Secondary Raw Materials from Residual Carbon Fiber-Reinforced Composites by An Upgraded Pyrolysis Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Components and Manufacturing of Virgin CFRP and Recycled CFRP
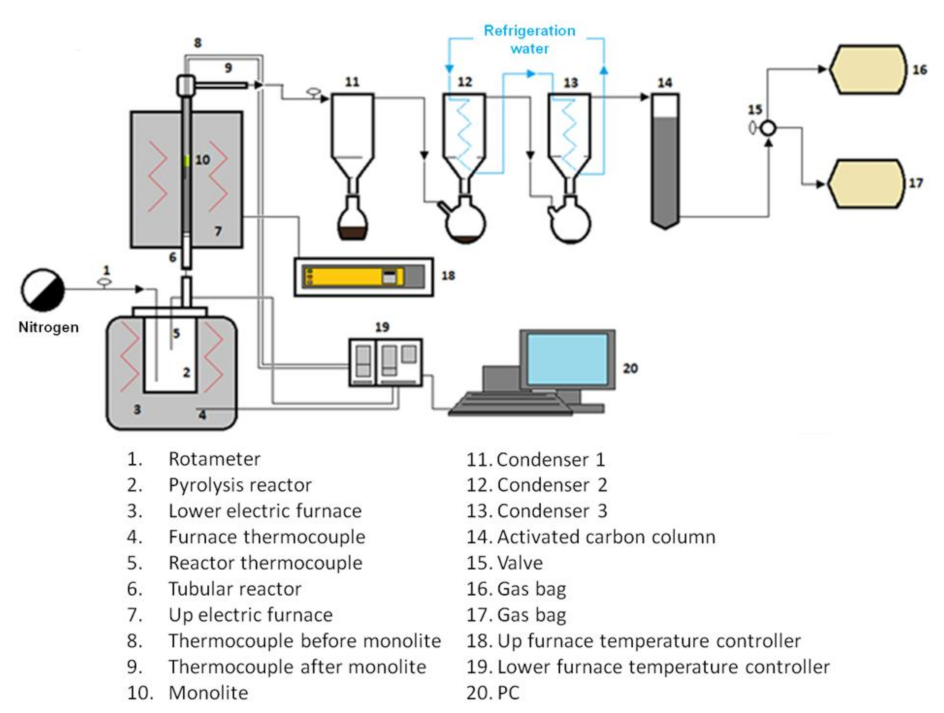
2.2. Carbon Fiber Recovery Process
2.3. Analytical Techniques
2.3.1. Analysis of Liquid and Gaseous Products
2.3.2. Characterization of Carbon Fibers
2.3.3. Characterization of vCFRP and rCFRP
3. Results and Discussion
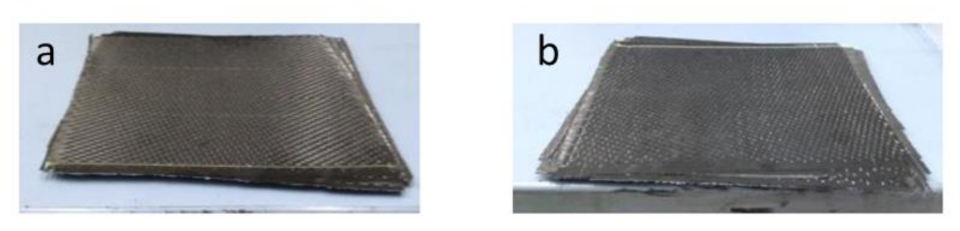
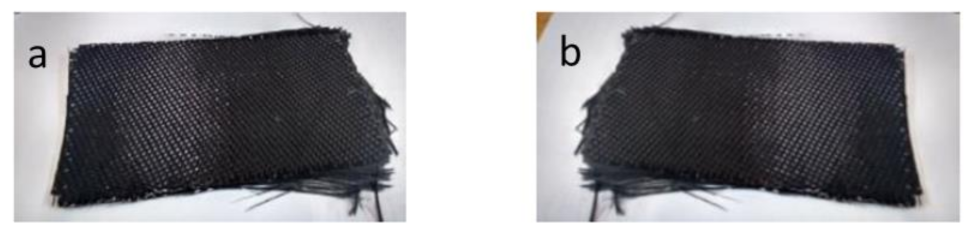
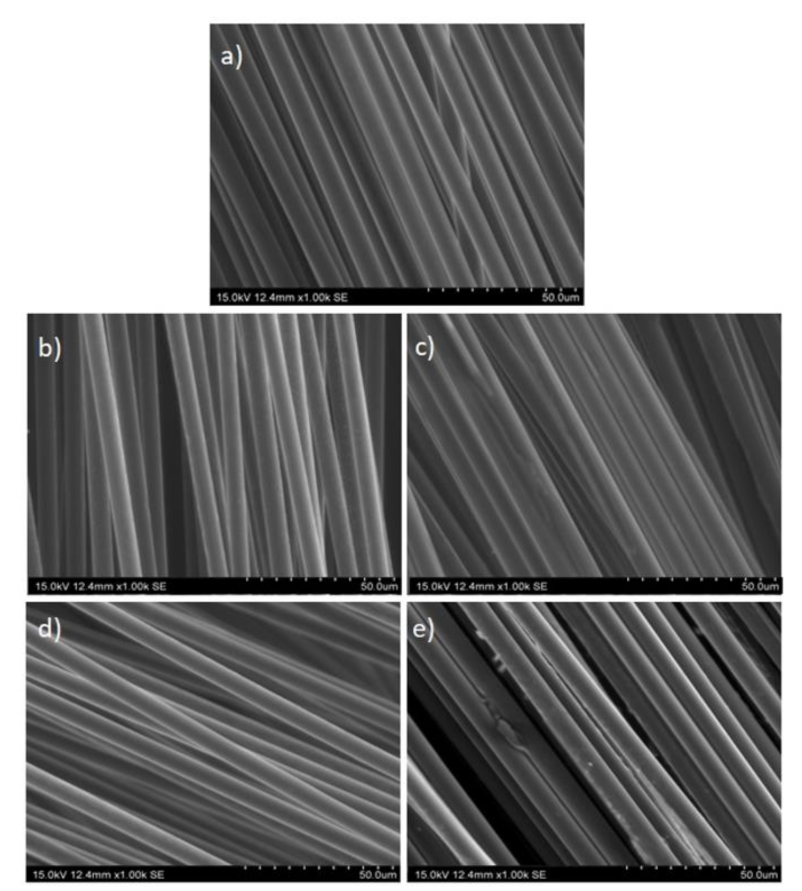
3.1. Recovery of Carbon Fibers
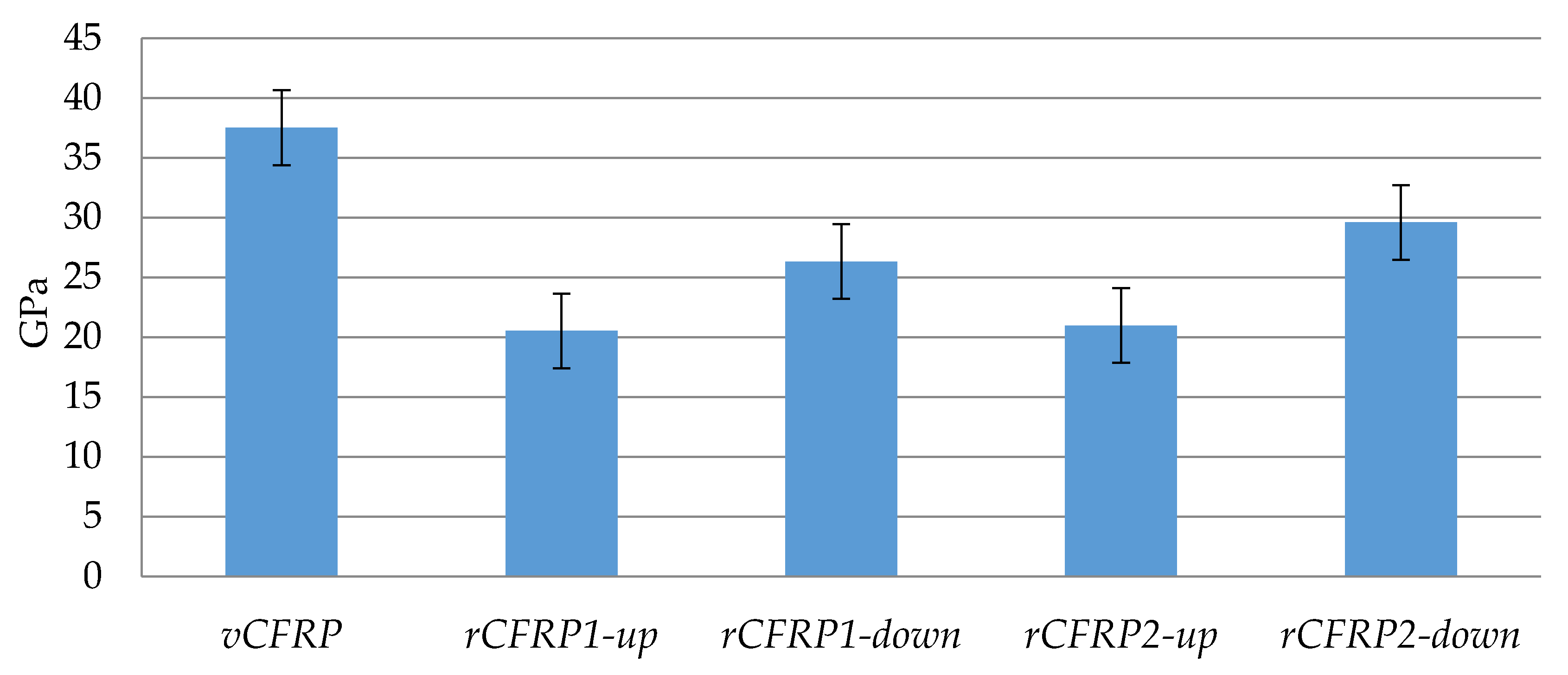
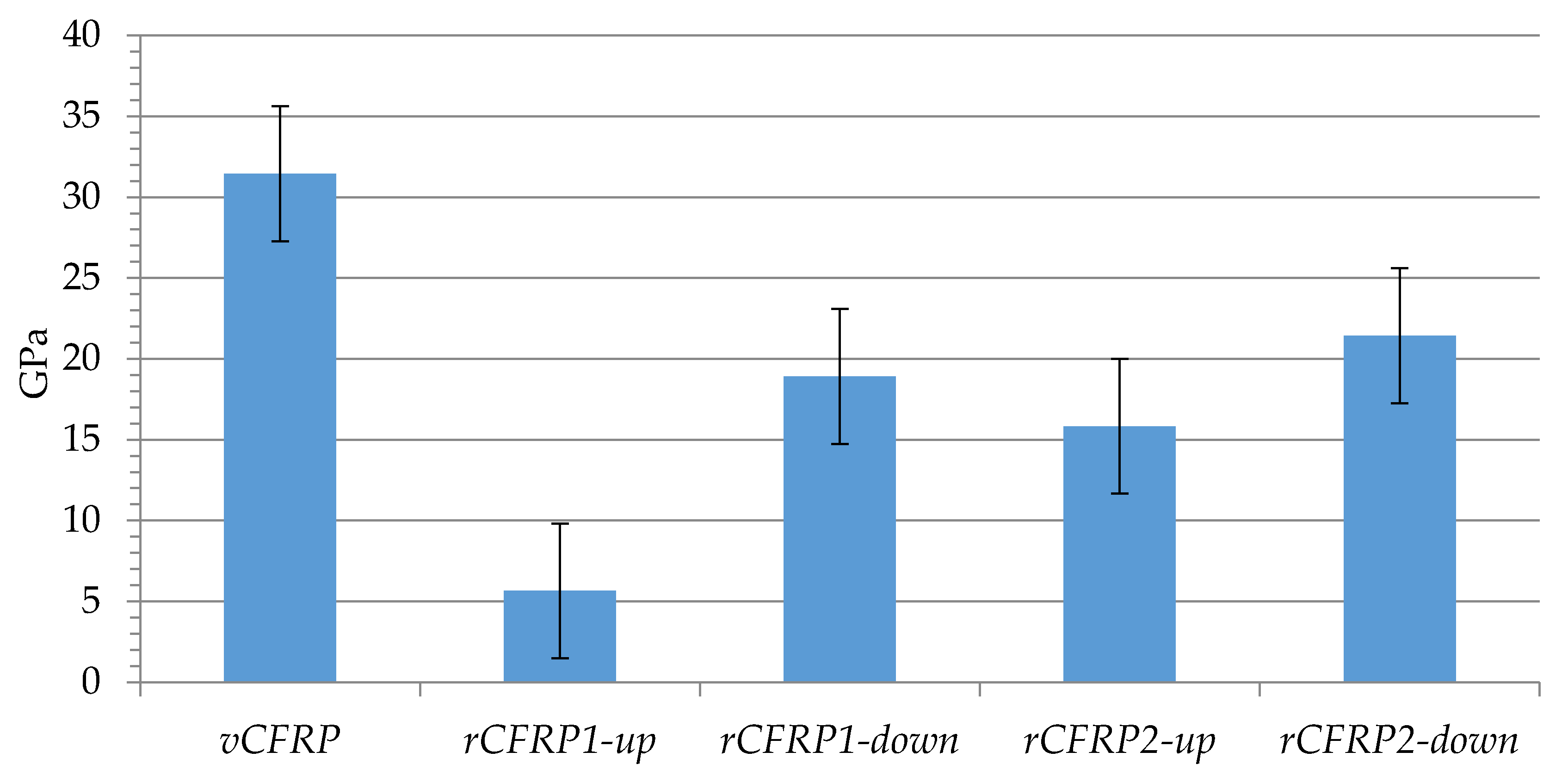
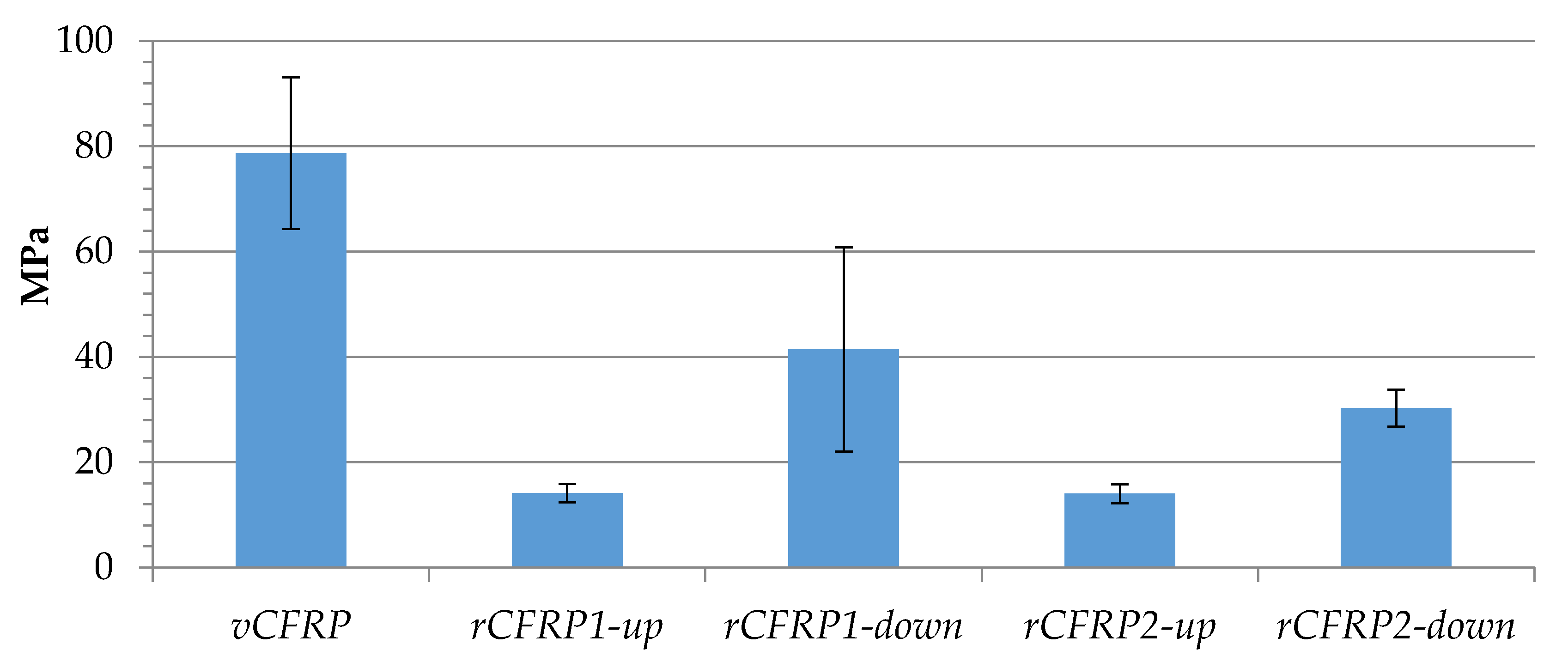
3.2. Characterization and Performance of Recycled CFRP
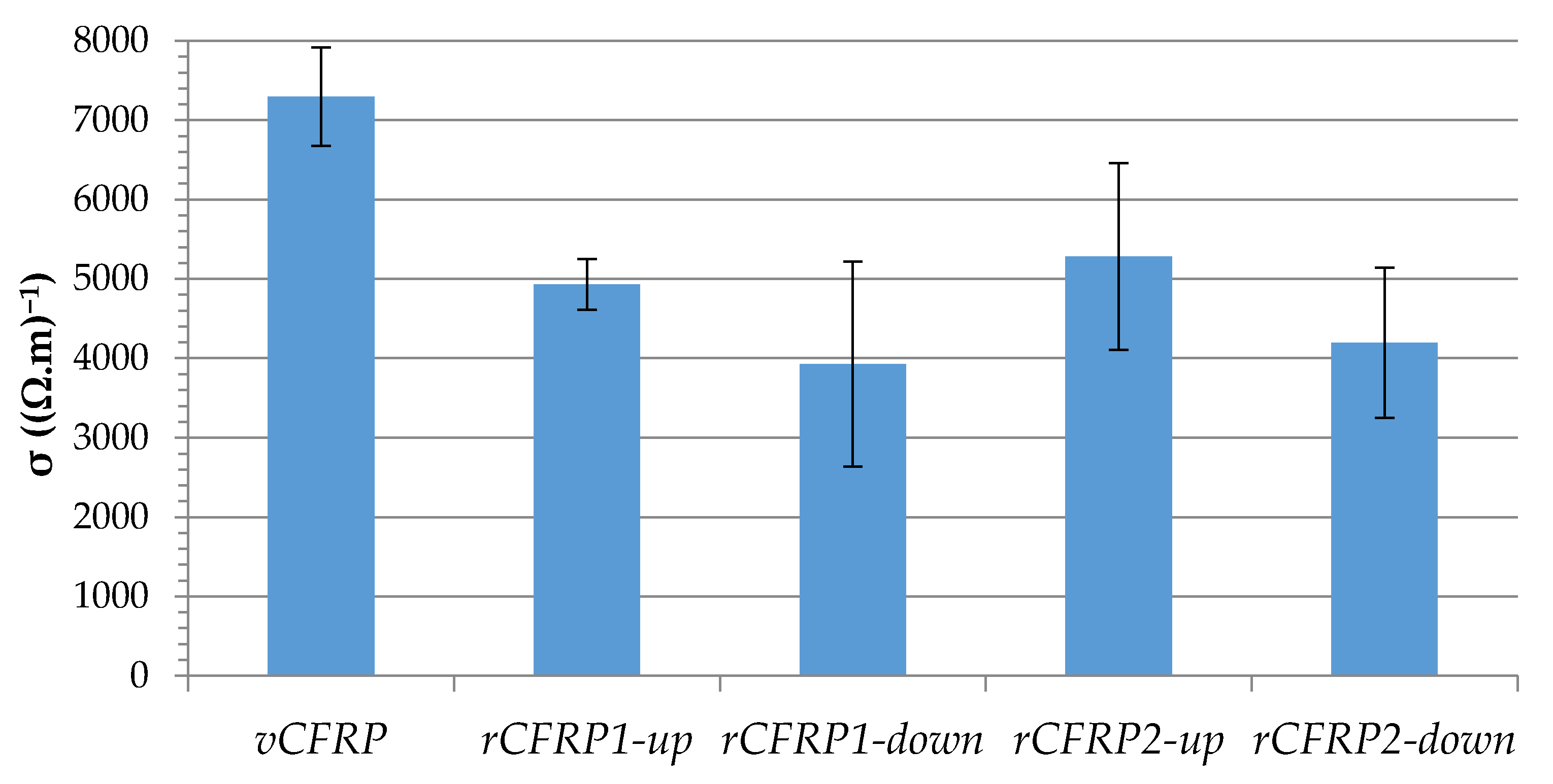
3.2.1. Electrical Conductivity
3.2.2. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, A. Building and Constructions Plastic Market—An Analysis. Reinf. Plast. 2021, 65, 194–198. [Google Scholar] [CrossRef]
- Francis, S. The State of Recycled Carbon Fiber. Compos. World. 2019, 64–71. Available online: https://www.compositesworld.com/articles/the-state-of-recycled-carbon-fiber (accessed on 20 July 2021).
- Mazumdar, S.; Pichler, D.; GangaRao, H.; Benevento, M.; Liang, R.; Witten, E. Industry Report. Materials and Markets in Composites Industry. Compos. Manuf. 2019. Available online: http://compositesmanufacturingmagazine.com/digital/2019/CM-issue-january-february-2019.pdf (accessed on 20 July 2021).
- Moyer, K.; Meng, C.; Marshall, B.; Assal, O.; Eaves, J.; Perez, D.; Karkkainen, R.; Roberson, L.; Pint, C.L. Carbon fiber reinforced structural lithium-ion battery composite: Multifunctional power integration for CubeSats. Energy Storage Mater. 2020, 24, 676–681. [Google Scholar] [CrossRef]
- Javaid, A.; Khalid, O.; Shakeel, A.; Noreen, S. Multifunctional structural supercapacitors based on polyaniline deposited carbon fiber reinforced epoxy composites. J. Energy Storage 2021, 33, 102168. [Google Scholar] [CrossRef]
- van de Werken, N.; Tekinalp, H.; Khanbolouki, P.; Ozcan, S.; Williams, A.; Tehrani, M. Additively manufactured carbon fiber-reinforced composites: State of the art and perspective. Addit. Manuf. 2020, 31, 100962. [Google Scholar] [CrossRef]
- Zhu, S.; Shi, R.; Qu, M.; Zhou, J.; Ye, C.; Zhang, L.; Cao, H.; Ge, D.; Chen, Q. Simultaneously improved mechanical and electromagnetic interference shielding properties of carbon fiber fabrics/epoxy composites via interface engineering. Compos. Sci. Technol. 2021, 207, 108696. [Google Scholar] [CrossRef]
- Hiremath, N.; Young, S.; Ghossein, H.; Penumadu, D.; Vaidya, U.; Theodore, M. Low cost textile-grade carbon-fiber epoxy composites for automotive and wind energy applications. Compos. Part B Eng. 2020, 198, 108156. [Google Scholar] [CrossRef]
- Si, H.; Zhou, L.; Wu, Y.; Song, L.; Kang, M.; Zhao, X.; Chen, M. Rapidly reprocessable, degradable epoxy vitrimer and recyclable carbon fiber reinforced thermoset composites relied on high contents of exchangeable aromatic disulfide crosslinks. Compos. Part B Eng. 2020, 199, 108278. [Google Scholar] [CrossRef]
- Black, S. Composites recycling: Gaining traction. Compos. World 2017, 3, 46–55. [Google Scholar]
- Holmes, M. Recycled carbon fiber composites become a reality. Reinf. Plast. 2018, 62, 148–153. [Google Scholar] [CrossRef]
- Hermansson, F.; Janssen, M.; Svanström, M. Prospective study of lignin-based and recycled carbon fibers in composites through meta-analysis of life cycle assessments. J. Clean. Prod. 2019, 223, 946–956. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, L.; Huang, Y.; Du, J. Recycling of carbon/epoxy composites. J. Appl. Polym. Sci. 2004, 94, 1912–1916. [Google Scholar] [CrossRef]
- Pegoretti, A. Towards sustainable structural composites: A review on the recycling of continuous-fiber-reinforced thermoplastics. Adv. Ind. Eng. Polym. Res. 2021, 4, 105–115. [Google Scholar] [CrossRef]
- Karuppannan Gopalraj, S.; Kärki, T. A review on the recycling of waste carbon fibre/glass fibre-reinforced composites: Fibre recovery, properties and life-cycle analysis. SN Appl. Sci. 2020, 2, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Rani, M.; Choudhary, P.; Krishnan, V.; Zafar, S. A review on recycling and reuse methods for carbon fiber/glass fiber composites waste from wind turbine blades. Compos. Part B Eng. 2021, 215, 108768. [Google Scholar] [CrossRef]
- Utekar, S.; Suriya, V.K.; More, N.; Rao, A. Comprehensive study of recycling of thermosetting polymer composites—Driving force, challenges and methods. Compos. Part B Eng. 2021, 207, 108596. [Google Scholar] [CrossRef]
- Pakdel, E.; Kashi, S.; Varley, R.; Wang, X. Recent progress in recycling carbon fibre reinforced composites and dry carbon fibre wastes. Resour. Conserv. Recycl. 2021, 166, 105340. [Google Scholar] [CrossRef]
- Pillain, B.; Loubet, P.; Pestalozzi, F.; Woidasky, J.; Erriguible, A.; Aymonier, C.; Sonnemann, G. Positioning supercritical solvolysis among innovative recycling and current waste management scenarios for carbon fiber reinforced plastics thanks to comparative life cycle assessment. J. Supercrit. Fluids 2019, 154, 104607. [Google Scholar] [CrossRef]
- He, D.; Soo, V.K.; Kim, H.C.; Compston, P.; Doolan, M. Comparative life cycle energy analysis of carbon fibre pre-processing, processing and post-processing recycling methods. Resour. Conserv. Recycl. 2020, 158. [Google Scholar] [CrossRef]
- Rybicka, J.; Tiwari, A.; Leeke, G.A. Technology readiness level assessment of composites recycling technologies. J. Clean. Prod. 2016, 112, 1001–1012. [Google Scholar] [CrossRef] [Green Version]
- Nickels, L. Closing the circle with recycled carbon fiber. Reinf. Plast. 2020, 64, 40–43. [Google Scholar] [CrossRef]
- Nickels, L. Smooth sailing with recycled fibers. Reinf. Plast. 2019, 63, 322–325. [Google Scholar] [CrossRef]
- Fernández, A.; Santangelo-Muro, M.; Fernández-Blázquez, J.P.; Lopes, C.S.; Molina-Aldareguia, J.M. Processing and properties of long recycled-carbon-fibre reinforced polypropylene. Compos. Part B Eng. 2021, 211. [Google Scholar] [CrossRef]
- Liu, W.; Huang, H.; Zhu, L.; Liu, Z. Integrating carbon fiber reclamation and additive manufacturing for recycling CFRP waste. Compos. Part B Eng. 2021, 215. [Google Scholar] [CrossRef]
- Tapper, R.J.; Longana, M.L.; Norton, A.; Potter, K.D.; Hamerton, I. An evaluation of life cycle assessment and its application to the closed-loop recycling of carbon fibre reinforced polymers. Compos. Part B Eng. 2020, 184, 107665. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; Gastelu, N.; Acha, E.; Caballero, B.M.; Orue, A.; Jiménez-Suárez, A.; Prolongo, S.G.; de Marco, I. Reclamation of carbon fibers and added-value gases in a pyrolysis-based composites recycling process. J. Clean. Prod. 2020, 273, 123173. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; Gastelu, N.; Acha, E.; Caballero, B.M.; de Marco, I. Production of hydrogen-rich gases in the recycling process of residual carbon fiber reinforced polymers by pyrolysis. Waste Manag. 2021, 128, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Gastelu, N.; Lopez-Urionabarrenechea, A.; Acha, E.; Caballero, B.M.; de Marco, I. Evaluation of HZSM-5 Zeolite as Cracking Catalyst for Upgrading the Vapours Generated in the Pyrolysis of an Epoxy-Carbon Fibre Waste Composite. Top. Catal. 2019, 62, 479–490. [Google Scholar] [CrossRef]
- Gastelu, N.; Lopez-Urionabarrenechea, A.; Solar, J.; Acha, E.; Caballero, B.M.; López, F.A.; de Marco, I. Thermo-Catalytic Treatment of Vapors in the Recycling Process of Carbon Fiber-Poly (Benzoxazine) Composite Waste by Pyrolysis. Catalysts 2018, 8, 523. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Urionabarrenechea, A.; de Marco, I.; Caballero, B.M.; Gastelu, N.; Hernández, A.; Adrados, A.; Solar, J. Method for Treating Vapours Generated during the Process for Recovering Carbon Fibres from Composites by Pyrolysis. WO/2016/135359. 2016. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=EP209578786&tab=NATIONALBIBLIO (accessed on 20 July 2021).
- Irisawa, T.; Aratake, R.; Hanai, M.; Sugimoto, Y.; Tanabe, Y. Elucidation of damage factors to recycled carbon fibers recovered from CFRPs by pyrolysis for finding optimal recovery conditions. Compos. Part B Eng. 2021, 218, 108939. [Google Scholar] [CrossRef]
- Tranchard, P.; Duquesne, S.; Samyn, F.; Estèbe, B.; Bourbigot, S. Kinetic analysis of the thermal decomposition of a carbon fibre-reinforced epoxy resin laminate. J. Anal. Appl. Pyrolysis 2017, 126, 14–21. [Google Scholar] [CrossRef]
- Mazzocchetti, L.; Benelli, T.; D’Angelo, E.; Leonardi, C.; Zattini, G.; Giorgini, L. Validation of carbon fibers recycling by pyro-gasification: The influence of oxidation conditions to obtain clean fibers and promote fiber/matrix adhesion in epoxy composites. Compos. Part A Appl. Sci. Manuf. 2018, 112, 504–514. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; Acha, E.; Adrados, A.; Solar, J.; Caballero, B.M.; de Marco, I. Use of a reforming catalyst for hydrogen production in the carbonization process of torrefied biomass. Catalysts 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Jiang, J.; Deng, G.; Chen, X.; Gao, X.; Guo, Q.; Xu, C.; Zhou, L. On the successful chemical recycling of carbon fiber/epoxy resin composites under the mild condition. Compos. Sci. Technol. 2017, 151, 243–251. [Google Scholar] [CrossRef]
- Qazi, H.; Lin, R.; Jayaraman, K. Fibre structure preservation in composite recycling using thermolysis process. Resour. Conserv. Recycl. 2021, 169, 105482. [Google Scholar] [CrossRef]
- Abdou, T.R.; Botelho Junior, A.B.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of polymeric composites from industrial waste by pyrolysis: Deep evaluation for carbon fibers reuse. Waste Manag. 2021, 120, 1–9. [Google Scholar] [CrossRef]
- López, F.A.; Rodríguez, O.; Alguacil, F.J.; García-Díaz, I.; Centeno, T.A.; García-Fierro, J.L.; González, C. Recovery of carbon fibres by the thermolysis and gasification of waste prepreg. J. Anal. Appl. Pyrolysis 2013, 104, 675–683. [Google Scholar] [CrossRef]
- Giorgini, L.; Benelli, T.; Mazzocchetti, L.; Leonardi, C.; Zattini, G.; Minak, G.; Dolcini, E.; Cavazzoni, M.; Montanari, I.; Tosi, C. Recovery of carbon fibers from cured and uncured carbon fiber reinforced composites wastes and their use as feedstock for a new composite production. Polym. Compos. 2015, 36, 1084–1095. [Google Scholar] [CrossRef]
- Park, J.M.; Kwon, D.J.; Wang, Z.J.; Gu, G.Y.; Devries, K.L. Effect of thermal treatment temperatures on the reinforcing and interfacial properties of recycled carbon fiber-phenolic composites. Compos. Part A Appl. Sci. Manuf. 2013, 47, 156–164. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Liu, W.; Wang, J.; Tang, T. Recycling of carbon fibre reinforced epoxy resin composites under various oxygen concentrations in nitrogen–oxygen atmosphere. J. Anal. Appl. Pyrolysis 2015, 112, 253–261. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Miskolczi, N.; Nagy, T.; Lipóczi, G. Recovery of glass fibre and carbon fibres from reinforced thermosets by batch pyrolysis and investigation of fibre re-using as reinforcement in LDPE matrix. Compos. Part B Eng. 2016, 91, 154–161. [Google Scholar] [CrossRef]
- Baek, Y.M.; Shin, P.S.; Kim, J.H.; Park, H.S.; Kwon, D.J.; DeVries, K.L.; Park, J.M. Investigation of interfacial and mechanical properties of various thermally-recycled carbon fibers/recycled PET composites. Fibers Polym. 2018, 19, 1767–1775. [Google Scholar] [CrossRef]
- Li, Z.; Wu, S.; Zhao, Z.; Xu, L. Influence of surface properties on the interfacial adhesion in carbon fiber/epoxy composites. Surf. Interface Anal. 2014, 46, 16–23. [Google Scholar] [CrossRef]
| Carbon Fiber | Tensile Strength (MPa) | Tensile Modulus (GPa) | Elongation at Break (%) | Density (g cm−3) |
|---|---|---|---|---|
| AS4C 3K | 4385 | 231 | 1.8 | 1.78 |
| Component | Viscosity (25 °C) (mPa·s) | Density (g cm−3) | Gel Time (140 °C) (Min) |
|---|---|---|---|
| Araldite® LY556 | 10,000–12,000 | 1.15–1.2 | − |
| Araldite® XB3473 | 95–145 | 0.99–1.02 | − |
| LY556/XB3473 (100/23 wt.%) | 6000 | 1.12–1.16 | 35–43 |
| Sample Name | vCFRP1 | vCFRP2 | rCFRP1 | rCFRP2 |
|---|---|---|---|---|
| Dimensions (cm) | 13 × 20 | 13 × 20 | 13 × 20 | 13 × 20 |
| Fiber content (wt. %) | 65.8 | 67.6 | 67.3 | 65.3 |
| Fiber content (vol. %) | 57.7 | 59.9 | 58.8 | 56.6 |
| Initial Sample | vCFRP1 | vCFRP2 |
|---|---|---|
| Pyrolysis step | P1 | P2 |
| Tank reactor final temperature (°C) | 500 | 500 |
| Tank reactor heating rate (°C min−1) | 3 | 3 |
| Tubular reactor temperature (°C) | 700 | 900 |
| Oxidation step | O1 | O2 |
| Mass of sample after pyrolysis (g) | 77.1 | 79.8 |
| Mass to be burned (g) | 11.3 | 12.2 |
| T (°C) | 500 | 500 |
| t (min) | 120 | 30 |
| Air flowrate (NL min−1) | 1.9 | 4.0 |
| Air excess (%) | 40 | 0 |
| Sample | vCFRP1 | vCFRP2 |
|---|---|---|
| Pyrolysis Step | P1 (500/700) | P2 (500/900) |
| Pyrolysis yields | wt. % | |
| Solid | 72.6 | 75.9 |
| Condensates | 23.4 | 14.2 |
| Collected liquids | 8.9 | 1.7 |
| Other condensates | 14.5 | 12.5 |
| Gases 1 | 4.0 | 9.9 |
| Pyrolysis gas composition | vol. % | |
| H2 | 81.7 | 66.4 |
| CO | 15.5 | 18.0 |
| CO2 | <0.1 | <0.1 |
| CH4 | 2.8 | 15.6 |
| HHV (MJ Nm−3) | 12.4 | 15.5 |
| Pyrolysis liquids’ composition | % area | |
| Water | 2.3 | 81.4 |
| Phenol | 50.3 | 6.8 |
| Phenol derivatives | 21.0 | 2.5 |
| Aromatic hydrocarbons | 16.0 | n.d. 2 |
| Other identified compounds | 5.3 | n.d. 2 |
| Not identified | 5.1 | 9.3 |
| Oxidation step | O1 (120/1.9/40) | O2 (30/4/0) |
| Remaining solid after oxidation (wt. %) 3 | 54.7 | 69.3 |
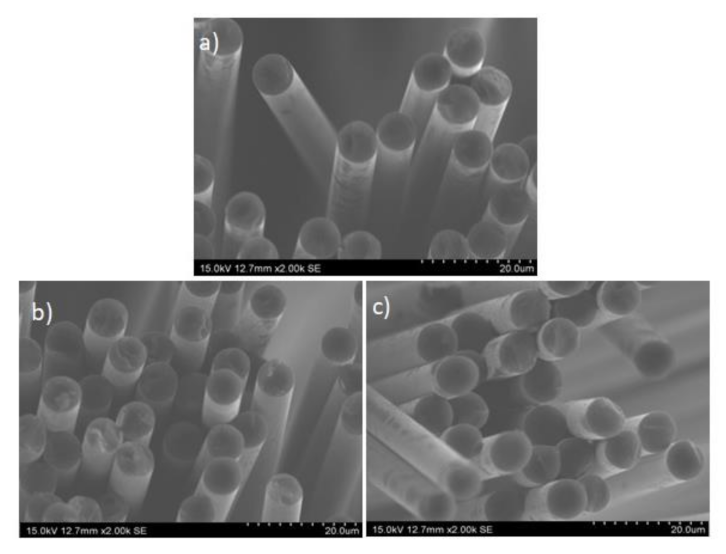
| Sample | Average Cross-Sectional Diameter (µm) |
|---|---|
| vCF | 7.25 ± 0.34 |
| rCF1-down | 7.06 ± 0.14 |
| rCF2-down | 7.15 ± 0.19 |
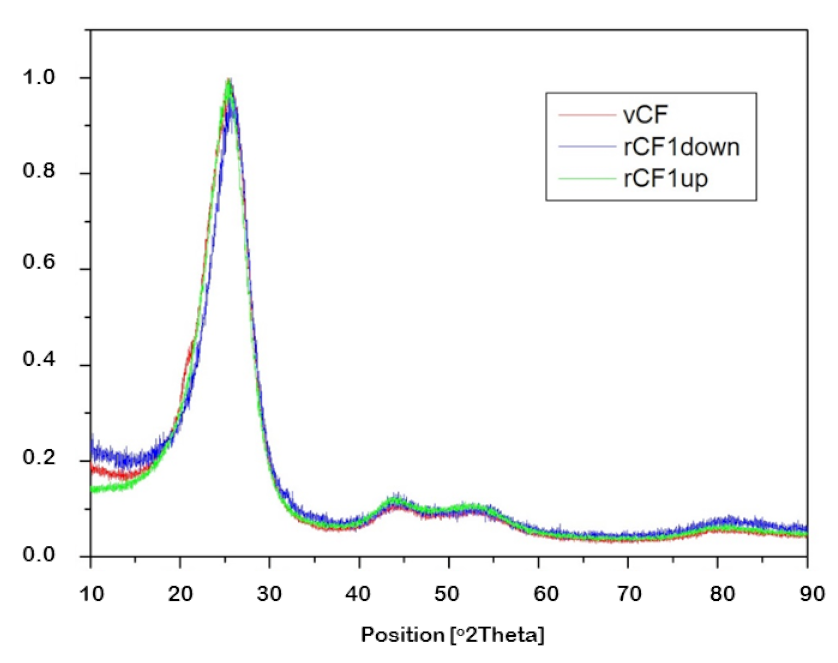
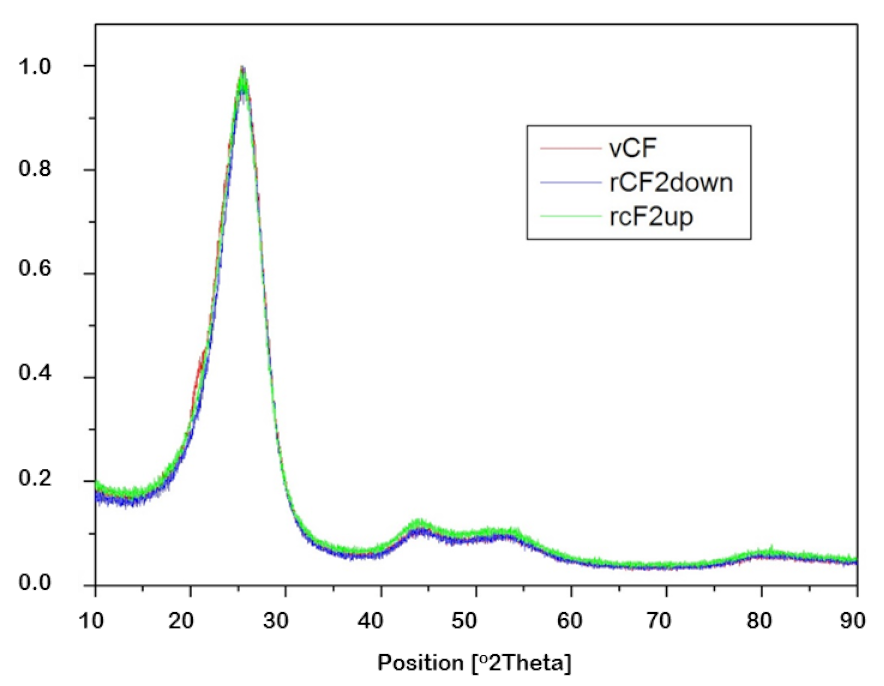
| Sample | FWHM | 2θ |
|---|---|---|
| vCF | 4.6754 | 25.5301 |
| rCF1-down | 4.09411 | 25.9785 |
| rCF1-up | 4.1091 | 25.7940 |
| rCF2-down | 4.3956 | 25.8036 |
| rCF2-up | 4.5776 | 25.5824 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Urionabarrenechea, A.; Gastelu, N.; Jiménez-Suárez, A.; Prolongo, S.G.; Serras-Malillos, A.; Acha, E.; Caballero, B.M. Secondary Raw Materials from Residual Carbon Fiber-Reinforced Composites by An Upgraded Pyrolysis Process. Polymers 2021, 13, 3408. https://doi.org/10.3390/polym13193408
Lopez-Urionabarrenechea A, Gastelu N, Jiménez-Suárez A, Prolongo SG, Serras-Malillos A, Acha E, Caballero BM. Secondary Raw Materials from Residual Carbon Fiber-Reinforced Composites by An Upgraded Pyrolysis Process. Polymers. 2021; 13(19):3408. https://doi.org/10.3390/polym13193408
Chicago/Turabian StyleLopez-Urionabarrenechea, Alexander, Naia Gastelu, Alberto Jiménez-Suárez, Silvia G. Prolongo, Adriana Serras-Malillos, Esther Acha, and Blanca María Caballero. 2021. "Secondary Raw Materials from Residual Carbon Fiber-Reinforced Composites by An Upgraded Pyrolysis Process" Polymers 13, no. 19: 3408. https://doi.org/10.3390/polym13193408
APA StyleLopez-Urionabarrenechea, A., Gastelu, N., Jiménez-Suárez, A., Prolongo, S. G., Serras-Malillos, A., Acha, E., & Caballero, B. M. (2021). Secondary Raw Materials from Residual Carbon Fiber-Reinforced Composites by An Upgraded Pyrolysis Process. Polymers, 13(19), 3408. https://doi.org/10.3390/polym13193408









