Impact of Titanium Dioxide in the Mechanical Recycling of Post-Consumer Polyethylene Terephthalate Bottle Waste: Tensile and Fracture Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. rPET Sheet Manufacturing
2.3. Molecular Characterization
2.4. Differential Scanning Calorimetry (DSC)
2.5. Mechanical and Fracture Characterization
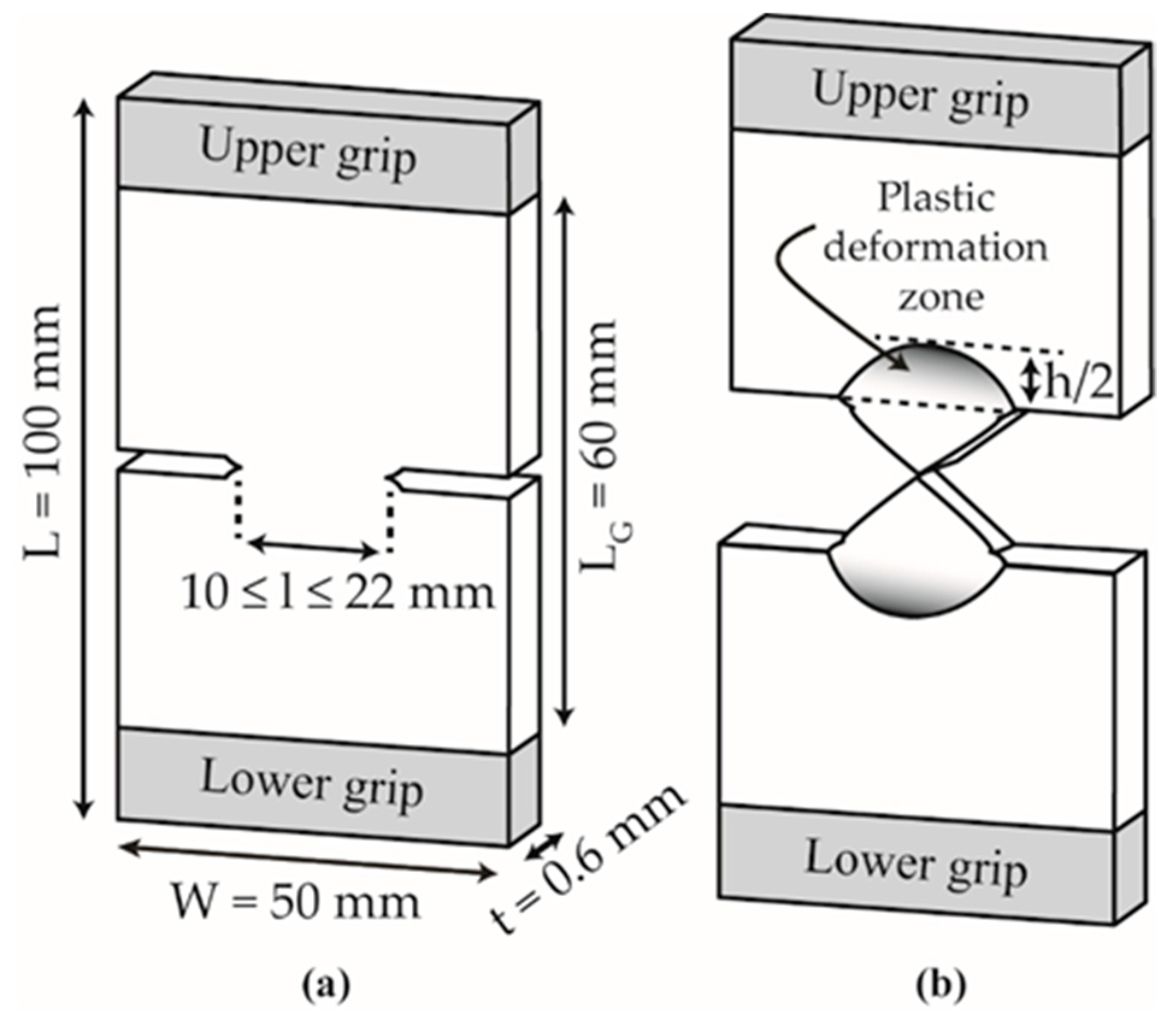
2.5.1. Tensile Tests
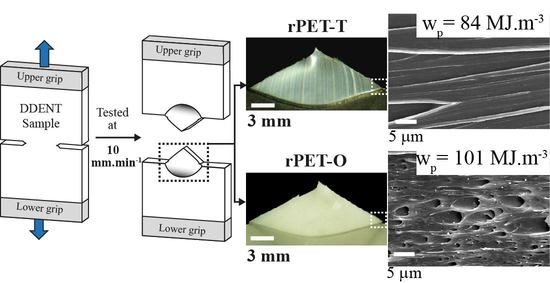
2.5.2. Fracture Behavior
2.6. Fractography Characterization
3. Results
3.1. Intrinsic Viscosity Measurements of Unprocessed Flakes and Homogenized Pellets
3.2. Thermal Properties of the rPET Calendered Sheets
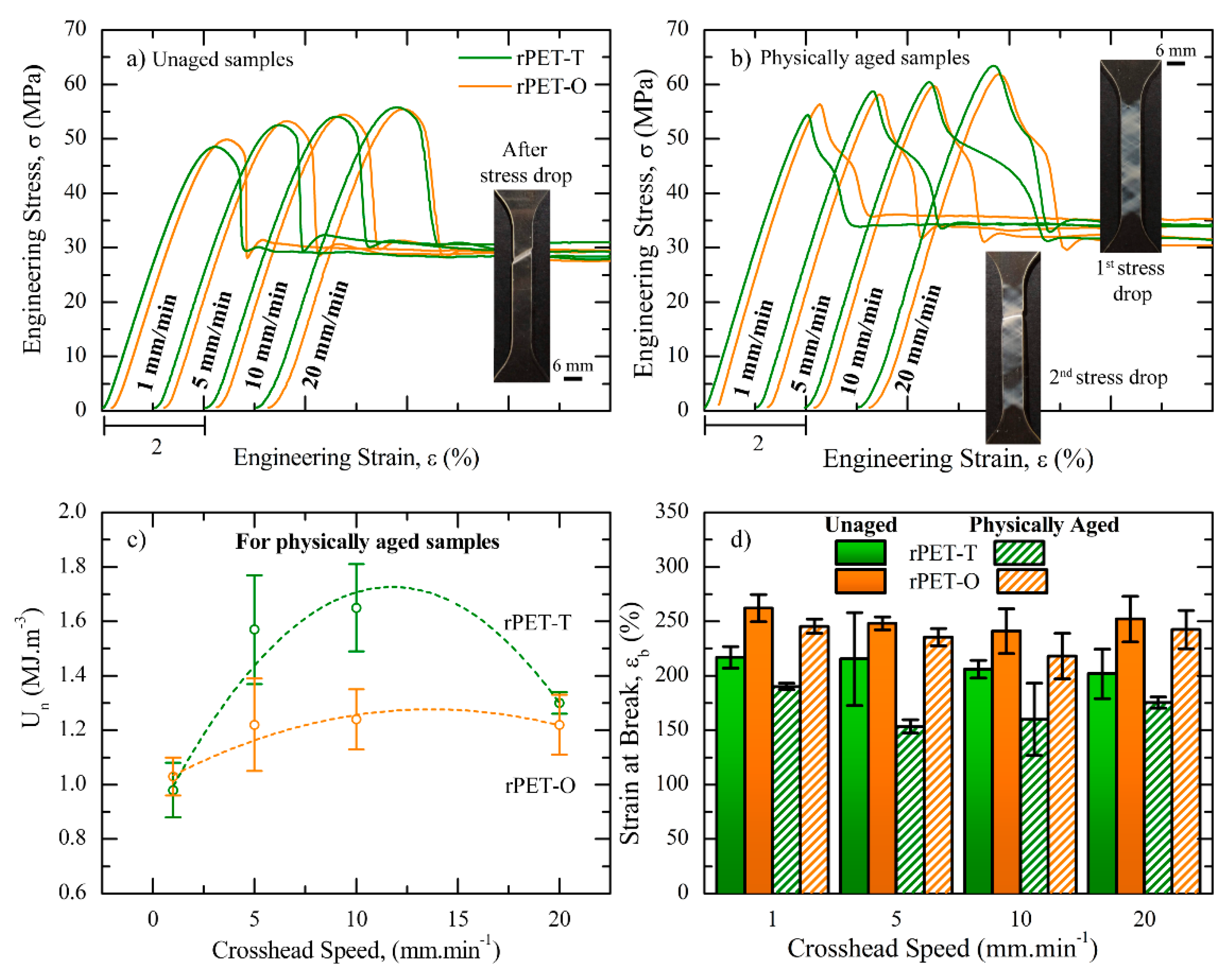
3.3. Mechanical Behavior
3.4. Fracture Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- PlasticsEurope. Plastics 2030: PlasticsEurope’s Voluntary Commitment to Increasing Circularity and Resource Efficiency. Available online: https://www.plasticseurope.org/application/files/6115/1700/8779/PlasticsEurope_Voluntary_Commitment_16012018.pdf (accessed on 14 July 2020).
- PlasticsEurope. Plastics—The Facts 2019: An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://www.plasticseurope.org/en/resources/publications/1804-plastics-facts-2019 (accessed on 13 July 2020).
- Welle, D.F. The Facts about PET. Available online: https://www.petcore-europe.org/images/news/pdf/factsheet_the_facts_about_pet_dr_frank_welle_2018.pdf (accessed on 22 July 2020).
- Eunomia. PET Market in Europe: State of Play. Porduction, Collection and Sorting Data. Available online: https://743c8380-22c6-4457-9895-11872f2a708a.filesusr.com/ugd/dda42a_0f5a9363bfd44caa8b890d6f43529aa9.pdf (accessed on 23 July 2020).
- Shen, L.; Worrell, E.; Patel, M.K. Open-loop recycling: A LCA case study of PET bottle-to-fibre recycling. Resour. Conserv. Recycl. 2010, 55, 34–52. [Google Scholar] [CrossRef]
- (Cotrep) Comité Technique pour le Recyclage des Emballages Plastiques. Note Préliminaire 2: Impact du Développement des Emballages en PET Opaque sur le Recyclage des Emballages en PET Coloré. Available online: https://www.cotrep.fr/content/uploads/sites/3/2018/11/np02-note-preliminaire-impact-du-pet-opaque-sur-le-recyclage-du-pet-colore.pdf (accessed on 14 July 2020).
- France, Z.W. PET Opaque—Dossier Complet. Available online: https://www.eco-conception.fr/data/sources/users/7/pet-opaque---dossier-complet.pdf (accessed on 14 July 2020).
- Taniguchi, A.; Cakmak, M. The suppression of strain induced crystallization in PET through sub micron TiO2 particle incorporation. Polymer 2004, 45, 6647–6654. [Google Scholar] [CrossRef]
- Vincent, L.; Connolly, S.; Dolan, F.; Willcocks, P.; Jayaweera, S.; Pendlebury, R. Determination and comparison of the plane stress essentialwork of fracture of three polyesters PET, PPT and PBT. J. Therm. Anal. Calorim. 2006, 86, 147–154. [Google Scholar] [CrossRef]
- Martínez, A.B.; Gamez-Perez, J.; Sanchez-Soto, M.; Velasco, J.I.; Santana, O.O.; Maspoch, M.L. The Essential Work of Fracture (EWF) method—Analyzing the Post-Yielding Fracture Mechanics of polymers. Eng. Fail. Anal. 2009, 16, 2604–2617. [Google Scholar] [CrossRef]
- Clutton, E.Q. Essential Work of Fracture. In Fracture Mechanics Testing Methods for Polymers, Adhesive and Composites; Moore, D.R., Pavan, A., Williams, J.G., Eds.; Elsevier: Oxford, UK, 2001; Volume 28, pp. 177–202. [Google Scholar]
- Hashemi, S. Fracture toughness evaluation of ductile polymeric films. J. Mater. Sci. 1997, 32, 1563–1573. [Google Scholar] [CrossRef]
- Mouzakis, D.E.; Papke, N.; Wu, J.; Karger-Kocsis, J. Fracture toughness assessment of poly (ethylene terephthalate) blends with glycidyl methacrylate modified polyolefin elastomer using essential work of fracture method. J. Appl. Polym. Sci. 2001, 79, 842–852. [Google Scholar] [CrossRef]
- Bárány, T.; Ronkay, F.; Karger-Kocsis, J.; Czigany, T. In-plane and out-of-plane fracture toughness of physically aged polyesters as assessed by the essential work of fracture (EWF) method. Int. J. Fract. 2005, 135, 251–265. [Google Scholar] [CrossRef]
- Bárány, T.; Földes, E.; Czigány, T. Effect of thermal and hygrothermal aging on the plane stress fracture toughness of poly(ethylene terephthalate) sheets. Express Polym. Lett. 2007, 1, 180–187. [Google Scholar] [CrossRef]
- Hashemi, S.; Xu, Y. Thermal effects on fracture of biaxial-oriented poly(ethylene terephthalate) (BOPET) film. J. Mater. Sci. 2007, 42, 6197–6204. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Czigany, T. On the essential and non-essential work of fracture of biaxial-oriented filled PET film. Polymer 1996, 37, 2433–2438. [Google Scholar] [CrossRef]
- Maspoch, M.L.; Hénault, V.; Ferrer-Balas, D.; Velasco, J.; Santana, O. Essential work of fracture on PET films: Influence of the thickness and the orientation. Polym. Test. 2000, 19, 559–568. [Google Scholar] [CrossRef]
- Chen, H.; Karger-Kocsis, J.; Wu, J. Effects of molecular structure on the essential work of fracture of amorphous copolyesters at various deformation rates. Polymer 2004, 45, 6375–6382. [Google Scholar] [CrossRef]
- Light, M.E.; Lesser, A.J. Effect of test conditions on the essential work of fracture in polyethylene terephthalate film. J. Mater. Sci. 2005, 40, 2861–2866. [Google Scholar] [CrossRef]
- Matxinandiarena, E.; Múgica, A.; Zubitur, M.; Yus, C.; Sebastián, V.; Irusta, S.; Loaeza, A.D.; Santana, O.; Maspoch, M.L.; Puig, C.; et al. The Effect of Titanium Dioxide Surface Modification on the Dispersion, Morphology, and Mechanical Properties of Recycled PP/PET/TiO2 PBNANOs. Polymers 2019, 11, 1692. [Google Scholar] [CrossRef]
- ISO. ISO 3451-1 Standard: Plastics—Determination of Ash—Part1: General Methods; ISO: Geneva, Switzerland, 2019. [Google Scholar]
- Sanches, N.; Dias, M.; Pacheco, E. Comparative techniques for molecular weight evaluation of poly (ethylene terephthalate) (PET). Polym. Test. 2005, 24, 688–693. [Google Scholar] [CrossRef]
- Abt, T.; Álvarez, G.; Rodríguez, C.; Maspoch, M.L. Using the small punch test to analyse the influence of ultraviolet radiation on the mechanical behaviour of recycled polyethylene terephthalate. J. Strain Anal. Eng. Des. 2019, 54, 401–407. [Google Scholar] [CrossRef]
- López, M.d.M.C.; Pernas, A.I.A.; López, M.J.A.; Latorre, A.L.; Vilariño, J.L.; Rodríguez, M.V.G. Assessing changes on poly(ethylene terephthalate) properties after recycling: Mechanical recycling in laboratory versus postconsumer recycled material. Mater. Chem. Phys. 2014, 147, 884–894. [Google Scholar] [CrossRef]
- ISO. ISO 527-3 Standard: Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- Haralabakopoulos, A.A.; Tsiourvas, D.; Paleos, C.M. Chain extension of poly(ethylene terephthalate) by reactive blending using diepoxides. J. Appl. Polym. Sci. 1999, 71, 2121–2127. [Google Scholar] [CrossRef]
- Awaja, F.; Daver, F.; Kosior, E. Recycled poly(ethylene terephthalate) chain extension by a reactive extrusion process. Polym. Eng. Sci. 2004, 44, 1579–1587. [Google Scholar] [CrossRef]
- Incarnato, L.; Scarfato, P.; Di Maio, L.; Acierno, D. Structure and rheology of recycled PET modified by reactive extrusion. Polymer 2000, 41, 6825–6831. [Google Scholar] [CrossRef]
- Japon, S.; Boogh, L.; Leterrier, Y.; Månson, J.-A.E. Reactive processing of poly(ethylene terephthalate) modified with multifunctional epoxy-based additives. Polymer 2000, 41, 5809–5818. [Google Scholar] [CrossRef]
- Badía, J.; Strömberg, E.; Ribes-Greus, A.; Karlsson, S. A statistical design of experiments for optimizing the MALDI-TOF-MS sample preparation of polymers. An application in the assessment of the thermo-mechanical degradation mechanisms of poly (ethylene terephthalate). Anal. Chim. Acta 2011, 692, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Pawlak, A.; Pluta, M.; Morawiec, J.; Galeski, A.; Pracella, M. Characterization of scrap poly(ethylene terephthalate). Eur. Polym. J. 2000, 36, 1875–1884. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.H. Poly (ethylene terephthalate) recycling for high value added textiles. Fash. Text. 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Badia, J.; Vilaplana, F.; Karlsson, S.; Ribes-Greus, A. Thermal analysis as a quality tool for assessing the influence of thermo-mechanical degradation on recycled poly(ethylene terephthalate). Polym. Test. 2009, 28, 169–175. [Google Scholar] [CrossRef]
- Masmoudi, F.; Fenouillot, F.; Mehri, A.; Jaziri, M.; Ammar, E. Characterization and quality assessment of recycled post-consumption poly(ethylene terephthalate) (PET). Environ. Sci. Pollut. Res. 2018, 25, 23307–23314. [Google Scholar] [CrossRef]
- Kong, Y.; Hay, J. Multiple melting behaviour of poly(ethylene terephthalate). Polymer 2003, 44, 623–633. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Hsiao, B.; Sauer, B.; Kampert, W. The nature of secondary crystallization in poly(ethylene terephthalate). Polymer 1999, 40, 4615–4627. [Google Scholar] [CrossRef]
- Righetti, M.C.; Laus, M.; Di Lorenzo, M.L. Temperature dependence of the rigid amorphous fraction in poly(ethylene terephthalate). Eur. Polym. J. 2014, 58, 60–68. [Google Scholar] [CrossRef]
- Wu, H.; Lv, S.; He, Y.; Qu, J.-P. The study of the thermomechanical degradation and mechanical properties of PET recycled by industrial-scale elongational processing. Polym. Test. 2019, 77, 105882. [Google Scholar] [CrossRef]
- Hay, J.N. The physical ageing of amorphous and crystalline polymers. Pure Appl. Chem. 1995, 67, 1855–1858. [Google Scholar] [CrossRef]
- Leonov, A. A theory of necking in semi-crystalline polymers. Int. J. Solids Struct. 2002, 39, 5913–5926. [Google Scholar] [CrossRef]
- Grellmann, W.; Langer, B. Deformation and Fracture Behaviour of Polymer Materials; Springer: Berlin/Heidelberg, Germany, 2017; Volume 247. [Google Scholar]
- Van Breemen, L.C.; Engels, T.A.; Klompen, E.T.; Senden, D.J.; Govaert, L.E. Rate- and temperature-dependent strain softening in solid polymers. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1757–1771. [Google Scholar] [CrossRef]
- Kechekyan, P.A.; Arzhakova, O.V.; Kechekyan, A.S.; Dolgova, A.A.; Volynskii, A.L. The effect of characteristic self-generated defects on the mechanical behavior of poly(ethylene terephthalate) fibers and films. Polymer 2019, 176, 11–19. [Google Scholar] [CrossRef]
- Caro-Bretelle, A.; Ienny, P.; Nait-Ali, L.; Bergeret, A. Dissipative Behaviour Analysis of Reprocessed Polyethylene Terephthalate using Digital Image Correlation and Updated Finite Element Analysis. Strain 2013, 49, 135–146. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Moskala, E.J. Molecular dependence of the essential and non-essential work of fracture of amorphous films of poly(ethylene-2,6-naphthalate) (PEN). Polymer 2000, 41, 6301–6310. [Google Scholar] [CrossRef]
- Chen, H.B.; Wu, J.S. Understanding the underlying physics of the essential work of fracture on the molecular level. Macromolecules 2007, 40, 4322–4326. [Google Scholar] [CrossRef]
- Al-Jabareen, A.; Illescas, S.; Maspoch, M.L.; Santana, O.O. Essential work of fracture testing of PC-rich PET/PC blends with and without transesterification catalysts. J. Mater. Sci. 2010, 45, 2907–2915. [Google Scholar] [CrossRef]
- Ferrer-Balas, D.; Maspoch, M.L.; Martinez, A.B.; Santana, O.O. Influence of annealing on the microstructural, tensile and fracture properties of polypropylene films. Polymer 2001, 42, 1697–1705. [Google Scholar] [CrossRef]






| Temperature Profile | Temperature From Zone 1 to Die (°C) |
|---|---|
| High | 200/240/255/260/265/270/270 |
| Medium | 190/230/245/250/255/260/260 |
| Low | 180/215/235/240/240/245/245 |
| Material | Temperature Profile | [η] (dL·g−1) | Mn (kg·mol−1) | Mw (kg·mol−1) | Mw/Mn |
|---|---|---|---|---|---|
| rPET-T flakes | - | 0.875 | 41.5 | 64.8 | 1.56 |
| rPET-T pellets | High | 0.677 (−23%) 1 | 29.2 | 44.4 | 1.52 |
| Medium | 0.717 (−18%) 1 | 31.6 | 48.3 | 1.53 | |
| Low | 0.790 (−10%) 1 | 36.1 | 55.7 | 1.54 | |
| rPET-O flakes | - | 0.792 | 36.2 | 55.9 | 1.54 |
| rPET-O pellets | Low | 0.726 (−8%) 1 | 32.2 | 49.2 | 1.53 |
| 1st Heating | Cooling | 2nd Heating | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Material | Tg (°C) | Tcc (°C) | Tm (°C) | Xc (%) | Tc (°C) | Xc 1 (%) | Tg (°C) | Tm1 (°C) | Tm2 (°C) | Tm3 (°C) |
| rPET-T | 70 | 124 | 248 | 7 | 200 | 34 | 81 | 204 | 240 | 247 |
| rPET-O | 69 | 122 | 247 | 8 | 198 | 34 | 79 | 204 | 239 | 246 |
| Property | Sample | Crosshead Speed (mm·min−1) | ||||
|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 20 | |||
| E(GPa) | T | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.1 ± 0.2 | |
| O | 2.0 ± 0.1 | 2.1 ± 0.2 | 2.2 ± 0.1 | 2.1 ± 0.2 | ||
| P.A. | T | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.2 | 2.2 ± 0.2 | |
| O | 2.2 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.2 | 2.3 ± 0.2 | ||
(MPa) | T | 48 ± 4 | 53 ± 1 | 54 ± 1 | 56 ± 5 | |
| O | 50 ± 1 | 53 ± 1 | 54 ± 1 | 55 ± 2 | ||
| P.A. | T | 54 ± 3 | 59 ± 5 | 61 ± 4 | 64 ± 2 | |
| O | 55 ± 1 | 58 ± 2 | 60 ± 2 | 62 ± 1 | ||
(%) | T | 2.7 ± 0.2 | 2.9 ± 0.1 | 3.1 ± 0.1 | 3.2 ± 0.1 | |
| O | 2.7 ± 0.2 | 2.9 ± 0.1 | 3.0 ± 0.1 | 3.0 ± 0.1 | ||
| P.A. | T | 2.7 ± 0.2 | 4.0 ± 0.6 | 4.2 ± 0.4 | 3.4 ± 0.1 | |
| O | 3.0 ± 0.3 | 3.3 ± 0.4 | 3.3 ± 0.2 | 3.3 ± 0.2 | ||
(kJ·m−3) | T | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | |
| O | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | ||
| P.A. | T | 1.0 ± 0.1 | 1.6 ± 0.3 | 1.7 ± 0.2 | 1.3 ± 0.1 | |
| O | 1.1 ± 0.1 | 1.2 ± 0.2 | 1.2 ± 0.1 | 1.2 ± 0.1 | ||
| Sample Nomenclature | we (kJ·m−2) | βwp (MJ·m−3) | β (x10−2) | wp (MJ·m−3) | R2 (wf vs. l) |
|---|---|---|---|---|---|
| Unaged | |||||
| rPET-T | 65 ± 4 | 11.3 ± 0.2 | 13.4 ± 0.3 | 84 ± 4 | 0.9945 |
| rPET-O | 56 ± 4 | 11.6 ± 0.3 | 11.5 ± 0.4 | 101 ± 7 | 0.9927 |
| Physically aged | |||||
| rPET-T | 48 ± 4 | 11.3 ± 0.2 | 11.1 ± 0.6 | 102 ± 1 | 0.9938 |
| rPET-O | 56 ± 5 | 10.5 ± 0.3 | 8.8 ± 0.4 | 126 ± 2 | 0.9895 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loaeza, D.; Cailloux, J.; Santana Pérez, O.; Sánchez-Soto, M.; Maspoch, M.L. Impact of Titanium Dioxide in the Mechanical Recycling of Post-Consumer Polyethylene Terephthalate Bottle Waste: Tensile and Fracture Behavior. Polymers 2021, 13, 310. https://doi.org/10.3390/polym13020310
Loaeza D, Cailloux J, Santana Pérez O, Sánchez-Soto M, Maspoch ML. Impact of Titanium Dioxide in the Mechanical Recycling of Post-Consumer Polyethylene Terephthalate Bottle Waste: Tensile and Fracture Behavior. Polymers. 2021; 13(2):310. https://doi.org/10.3390/polym13020310
Chicago/Turabian StyleLoaeza, David, Jonathan Cailloux, Orlando Santana Pérez, Miguel Sánchez-Soto, and Maria Lluïsa Maspoch. 2021. "Impact of Titanium Dioxide in the Mechanical Recycling of Post-Consumer Polyethylene Terephthalate Bottle Waste: Tensile and Fracture Behavior" Polymers 13, no. 2: 310. https://doi.org/10.3390/polym13020310
APA StyleLoaeza, D., Cailloux, J., Santana Pérez, O., Sánchez-Soto, M., & Maspoch, M. L. (2021). Impact of Titanium Dioxide in the Mechanical Recycling of Post-Consumer Polyethylene Terephthalate Bottle Waste: Tensile and Fracture Behavior. Polymers, 13(2), 310. https://doi.org/10.3390/polym13020310