Synthesis of π-Conjugated Polymers Containing Benzotriazole Units via Palladium-Catalyzed Direct C-H Cross-Coupling Polycondensation for OLEDs Applications
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Instruments
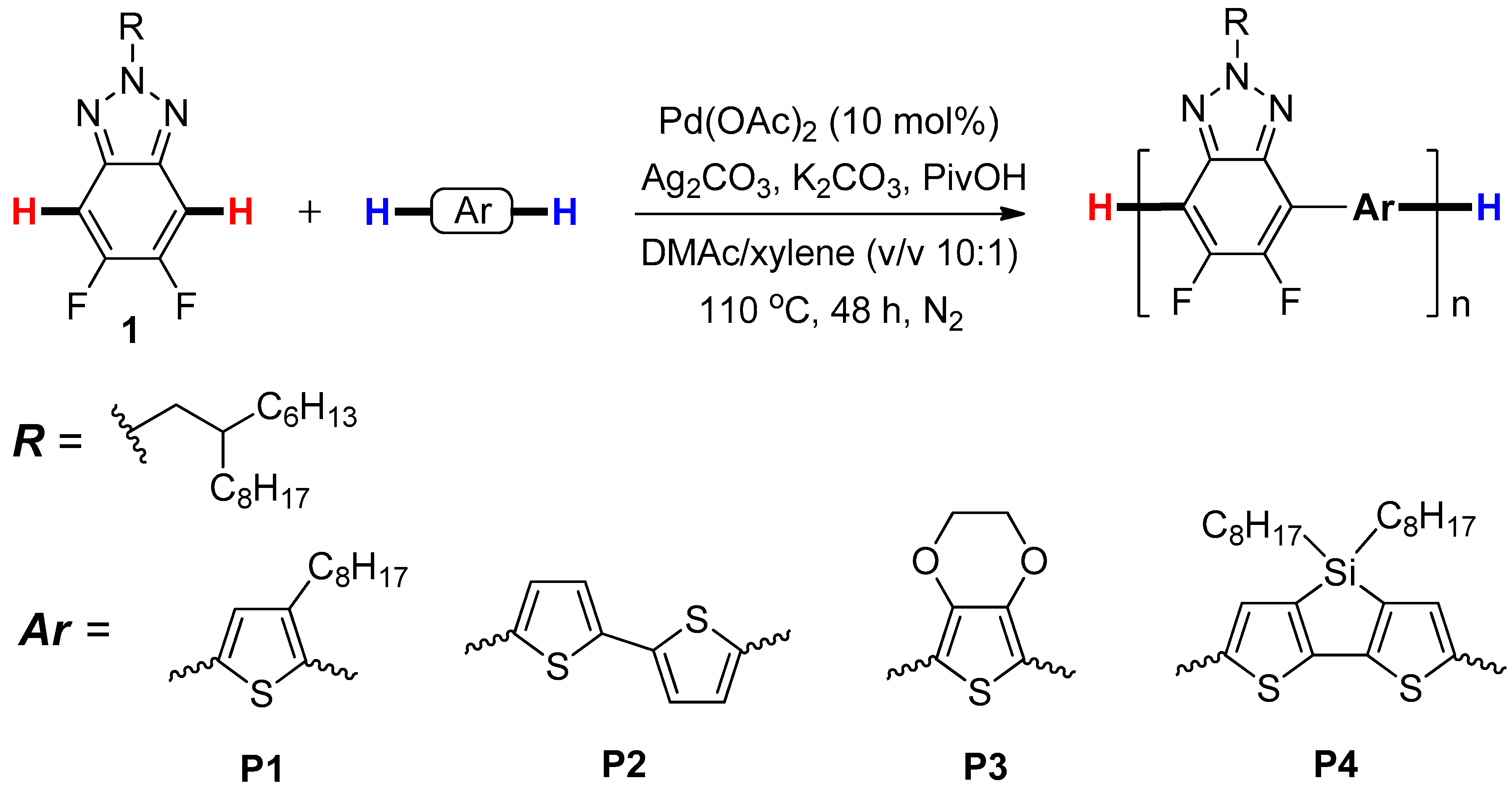
2.3. Synthesis of Polymer
2.4. Device Fabrication and Characterization
3. Results and Discussion
3.1. Synthesis and Characterization of Polymers
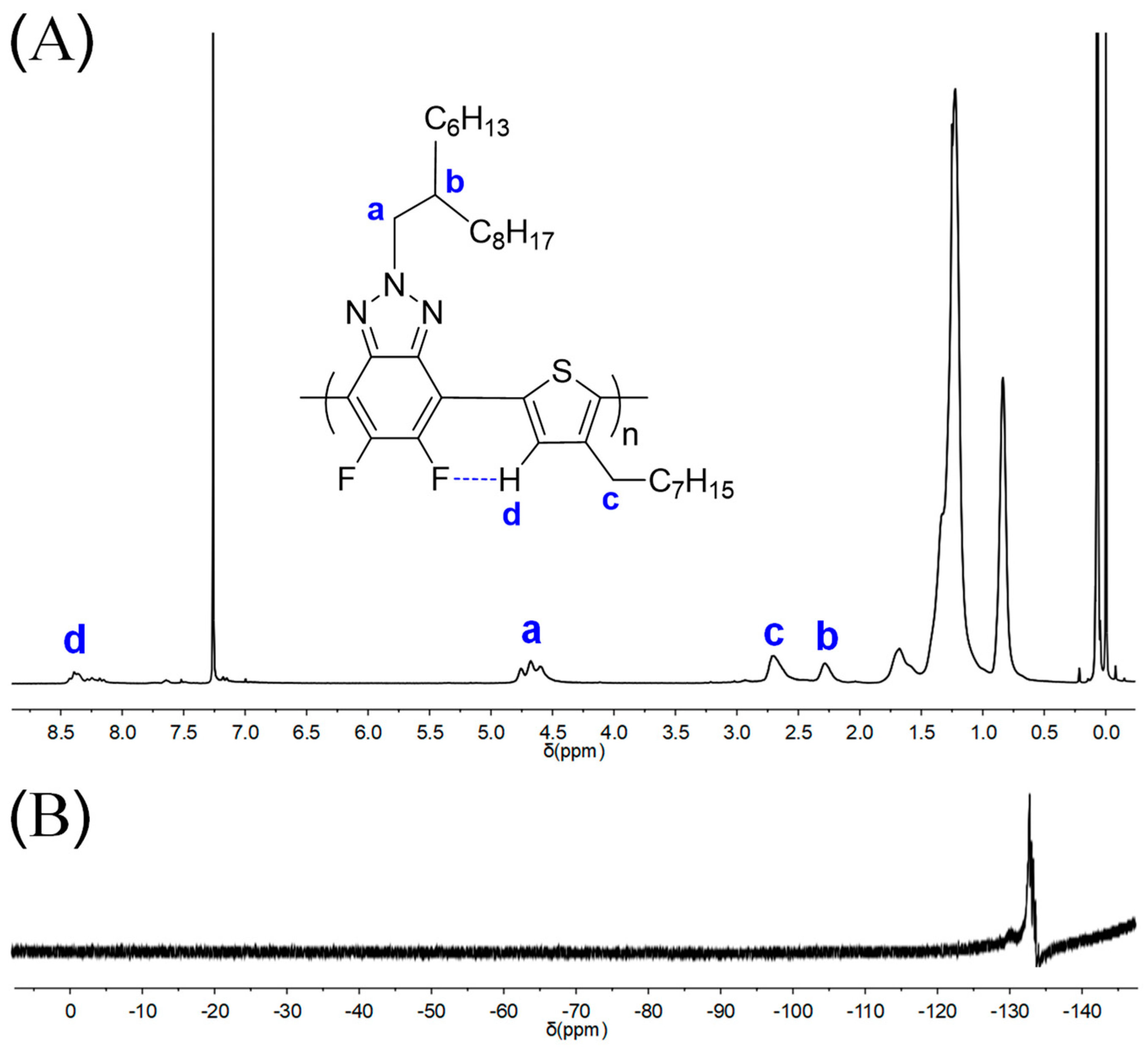
3.2. Structural Characterization
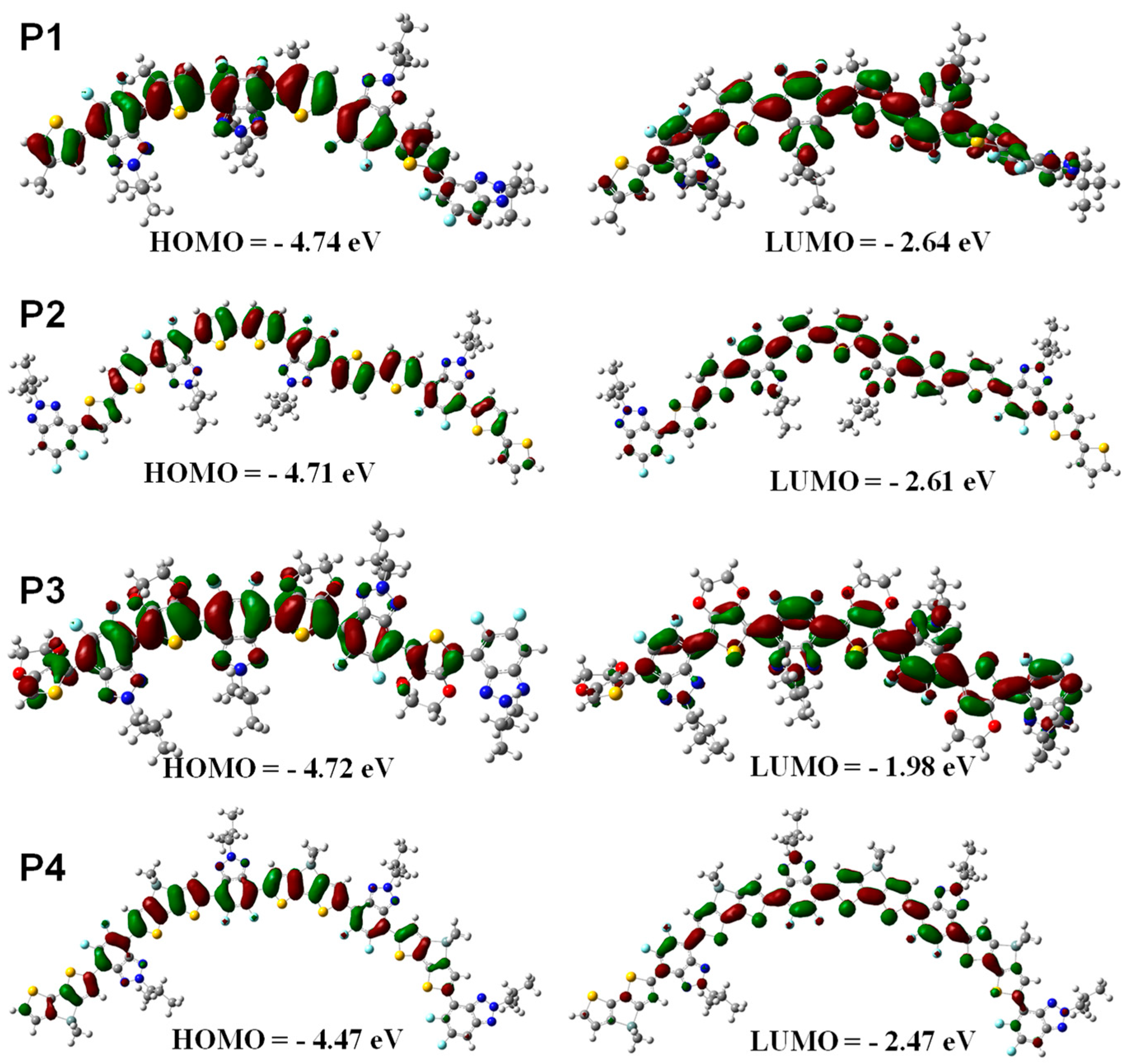
3.3. DFT Simulation
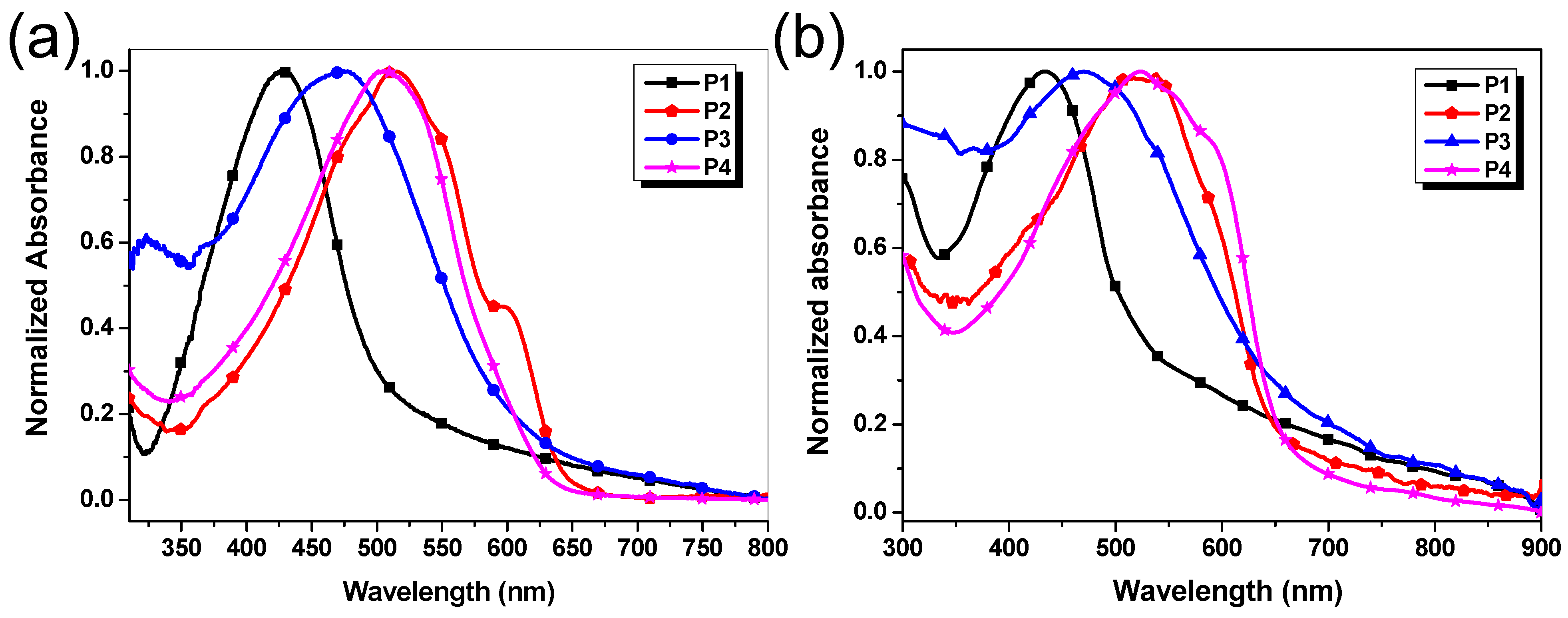
3.4. UV–vis Absorption Spectra
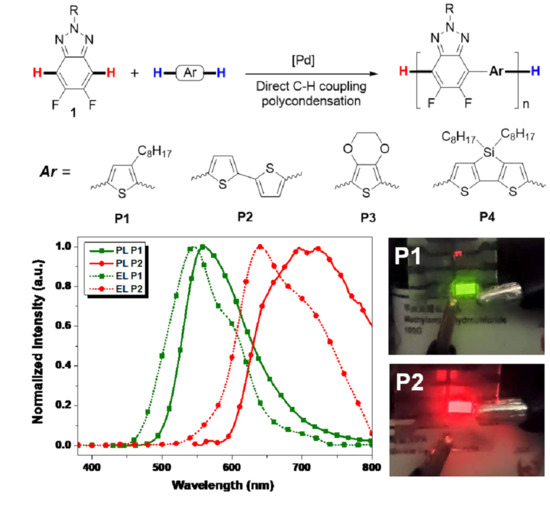
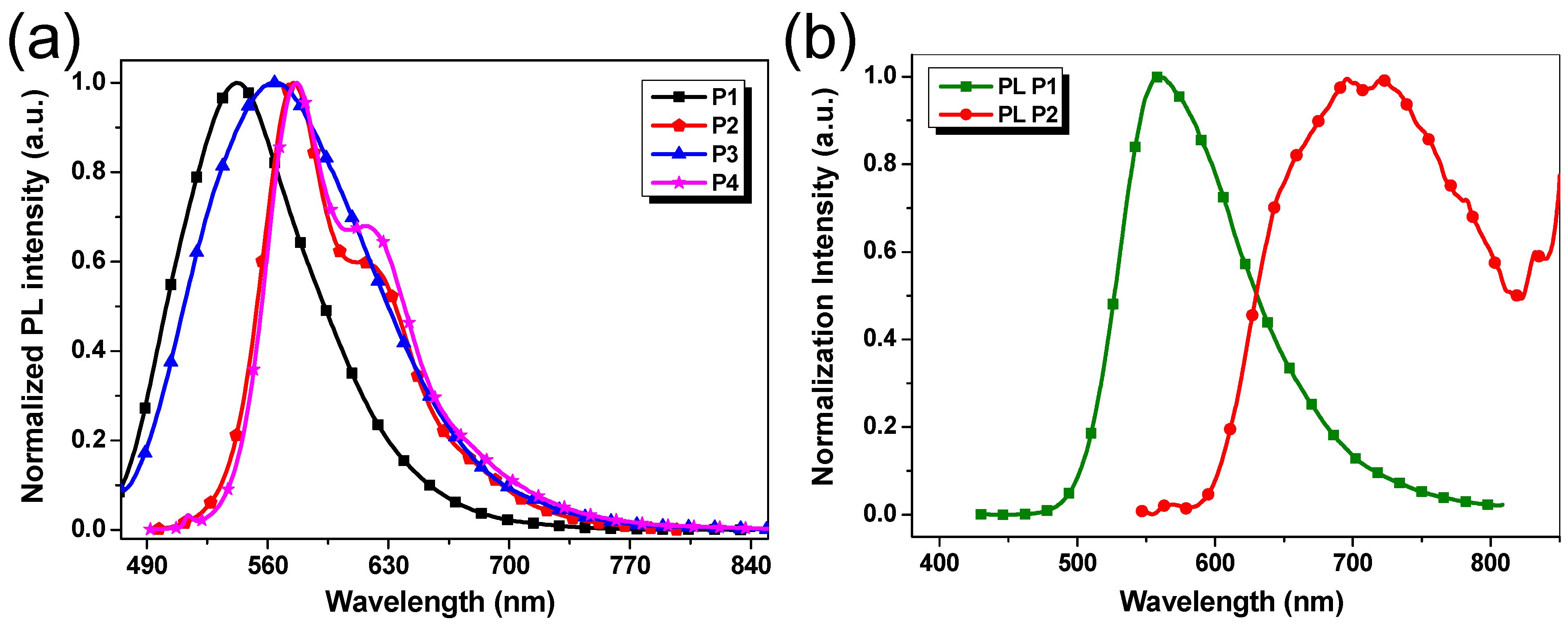
3.5. Photoluminescence Property
3.6. Electrochemical Property
3.7. Electroluminescence Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hildner, R.; Köhler, A.; Müller-Buschbaum, P.; Panzer, F.; Thelakkat, M. π-Conjugated Donor Polymers: Structure Formation and Morphology in Solution, Bulk and Photovoltaic Blends. Adv. Energy Mater. 2017, 7, 1700314. [Google Scholar] [CrossRef]
- Wang, C.; Dong, H.; Hu, W.; Liu, Y.; Zhu, D. Semiconducting π-Conjugated Systems in Field-Effect Transistors: A Material Odyssey of Organic Electronics. Chem. Rev. 2012, 112, 2208–2267. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Abdalla, H.; Kemerink, M. Conjugated Polymer Blends for Organic Thermoelectrics. Adv. Electron. Mater. 2019, 5, 1800821. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting Polymers: The Third Generation. Chem. Soc. Rev. 2010, 39, 2354–2371. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Brasiunas, B.; Damaskaite, A.; Plikusiene, I.; Ramanavicius, A.; Ramanaviciene, A. Electrodeposited Gold Nanostructures for the Enhancement of Electrochromic Properties of PANI-PEDOT Film Deposited on Transparent Electrode. Polymers 2020, 12, 2778. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Mikoliunaite, L.; Bagdziunas, G.; Ramanavicius, A.; Ramanaviciene, A. Comparative Study of Polyaniline (PANI), Poly(3,4-ethylenedioxythiophene) (PEDOT) and PANI-PEDOT Films Electrochemically Deposited on Transparent Indium Thin Oxide Based Electrodes. Polymer 2019, 172, 133–141. [Google Scholar] [CrossRef]
- Sakamoto, J.; Rehahn, M.; Wegner, G.; Schlüter, A.D. Suzuki Polycondensation: Polyarylenes à la Carte. Macromol. Rapid Commun. 2009, 30, 653–687. [Google Scholar] [CrossRef]
- Carsten, B.; He, F.; Son, H.J.; Xu, T.; Yu, L. Stille Polycondensation for Synthesis of Functional Materials. Chem. Rev. 2011, 111, 1493–1528. [Google Scholar] [CrossRef]
- Yokozawa, T.; Yokoyama, A. Chain-Growth Condensation Polymerization for the Synthesis of Well-Defined Condensation Polymers and π-Conjugated Polymers. Chem. Rev. 2009, 109, 5595–5619. [Google Scholar] [CrossRef]
- Todd, A.D.; Bielawski, C.W. Controlled Synthesis of an Alternating Donor-Acceptor Conjugated Polymer via Kumada Catalyst-Transfer Polycondensation. ACS Macro Lett. 2015, 4, 1254–1258. [Google Scholar] [CrossRef]
- Yum, S.; An, T.K.; Wang, X.; Lee, W.; Uddin, M.A.; Kim, Y.J.; Nguyen, T.L.; Xu, S.; Hwang, S.; Park, C.E.; et al. Benzotriazole-Containing Planar Conjugated Polymers with Noncovalent Conformational Locks for Thermally Stable and Efficient Polymer Field-Effect Transistors. Chem. Mater. 2014, 26, 2147–2154. [Google Scholar] [CrossRef]
- Scott, C.N.; Bisen, M.D.; Stemer, D.M.; McKinnon, S.; Luscombe, C.K. Direct Arylation Polycondensation of 2,5-Dithienylsilole with a Series of Difluorobenzodiimine-Based Electron Acceptors. Macromolecules 2017, 50, 4623–4628. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, X.; Lu, Y.; Li, Y.; Li, Y.; Li, C.; Wu, H.; Chen, Y. The Synthesis of 5-Alkyl[3,4-c]thienopyrrole-4,6-dione-Based Polymers Using a Pd-catalyzed Oxidative C-H/C-H Homopolymerization Reaction. Chem. Commun. 2014, 50, 12497–12499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Lu, Y.; Zhang, H.; Li, M.; Yang, Y.; Wang, J.; Chen, Y.; Li, C. Pd-catalysed Oxidative C-H/C-H Coupling Polymerization for Polythiazole-Based Derivatives. Polymer 2015, 68, 227–233. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, M.; Lu, Y.; Sun, Y.; Li, C.; Yang, X.; Zhang, M.; Chen, Y. A Direct C-H Coupling Method for Preparing π-Conjugated Functional Polymers with High Regioregularity. Macromolecules 2018, 51, 379–388. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Deng, L.; Zhao, L.; Li, C.; Lu, Y. Synthesis of Highly Regioregular, Head-to-Tail Coupled Poly(3-octylesterthiophene) via C-H/C-H Coupling Polycondensation. Chin. J. Polym. Sci. 2018, 36, 1019–1026. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, Q.; Zhao, L.; Lu, Y. Direct C-H Coupling Polymerization of Asymmetric Monomer: Synthesis and Properties of Regioregular Ppoly(alkyl thiophene-3-carboxylates). Eur. Polym. J. 2018, 109, 72–81. [Google Scholar] [CrossRef]
- Gobalasingham, N.S.; Noh, S.; Thompson, B.C. Palladium-Catalyzed Oxidative Direct Arylation Polymerization (Oxi-DArP) of an Ester-Functionalized Thiophene. Polym. Chem. 2016, 7, 1623–1631. [Google Scholar] [CrossRef]
- Gobalasingham, N.S.; Pankow, R.M.; Thompson, B.C. Synthesis of Random Poly(hexyl thiophene-3-carboxylate) Copolymers via Oxidative Direct Arylation Polymerization (oxi-DArP). Polym. Chem. 2017, 8, 1963–1971. [Google Scholar] [CrossRef]
- Gobalasingham, N.S.; Thompson, B.C. Direct Arylation Polymerization: A Guide to Optimal Conditions for Effective Conjugated Polymers. Prog. Polym. Sci. 2018, 83, 135–201. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, J.; You, J. Oxidative C-H/C-H Coupling Reactions between Two (Hetero)arenes. Chem. Rev. 2017, 117, 8787–8863. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.J.; Xing, L.; Luscombe, C.K. Exploration and Development of Gold- and Silver-Catalyzed Cross Dehydrogenative Coupling toward Donor-Acceptor π-Conjugated Polymer Synthesis. Polym. Chem. 2019, 10, 486–493. [Google Scholar] [CrossRef]
- Collier, G.S.; Reynolds, J.R. Exploring the Utility of Buchwald Ligands for C–H Oxidative Direct Arylation Polymerizations. ACS Macro Lett. 2019, 8, 931–936. [Google Scholar] [CrossRef]
- Aoki, H.; Saito, H.; Shimoyama, Y.; Kuwabara, J.; Yasuda, T.; Kanbara, T. Synthesis of Conjugated Polymers Containing Octafluorobiphenylene Unit via Pd-Catalyzed Cross-Dehydrogenative-Coupling Reaction. ACS Macro Lett. 2018, 7, 90–94. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, D.; You, J. Oxidative Direct Arylation Polymerization Using Oxygen as the Sole Oxidant: Facile, Green Access to Bithiazole-Based Polymers. ChemSusChem 2016, 9, 2765–2768. [Google Scholar] [CrossRef]
- Guo, Q.; Jiang, R.; Wu, D.; You, J. Rapid Access to 2,2′-Bithiazole-Based Copolymers via Sequential Palladium-Catalyzed C-H/C-X and C-H/C-H Coupling Reactions. Macromol. Rapid Commun. 2016, 37, 794–798. [Google Scholar] [CrossRef]
- Huang, Q.; Qin, X.; Li, B.; Lan, J.; Guo, Q.; You, J. Cu-catalysed Oxidative C-H/C-H Coupling Polymerisation of Benzodiimidazoles: An Efficient Approach to Regioregular Polybenzodiimidazoles for Blue-Emitting Materials. Chem. Commun. 2014, 50, 13739–13741. [Google Scholar] [CrossRef]
- Yang, Y.; Nishiura, M.; Wang, H.; Hou, Z. Metal-Catalyzed C-H Activation for Polymer Synthesis and Functionalization. Coord. Chem. Rev. 2018, 376, 506–532. [Google Scholar] [CrossRef]
- Li, J.; Han, D.; Zhang, Q.; HE, Z.; Lu, Y. Synthesis and Properties of Fluorinated Benzotriazole-Based Donor-Acceptor-Type Conjugated Polymers via Pd-Catalyzed Direct C-H/C-H Coupling Polymerization. J. Polym. Sci. 2021. [Google Scholar] [CrossRef]
- Pankow, R.M.; Ye, L.; Thompson, B.C. Influence of An Ester Directing-Group on Defect Formation in the Synthesis of Conjugated Polymers via Direct Arylation Polymerization (DArP) Using Sustainable Solvents. Polym. Chem. 2019, 10, 4561–4572. [Google Scholar] [CrossRef]
- Lombeck, F.; Marx, F.; Strassel, K.; Kunz, S.; Lienert, C.; Komber, H.; Friend, R.; Sommer, M. To Branch or not to Branch: C-H Selectivity of Thiophene-Based Donor-Acceptor-Donor Monomers in Direct Arylation Polycondensation Exemplified by PCDTBT. Polym. Chem. 2017, 8, 4738–4745. [Google Scholar] [CrossRef]
- Adachi, Y.; Ooyama, Y.; Ren, Y.; Yin, X.; Jäkle, F.; Ohshita, J. Hybrid Conjugated Polymers with Alternating Dithienosilole or Dithienogermole and Tricoordinate Boron Units. Polym. Chem. 2018, 9, 291–299. [Google Scholar] [CrossRef]
- Fei, Z.; Kim, Y.; Smith, J.; Domingo, E.B.; Stingelin, N.; McLachlan, M.A.; Song, K.; Anthopoulos, T.D.; Heeney, M. Comparative Optoelectronic Study between Copolymers of Peripherally Alkylated Dithienosilole and Dithienogermole. Macromolecules 2012, 45, 735–742. [Google Scholar] [CrossRef]
- Zhong, L.; Bin, H.; Angunawela, I.; Jia, Z.; Qiu, B.; Sun, C.; Li, X.; Zhang, Z.; Ade, H.; Li, Y. Effect of Replacing Thiophene by Selenophene on the Photovoltaic Performance of Wide Bandgap Copolymer Donors. Macromolecules 2019, 52, 4776–4784. [Google Scholar] [CrossRef]
- Hu, H.; Ye, L.; Ghasemi, M.; Balar, N.; Rech, J.J.; Stuard, S.J.; You, W.; O’Connor, B.T.; Ade, H. Highly Efficient, Stable, and Ductile Ternary Nonfullerene Organic Solar Cells from a Two-Donor Polymer Blend. Adv. Mater. 2019, 31, 1808279. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Song, W.; Xiao, B.; Guo, J.; Min, J.; Ge, Z.; Zhang, J.; Wei, Z.; Zhou, E. Benzotriazole-Based Acceptor and Donors, Coupled with Chlorination, Achieve a High Voc of 1.24 V and an Efficiency of 10.5% in Fullerene-Free Organic Solar Cells. Chem. Mater. 2019, 31, 3941–3947. [Google Scholar] [CrossRef]
- Livi, F.; Gobalasingham, N.S.; Bundgaard, E.; Thompson, B.C. Influence of Functionality on Direct Arylation of Model Systems as a Route Toward Fluorinated Copolymers via Direct Arylation Polymerization (DArP). J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2598–2605. [Google Scholar] [CrossRef]
- Wu, W.-C.; Liu, C.-L.; Chen, W.-C. Synthesis and Characterization of New Fluorene-Acceptor Alternating and Random Copolymers for Light-Emitting Applications. Polymer 2006, 47, 527–538. [Google Scholar] [CrossRef]
- Torres-Moya, I.; Vázquez-Guilló, R.; Fernández-Palacios, S.; Carrillo, J.R.; Díaz-Ortiz, Á.; López Navarrete, J.T.; Ponce Ortiz, R.; Ruiz Delgado, M.C.; Mallavia, R.; Prieto, P. Fluorene-Based Donor-Acceptor Copolymers Containing Functionalized Benzotriazole Units: Tunable Emission and their Electrical Properties. Polymers 2020, 12, 256. [Google Scholar] [CrossRef]
- Tutuncu, E.; Cihaner, A.; Icli Ozkut, M. Synthesis and Electropolymerization of a Donor-Acceptor-Donor Trimeric Monomer Containing 3,4-Propylenedioxythiophene and Dithienosilole Units. Eur. Polym. J. 2019, 118, 239–243. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, J.B.; Kim, H.U.; Kang, I.-N.; Hwang, D.-H. High Open-Circuit Voltage Organic Photovoltaic Cells Fabricated Using Semiconducting Copolymers Consisting of Bithiophene and Fluorinated Quinoxaline or Triazole Derivatives. Synth. Met. 2014, 194, 88–96. [Google Scholar] [CrossRef]
- Chen, J.; Yan, Z.; Tang, L.; Uddin, M.A.; Yu, J.; Zhou, X.; Yang, K.; Tang, Y.; Shin, T.J.; Woo, H.Y.; et al. 1,4-Di(3-alkoxy-2-thienyl)-2,5-Difluorophenylene: A Building Block Enabling High-Performance Polymer Semiconductors with Increased Open-Circuit Voltages. Macromolecules 2018, 51, 5352–5363. [Google Scholar] [CrossRef]


| Entry | Polymer a | Mnb | Mwb | Mw/Mnb | Yield/% c | Tdd |
|---|---|---|---|---|---|---|
| 1 | P1 | 22,300 | 40,580 | 1.82 | 93 | 317 |
| 2 | P2 | 28,100 | 34,280 | 1.22 | 82 | 385 |
| 3 | P3 | 15,000 | 23,850 | 1.59 | 10 | 256 |
| 4 | P4 | 3500 | 4690 | 1.34 | 20 | 392 |
| Entry | UV–vis | PL | CV | |||||
|---|---|---|---|---|---|---|---|---|
| λmaxSol (nm) | λmaxFilm (nm) | (eV) a | λmaxSol (nm) | λmaxFilm (nm) | HOMO (eV) b | LUMO (eV) b | Egcv (eV) b | |
| P1 | 427 | 428 | 2.16 | 542 | 560 | −5.57 | −3.60 | 1.97 |
| P2 | 515, 596 | 510 | 1.81 | 575, 614 | 723 | −5.69 | −3.68 | 2.01 |
| P3 | 471 | 468 | 1.85 | 564 | - | −5.45 | −3.59 | 1.86 |
| P4 | 506 | 523 | 1.88 | 577, 616 | - | −5.67 | −3.58 | 2.09 |
| Polymer | λmaxEL (nm) | Von (V) | Luminance (cd/m2) | ηc (cd/A) | ηp (lm/W) | EQEmax (%) | CIE (x, y) |
|---|---|---|---|---|---|---|---|
| P1 | 544 | 4.0 | 774 | 0.12 | 0.059 | 0.07 | (0.40, 0.52) |
| P2 | 640 | 3.0 | 1178 | 0.25 | 0.13 | 0.14 | (0.66, 0.33) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Li, J.; Zhang, Q.; He, Z.; Wu, Z.; Chu, J.; Lu, Y. Synthesis of π-Conjugated Polymers Containing Benzotriazole Units via Palladium-Catalyzed Direct C-H Cross-Coupling Polycondensation for OLEDs Applications. Polymers 2021, 13, 254. https://doi.org/10.3390/polym13020254
Han D, Li J, Zhang Q, He Z, Wu Z, Chu J, Lu Y. Synthesis of π-Conjugated Polymers Containing Benzotriazole Units via Palladium-Catalyzed Direct C-H Cross-Coupling Polycondensation for OLEDs Applications. Polymers. 2021; 13(2):254. https://doi.org/10.3390/polym13020254
Chicago/Turabian StyleHan, Dong, Jingwen Li, Qiang Zhang, Zewang He, Zhiwei Wu, Jingting Chu, and Yan Lu. 2021. "Synthesis of π-Conjugated Polymers Containing Benzotriazole Units via Palladium-Catalyzed Direct C-H Cross-Coupling Polycondensation for OLEDs Applications" Polymers 13, no. 2: 254. https://doi.org/10.3390/polym13020254
APA StyleHan, D., Li, J., Zhang, Q., He, Z., Wu, Z., Chu, J., & Lu, Y. (2021). Synthesis of π-Conjugated Polymers Containing Benzotriazole Units via Palladium-Catalyzed Direct C-H Cross-Coupling Polycondensation for OLEDs Applications. Polymers, 13(2), 254. https://doi.org/10.3390/polym13020254