1. Introduction
Microphase separation of the diblock copolymer, A-
b-B, can generate a variety of long-range ordered microdomains, including one-dimensionally stacked lamellae (
LAM), hexagonally packed cylinders (
HEX), double gyroids and body-centered cubic (BCC)-packed spheres [
1,
2]. The type of structure formed is governed by the interfacial free energy and the conformational free energy associated with the stretching of the block chains [
3,
4]. These effects are parameterized into the segregation strength expressed by
χN (with
χ and
N being the Flory–Huggins interaction parameter and the overall degree of polymerization of the copolymer, respectively) and the constituent volume fraction for constructing the universal phase diagram [
3,
4]. At sufficiently high compositional asymmetry, the microdomain interface becomes hyperbolically curved to alleviate the stretching of the majority blocks, leading to the formation of cylindrical or spherical domains by the minority blocks.
Another way to tune the microdomain morphology of diblock copolymer is through blending A-
b-B with the corresponding homopolymer, h-A, to form a thermodynamically single-phase mixture exhibiting only microphase separation [
2,
5,
6,
7,
8]. When the h-A chains are allowed to enter microdomain A and mix uniformly with A blocks (called “uniform solubilization”), the nearest-neighbor distance between the junction points (called “junction point separation”) locating in the interface will be swollen to alleviate the stretching of A blocks and h-A chains [
5,
6]. The increase of junction point separation increases the interfacial free energy, and B block chains will be compressed along the domain interface to maintain their normal segmental density. Once the entropic penalty associated with such a chain compression is too large, a morphological transformation from microdomain with lower curvature (e.g., lamellae) to the one with higher curvature (e.g., cylinder) will take place to relieve the conformational entropy loss.
Consequently, an essential condition for achieving domain morphology transformation via homopolymer blending is the uniform solubilization of h-A in microdomain A to form the so-called “wet-brush” mixture of A blocks and h-A chains [
5,
6,
7]. Achieving such a condition is not trivial due to the need to overcome the free energy penalty associated with the perturbation of conformational free energy as well as the increase of interfacial free energy. It has been established that wet-brush blending can be attained when the molecular weight of h-A (
Mh-A) is smaller than that of A block (
Mb-A), i.e.,
r =
Mh-A/
Mb-A < 1 [
5,
6,
7,
8,
9,
10,
11,
12]. If h-A and block A have approximately the same molecular weight (i.e.,
r ~ 1), h-A chains may still enter microdomain A, but they tend to be segregated into the middle region of the domain, forming the “dry-brush” mixture with A blocks [
5,
6,
13]. Dry-brush type of blending is unable to induce transformation of microdomain morphology, since it only causes a continuous swelling of the size of domain A without altering the junction point separation and the local interfacial curvature [
5].
It should be noted that the molecular weight criteria for homopolymer solubilization were established using the diblock copolymer system (e.g., polystyrene-
block-polyisoprene (PS-
b-PI)) showing a reduction of segregation strength with increasing temperature [
5,
6]. This type of diblock system is said to exhibit the “upper critical ordering transition (UCOT)”, where the order–disorder transition (ODT) occurred on heating. The free energy components of the copolymer blends displaying UCOT behavior compose the interfacial free energy, the conformational free energy of block and homopolymer chains, the free energy of mixing of h-A and A block, and the free energy associated with the translational entropy the junction points [
5]. Moreover, this type of system is considered to follow the melt incompressibility condition, where the sum of the local number densities of A and B segments is constant when they mix in the disordered melt or in the microdomain interface [
1,
3,
4].
There is another class of diblock copolymer showing opposite temperature dependence of segregation strength, namely, the net repulsion between A and B segments becomes stronger at higher temperature, such that the ODT may take place on cooling. The copolymer is then said to display the “lower critical ordering transition (LCOT)” [
14,
15,
16,
17,
18,
19]. LCOT of diblock copolymer was first identified by Russell et al. [
14]. and its origin was attributed to the disparity in the thermal expansivities of the constituent blocks, which is identical to that of the homopolymer blends exhibiting lower critical solution temperature (LCST) phase diagram [
14,
20,
21]. In this case, the constraint of the free volume of the more expansive component by the less expansive one results in an entropy loss when they mix intimately with each other. As a result, a repulsive force with entropic in origin develops to trigger the microphase separation at elevated temperature. The equation of state theories that take account of the melt compressibility and the volume of mixing provide the appropriate theoretical framework for predicting the LCOT phase behavior [
20,
21,
22,
23].
Considering the more complex interplay among the free energy components, it is of fundamental significance to examine if the blend of a diblock copolymer showing the feature of LCOT behavior with its corresponding homopolymer exhibits different phase structure from that of the conventional UCOT blend. The present work sheds light on the solubilization behavior of homopolymer in such a blend system through studying the blends of poly(4-vinyl pyridine) homopolymers (h-P4VP) with different molecular weights and a lamellae-forming poly(ethylene oxide)-block-poly(4-vinyl pyridine) (PEO-b-P4VP) showing the signature of LCOT behavior in terms of weaker segregation at lower temperature. It will be shown that, while the wet-brush criterion established for the conventional UCOT blend was still applicable to the LCOT system, the LCOT blend showed a higher degree of swelling of the junction point separation upon the addition of homopolymer, even resulting in a decrease of interdomain distance with increasing h-P4VP composition. The excess swelling of junction point separation will be discussed in connection with the reduction of the interfacial free energy upon incorporating h-P4VP segments into the microdomain interface. The excess swelling of junction point separation constitutes another anomalous feature of LCOT block copolymer, besides the opposite temperature variation of segregation strength, compared with the UCOT system.
3. Results and Discussion
Our previous study demonstrated that poly(ethylene oxide)-
block-poly(2-vinyl pyridine) (PEO-
b-P2VP) displayed LCOT phase diagram [
18]. As the chemical analogue of PEO-
b-P2VP, PEO-
b-P4VP would be expected to show similar phase behavior.
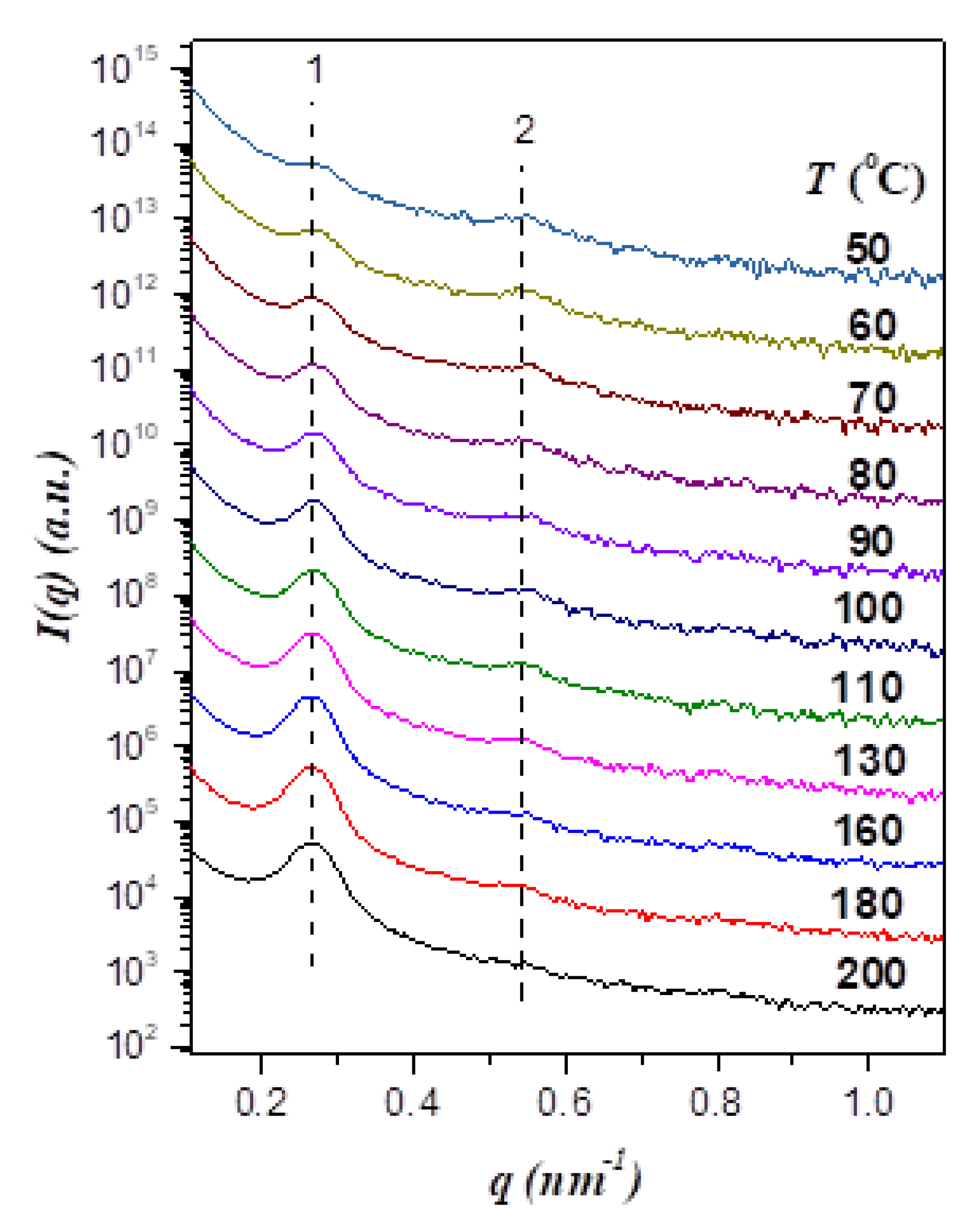
Figure 1 displays the temperature-dependent SAXS profiles of neat EO4VP, where the sample was first heated to 200 °C to erase the previous solvent history followed by collecting the scattering profiles in a cooling cycle. The SAXS curve at 200 °C displayed 2 peaks with the position ratio of 1:2, indicating that the copolymer formed a lamellar morphology with the interdomain distance (
D) of 23.4 nm. The intensity of the primary peak was found to drop progressively with decreasing temperature, which seemed to suggest a reduction of segregation strength in the cooling process. Nevertheless, the difference in electron density between PEO and P4VP was found to decrease with decreasing temperature because of the disparity in their thermal expansion coefficients, as demonstrated in
Figure S1 of the Supporting Information. Since the reduction of electron density contrast between PEO and P4VP domains could also cause the diminishment of peak intensity, identifying the phase behavior of EO4VP based solely on the temperature variation of scattering intensity was not unambiguous.
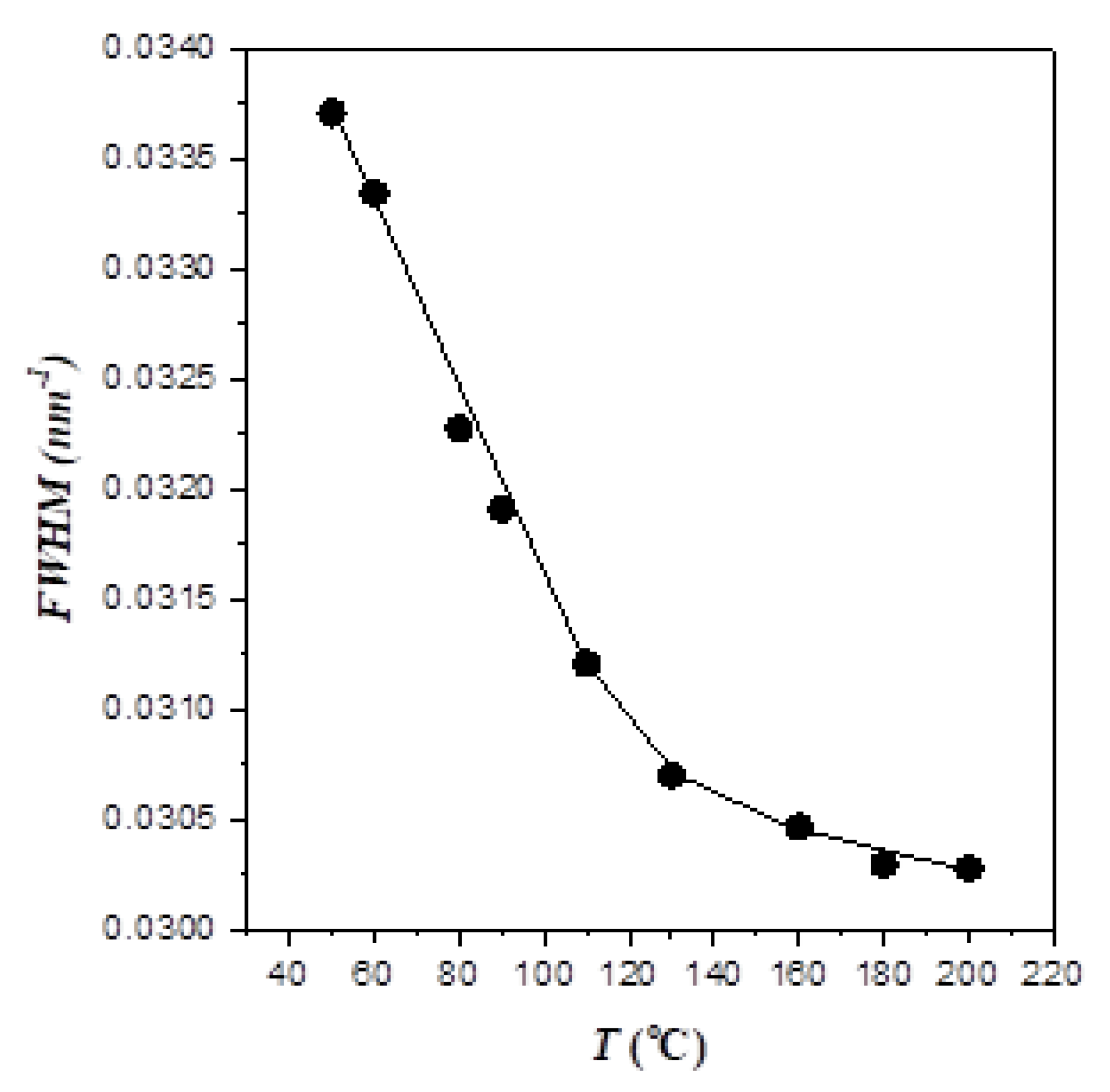
The effect of temperature on the segregation strength was alternatively elucidated from the change of the primary peak width in the cooling process, as demonstrated in
Figure 2 showing the width at the half-height of this peak as a function of temperature. It can be seen that the peak broadened progressively with decreasing temperature, signaling a reduction of segregation strength with decreasing temperature. Moreover, we found that the blend of another symmetric PEO-
b-P4VP (
Mb-PEO = 4000 g/mol;
Mb-P4VP = 6000 g/mol) and h-P4VP1 with f
P4VP = 0.68 exhibited an order–order transition from
LAM to
HEX phase on cooling (see
Figure S2 of the Supporting Information), which was in the opposite direction to that displayed by the conventional UCOT system. These experimental observations attested that the segregation strength of EO4VP became weaker at a lower temperature. That is, this copolymer system displayed the feature of LCOT behavior, though the disordered state was not accessible before the occurrence of the crystallization of PEO block below its melting point.
The morphology and phase behavior of EO4VP/h-P4VP blends were also probed by SAXS. Here we used EO4VP/h-P4VP1 blend with the lowest molecular weight ratio r = Mh-P4VP/Mb-P4VP = 0.14 as the representative to demonstrate the LCOT characteristic of the blends, since the temperature-dependent SAXS results of the other blends basically showed the same features.
Figure 3 presents the temperature-dependent SAXS profiles of the EO4VP/h-P4VP1 blends with the overall volume fraction of P4VP,
fP4VP, ranging from 0.66 to 0.71. The SAXS curves of all samples displayed two peaks with integral position ratio, showing that the blends also formed lamellar morphology over the temperature range studied. Similar to neat EO4VP, the primary peak diminished and broadened with decreasing temperature; consequently, EO4VP/h-P4VP1 blends also exhibited the feature of LCOT type of phase behavior.
Over the range of h-P4VP molecular weight studied, all blends were found to show weaker segregation strength at lower temperature; nevertheless, the perturbations of interdomain distance and junction point separation upon incorporating h-P4VP into the P4VP lamellar microdomain depended on h-P4VP molecular weight.
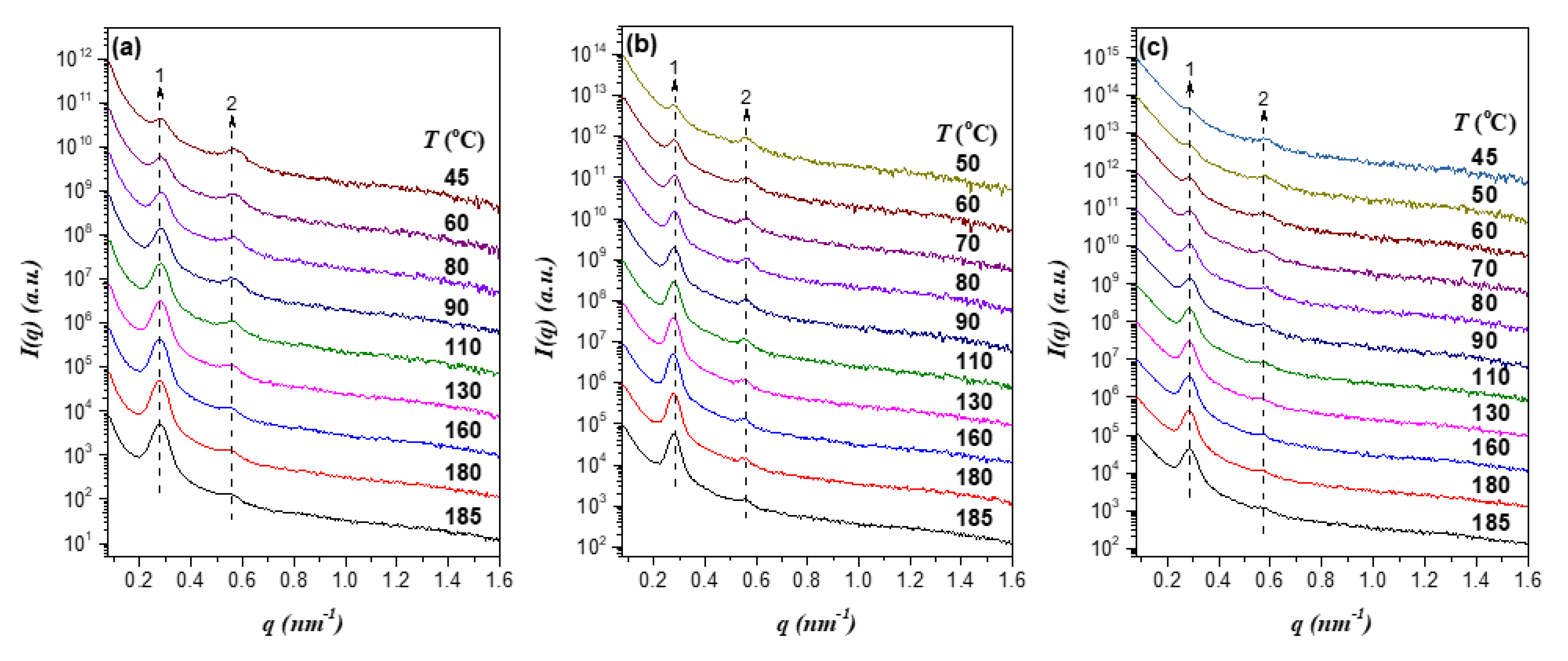
Figure 4a shows the SAXS profiles of EO4VP/h-P4VP1 blends (
r = 0.14) with various compositions at a representative temperature, 180 °C. The scattering peaks associated with the lamellar structure were found to shift to higher
q with increasing
fP4VP, indicating that the interdomain distance
D became smaller with the addition of more h-P4VP. This was rather unexpected, as the previous studies of diblock copolymer blends had predominantly observed the swelling of interdomain distance once the homopolymer was solubilized in the corresponding microdomain [
5,
6].
The composition-dependent SAXS profiles of EO4VP/h-P4VP2 blends (
r = 0.24) displayed in
Figure 4b showed the formation of lamellar morphology; the scattering peaks were again found to shift towards higher
q with increasing
fP4VP, but the shift was less pronounced compared to that displayed by EO4VP/h-P4VP1 blend. The SAXS curves of EO4VP/h-P4VP3 blends (
r = 0.23) shown in
Figure 4c demonstrated that, while the blends still formed lamellar structure, the positions of the scattering peaks appeared to be fixed, implying that the interdomain distance remained largely unperturbed upon blending with the homopolymer. For the blend with h-P4VP4 bearing the highest molecular weight (
r = 0.60) among the h-P4VP samples used, the SAXS profiles in
Figure 4d revealed the shift of scattering peaks to lower
q with the increase of P4VP composition. In this case, blending with h-P4VP tended to swell the interdomain distance, which was in clear contrast to the composition dependence of
D displayed by the other three blends.
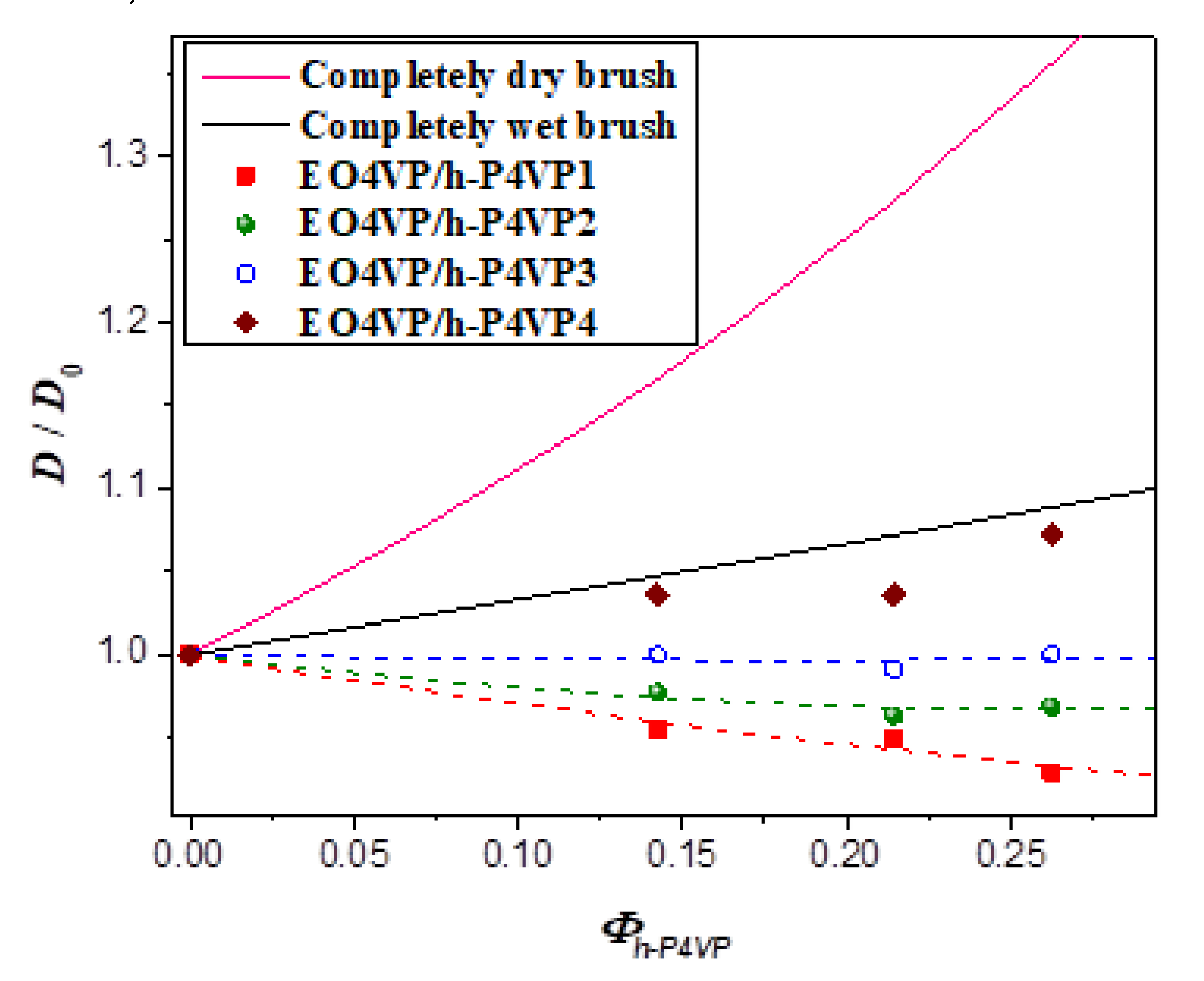
Figure 5 plots the interlamellar distance of the blend normalized by that of neat EO4VP (
D0),
D/
D0, as a function of the total volume fraction of h-P4VP,
Φh-P4VP = 1 −
ΦEO4VP, in the blends at 180 °C. The composition dependence of
D/
D0 was seen to depend on the molecular weight of h-P4VP. For the blend with h-P4VP1 bearing the lowest molecular weight (
r = 0.14), the interdomain distance decreased with increasing h-P4VP content, implying that the junction point separation was swollen significantly by h-P4VP, making the lamellar thickness even smaller than that in neat EO4VP. The interdomain distance of the blend with h-P4VP2 (
r = 0.24) also showed similar composition dependence, but the drop of
D/
D0 was less pronounced. The interdomain distance remained virtually constant for h-P4VP3 blend (
r = 0.32), whereas it increased monotonically with increasing homopolymer composition when the molecular weight of h-P4VP was increased to 4300 g/mol (
r = 0.60).
The reduction of interdomain distance with increasing homopolymer composition observed for the blends with h-P4VP1 and h-P4VP2 offered clear-cut evidence of wet-brush mixing between P4VP blocks and h-P4VP. It was, however, unclear if h-P4VP4 blend displayed wet-brush or dry-brush behavior because both may lead to swelling of interdomain distance. This problem was resolved by calculating the junction point separation
aj in the blend normalized by that in the neat copolymer,
aj/
aj0 via [
5]
Equation (1) assumes that all added homopolymer was solubilized into the microdomain. This was a reasonable assumption for the blend systems studied here, in that no sign of macrophase separation was observed.
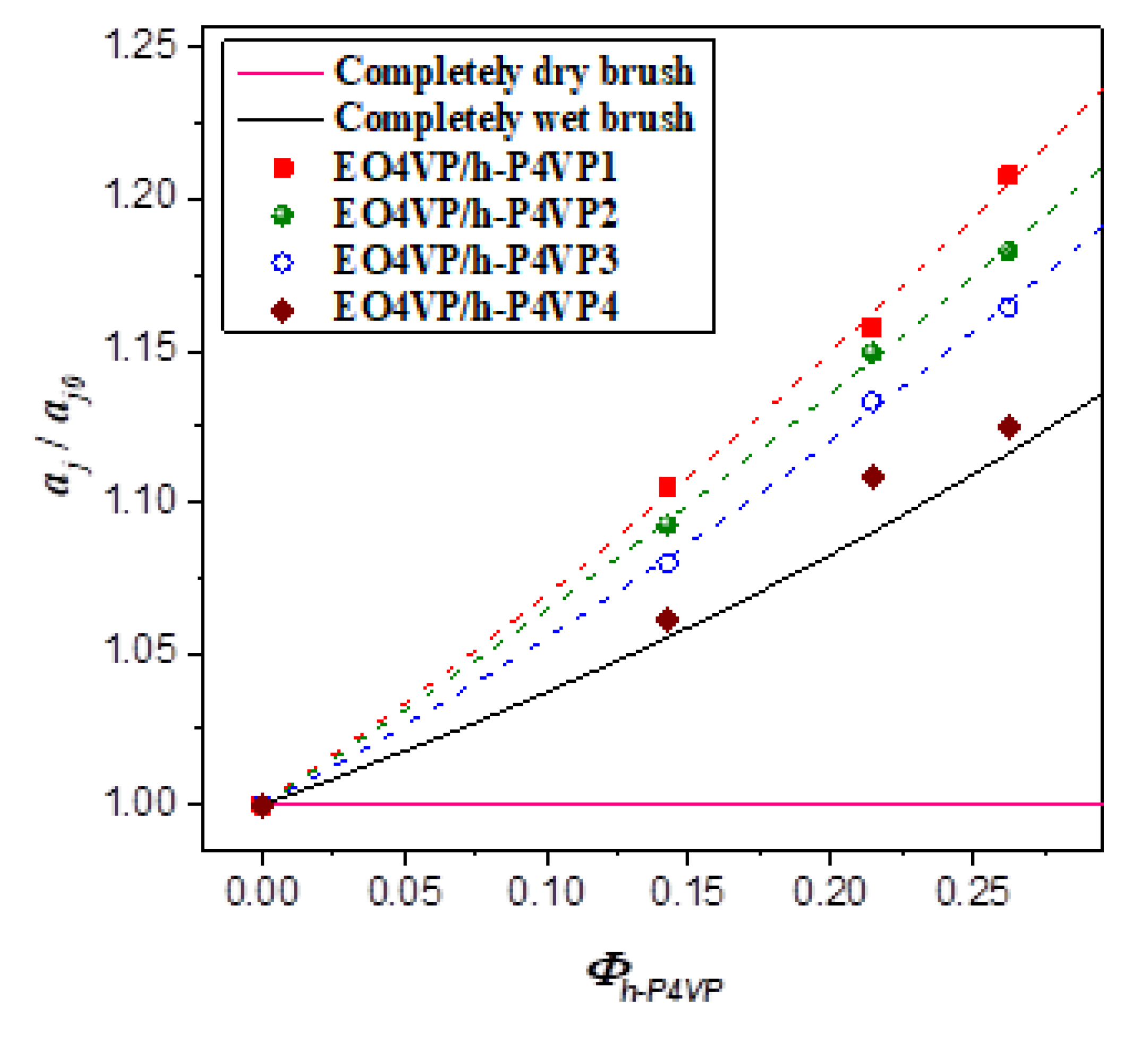
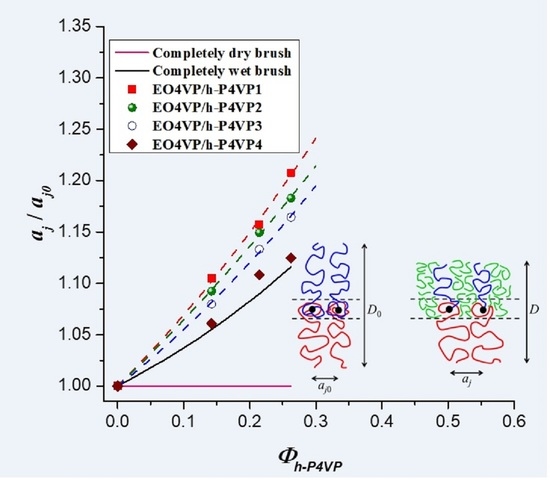
Figure 6 shows the calculated
aj/
aj0 as a function of
Φh-P4VP. For the four blend systems studied, the values of
aj/
aj0 situated above 1.0 (
aj/
aj0 = 1 for dry-brush mixing) and increased monotonically with increasing h-P4VP composition, indicating that they all exhibited wet-brush behavior, where h-P4VP was uniformly solubilized in P4VP microdomains. Since the values of
r associated with the four blends all fell below 1.0, the results suggested that the classical criterion of molecular weight ratio for wet-brush behavior, i.e.,
r < 1, was applicable to the blends showing the feature of LCOT behavior.
Although all h-P4VP samples formed wet-brush mixture with P4VP blocks, the degree of swelling of the junction point separation was found to decrease with increasing h-P4VP molecular weight. If the added homopolymer was uniformly solubilized in P4VP microdomain, swelling of the junction point separation would alleviate the stretching of block and homopolymer chains; however, this effect was counteracted by the increase of interfacial free energy arising from the enlargement of interfacial area. The fact that aj/aj0 was larger in the blend implied that the corresponding interfacial free energy per unit area (i.e., the surface free energy) of the lamellar phase was lower. In other words, the surface free energy was modified by the presence of h-P4VP in a molecular weight-dependent manner.
Here, we further compared the experimentally observed
D/
D0 and
aj/
aj0 with the values expected for completely dry brush and completely wet brush behavior. Because the thickness of PEO microdomain was unperturbed in dry-brush blend, the expected values are
aj/
aj0 = 1 and
D/
D0 = (1 −
fP4VP0)/(1 −
fP4VP) with
fP4VP0 being the volume fraction of P4VP in neat EO4VP. The calculations of
aj/
aj0 and
D/
D0 for wet-brush behavior were not straightforward. Here we adopted the model developed by Tanaka et al., originally derived for the ternary blend of a diblock copolymer with the two corresponding homopolymers [
5], to obtain the formula of
aj/
aj0 and
D/
D0 of wet-brush blend.
For the binary mixture of A-
b-B and h-A forming the lamellar morphology, the free energy per chain
Fchain is given by [
5]
where
Di,
a,
Ni,
PA,
Σ,
γ and
ϕh-A are thickness of microdomain
i (
i = A or B), Kuhn length (assuming the same for A and B blocks), degree of polymerization (DP) of block
i, DP of homopolymer A uniformly solubilized in its microdomain, the cross-sectional area per junction point, the surface free energy, and the volume fraction of homopolymer A in microdomain A, respectively. The three terms at the right-hand side of Equation (2) correspond to the interfacial free energy, the conformational free energy of the block chains and the free energy of mixing of h-A and A block in microdomain A, respectively.
Neglecting the volume of mixing that might exist in the interfacial region in which A and B segments mix with one another, the volume of one block chain is expressed in terms of the cross-sectional area of the junction point as
Substituting Equation (3) and Equation (4) into Equation (2) yields
where
C is a constant. The equilibrium cross-sectional area of the blend,
Σeq, is obtained by minimizing the total free energy to yield
The cross-sectional area per junction point in neat block is given by
where γ
0 is the surface free energy of the lamellar microdomain in neat diblock copolymer. Combining Equation (6) and Equation (7), the normalized junction point separation is given by
where
fi is the overall volume fraction of component
i in the blend. The corresponding normalized interdomain distance is obtained as
Figure 5 shows the comparison of the experimentally observed
D/
D0 with the values calculated by Equation (9) under the assumption of γ
0 = γ for wet-brush behavior and the equation for dry-brush blend. The calculated
D/
D0 of dry-brush blend was always much larger than the observed values under a given composition, confirming that the blends studied here did not show dry-brush behavior. The model predicted monotonic increase of
D/
D0 with increasing h-P4VP composition for wet-brush behavior. The composition variation of
D/
D0 of EO4VP/h-P4VP4 blend agreed quite well with the calculated result, as did the corresponding result of
aj/
aj0 (see
Figure 6), suggesting that the h-P4VP4 was uniformly solubilized in P4VP microdomains and its presence did not alter the surface free energy significantly.
On the other hand, the observed composition dependences of
D/
D0 and
aj/
aj0 of the blends with lower homopolymer molecular weights deviated obviously from the theoretical predictions, where the observed junction point separations were always larger than the calculated values (see
Figure 6). Consequently, an excess swelling of the junction point separation occurred in the blends with sufficiently low homopolymer molecular weight (< ca. 4000 g/mol). The comparison was relative to the junction point separation calculated by assuming that the surface free energy was unperturbed after blending, i.e.,
γ =
γ0. According to Equation (8),
aj becomes larger when
γ <
γ0; therefore, the excess swelling of
aj was attributed to the decrease of surface free energy under the presence of h-P4VP in the microdomain.
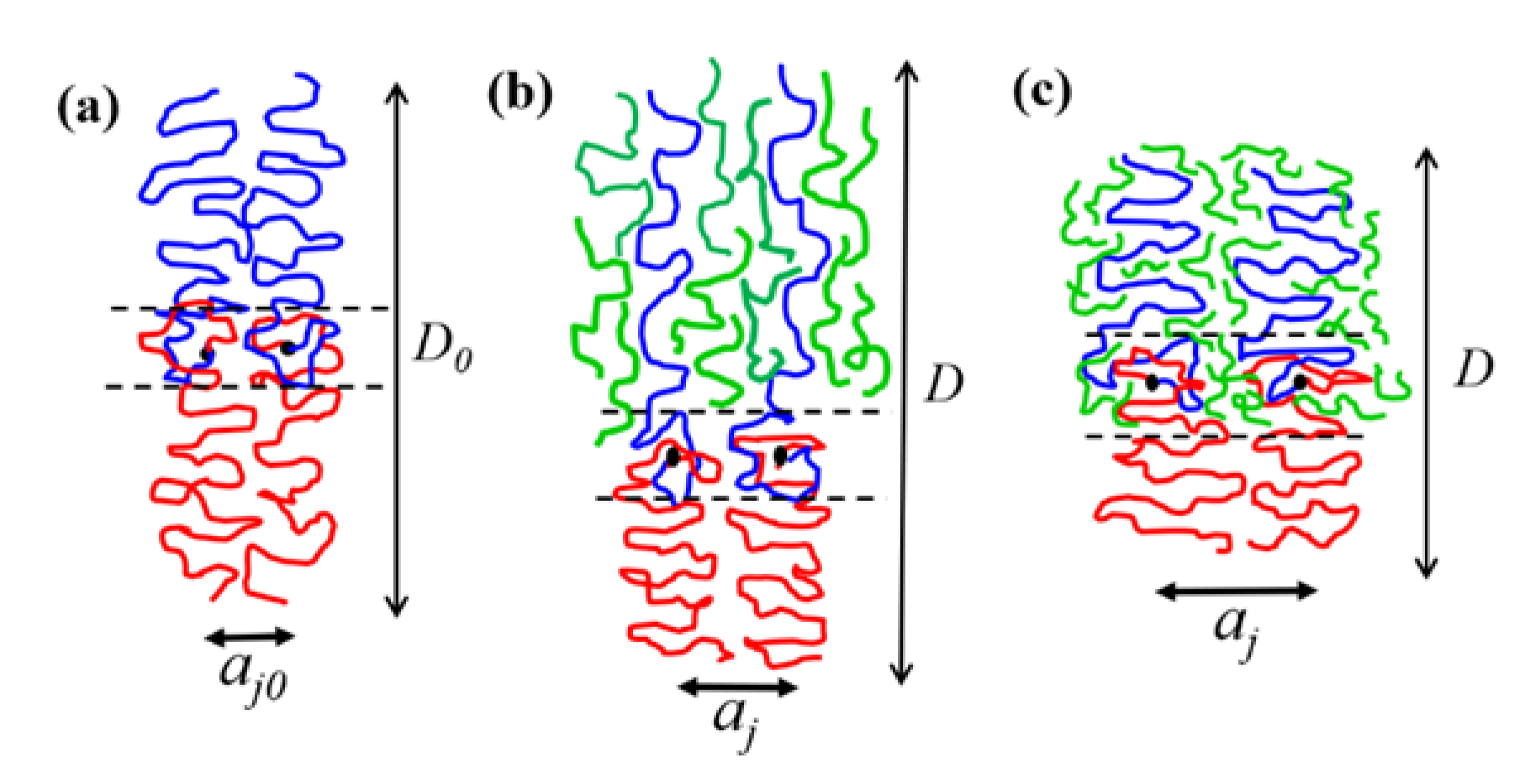
The interface between the PEO and P4VP lamellar domains was formed by the gradient mixing of EO and 4VP segments, as schematically illustrated in
Figure 7a, and the surface free energy composes both enthalpic and entropic components, i.e.,
γ =
γH +
γS. γ
H is related to the interaction energy between EO and 4VP segments (i.e.,
γH ~
χ1/2);
γS consists of the contributions from (a) the gain of entropy from the combinatorial mixing of dissimilar segments, (b) the loss of entropy arising from the confinement of A (B) blocks in the interface, and (c) the loss of entropy stemming from the reduction of free volume of EO segments upon mixing with 4VP segments in the interface [
24]. These three entropic terms are denoted as
γS,mix,
γS,conf and
γS,fv, respectively;
γS,fv is a term that is particularly significant in the LCOT system.
We propose that the solubilization of h-P4VP in the microdomain may effectively reduce
γS,fv, which in turn caused the excess swelling of the junction point separation. When h-P4VP was solubilized in P4VP microdomain, there were two ways to establish the interfacial composition profile. In the first scenario, h-P4VP chains were segregated out of the interfacial region completely, such that the interface composed the mixture of the segments associated with PEO and P4VP block chains, as schematically illustrated in
Figure 7b. In this case, the surface free energy of the blend should approximately equal that of the neat copolymer, i.e.,
γ ≈
γ0. This was likely the case for the h-P4VP4 blend, where the composition variation of
aj/
aj0 agreed well with that predicted by Equation (8) assuming unperturbed surface free energy.
In the second scenario, as schematically illustrated in
Figure 7c, a fraction of h-P4VP chains or their segments entered the interfacial region to replace some segments of P4VP block chains in establishing the composition profile in the interface. The interface then composed the segments of PEO blocks, P4VP blocks and h-P4VP chains. The replacement of P4VP block segments by the homopolymer segments may likely alleviate the free volume constraint imposed on PEO, because the short h-P4VP chains have higher free volume than the longer P4VP blocks due to the absence of junction point constraint and lower molecular weight. In this case, EO segments might have a higher free volume if they mix with h-P4VP chains. The alleviation of the free volume constraint reduced
γS,fv, and hence allowed a greater swelling of the junction point separation.