. Appl. Nanosci. 2020, 10, 917–930. [Google Scholar] [CrossRef]
- Qureshi, U.A.; Khatri, Z.; Ahmed, F.; Ibupoto, A.S.; Khatri, M.; Mahar, F.K.; Brohi, R.Z.; Kim, I.S. Highly efficient and robust electrospun nanofibers for selective removal of acid dye. J. Mol. Liq. 2017, 244, 478–488. [Google Scholar] [CrossRef]
- Khatri, M.; Ahmed, F.; Shaikh, I.; Phan, D.-N.; Khan, Q.; Khatri, Z.; Lee, H.; Kim, I.S. Dyeing and characterization of regenerated cellulose nanofibers with vat dyes. Carbohydr. Polym. 2017, 174, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Li, X.; Gao, W.; Fu, X.; Fang, R.H.; Zhang, L.; Zhang, K. Tissue repair and regeneration with endogenous stem cells. Nat. Rev. Mater. 2018, 3, 174–193. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent advances in natural gum-based biomaterials for tissue engineering and regenerative medicine: A review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef] [Green Version]
- Khajavi, R.; Abbasipour, M.; Bahador, A. Electrospun biodegradable nanofibers scaffolds for bone tissue engineering. J. Appl. Polym. Sci. 2016, 133, 42883. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Xun, X.; Wan, Y.; Zhang, Q.; Gan, D.; Hu, J.; Luo, H. Low adhesion superhydrophobic AZ31B magnesium alloy surface with corrosion resistant and anti-bioadhesion properties. Appl. Surf. Sci. 2020, 505, 144566. [Google Scholar] [CrossRef]
- Dou, X.-Q.; Zhang, D.; Feng, C.-L. Wettability of supramolecular nanofibers for controlled cell adhesion and proliferation. Langmuir 2013, 29, 15359–15366. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tu, Q.; Shen, X.; Pan, M.; Jiang, C.; Zhu, P.; Li, Y.; Li, P.; Hu, C. Surface modification of Poly (p-phenylene terephthalamide) fibers by polydopamine-polyethyleneimine/graphene oxide multilayer films to enhance interfacial adhesion with rubber matrix. Polym. Test. 2019, 78, 105985. [Google Scholar] [CrossRef]
- Rezaei, S.M.; Ishak, Z.A.M. The biocompatibility and hydrophilicity evaluation of collagen grafted poly (dimethylsiloxane) and poly (2-hydroxyethylmethacrylate) blends. Polym. Test. 2011, 30, 69–75. [Google Scholar] [CrossRef]
- Ngiam, M.; Liao, S.; Patil, A.J.; Cheng, Z.; Chan, C.K.; Ramakrishna, S. The fabrication of nano-hydroxyapatite on PLGA and PLGA/collagen nanofibrous composite scaffolds and their effects in osteoblastic behavior for bone tissue engineering. Bone 2009, 45, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, T.; Kobayashi, H.; Taguchi, T.; Saito, H.; Kamatsu, Y.; Kataoka, K.; Tanaka, J. Synthesis of activated poly (α, β-malic acid) using N-hydroxysuccinimide and its gelation with collagen as biomaterials. Mater. Sci. Eng. C 2004, 24, 815–819. [Google Scholar] [CrossRef]
- El-Ghazali, S.; Khatri, M.; Mehdi, M.; Kharaghani, D.; Tamada, Y.; Katagiri, A.; Kobayashi, S.; Kim, I.S. Fabrication of Poly (Ethylene-glycol 1, 4-Cyclohexane Dimethylene-Isosorbide-Terephthalate) Electrospun Nanofiber Mats for Potential Infiltration of Fibroblast Cells. Polymers 2021, 13, 1245. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Ghazali, S.; Kobayashi, H.; Khatri, M.; Phan, D.-N.; Khatri, Z.; Mahar, S.K.; Kobayashi, S.; Kim, I.-S. Preparation of a Cage-Type Polyglycolic Acid/Collagen Nanofiber Blend with Improved Surface Wettability and Handling Properties for Potential Biomedical Applications. Polymers 2021, 13, 3458. https://doi.org/10.3390/polym13203458
El-Ghazali S, Kobayashi H, Khatri M, Phan D-N, Khatri Z, Mahar SK, Kobayashi S, Kim I-S. Preparation of a Cage-Type Polyglycolic Acid/Collagen Nanofiber Blend with Improved Surface Wettability and Handling Properties for Potential Biomedical Applications. Polymers. 2021; 13(20):3458. https://doi.org/10.3390/polym13203458
Chicago/Turabian StyleEl-Ghazali, Sofia, Hisatoshi Kobayashi, Muzamil Khatri, Duy-Nam Phan, Zeeshan Khatri, Sheeraz Khan Mahar, Shunichi Kobayashi, and Ick-Soo Kim. 2021. "Preparation of a Cage-Type Polyglycolic Acid/Collagen Nanofiber Blend with Improved Surface Wettability and Handling Properties for Potential Biomedical Applications" Polymers 13, no. 20: 3458. https://doi.org/10.3390/polym13203458
APA StyleEl-Ghazali, S., Kobayashi, H., Khatri, M., Phan, D.-N., Khatri, Z., Mahar, S. K., Kobayashi, S., & Kim, I.-S. (2021). Preparation of a Cage-Type Polyglycolic Acid/Collagen Nanofiber Blend with Improved Surface Wettability and Handling Properties for Potential Biomedical Applications. Polymers, 13(20), 3458. https://doi.org/10.3390/polym13203458