pH-Induced Electrostatic Interaction between Polyacrylates and Amino-Functionalized Graphene Oxide on Stability and Coating Performances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
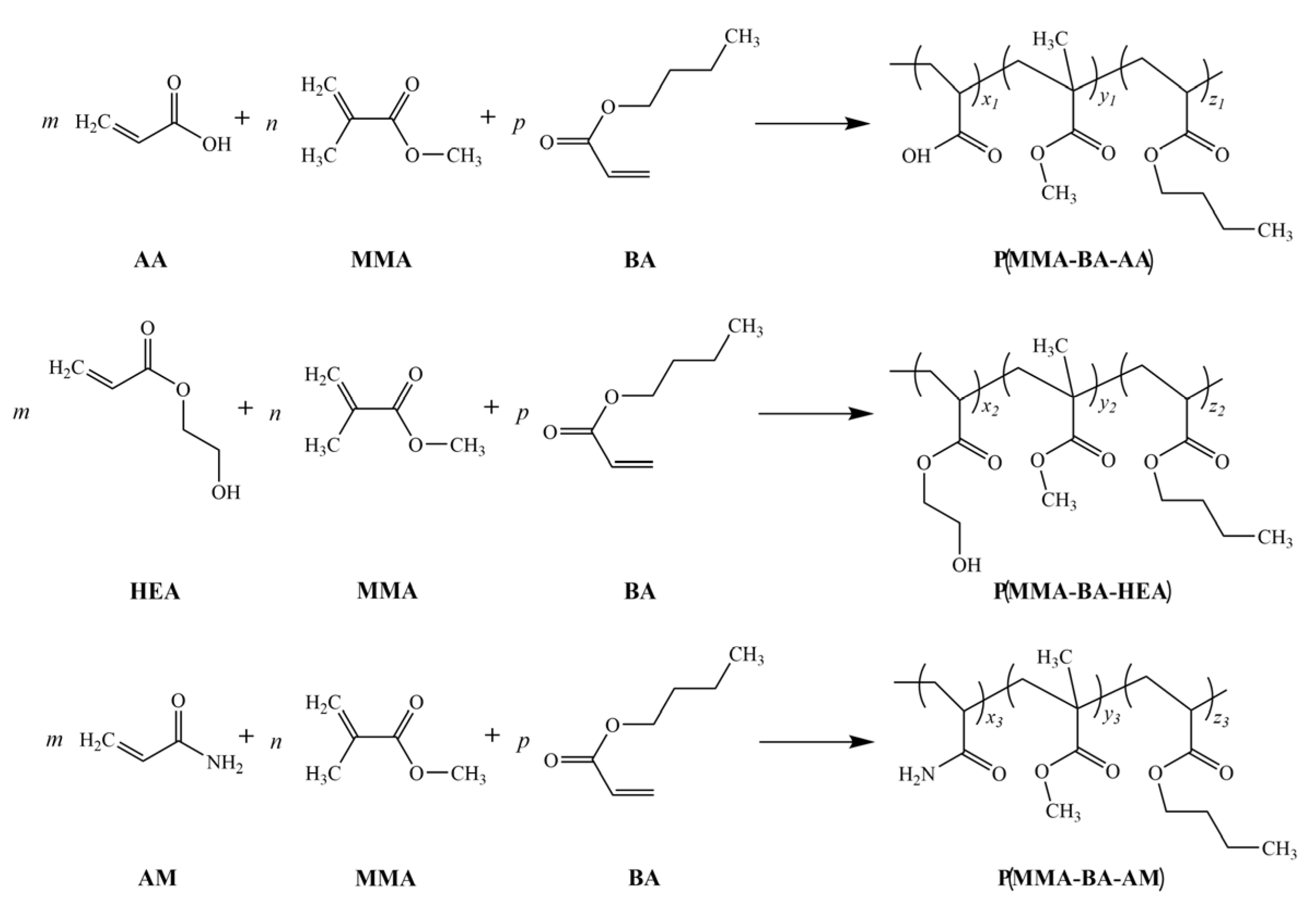
2.2. Preparation of Polyacrylate with Different Functional Groups
2.3. Preparation of Polyacrylate/NGO Composites
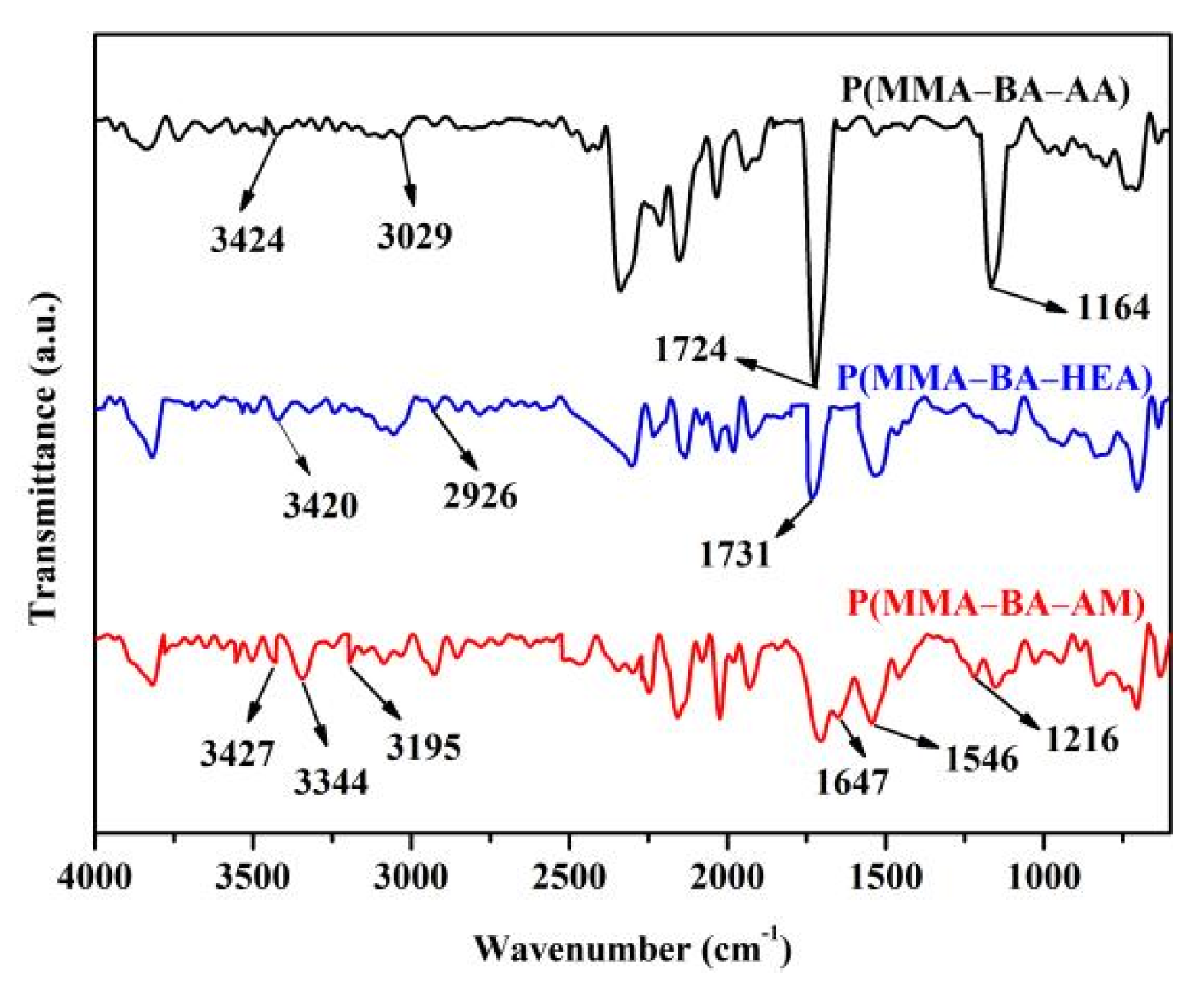
2.4. Characterization
3. Results and Discussion
3.1. Structural Analysis of Polyacrylate with Different Functional Groups
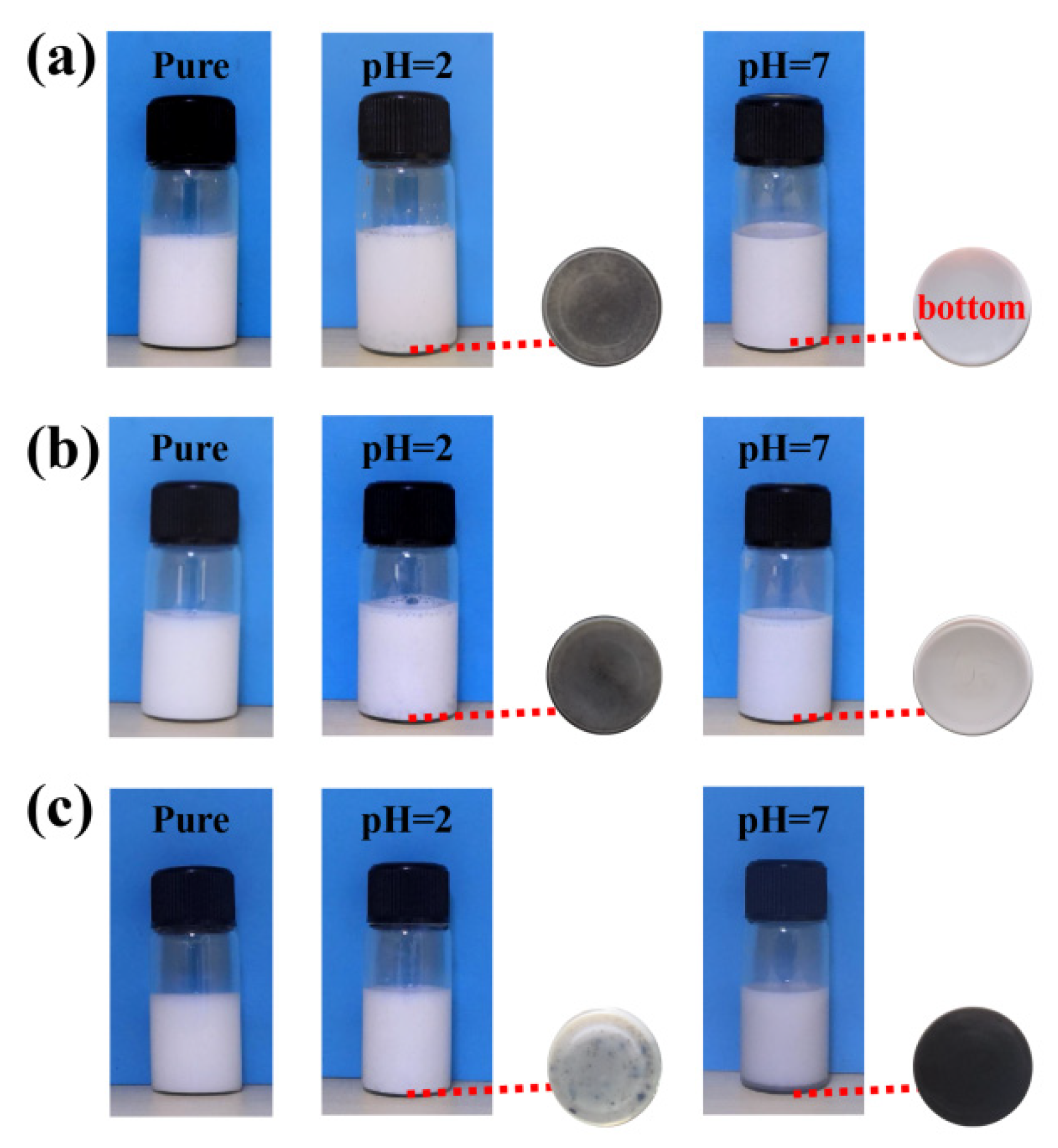
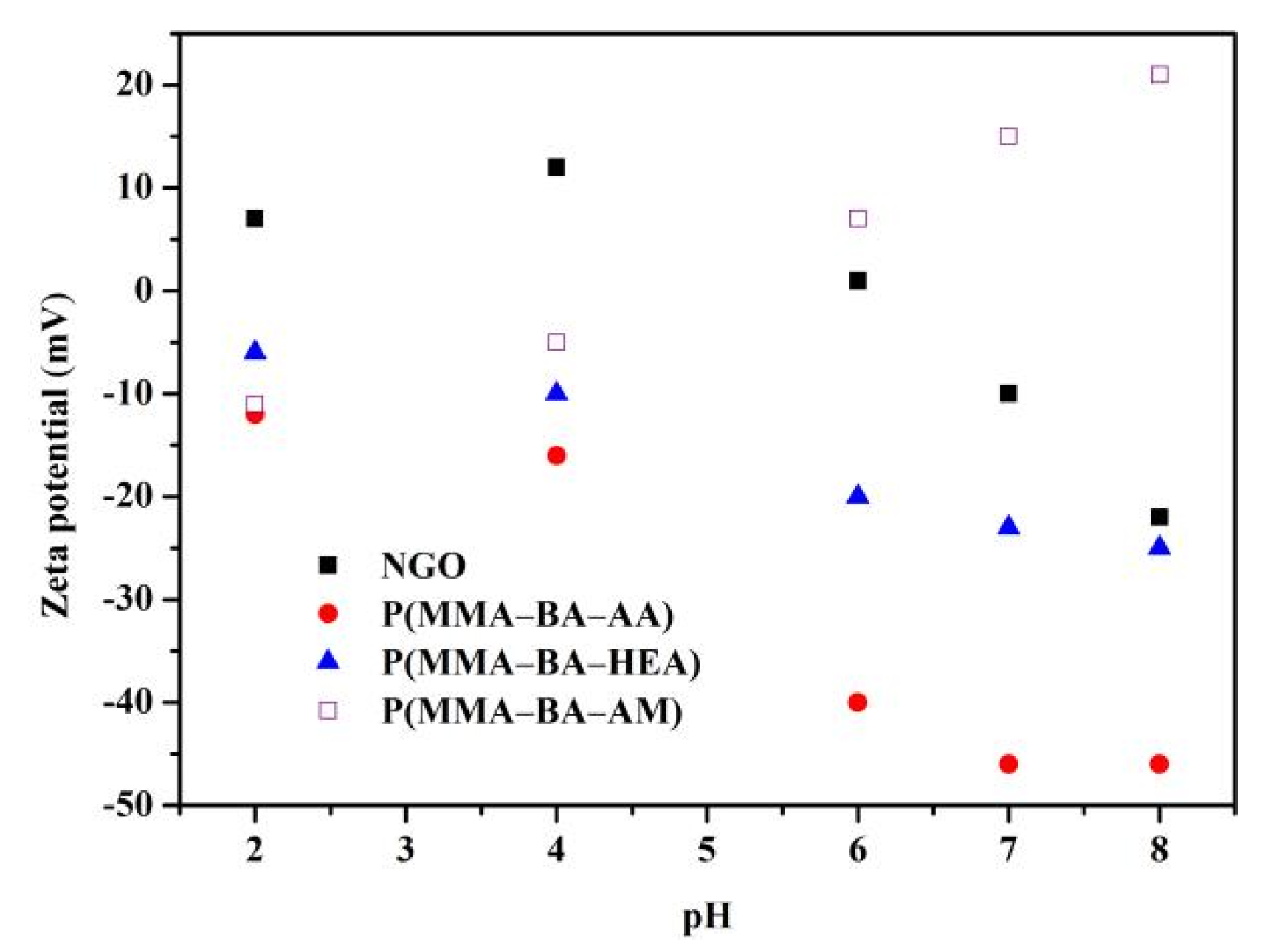
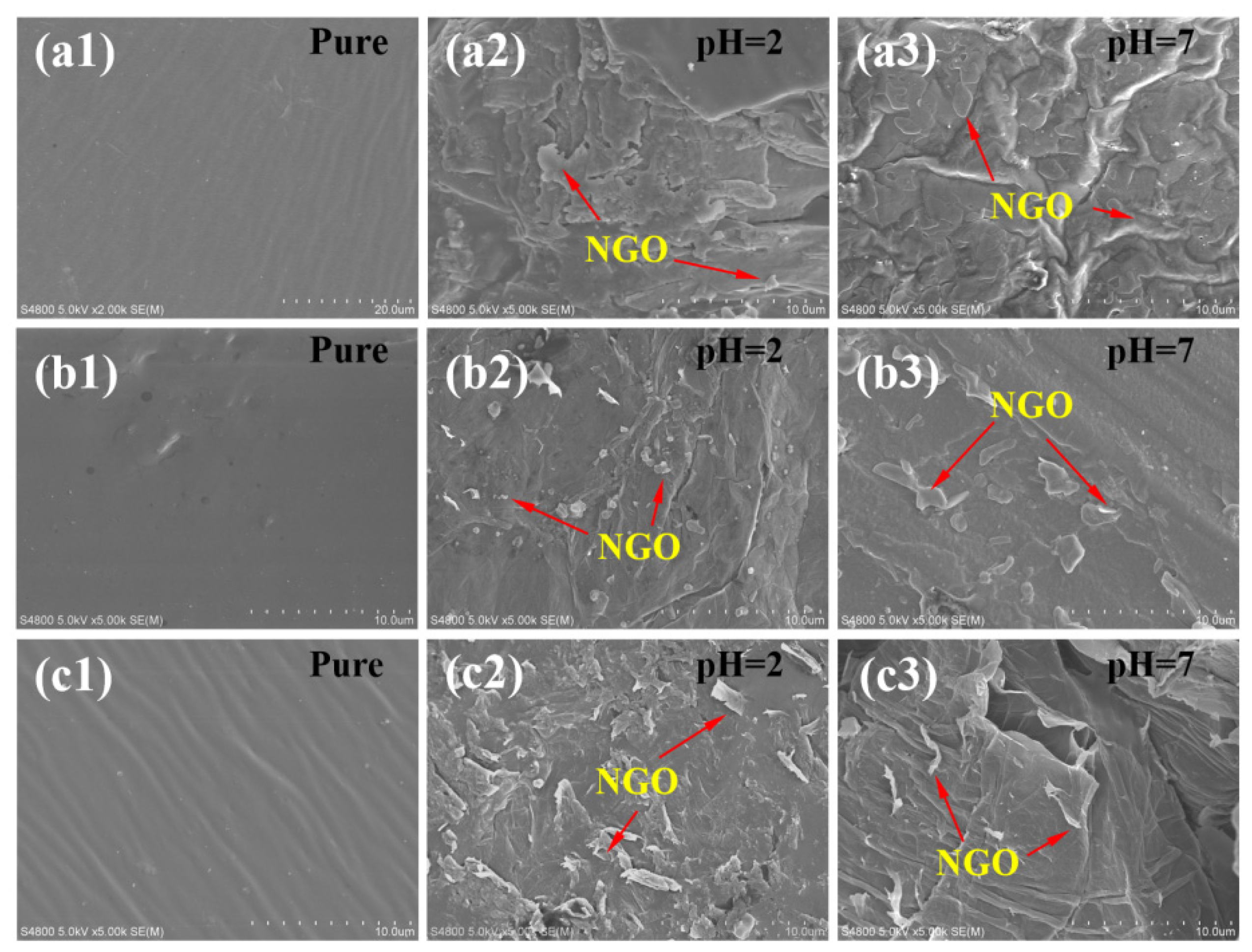
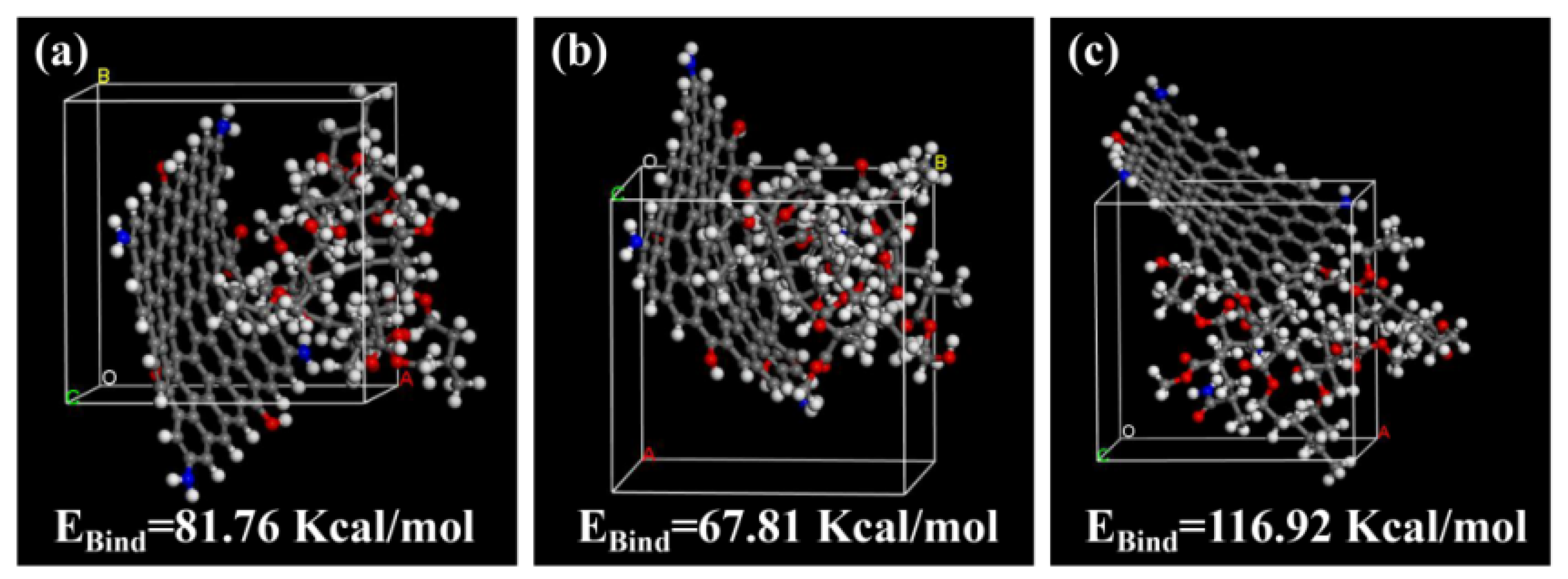
3.2. Stability of Polyacrylate/NGO Composite Material
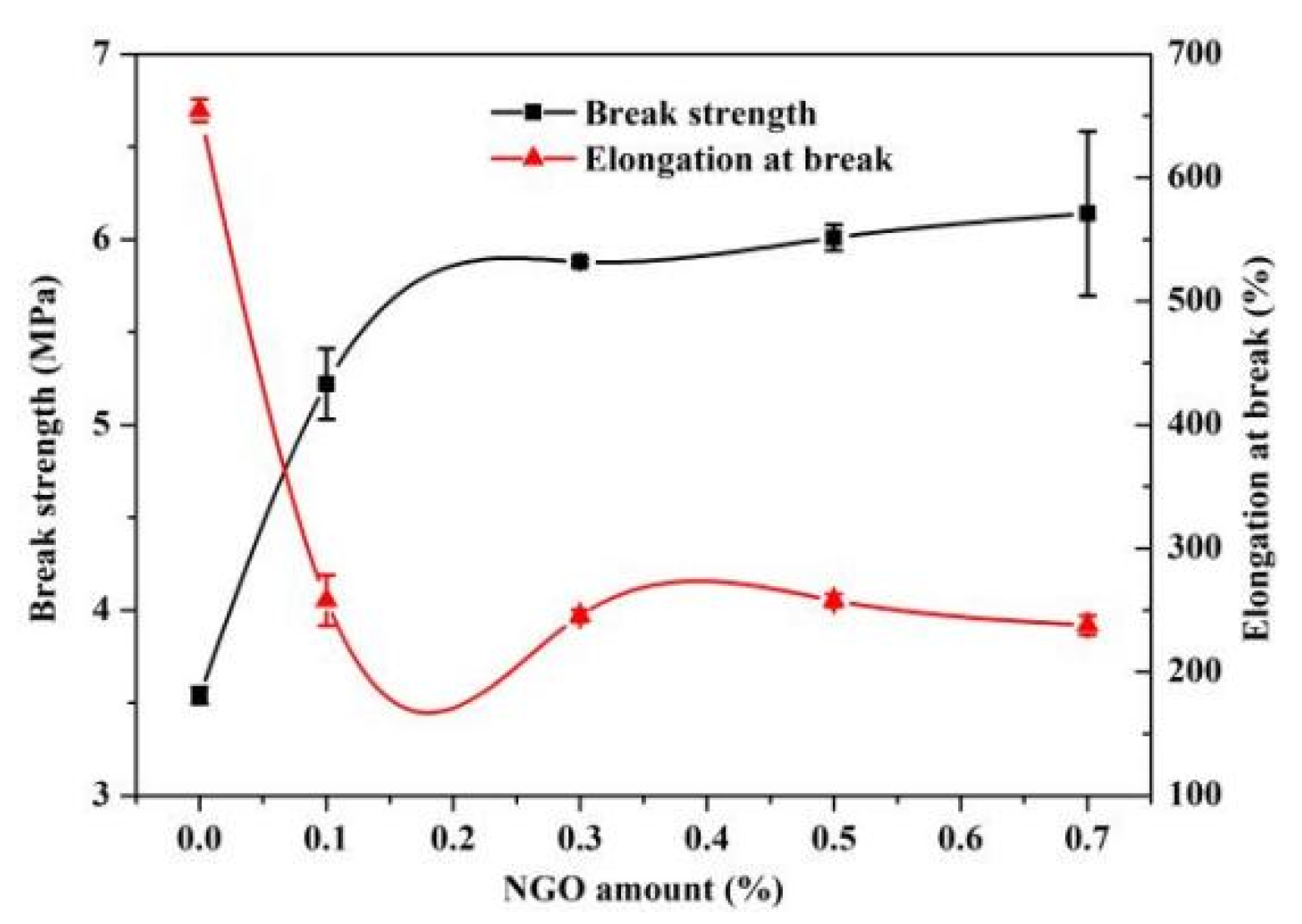
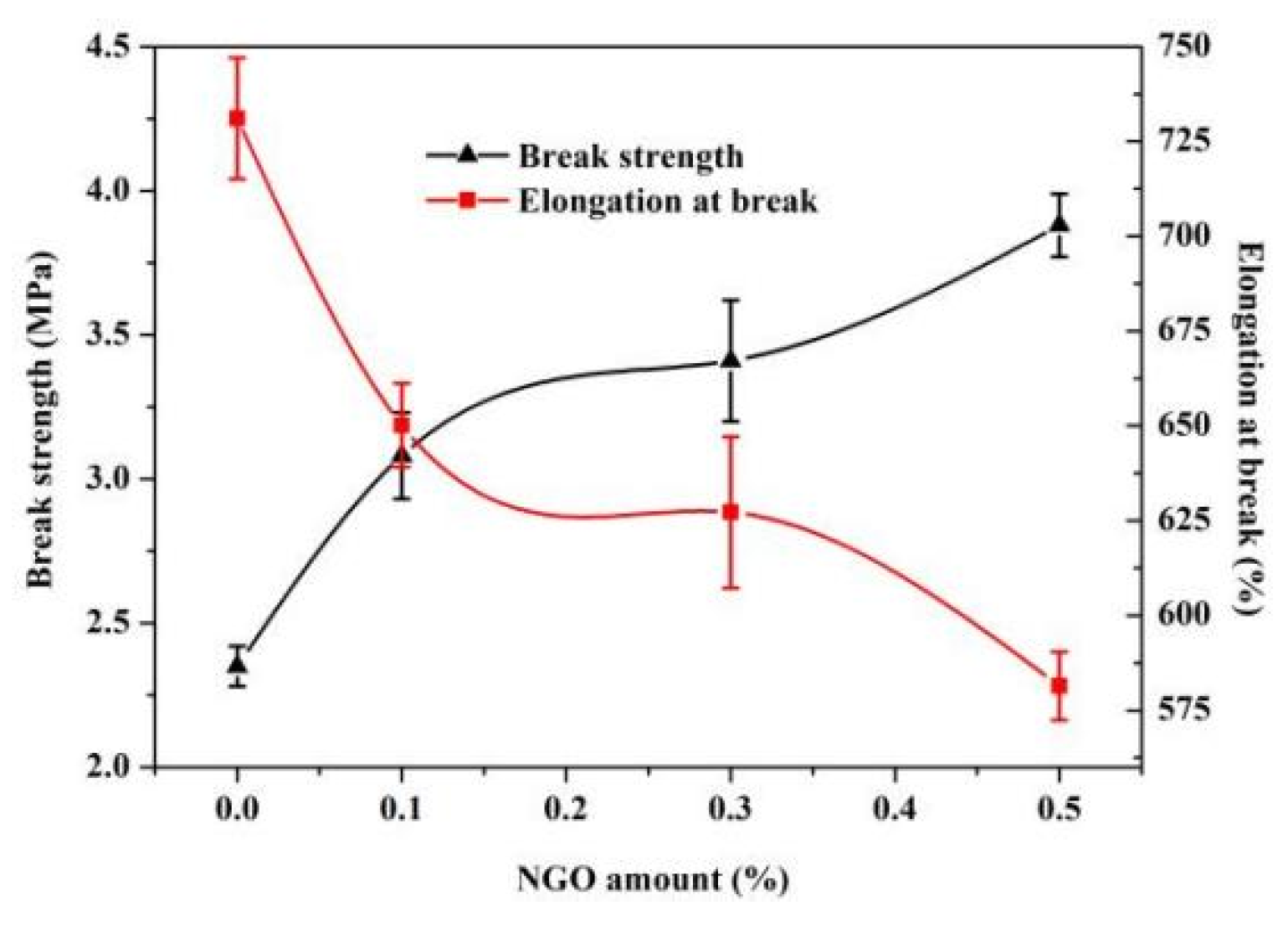
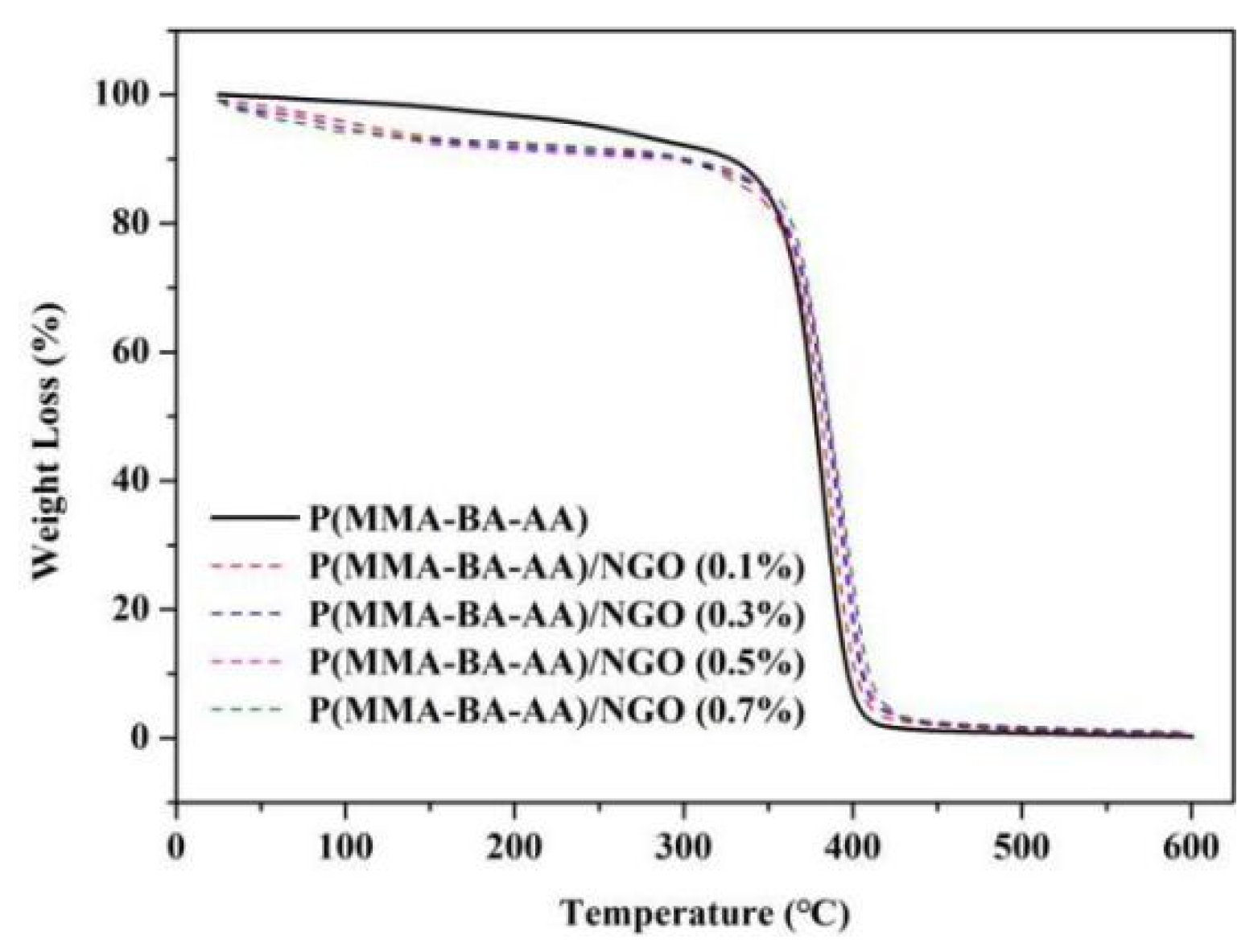
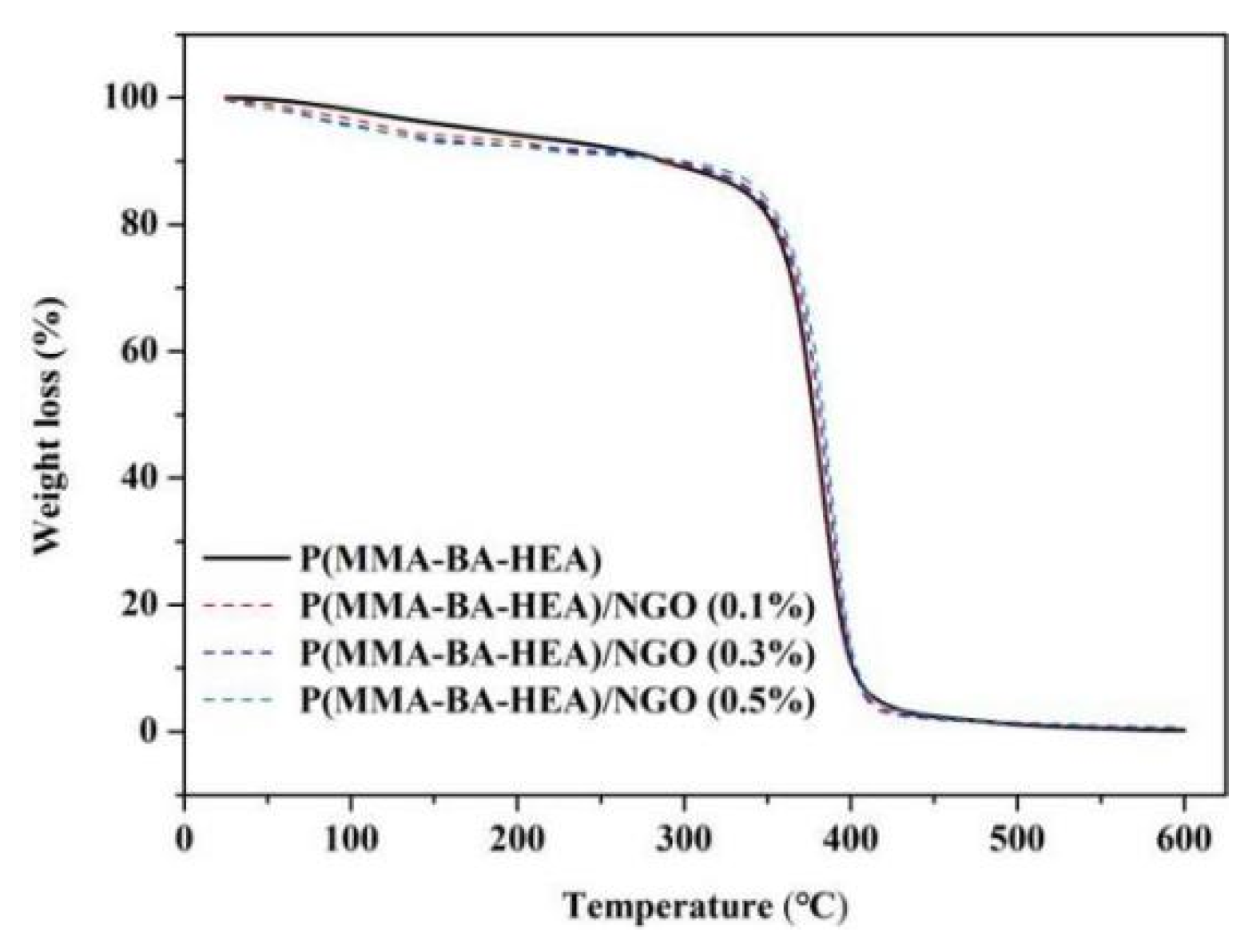
3.3. Mechanical and Thermal Properties of Polyacrylate/NGO Composite Material
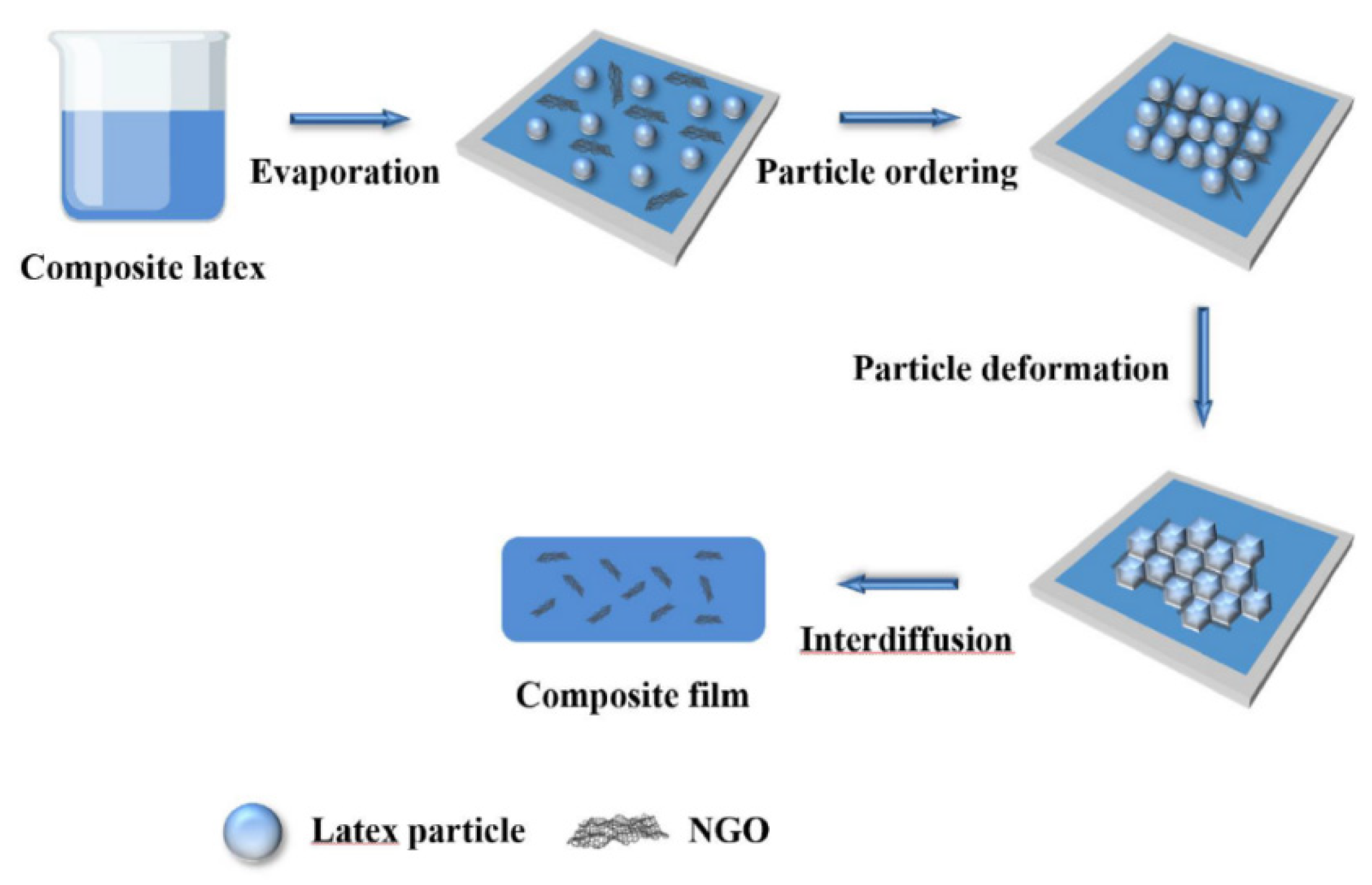
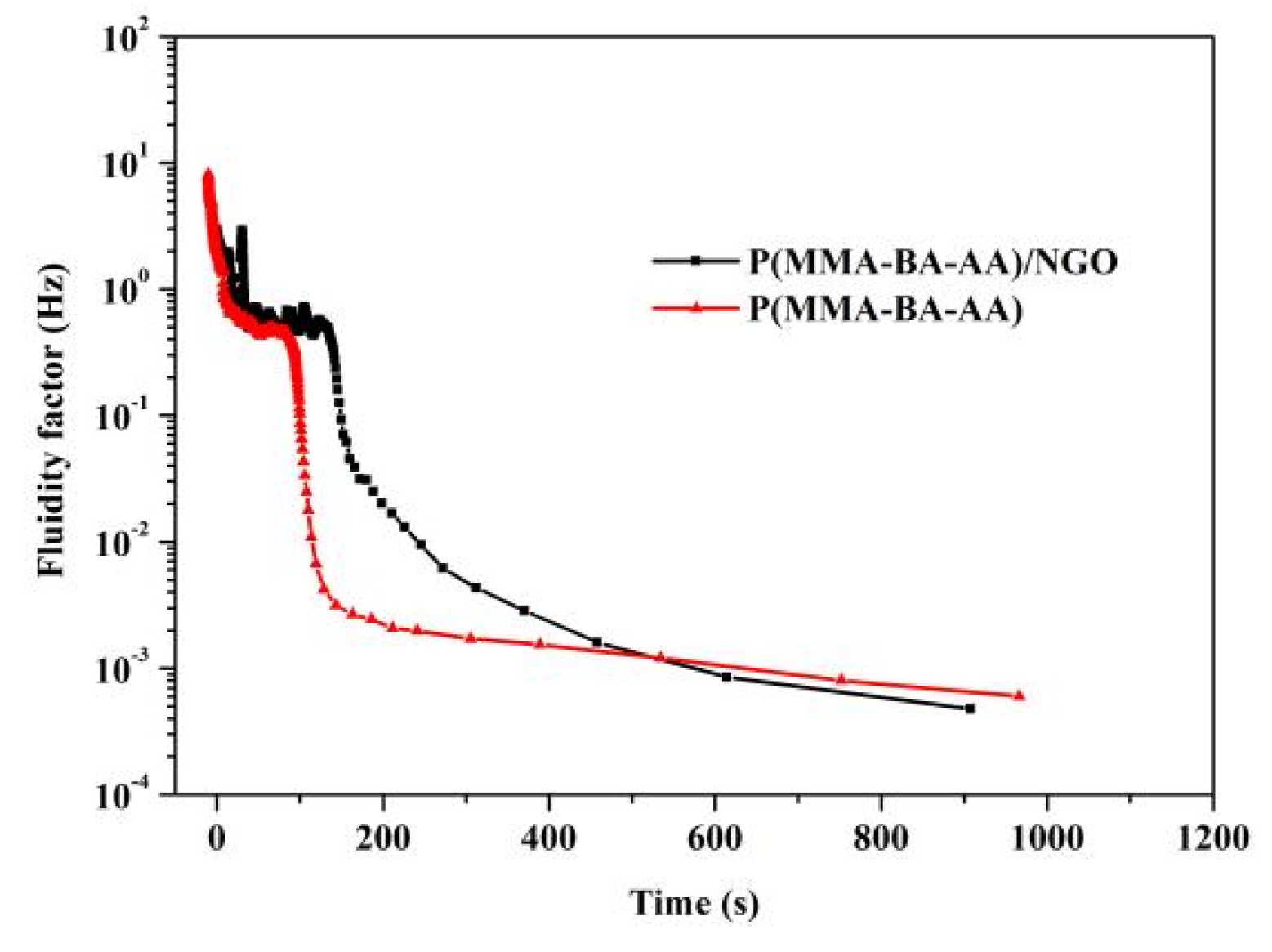
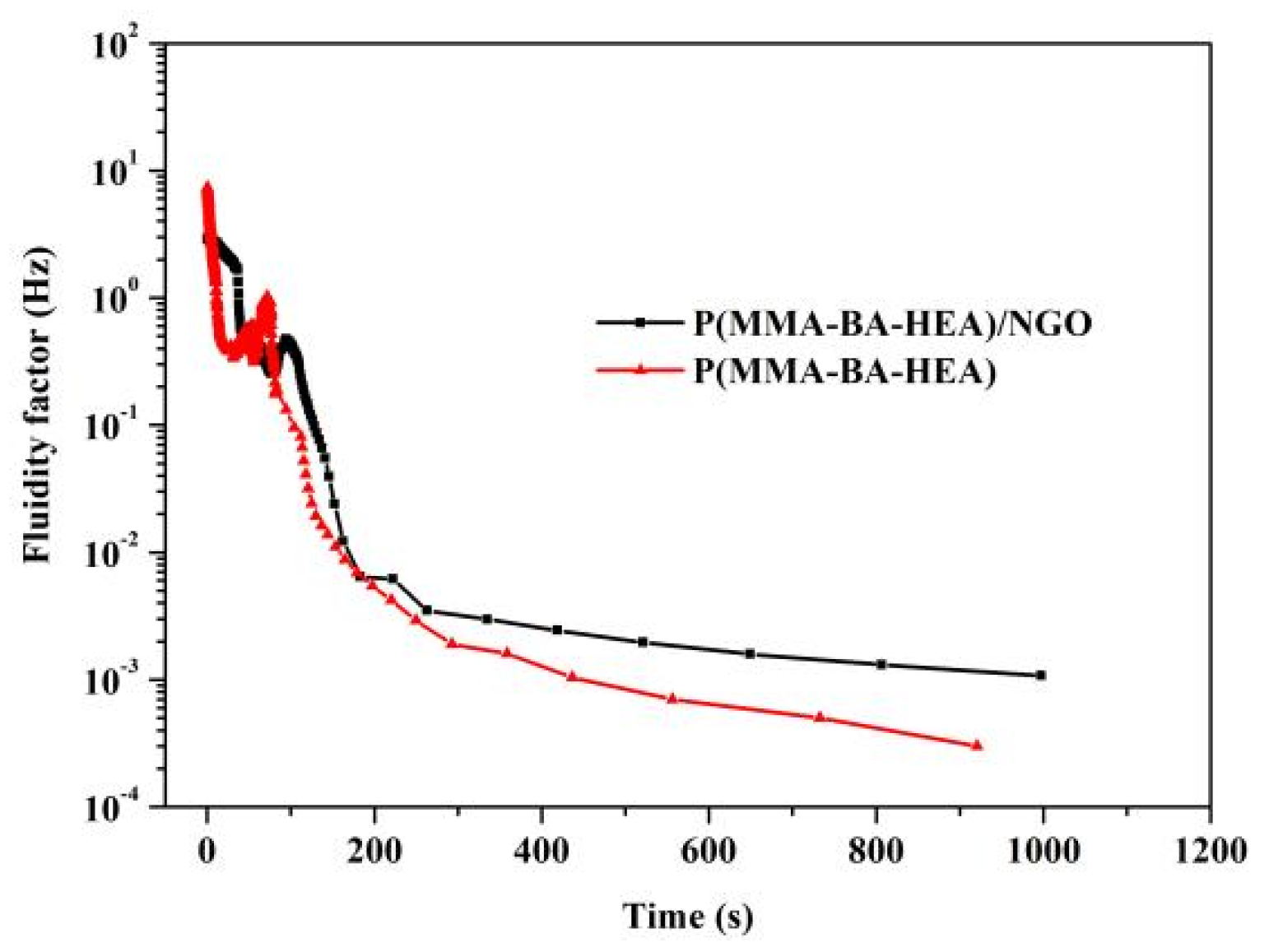
3.4. Influence of NGO on Polyacrylate/NGO Composite Film-Forming Processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Peng, F.; Wang, H.; Huang, F.; Meng, F.; Hui, D.; Zhou, Z. Intercalation polymerization approach for preparing graphene/polymer composites. Polymers 2018, 10, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Jing, Y.; Hu, W.; Lin, J.; Cheng, Y.; Yang, X.; Zhang, K.; Lu, C. Regulation mechanism of graphene oxide on the structure and mechanical properties of bio-based gel-spun lignin/poly (vinyl alcohol) fibers. Cellulose 2021, 28, 4745–4760. [Google Scholar] [CrossRef]
- Vallés, C.; Zhang, X.; Cao, J.; Lin, F.; Young, R.; Lombardo, A.; Ferrari, A.; Burk, L.; Mülhaupt, R.; Kinloch, I. Graphene/polyelectrolyte layer-by-layer coatings for electromagnetic interference shielding. ACS Appl. Nano Mater. 2019, 2, 5272–5281. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wei, L.; Ma, J.; Bai, S. Exfoliation and defect control of graphene oxide for waterborne electromagnetic interference shielding coatings. Compos. Part A-Appl. Sci. 2020, 132, 105838. [Google Scholar] [CrossRef]
- Li, Q.; Tian, X.; Wu, N.; Li, Y.; Pan, T.; Zhang, B.; Duan, Y.; Wang, S.; Li, Y. Enhanced thermal conductivity and isotropy of polymer composites by fabricating 3D network structure from carbon-based materials. J. Appl. Polym. Sci. 2021, 138, 49781. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Xiao, S.; Zhu, M.; Yang, J. Preparation of p-Phenylenediamine Modified Graphene Foam/Polyaniline@Epoxy Composite with Superior Thermal and EMI Shielding Performance. Polymers 2021, 13, 2324. [Google Scholar] [CrossRef]
- Adak, B.; Joshi, M.; Butola, B.S. Polyurethane/functionalized-graphene nanocomposite films with enhanced weather resistance and gas barrier properties. Compos. Part B-Eng. 2019, 176, 107303. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Sun, Y.; Zhang, T.; Wang, L.; Wang, J.; Liang, Y.; Hao, M.; Fu, Q. Green preparation and enhanced gas barrier property of rubber nanocomposite film based on graphene oxide-induced chemical crosslinking. Polymers 2021, 225, 123756. [Google Scholar] [CrossRef]
- Wang, G.; Xu, W.; Chen, R.; Li, W.; Liu, Y.; Yang, K. Synergistic effect between zeolitic imidazolate framework-8 and expandable graphite to improve the flame retardancy and smoke suppression of polyurethane elastomer. J. Appl. Polym. Sci. 2020, 137, 48048. [Google Scholar] [CrossRef]
- Yao, Y.; Jin, S.; Ma, X.; Yu, R.; Zou, H.; Wang, H.; Lu, X.; Shu, Q. Graphene-containing flexible polyurethane porous composites with improved electromagnetic shielding and flame retardancy. Compos. Sci. Technol. 2020, 200, 108457. [Google Scholar] [CrossRef]
- Kumar, S.; Raj, S.; Jain, S.; Chatterjee, K. Multifunctional biodegradable polymer nanocomposite incorporating graphene-silver hybrid for biomedical applications. Mater. Des. 2016, 108, 319–332. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, T.; Zhao, K.; Deng, A.; Li, J. A novel electrochemiluminescent immunoassay for diclofenac using conductive polymer functionalized graphene oxide as labels and gold nanorods as signal enhancers. Talanta 2019, 193, 184–191. [Google Scholar] [CrossRef]
- Vasile, E.; Pandele, A.; Andronescu, C.; Selaru, A.; Dinescu, S.; Costache, M.; Hanganu, A.; Raicopol, M.; Teodorescu, M. Hema-functionalized graphene oxide: A versatile nanofiller for poly (propylene fumarate)-based hybrid materials. Sci. Rep. 2019, 9, 18685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Yang, X.; Wickramasinghe, D.; Sundhoro, M.; Orbey, N.; Chow, K.; Yan, M. Functionalization of pristine graphene for the synthesis of covalent graphene–polyaniline nanocomposite. RSC Adv. 2020, 10, 26486–26493. [Google Scholar] [CrossRef]
- Wu, W.; Tu, J.; Li, H.; Zhan, Z.; Huang, L.; Cai, Z.; Li, Q.; Jiang, M.; Huang, J. Suppressed dielectric loss and enhanced thermal conductivity in poly (vinylidene fluoride) nanocomposites using polyethylene glycol-grafted graphene oxide. J. Mater. Sci. Mater. Electron. 2020, 31, 807–813. [Google Scholar] [CrossRef]
- Firdaus, S.; Anasyida, A.; Zubir, S.; Mariatti, M. Graphene/polyaniline nanocomposites: Effect of in-situ polymerization and solvent blending methods with dodecylbenzene sulfonic acid surfactant. J. Mater. Sci. Mater. Electron. 2020, 31, 15805–15821. [Google Scholar] [CrossRef]
- Li, S.; Gao, A.; Yi, F.; Shu, D.; Cheng, H.; Zhou, X.; He, C.; Zeng, D.; Zhang, F. Preparation of carbon dots decorated graphene/polyaniline composites by supramolecular in-situ self-assembly for high-performance supercapacitors. Electrochim. Acta 2019, 297, 1094–1103. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Xu, X.; Wang, C. Facile route for the preparation of functionalized reduced graphene oxide/polyaniline composite and its enhanced electrochemical performance. ECS J. Solid. State Sci. 2021, 10, 031003. [Google Scholar] [CrossRef]
- Wang, J.; Fei, G.; Pan, Y.; Zhang, K.; Hao, S.; Zheng, Z.; Xia, H. Simultaneous reduction and surface functionalization of graphene oxide by cystamine dihydrochloride for rubber composites. Compos. Part Appl. Sci. 2019, 122, 18–26. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, J.; Gao, D.; Zhou, Y.; Li, C.; Zha, J.; Zhang, J. Preparation of amino-functionalized graphene oxide by Hoffman rearrangement and its performances on polyacrylate coating latex. Prog. Org. Coat. 2016, 94, 9–17. [Google Scholar] [CrossRef]
- Wei, L.; Ma, J.; Zhang, W.; Bai, S.; Ren, Y.; Zhang, L.; Wu, Y.; Qin, J. pH triggered hydrogen bonding for preparing mechanically strong, electromagnetic interference shielding and thermally conductive waterborne polymer/graphene@ polydopamine composites. Carbon 2021, 181, 212–224. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Ma, G.; Yang, D. Waterborne Cross-Linkable Polyacrylate Latex Coatings with Good Water Resistance and Strength Stabilized by Modified Hectorite. Polymers 2021, 13, 2470. [Google Scholar] [CrossRef]
- Bao, Y.; Feng, C.; Wang, C.; Ma, J.; Tian, C. Hygienic, antibacterial, UV-shielding performance of polyacrylate/ZnO composite coatings on a leather matrix. Colloid. Surf. 2017, 518, 232–240. [Google Scholar] [CrossRef]
- Garay-Jimenez, J.; Turos, E. A convenient method to prepare emulsified polyacrylate nanoparticles from for drug delivery applications. Bioorg. Med. Chem. Lett. 2011, 21, 4589–4591. [Google Scholar] [CrossRef]
- Fan, J.; Yang, J.; Li, H.; Tian, J.; Wang, M.; Zhao, Y. Cryogenic mechanical properties of graphene oxide/epoxy nanocomposites: Influence of graphene oxide with different oxidation degrees. Polym. Test. 2021, 96, 107074. [Google Scholar] [CrossRef]
- Wang, G.; Dai, Z.; Liu, L.; Hu, H.; Dai, Q.; Zhang, Z. Tuning the interfacial mechanical behaviors of monolayer graphene/PMMA nanocomposites. ACS Appl. Mater. Int. 2016, 8, 22554–22562. [Google Scholar] [CrossRef] [PubMed]
- Alhusaiki-Alghamdi, H. The spectroscopic and physical properties of PMMA/PCL blend incorporated with graphene oxide. Results Phys. 2021, 24, 104125. [Google Scholar] [CrossRef]
- Maio, A.; Fucarino, R.; Khatibi, R.; Rosselli, S.; Bruno, M.; Scaffaro, R. A novel approach to prevent graphene oxide re-aggregation during the melt compounding with polymers. Compos. Sci. Technol. 2015, 119, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Wang, T.; Tsai, J. Mechanical properties of nanocomposites with functionalized graphene. J. Compos. Mater. 2016, 23, 54–61. [Google Scholar] [CrossRef]
- Mi, X.; Huang, G.; Xie, W.; Wang, W.; Liu, Y.; Gao, J. Preparation of graphene oxide aerogel and its adsorption for Cu2+ ions. Carbon 2012, 50, 4856. [Google Scholar] [CrossRef]
- Brun, A.; Dihang, H.; Brunel, L. Film formation of coatings studied by diffusing-wave spectroscopy. Prog. Org. Coat. 2008, 61, 181–191. [Google Scholar] [CrossRef]
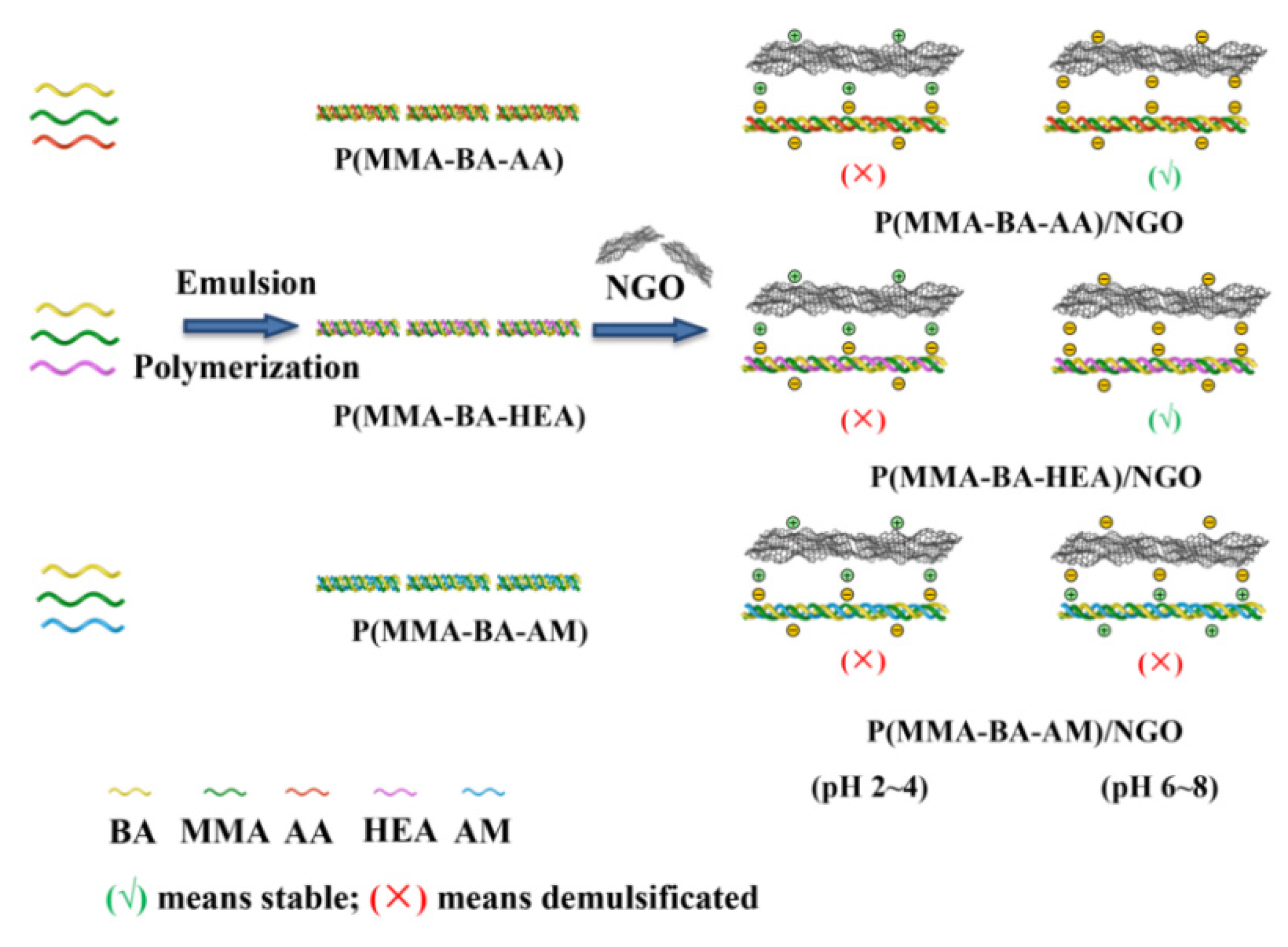
| pH Value | 2.0 | 4.0 | 6.0 | 7.0 | 8.0 |
|---|---|---|---|---|---|
| P(MMA-BA-AA)/NGO | × | × | √ | √ | √ |
| P(MMA-BA-HEA)/NGO | × | × | √ | √ | √ |
| P(MMA-BA-AM)/NGO | × | × | × | × | × |
| Samples | Open Time (s) | Dust Free (s) | Set to Touch (s) | Touch Dry (s) |
|---|---|---|---|---|
| P(MMA-BA-AA) | 18 | 71 | 233 | 667 |
| P(MMA-BA-AA)/NGO | 34 | 98 | 134 | 575 |
| P(MMA-BA-HEA) | 21 | 60 | 130 | 714 |
| P(MMA-BA-HEA)/NGO | 39 | 67 | 112 | 608 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Li, S.; Ma, J.; Wu, Y.; Liu, C.; Yan, H. pH-Induced Electrostatic Interaction between Polyacrylates and Amino-Functionalized Graphene Oxide on Stability and Coating Performances. Polymers 2021, 13, 3406. https://doi.org/10.3390/polym13193406
Zhang W, Li S, Ma J, Wu Y, Liu C, Yan H. pH-Induced Electrostatic Interaction between Polyacrylates and Amino-Functionalized Graphene Oxide on Stability and Coating Performances. Polymers. 2021; 13(19):3406. https://doi.org/10.3390/polym13193406
Chicago/Turabian StyleZhang, Wenbo, Sichun Li, Jianzhong Ma, Yingke Wu, Chao Liu, and Hongxia Yan. 2021. "pH-Induced Electrostatic Interaction between Polyacrylates and Amino-Functionalized Graphene Oxide on Stability and Coating Performances" Polymers 13, no. 19: 3406. https://doi.org/10.3390/polym13193406
APA StyleZhang, W., Li, S., Ma, J., Wu, Y., Liu, C., & Yan, H. (2021). pH-Induced Electrostatic Interaction between Polyacrylates and Amino-Functionalized Graphene Oxide on Stability and Coating Performances. Polymers, 13(19), 3406. https://doi.org/10.3390/polym13193406








