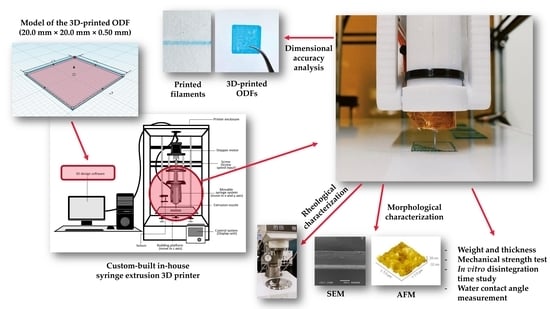
Characterization of Hydrophilic Polymers as a Syringe Extrusion 3D Printing Material for Orodispersible Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
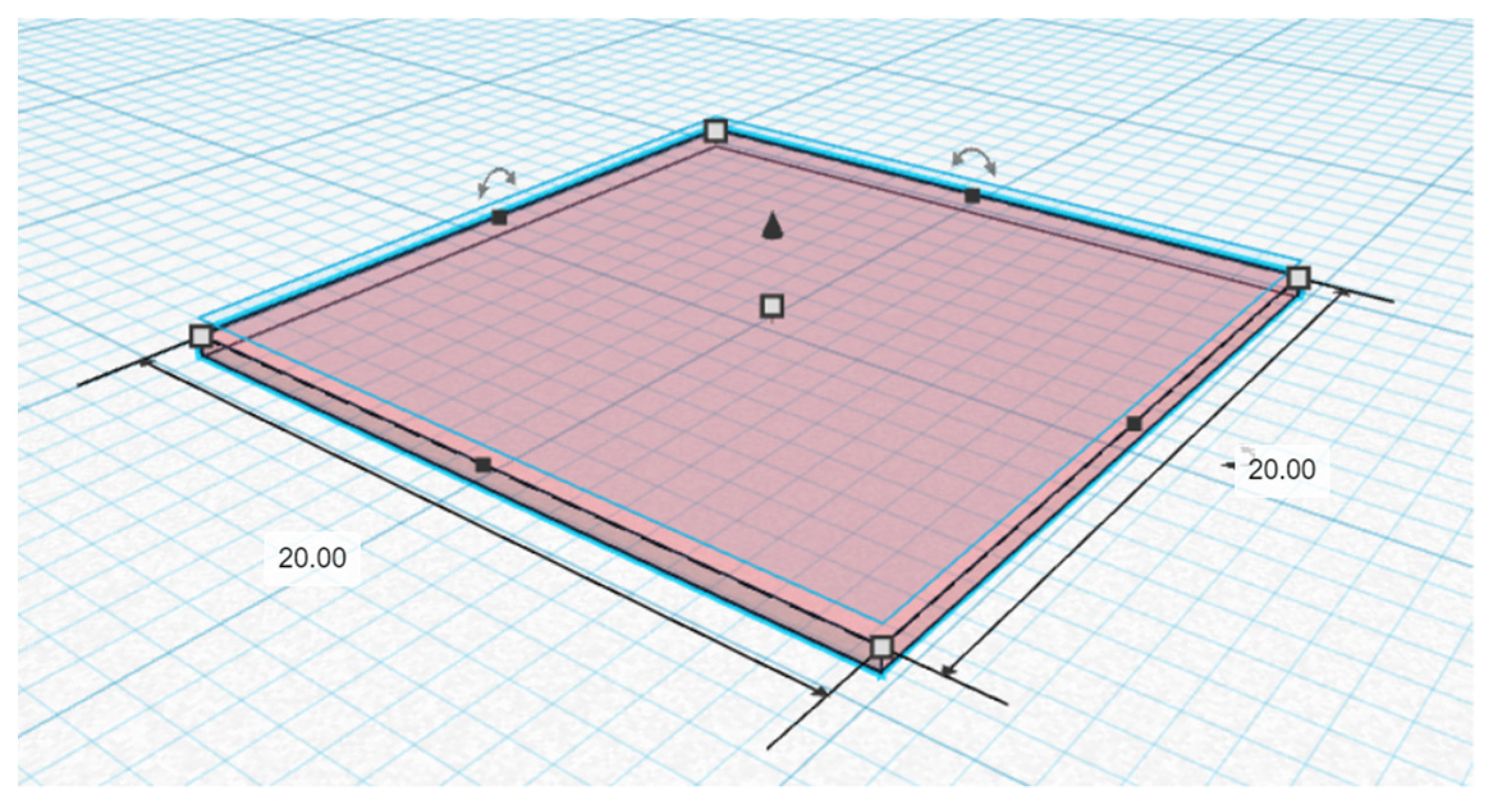
2.2. Fabrication of 3D-Printed Orodispersible Films
2.3. Rheological Characterization
2.4. Dimensional Accuracy Analysis
2.5. Morphological Characterizations
2.6. Weight and Thickness
2.7. Mechanical Strength Test of 3D-Printed ODFs
2.8. In Vitro Disintegration Time Study
2.9. Water Contact Angle Measurement
2.10. Statistical Analysis
3. Results
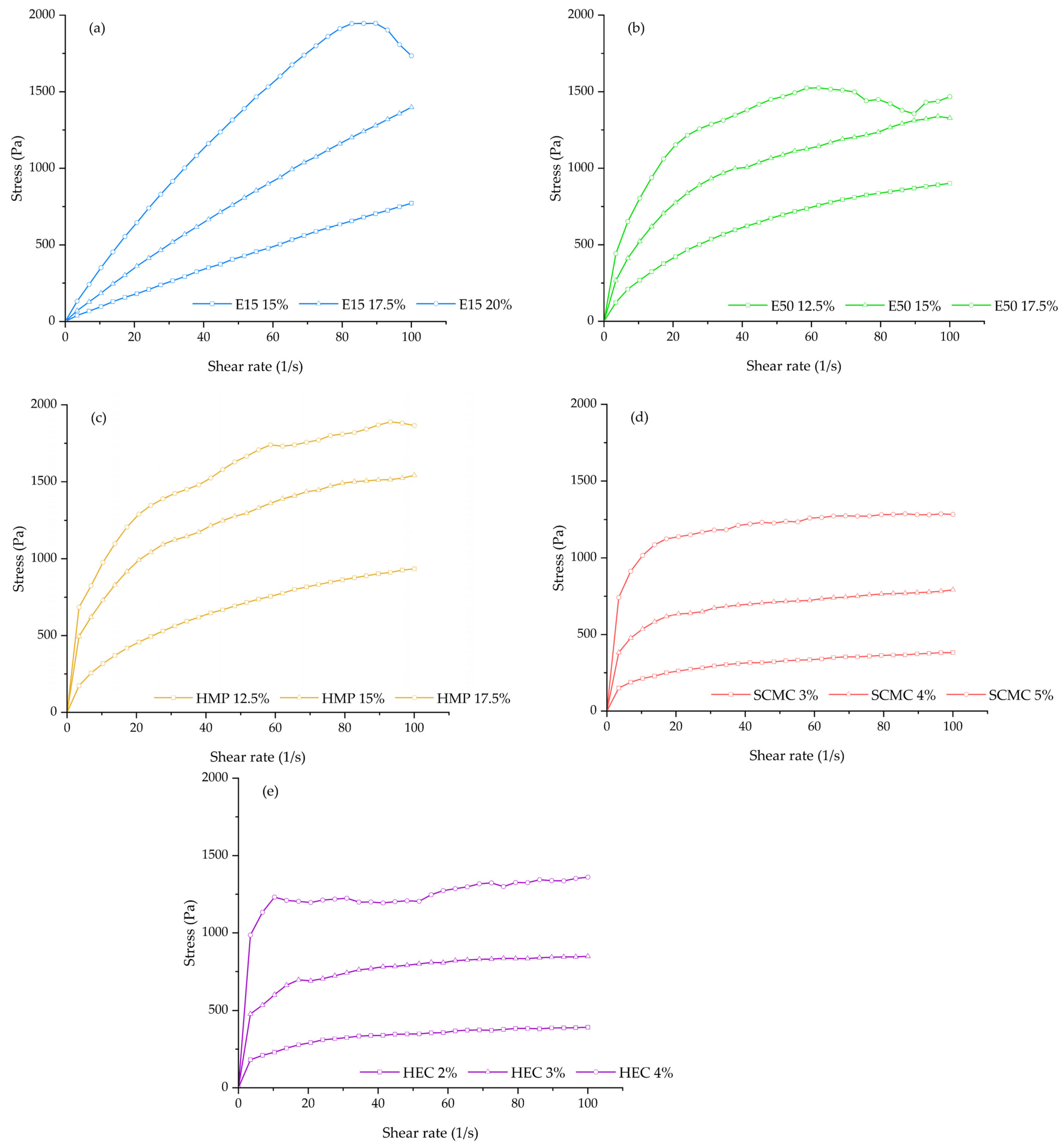
3.1. Rheological Behaviors and Dimensional Accuracy Analysis
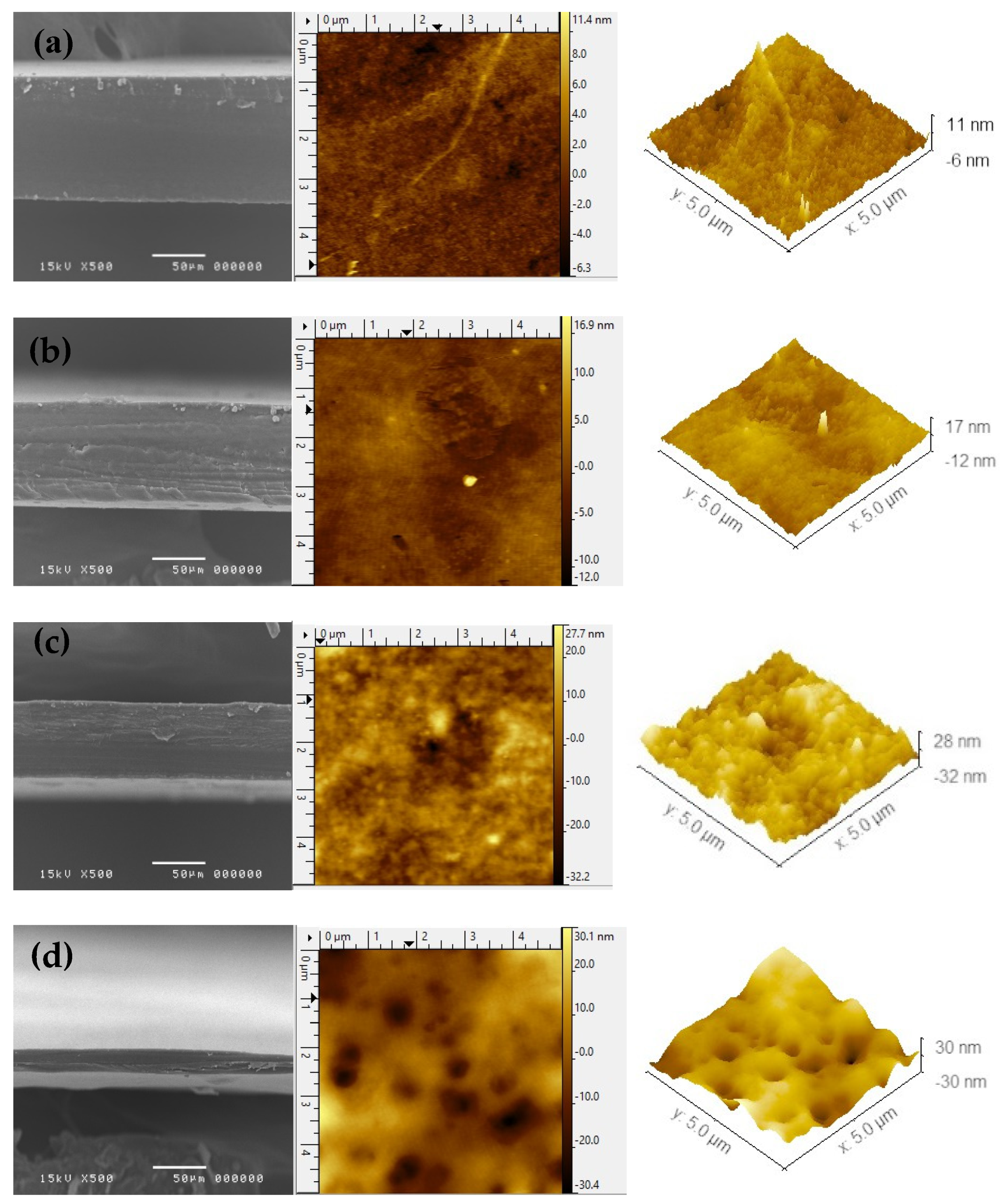
3.2. Morphology of 3D-Printed ODFs
3.3. Thickness and Weight Variation of 3D-Printed ODFs
3.4. Mechanical Characteristics of 3D-Printed ODFs
3.5. In Vitro Disintegration Time
3.6. Water Contact Angle
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Souto, E.B.; Campos, J.C.; Filho, S.C.; Teixeira, M.C.; Martins-Gomes, C.; Zielinska, A.; Carbone, C.; Silva, A.M. 3D printing in the design of pharmaceutical dosage forms. Pharm. Dev. Technol 2019, 24, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Li, S.W.; Kowsari, K.; Shetty, A.; Sorrells, L.; Sen, K.; Ma, A.W. Binder-jet 3D printing of indomethacin-laden pharmaceutical dosage forms. J. Pharm. Sci. 2020, 109, 3054–3063. [Google Scholar] [CrossRef]
- Karakurt, I.; Aydoğdu, A.; Çıkrıkcı, S.; Orozco, J.; Lin, L. Stereolithography (SLA) 3D printing of ascorbic acid loaded hydrogels: A controlled release study. Int. J. Pharm. 2020, 584, 119428. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.M.; Ali, S.F.B.; Rahman, Z.; Dharani, S.; Ozkan, T.; Kuttolamadom, M.A.; Khan, M.A. Formulation optimization of selective laser sintering 3D-printed tablets of clindamycin palmitate hydrochloride by response surface methodology. AAPS PharmSciTech 2020, 21, 1–15. [Google Scholar] [CrossRef]
- Kyobula, M.; Adedeji, A.; Alexander, M.R.; Saleh, E.; Wildman, R.; Ashcroft, I.; Roberts, C.J. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J. Control. Release 2017, 261, 207–215. [Google Scholar] [CrossRef]
- Lepowsky, E.; Tasoglu, S. 3D printing for drug manufacturing: A perspective on the future of pharmaceuticals. Int. J. Bioprint. 2018, 4, 119. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Pan, W. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Algahtani, M.S.; Mohammed, A.A.; Ahmad, J. Extrusion-based 3D printing for pharmaceuticals: Contemporary research and applications. Curr. Pharm. Des. 2018, 24, 4991–5008. [Google Scholar] [CrossRef]
- Placone, J.K.; Engler, A.J. Recent advances in extrusion-based 3D printing for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1701161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaled, S.A.; Alexander, M.R.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int. J. Pharm. 2018, 538, 223–230. [Google Scholar] [CrossRef]
- Yi, H.G.; Choi, Y.J.; Kang, K.S.; Hong, J.M.; Pati, R.G.; Park, M.N.; Shim, I.K.; Lee, C.M.; Kim, S.C.; Cho, D.W. A 3D-printed local drug delivery patch for pancreatic cancer growth suppression. J. Control. Release 2016, 238, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, X.; Jin, Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int. J. Pharm. 2018, 539, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.T.; Lv, Z.F.; Tian, P.; Lin, M.M.; Lin, W.; Huang, S.Y.; Chen, Y.Z. Semi-solid extrusion 3d printing ODFs: An individual drug delivery system for small scale pharmacy. Drug Dev. Ind. Pharm. 2020, 46, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, G.K.; Fatouros, D.G. Haptic Evaluation of 3D-Printed Braille-Encoded Intraoral Films. Eur. J. Pharm. Sci. 2021, 157, 105605. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Katsiotis, C.S.; Andreadis, D.A.; Tzetzis, D.; Ritzoulis, C.; Bouropoulos, N.; Fatouros, D.G. Inkjet printing of a thermolabile model drug onto FDM-printed substrates: Formulation and evaluation. Drug Dev. Ind. Pharm. 2020, 46, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Yoshimura, N.; Goto, E.; Noda, T.; Ozeki, T. Fabrication of muco-adhesive oral films by the 3D printing of hydroxypropyl methylcellulose-based catechin-loaded formulations. Biol. Pharm. Bull. 2019, 42, 1898–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cilurzo, F.; Musazzi, U.M.; Franzé, S.; Selmin, F.; Minghetti, P. Orodispersible dosage forms: Biopharmaceutical improvements and regulatory requirements. Drug Discov. Today 2018, 23, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Scarpa, M.; Stegemann, S.; Hsiao, W.K.; Pichler, H.; Gaisford, S.; Bresciani, M.; Paudel, A.; Orlu, M. Orodispersible films: Towards drug delivery in special populations. Int. J. Pharm. 2017, 523, 327–335. [Google Scholar] [CrossRef]
- Jamróz, W.; Kurek, M.; Łyszczarz, E.; Szafraniec, J.; Knapik-Kowalczuk, J.; Syrek, K.; Paluch, M.; Jachowicz, R. 3D printed orodispersible films with Aripiprazole. Int. J. Pharm. 2017, 533, 413–420. [Google Scholar] [CrossRef]
- Ehtezazi, T.; Algellay, M.; Islam, Y.; Roberts, M.; Dempster, N.M.; Sarker, S.D. The application of 3D printing in the formulation of multilayered fast dissolving oral films. J. Pharm. Sci. 2018, 107, 1076–1085. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Kantarelis, E.; Monou, P.K.; Andriotis, E.G.; Bouropoulos, N.; Tzimtzimis, E.K.; Fatouros, D.G. Automated digital design for 3D-printed individualized therapies. Int. J. Pharm. 2021, 599, 120437. [Google Scholar] [CrossRef]
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D printing of multilayered orodispersible films with in-process drying. Int. J. Pharm. 2020, 575, 118883. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.; Sultana, T. Polymers for extrusion-based 3D printing of pharmaceuticals: A holistic materials–process perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panraksa, P.; Udomsom, S.; Rachtanapun, P.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. Hydroxypropyl Methylcellulose E15: A hydrophilic polymer for fabrication of orodispersible film using syringe extrusion 3D printer. Polymers 2020, 12, 2666. [Google Scholar] [CrossRef] [PubMed]
- Polamaplly, P.; Cheng, Y.; Shi, X.; Manikandan, K.; Kremer, G.E.; Qin, H. 3D printing and characterization of hydroxypropyl methylcellulose and methylcellulose for biodegradable support structures. Procedia Manuf. 2019, 34, 552–559. [Google Scholar] [CrossRef]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.O.; McConville, J.T. Manufacture and characterization of mucoadhesive buccal films. Eur. J. Pharm. Biopharm. 2011, 77, 187–199. [Google Scholar] [CrossRef]
- Preis, M.; Gronkowsky, D.; Grytzan, D.; Breitkreutz, J. Comparative study on novel test systems to determine disintegration time of orodispersible films. J. Pharm. Pharmacol. 2014, 66, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.R.; Loebenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Lewis, J.A. Direct ink writing of 3D functional materials. Adv. Funct. Mater. 2006, 16, 2193–2204. [Google Scholar] [CrossRef]
- Jiang, Z.; Diggle, B.; Tan, M.L.; Viktorova, J.; Bennett, C.W.; Connal, L.A. Extrusion 3D printing of polymeric materials with advanced properties. Adv. Sci. 2020, 7, 2001379. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.L.; Shi, X.L.; Jiang, X.P.; Wang, X.H.; Qin, H.T. Printability of a cellulose derivative for extrusion-based 3D printing: The application on a biodegradable support material. Front. Mater. 2020, 7, 86. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zhang, Y.; Han, L.; Che, S.; Terasaki, O. Structure characterization of mesoporous materials by electron microscopy. In The Enzymes; Tamanoi, F., Ed.; Academic Press: Waltham, MA, USA, 2018; pp. 11–30. [Google Scholar]
- Álvarez-Fernández, A.; Valdés-Bango, F.; Losada-Ambrinos, R.; Martín, J.I.; Vélez, M.; Alameda, J.M.; García Alonso, F.J. Polymer porous thin films obtained by direct spin coating. Polym. Int. 2018, 67, 393–398. [Google Scholar] [CrossRef]
- Kalosakas, G.; Martini, D. Drug release from slabs and the effects of surface roughness. Int. J. Pharm. 2015, 496, 291–298. [Google Scholar] [CrossRef]
- Dahiya, M.; Saha, S.; Shahiwala, A.F. A review on mouth dissolving films. Curr. Drug Deliv. 2009, 6, 469–476. [Google Scholar] [CrossRef]
- Scoutaris, N. AFM in advanced pharmaceutical technology. Pharm. Anal. Acta 2012, 3, 1–2. [Google Scholar] [CrossRef]
- Mohamed, M.I.; Haider, M.; Ali, M.M. Buccal mucoadhesive films containing antihypertensive drug: In vitro/in vivo evaluation. J. Chem. Pharm. Res. 2011, 3, 665–686. [Google Scholar]
- Sapkal, N.P.; Kilor, V.A.; Daud, A.S.; Bonde, M.N. Development of fast dissolving oral thin films of ambroxol hydrochloride: Effect of formulation variables. J. Adv. Pharm. Res. 2011, 2, 102–109. [Google Scholar]
- Maria, T.M.; De Carvalho, R.A.; Sobral, P.J.; Habitante, A.M.B.; Solorza-Feria, J. The effect of the degree of hydrolysis of the PVA and the plasticizer concentration on the color, opacity, and thermal and mechanical properties of films based on PVA and gelatin blends. J. Food Eng. 2008, 87, 191–199. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, K.; Kim, M.; Choi, D.H.; Jeong, S.H. Orally disintegrating films focusing on formulation, manufacturing process, and characterization. J. Pharm. Investig. 2017, 47, 183–201. [Google Scholar] [CrossRef]
- Drelich, J.W.; Boinovich, L.; Chibowski, E.; Della Volpe, C.; Hołysz, L.; Marmur, A.; Siboni, S. Contact angles: History of over 200 years of open questions. Surf. Innov. 2019, 8, 3–27. [Google Scholar] [CrossRef] [Green Version]
- Ryan, B.J.; Poduska, K.M. Roughness effects on contact angle measurements. Am. J. Phys. 2008, 76, 1074–1077. [Google Scholar] [CrossRef] [Green Version]
- Tran, P.A.; Webster, T.J. Understanding the wetting properties of nanostructured selenium coatings: The role of nanostructured surface roughness and air-pocket formation. Int. J. Nanomed. 2013, 8, 2001. [Google Scholar]
| Formulation Code | Polymer (% w/v) | Methylene Blue (% w/v) | ||||
|---|---|---|---|---|---|---|
| HPMC E15 | HPMC E50 | HMP | SCMC | HEC | ||
| E15-15 | 15.0 | - | - | - | - | 1.0 |
| E15-17.5 | 17.5 | - | - | - | - | 1.0 |
| E15-20 | 20.0 | - | - | - | - | 1.0 |
| E50-12.5 | - | 12.5 | - | - | - | 1.0 |
| E50-15 | - | 15.0 | - | - | - | 1.0 |
| E50-17.5 | - | 17.5 | - | - | - | 1.0 |
| HMP-12.5 | - | - | 12.5 | - | - | 1.0 |
| HMP-15 | - | - | 15.0 | - | - | 1.0 |
| HMP-17.5 | - | - | 17.5 | - | - | 1.0 |
| SCMC-3 | - | - | - | 3 | - | 1.0 |
| SCMC-4 | - | - | - | 4 | - | 1.0 |
| SCMC-5 | - | - | - | 5 | - | 1.0 |
| HEC-2 | - | - | - | - | 2 | 1.0 |
| HEC-3 | - | - | - | - | 3 | 1.0 |
| HEC-4 | - | - | - | - | 4 | 1.0 |
| Formulation Code | Viscosity | Flow Behavior Index | Consistency Coefficient | Shape Fidelity Factor | Diameter of Printed Filament (mm) |
|---|---|---|---|---|---|
| E15-15 | 8.20 ± 0.07 | 0.90 | 12.06 | 1.12 ± 0.03 | 1.32 ± 0.04 |
| E15-17.5 | 15.42 ± 0.19 | 0.89 | 23.80 | 1.09 ± 0.02 | 1.03 ± 0.07 |
| E15-20 | 26.27 ± 0.46 | 0.81 | 54.56 | 1.06 ± 0.01 | 0.57 ± 0.07 |
| E50-12.5 | 15.11 ± 0.26 | 0.57 | 72.16 | 1.11 ± 0.02 | 0.85 ± 0.12 |
| E50-15 | 26.03 ± 0.64 | 0.44 | 187.54 | 1.06 ± 0.03 | 0.53 ± 0.03 |
| E50-17.5 | 36.48 ± 0.72 | 0.31 | 402.90 | NA | NA |
| HMP-12.5 | 16.60 ± 0.69 | 0.49 | 100.95 | 1.06 ± 0.02 | 1.18 ± 0.09 |
| HMP-15 | 34.26 ± 0.94 | 0.34 | 341.66 | 1.06 ± 0.02 | 0.59 ± 0.01 |
| HMP-17.5 | 43.68 ± 1.13 | 0.30 | 494.08 | 1.03 ± 0.01 | 0.41 ± 0.02 |
| SCMC-3 | 9.26 ± 0.06 | 0.27 | 114.58 | 1.02 ± 0.02 | 1.25 ± 0.13 |
| SCMC-4 | 21.74 ± 1.19 | 0.19 | 341.90 | 1.08 ± 0.02 | 0.88 ± 0.08 |
| SCMC-5 | 39.44 ± 3.03 | 0.13 | 723.44 | 1.03 ± 0.01 | 0.56 ± 0.02 |
| HEC-2 | 10.25 ± 0.11 | 0.23 | 141.81 | 1.03 ± 0.02 | 1.03 ± 0.06 |
| HEC-3 | 24.69 ± 0.71 | 0.16 | 413.24 | 1.07 ± 0.02 | 0.77 ± 0.09 |
| HEC-4 | 44.46 ± 0.62 | 0.07 | 963.39 | 1.04 ± 0.01 | 0.56 ± 0.05 |
| AFM Parameters | Formulation | |||
|---|---|---|---|---|
| E15-20 | E50-15 | HMP-15 | SCMC-5 | |
| Average surface roughness (Sa, nm) | 1.51 | 2.12 | 6.83 | 8.83 |
| RMS roughness (Sq, nm) | 1.13 | 1.61 | 5.34 | 6.96 |
| Maximum peak height (Sp, nm) | 11.50 | 16.94 | 27.61 | 30.09 |
| Maximum pit depth (Sv, nm) | 6.33 | 12.02 | 30.39 | 32.28 |
| Formulation | Thickness (µm ± SD) | Weight (mg ± SD) |
|---|---|---|
| E15-20 | 88.93 ± 3.60 a | 40.46 ± 2.04 a |
| E50-15 | 63.87 ± 4.95 b | 29.64 ± 1.91 b |
| HMP-15 | 46.80 ± 5.47 c | 29.88 ± 2.61 b |
| SCMC-5 | 18.73 ± 2.25 d | 9.98 ± 0.54 c |
| Formulation | Normalized Tensile Strength (MPa) | Normalized Elongation (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| E15-20 | 2.52 ± 0.07 a | 2.10 ± 0.22 a | 245.63 ± 17.36 a |
| E50-15 | 2.96 ± 0.40 a | 2.91 ± 0.21 b | 279.99 ± 19.92 b |
| HMP-15 | 2.32 ± 0.32 a | 2.23 ± 0.23 a | 310.74 ± 14.36 c |
| SCMC-5 | 2.37 ± 0.37 a | 3.76 ± 0.38 c | 333.37 ± 4.16 c |
| Formulation | Disintegration Time (s) | Normalized Disintegration Time (s) | Water Contact Angle (°) | |
|---|---|---|---|---|
| Initial | After 30 s | |||
| E15-20 | 49.85 ± 14.28 a | 10.62 ± 2.83 a | 62.4 ± 6.2 | 61.8 ± 5.2 |
| E50-15 | 24.08 ± 5.88 b | 7.11 ± 1.25 b | 52.5 ± 0.2 | 52.4 ± 1.7 |
| HMP-15 | 6.48 ± 1.07 c | 2.63 ± 0.38 c | 58.4 ± 1.9 | 56.9 ± 1.3 |
| SCMC-5 | 2.02 ± 0.14 c | 2.08 ± 0.28 c | 51.5 ± 2.6 | 45.1 ± 2.6 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panraksa, P.; Qi, S.; Udomsom, S.; Tipduangta, P.; Rachtanapun, P.; Jantanasakulwong, K.; Jantrawut, P. Characterization of Hydrophilic Polymers as a Syringe Extrusion 3D Printing Material for Orodispersible Film. Polymers 2021, 13, 3454. https://doi.org/10.3390/polym13203454
Panraksa P, Qi S, Udomsom S, Tipduangta P, Rachtanapun P, Jantanasakulwong K, Jantrawut P. Characterization of Hydrophilic Polymers as a Syringe Extrusion 3D Printing Material for Orodispersible Film. Polymers. 2021; 13(20):3454. https://doi.org/10.3390/polym13203454
Chicago/Turabian StylePanraksa, Pattaraporn, Sheng Qi, Suruk Udomsom, Pratchaya Tipduangta, Pornchai Rachtanapun, Kittisak Jantanasakulwong, and Pensak Jantrawut. 2021. "Characterization of Hydrophilic Polymers as a Syringe Extrusion 3D Printing Material for Orodispersible Film" Polymers 13, no. 20: 3454. https://doi.org/10.3390/polym13203454
APA StylePanraksa, P., Qi, S., Udomsom, S., Tipduangta, P., Rachtanapun, P., Jantanasakulwong, K., & Jantrawut, P. (2021). Characterization of Hydrophilic Polymers as a Syringe Extrusion 3D Printing Material for Orodispersible Film. Polymers, 13(20), 3454. https://doi.org/10.3390/polym13203454