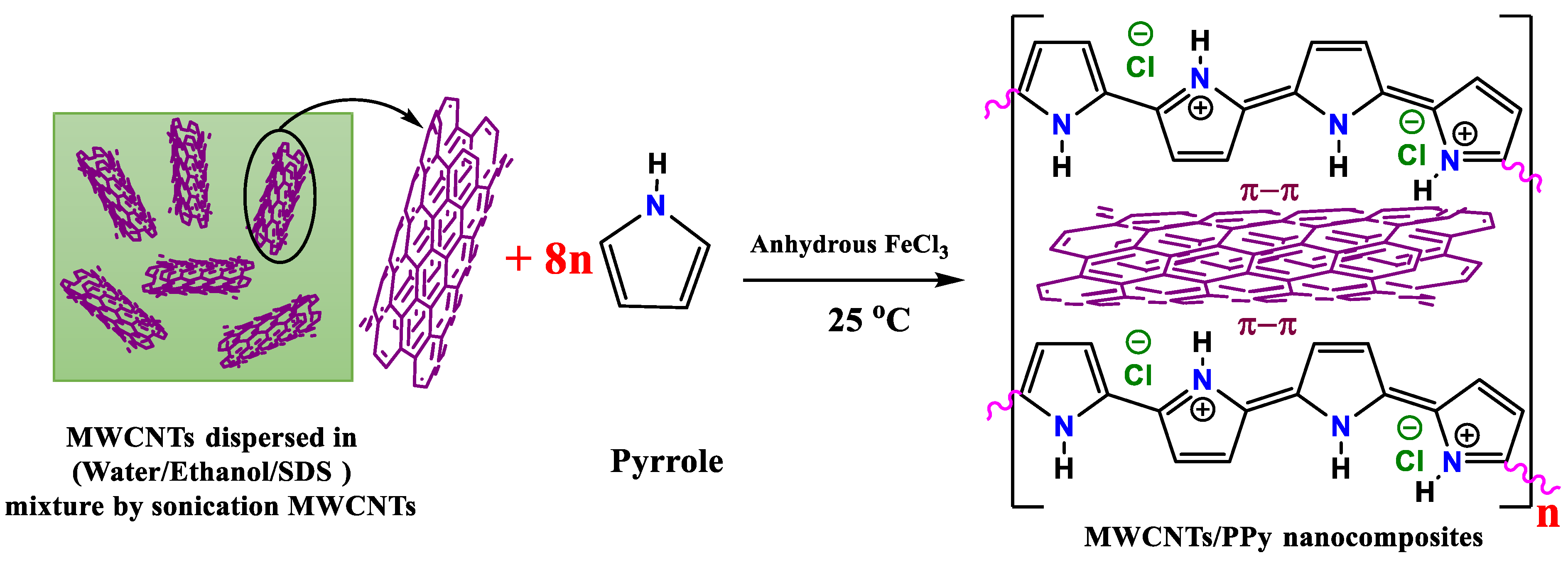
3.1. Structural Properties
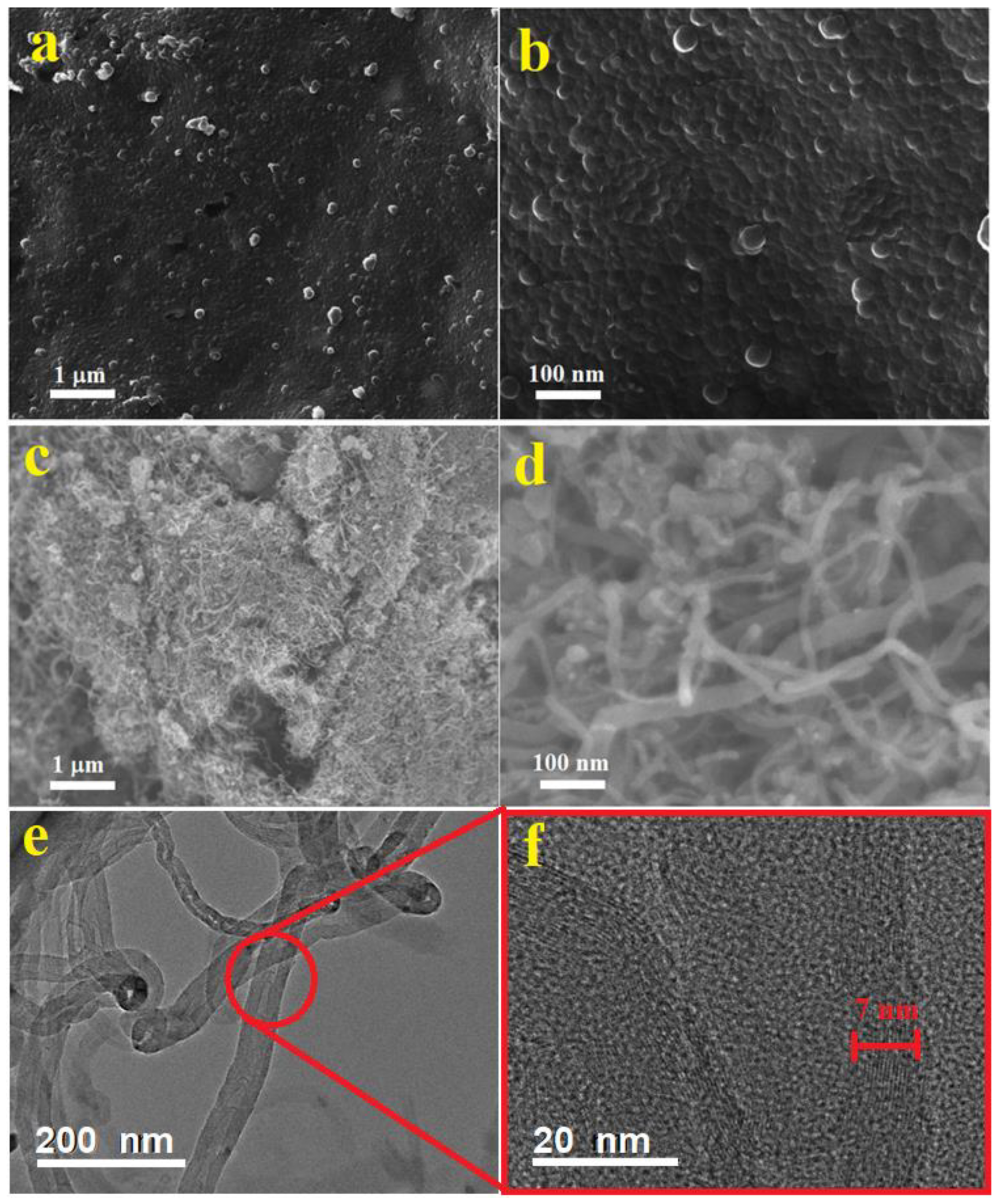
The surface morphology and nanostructure form of the synthesized pure PPy were investigated by SEM technique. The obtained SEM images are represented in
Figure 2a,b. These SEM images show that the PPy structures is produced in a 2-dimensional film. These films are composing fine nanoparticles with sizes in the range 15–25 nm. The shape and size of the resulting polymer is well-known to depends on the type of used surfactant during the polymerization process. The formation of PPy films in the presence of SDS and FeCl
3 was reported by Gangopadhyay [
15] (dissolved in a water medium), but it is stated that the films are too brittle to handle freely. This perhaps might be due to the absence of ethanol as a co-solvent, or may be due to the variations in the molar ratios of the pyrroles and surfactants/oxidants, which might affect the bonding between the formed PPy chains.
Figure 2c–e shows SEM and TEM images of the MWCNTs at different magnifications. Thin and thick nanotubes can be observed in bundle entangled forms (c). The diameters of these MWCNTs are in the range 10–40 nm. These nonuniform MWCNTs were used as received without further purification or catalysts removal, therefore a considerable defects and catalyst particles are noticeable in these nanotubes (
Figure 2d,e). The HRTEM image shows multi-walled nanotubes of around 15–18 walls with walls thicknesses around 7 nm as shown in
Figure 2f. Zigzagged walls can be observed in the thin and thick MWCNTs. Moreover, bamboo like structures is also noticed in some of the thin MWCNTs (e).
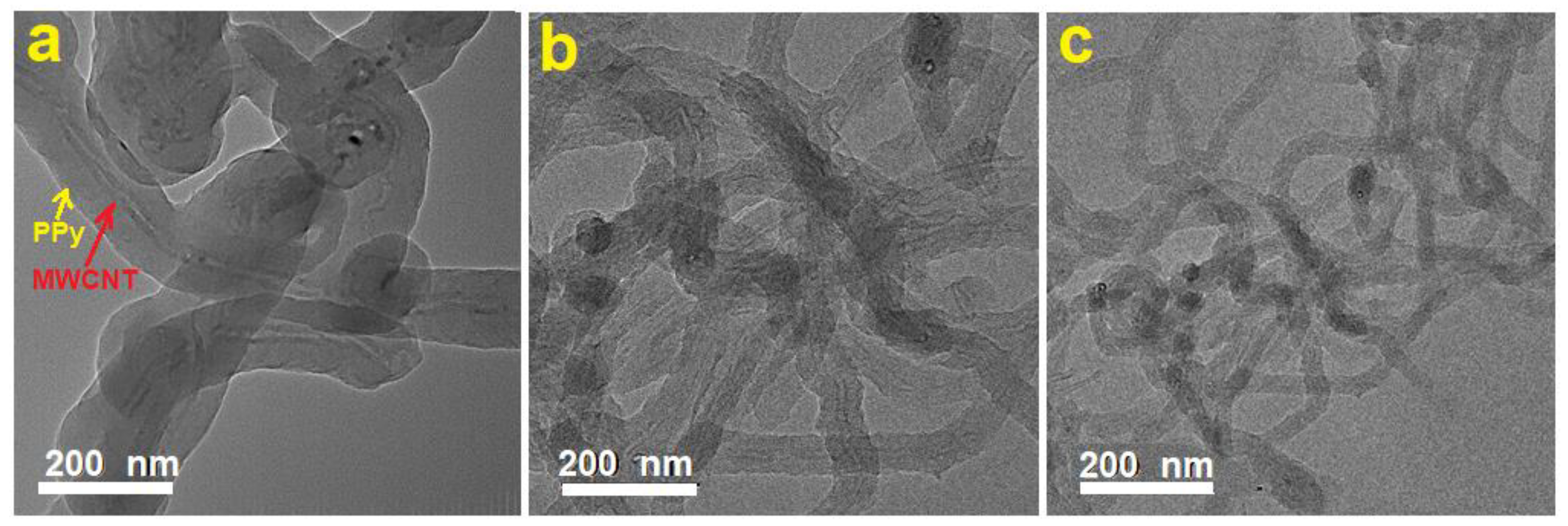
The morphology and microstructure of the MWCNTs/PPy composites were investigated by the SEM and TEM and the obtained results are presented in
Figure 3 and
Figure 4, respectively. It is clear that the PPy were grown on the side walls of these MWCNTs, making one dimensional (1D) nanocomposite. Perfect coating can be observed, but at different thicknesses (
Figure 3). Lower amounts of MWCNTs results on thicker shells/layers of PPy, while higher amounts of MWCNTs could produce thinner layers of PPy. These observations are clearly shown in
Figure 4. The coating was performed with different ratios between PPy and MWCNTs as mentioned in
Table 1. It is clear that with increasing the percentage of MWCNTs, the PPy layer thicknesses were decreased. As mentioned above the amount of Py was fixed, while that of MWCNTs vary from 1.1 to 14.6 wt.%. This perhaps could distribute the available monomers of PPy on the surface of MWCNTs, therefore the higher amounts of MWCNTs got thin layers of PPy. This results in forming MWCNTs/PPy nanocomposites with different diameters as shown in
Figure 3a–e.
Figure 4 shows TEM images at the same magnification of typical MWCNTs/PPy nanocomposites (a: 1.1 wt.%, b: 4.6 wt.%, c: 14.6wt.%). In this figure, it is clear that the MWCNTs are located at the cores of these 1D structures, and the PPy are forming the shells. With increasing the percentage of MWCNTs, the thickness of the coated PPy decreases and vice versa as illustrated in
Figure 4a–c. This result is remarkable, by means it is possible that the presence of SDS as a surfactant could favor the PPy growth smoothly in the side walls of MWCNTs, which themselves acted as 1D templates. This result is much better than that reported earlier [
14] on producing MWCNTs/PPy nanocomposites using p-toluenesulfonic acid (TSA) as a dopant and iron chloride (FeCl
3) as an oxidant. They obtained PPy nanoparticles at the surface of the MWCNTs, without a uniform coating [
14]. It is clear that the used surfactant in our case is the key factor on obtaining 1D core/shell MWCNTs/PPy nanocomposites.
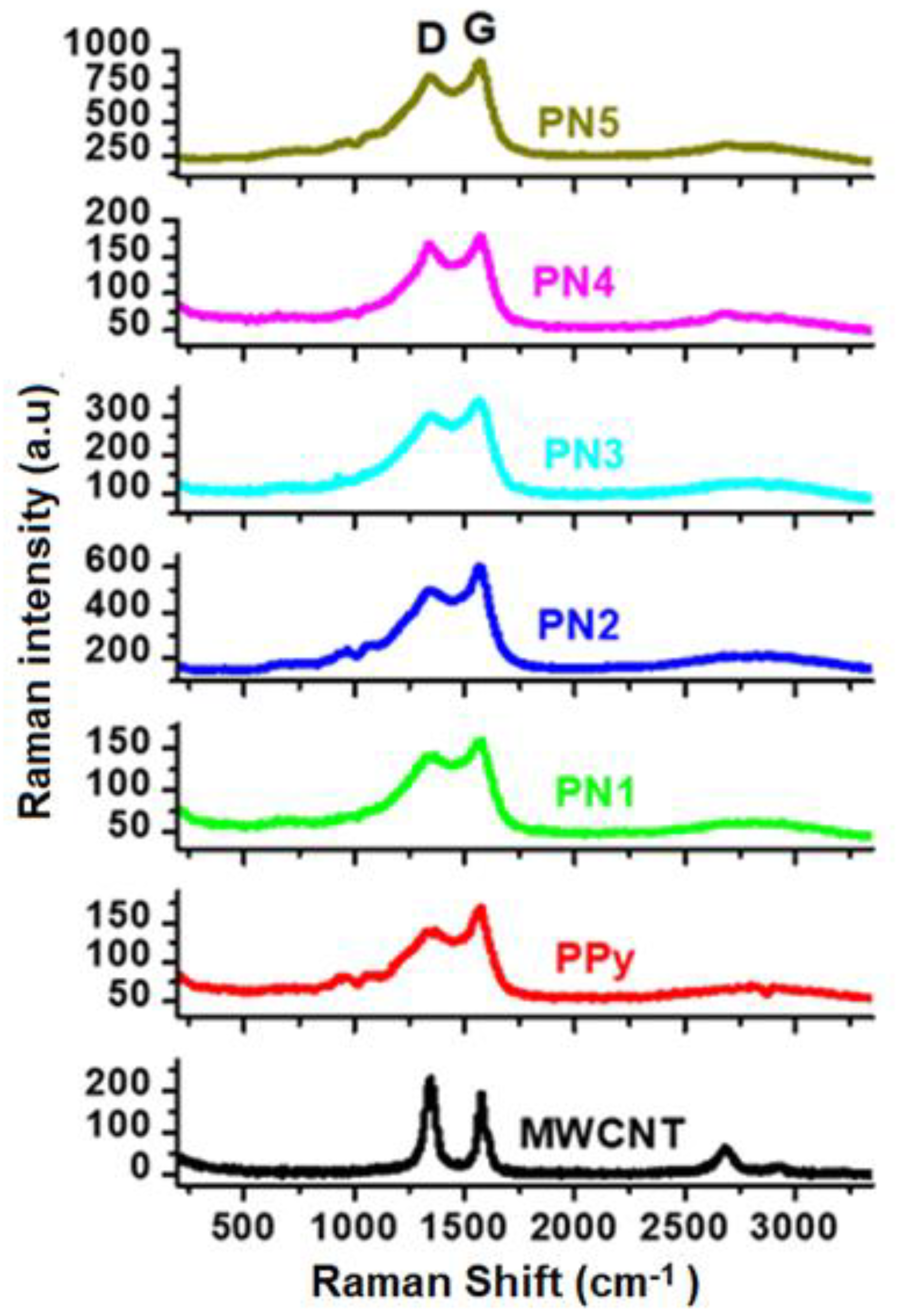
Figure 5 displays the Raman spectra of Nanocomposites MWCNTs, PPy and PPy/MWCNTs. 5. Two identified bands of 1598 and 1345 cm
−1 were seen in the neat MWCNTs. These are the vibration of the MWCNTs (G band) caused by the condition (D-band) and in-plane vibration. There were two large bands with a duration of approx. 1362 and 1571 cm
−1 corresponding to the vibration modes C–C and C=C in the Ring of the PPy backbone [
16,
17] respectively. Weak bands of 952 and 1081 cm
−1 were also present in the neat PPy. Both are the in-plan deformation of the ring associated with the di-polaron and C–H respectively [
18]. The MWCNTs/PPy spectrum is similar to the neat PPy spectrum, but the range of amplitude increases systematically by increasing the MWCNTs material. Furthermore, there is a shift in the composites from the 1571 cm
−1 tidy PPy band to the higher wave number line. This increase and band change means that MWCNTs are associated with PPy chains by moving the charge. A major decrease in the intensity of the band has contributed to a successful π–π stacking between the MWCNTs and PPy chains for composites with the smaller percentage of MWCNTs [
19].
FTIR spectra for PPY, MWCNTs, and /MWCNTs/PPy nanocomposites are presented in
Figure 6. This chart shows 1550 and 1450 cm
−1 bands. These fit the stretching pulse of C=C and C–N of PPy. The vibration of the pyrrole ring formed the band at 1170 cm
−1. The in-plane vibratory deformation of C–H and N–H occurred at 1040 cm
−1 and the out-plane deformation band at 860 cm
−1 [
20]. The 1130 cm
−1 band is PPy (doped with chloride ion) characteristic [
21]. Due to the overlap with the vibration of the pyrrole ring at 1170 cm
−1 the expected S=O expanded vibration at 1183 cm
−1 could not be detected [
22]. The PPy/MWCNTs composites with PPy have an FTIR spectrum similar to the neat MWCNTs and PPy. This confirms the existence in the composites of all characteristic bands of PPy and MWCNTs. With a growing percentage of MWCNTs, the intensity of the C–H band shifted considerably. This is can be credited to the π–π assembling MWCNTs and PPy backbones.
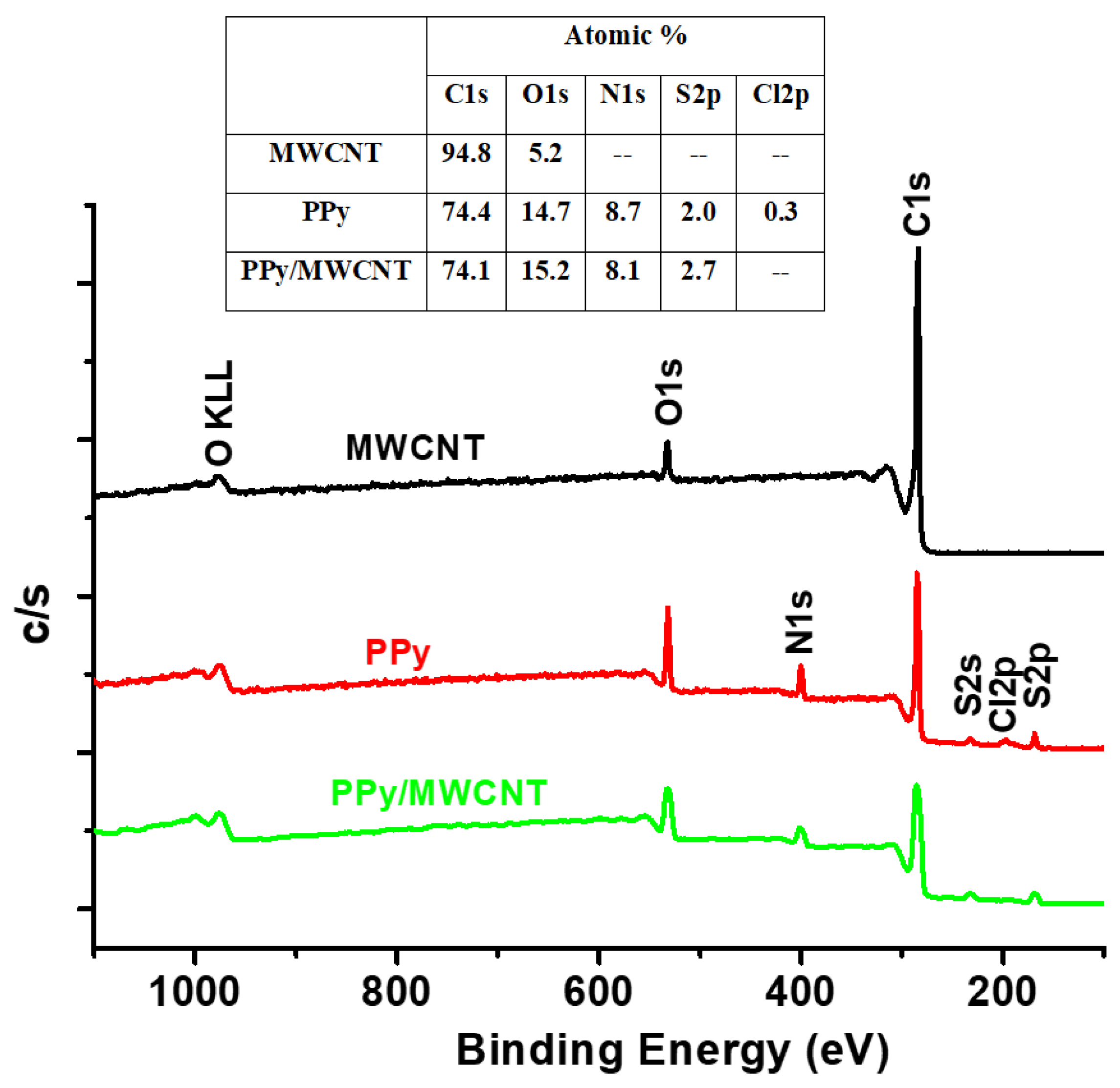
The XPS survey profiles of the MWCNTs, PPy, and MWCNTs/PPy nanocomposites are shown in
Figure 7. The C1s and O1s OKLL peaks could be observed in the spectra of the neat of both MWCNTs and PPy. A weak Cl2p and S2P bands were observed at around 200 eV for both neat PPy and PPy/MWCNTs composite. These peaks are due to the chloride ion-doped from FeCl
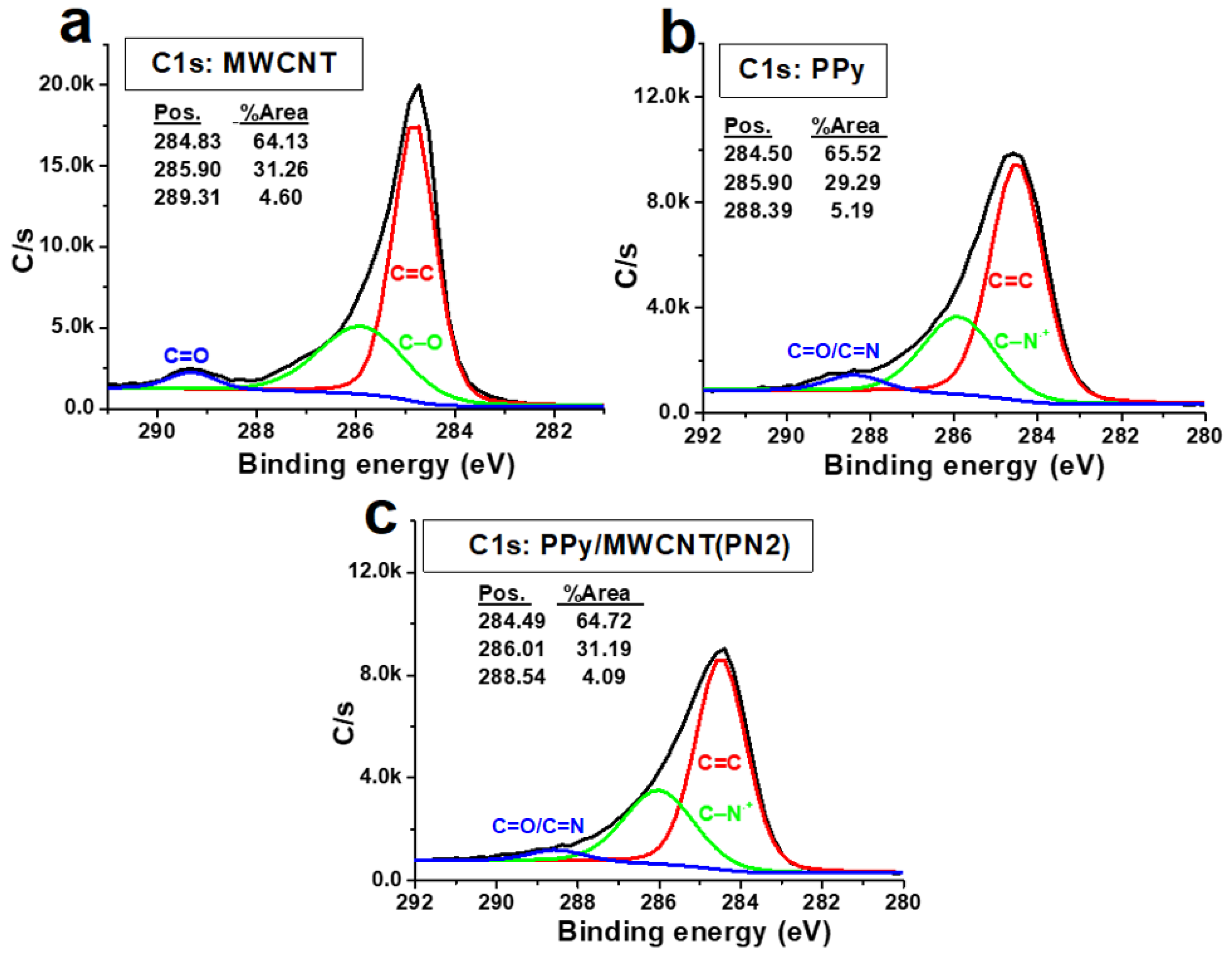
3 initiator and sulfur gained from SDS surfactant during the polymerization process. The C1s/O1s ratio of the neat CNTs is higher than that of neat PPy. The intensity of the O1s band decreased slightly for the PPy/MWCNTs composites with the appearance of a weak specified peak for chloride ion (Cl2p band at around 200 eV). This indicates the success of doping with chloride ions for the PPy chains. The C1s XPS profiles of MWCNTs, PPy, and MWCNTs/ PPy nanocomposites (PN2) are shown in
Figure 8(a-c). As shown in
Figure 8b, the observed peaks at 284.5 eV and 285.90 eV, which occupied areas of 65.52%.and 29.29% might be attributed to hybridization of carbon sp
2 and sp
3 type, respectively. The peak at 288.6 eV that occupied an area of 5.9% might be due to C-N of PPy. As shown in
Figure 8c, the observed three peaks at 284.49, 286.01 and 288.5 eV are occupied areas of 64.72%, 31.19%, and 4.09%, respectively. The C=C band is the most intense one in the spectrum of neat PPy; however, its intensity is lower than that of the C=C band in the MWCNTs (
Figure 8a). The spectra of the coated MWCNTs by PPy in the current composite (2.17% MWCNTs) is nearly similar to the spectra of the uncoated MWCNTs and neat PPy; though, the intensity of the resulting band of the composite to that of the neat MWCNTs and PPy (lower than that of the pure MWCNTs but higher than that of PPy). There are no significant changes are observed in the peak positions. This confirms that MWCNTs have been successfully coated by PPy with no extra bonds or crosslinking in the contact surfaces. The excellent π–π stacking between PPy backbones and MWCNTs might also assist in stabilizing.
3.2. Thermoelectric Properties
To investigate the TE properties of the above mentioned MWCNTs/PPy nanocomposites powder samples, they were initially pressed in pellets forms as described in the experimental section.
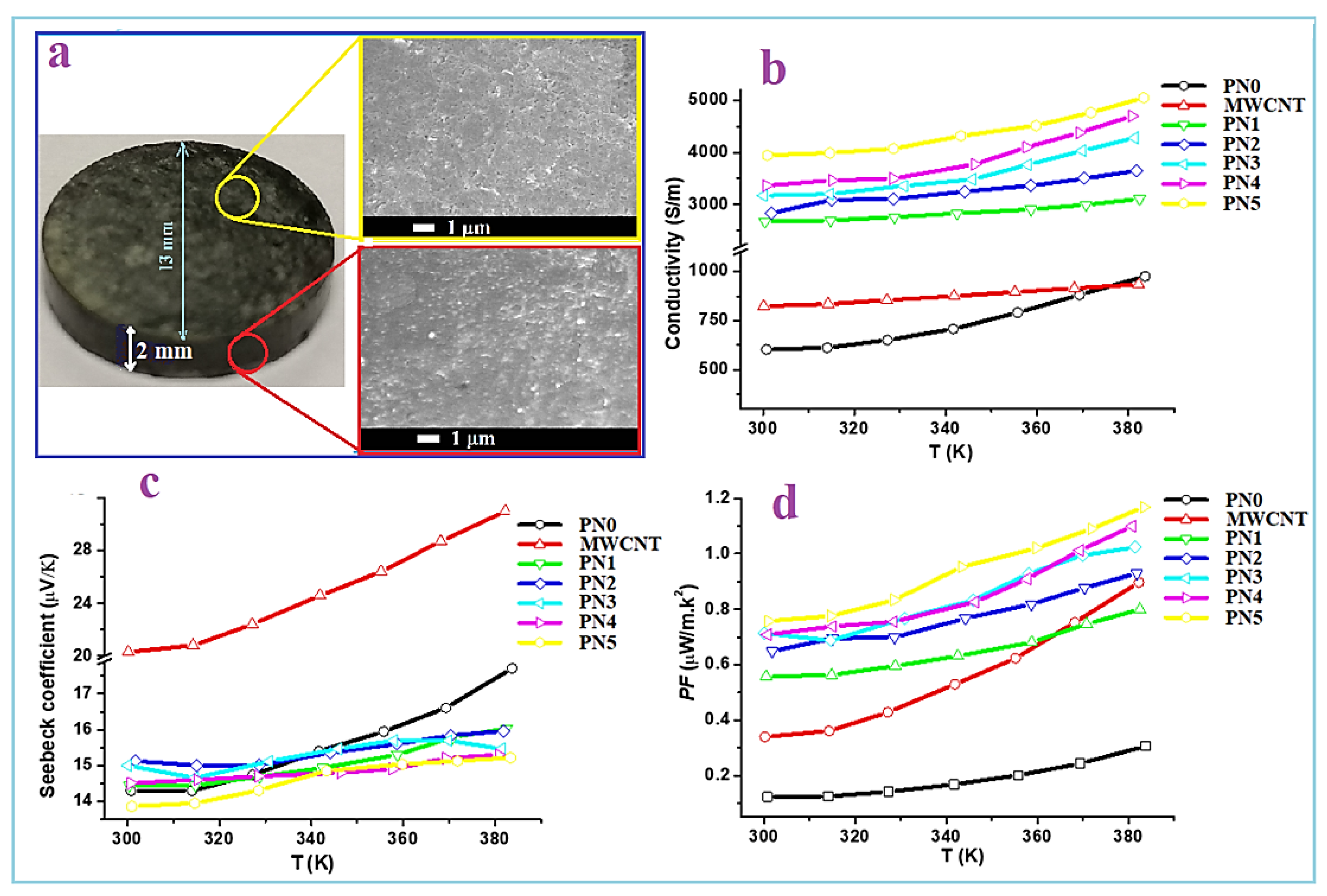
Figure 9a shows a picture of one of the formed MWCNTs/PPy pellets, which is of the PN3 sample. SEM images with a top and cross section view of this pellet are also shown. These images show well pressed pellet with almost no formation of cracks or defects. The produced 1D nanocomposites of MWCNTs/PPy were easily aligned under the applied press (~7 tons) making solid pellet with a good contact between its contents. The top and cross section view SEM images clearly show highly dense pellet with side-to-side contact of the 1D nanocomposite. This is an essential requirement to facilitate the charge transport across the whole pellet.
The TE properties of the pure PPy (PN0), neat MWCNTs and MWCNTs/PPy nanocomposites (PN1-PN5) are shown in
Figure 9b–d. The measured electrical conductivity at room temperature for PPy is around 600 S/m, while it is approximately 830 S/m for MWCNTs
Figure 9b. These values increased with an increase in temperature up to 383 K reaching to 930 S/m for the PPy and 900 S/m for MWCNTs. The relatively low electrical conductivity of the MWCNTs used in this study as compared to those of the MWCNTs reported in the literature [
23] might be attributed to their microstructure, as shown in
Figure 2c–f. The zigzagged structure and bamboo-like form of these MWCNTs in addition to the considerable amounts of defects/metal oxides are greatly affecting the electrical conductivity. The poor graphitization of the used MWCNTs (as reflected form the low ratio of G to D bands of Raman, shown in
Figure 5) is also another reason for the low electrical conductivity. These are responsible for hindered the mobility of charge carriers, thus reducing the electrical conductivity. It is well known that oxygen containing graphitic carbon materials are p-type semiconducting [
24]. In the present MWCNTs, the XPS results shown in
Figure 7 and
Figure 8 display limited amount of oxygen which might not be enough to enrich the MWCNTs with carrier holes, and thus showed only low electrical conductivity. In case of the PPy, its electrical conductivity is comparable to or even higher than those with similar structures or morphologies as reported by other research groups [
25,
26].
The measured electrical conductivity as a function of temperature of the MWCNTs/PPy nanocomposites (PN1-PN5) shown in
Figure 9a increased with an increase in the MWCNTs content. For PN0 with the rise in temperature, some electrons gain energy and then become free to conduct electricity (PPy is well known as a good conducting polymer). Therefore, polypyrrole’s electric conductivity increases with temperature. The increased conductivity of MWCNTs with temperature can be attributed to the decreasing in the contact resistance with temperature. At room temperature, the electrical conductivity was recorded around 2700 S/m for the MWCNTs/PPy nanocomposite at a MWCNTs content of 1.1 wt.% (PN1). This value was increased to approximately 4000 S/m by increasing the MWCNTs content to 14.6 wt.%. The measurements demonstrated that the electrical conductivity of the MWCNTs/PPy nanocomposites showed a semiconductor behavior, e.g., an increasing trend with an increase in temperature. At 383 K, the electrical conductivity values of all the nanocomposite samples were 15–25% higher than those recorded at room temperature. This significant enhancement in the electrical conductivity of the nanocomposites is remarkable, which might be attributed to the smooth and perfect PPy coating formed on the surface of the MWCNTs. Agglomerations of PPy or particles forming on the side walls of these nanotubes was not observed as shown in the SEM and TEM images.
Excellent 1D lamination may promote the shipment of charges and maximize the concentration of freight easily by incorporating both MWCNTs and PPy carriers. The results shown for PPy nanowire/graphene composites are compatible with previous works [
26]. In addition, the increase in the power conductivity of MWCNTs/PPy composites has been recorded in the literature [
14] and it is due to MWCNTs serving as a template to direct PPy’s self-assembly through the β-та interplacement of PPy with MWCNTs during production. In the formed layers and especially at interfaces, the concentration of the available transporters can be high, thereby improving electrical conductivity, with the effect on load mobility. The interfaces layers are higher in case of higher MWCNTs contents, therefore showing enhanced electrical conductivity. As temperature increases, some electrons acquire energy and then become free for electrical conduction. Hence, the electrical conductivity of polypyrrole increases with temperature.
The measured Seebeck coefficient of the pure PPy (PN0), MWCNTs and MWCNTs/PPy nanocomposites (PN1-PN5) are shown in
Figure 9b. In case of pure PPy, it has Seebeck value around 14.5 µV/K at RT, while the neat MWCNTs has a value of 20 µV/K. These values were increased by around 30% with an increase in the temperature to 383 K. These values for both PPy and MWCNTs are close to those reported previously [
19]. In case of the MWCNTs/PPy nanocomposites, the obtained Seebeck coefficient values are almost similar to that of the pure PPy. But these values are much higher than that reported for similar composite [
14]. As a result of the improved electrical conductivity and almost invariant Seebeck coefficient, the calculated power factor,
PF values of the nanocomposite samples are higher than those of the individuals PPy and MWCNTs as displayed in
Figure 9c. The maximum value is recorded by the nanocomposites of PN5 at MWCNTs content of 14.6 wt.%. It recorded 0.77 µW/mK
2 at room temperature, and this value increased to 1.3 µW/mK
2 at 383 K. Although the MWCNTs has low electrical conductivity as compared to those reported in the literature [
23], the
PF value of the composite samples are higher than that of MWCNTs/PPy composites [
14].
It is well known that in solid materials, heat is transferred mainly by phonons and electrons, so the total thermal conductivity is defined as
κtotal=
κp +
κe, where
κp and
κe are the phonon and the electron thermal conductivities, respectively. The value of
κe can be determined using the measured electrical conductivity σ and then the Wiedemann–Franz law on the assumption that the relaxation time of phonons and electron holes is identical [
27].
where
L is the Lorenz number,
kB is Boltzmann’s constant, and
e is the charge of an electron. The
κtotal,
κp and
κe of the pure MWCNTs, PPy and MWCNTs/PPy nanocomposites were plotted as the functions of temperature and the obtained result is presented in
Figure 10a–c. The values of
κp, were obtained by subtracting the values of
κe from those of
κtotal.
The
κtotal of the neat MWCNTs was approximately 0.16 W/mK at RT. This value increased to approximately 0.28 W/mK at 383 K (
Figure 10a). These values are low compared with that reported in the literature [
28]. This most probably can be attributed to the complex network, presence of defects and also to the zigzag and bamboo-like structure of the MWCNTs (
Figure 1), which might have generated extra scattering sites for the phonons. The defects present in such nanotubes along with their quality were reported to be important for their thermal performance [
29]. In case of the pure PPy its
κtotal is found to be around 0.14 W/mK at both RT as well as at high temperature (383 K). This value is close to those reported previously [
25]. The
κtotal of MWCNTs seems to be temperature dependent, which is not the case for PPy. This might be due to their intrinsic as well as network structures. The MWCNTs might expand by heating and becomes closers. This close contact between the adjacent nanotubes by heating might reduce the phonons scattering sites and facilitate the phonons transport and thus increasing
κtotal. In case of PPy, their polymerization resulted on forming film/sheet structures; this form might not be affected by heating and could maintain the same phonons scattering sites, even if expands.
The
κp of both MWCNTs and PPy are closer to those of
κtotal, and have almost the same trend with temperature, as shown in
Figure 10b. This indicate that the phonons are the major heat carriers. The
κe of both MWCNTs and PPy contributed only 3 and 4% of the
κtotal of both materials, respectively. With increasing the temperature up to 383 K there is a slight increase in
κe of both MWCNTs and PPy. This behavior with temperature is different than that of
κp of both materials. In case of MWCNTs the
κe is observed to be lesser temperature dependent than
κp, while in case of PPy, it is vice versa. This means PPy has electrical properties closer to perfect semiconductors than the used MWCNTs, which are impure. These MWCNTs with its considerable imperfections and low-quality graphitization exhibit poor electrical and thermal conductivities [
29], but this loss in the thermal conductivity is quite useful for TE materials to have better performance.
In case of the MWCNTs/PPy nanocomposite samples (PN1–PN5), the
κtotal at RT recorded higher values than those of the individuals MWCNTs and PPy (
Figure 10a). The PN1 sample recorded around 0.175 W/m·K at room temperature and with increasing the MWCNTs content to 14.6 wt.% (PN5) this value increased to 0.24 W/mK. As mentioned above due to the higher amounts of catalyst particles along with complex structure of the MWCNTs, the thermal conductivity is still low in these MWCNTs/PPy nanocomposites. The included MWCNTs were used without purification and still have a considerable oxide catalyst, therefore could significantly reduce the thermal conductivity of the pure MWCNTs as well as the MWCNTs/PPy nanocomposites. The interfacing sites between the MWCNTs and PPy can also add extra scattering site for the phonons. These all tougher are responsible for this low thermal conductivity. These low thermal conductivities are useful for better figure of merit and hence TE performance. The perfect coating of the MWCNTs by PPy might facilitate the charge transport, but might also make a smooth bath for the generated phonons thus increasing the electrical conductivity. However, the MWCNTs/PPy nanocomposites still have very low thermal conductivity as compared to similar composites reported in the literature [
25].
The
κp of the MWCNTs/PPy nanocomposite samples (PN1–PN5) are closer to those of
κtotal, and have almost the same trend with temperature as shown in
Figure 10b. This indicate that the phonons are the major heat carriers in these 1D nanocomposite samples. But, the
κe contribution seems to be higher in these nanocomposites than in case of the individual materials. This contribution reached to around 12% of
κtotal. The semiconducting behavior in these 1D nanocomposite is also prominent; when the temperature increased the
κe was observed to significantly increase (
Figure 10c). With increasing the MWCNTs content in these 1D nanocomposites, the
κe is further increased. Moreover, the rate of increases with temperature was also higher at higher MWCNTs content. This indicate that the formed 1D MWCNTs/PPy nanocomposite samples gained enhanced semiconducting properties, which might be due to the excellent 1D lamination, which promote the shipment of charges and maximize the concentration of freight easily by incorporating both MWCNTs and PPy carriers.
The perfect 1D coating of PPy on the side walls of MWCNTs significantly increased the figure of merit,
zT of the resulting 1D core shell nanocomposites. This result is presented in in
Figure 10d. The
zT value of the neat MWCNTs was 0.7 × 10
−3 at RT, while it slowly increased to 1.0 × 10
−3 at 383 K. PPy showed lower
zT values (than the pure MWCNTs), which are 0.25 × 10
−3 at RT and 0.75 × 10
−3 at 383 K. The
zT values of the MWCNTs/PPy nanocomposites were significantly higher than those of both the pure MWCNTs and PPy. The highest
zT value of 1.0 × 10
−3 was obtained for the MWCNTs/PPy nanocomposites at all MWCNTs contents at RT. This value increased by a factor of 2.5 at 383 K for the MWCNTs/PPy nanocomposite at MWCNTs content of 4.6 wt.%.
The adopted approach in this work on perfectly coating MWCNTs with smooth layers of PPy with no PPy agglomeration and maintaining 1D core shell nanocomposites seem to be effective on improving the TE performance. The substantial increase in electrical conductivity and Seebeck coefficient resulted for this development. This improvement has been rendered rather by an important element in the wide surface area of the MWCNTs, which were used as bridges or networks linking PPy leading domains [
30]. The second consideration is that MWCNTs serve as a basis for the self-assembly of PPy, which increases the electrical conductivity of the MWCNTs, in a more ordered crystalline alignment [
31]. The third aspect is related to the stability and the energy filtration effect at the Seebeck coefficient values at the MWCNT/PPy interfaces. These interfaces permitted the carriers with greater energy to pass through sufficient potential limit barriers, thereby increasing the average carrier energy in the flow [
25]. The low thermal conductivity of the resulting MWCNTs/PPy nanocomposites can have an important impact on dispersion of phonons due to the complex MWCNTs network and the interfaces between the MWCNT/PPy nanocomposites.
The presented results in this work demonstrated a new finding, which is the improvements in the electrical conductivity of MWCNTs by perfectly coating it with the conducting polymer, PPy to form 1D core shell nanocomposite. This finding might be a property of only conducting polymers such as PPy (using proper approach for polymerization with suitable precursors), but in case of other polymers the conductivity might decrease, as recently reported by Wang et al. [
32], who mentioned that “polymer/carbon nanotube composites have lower electrical conductivity than pristine CNTs”. The latest work on coating MWCNTs with PPy has shown a strong increase in electricity conductivity [
19]. The successful interaction between PPy and MWCNTs by staking the polymer chain with these MWICNTs as seen in
Figure 1 could accomplish this as above stated. The connectivity between the PPy and the MWCNTs may thus be substantially strong and simple. This clear approach with the adopted simple process might be quite useful guidelines to enhance the TE performance of TE based polymer materials. It does not involve long process or complex ternary composites.