The Application of Hollow Carbon Nanofibers Prepared by Electrospinning to Carbon Dioxide Capture
Abstract
:1. Introduction
2. Materials and Methods
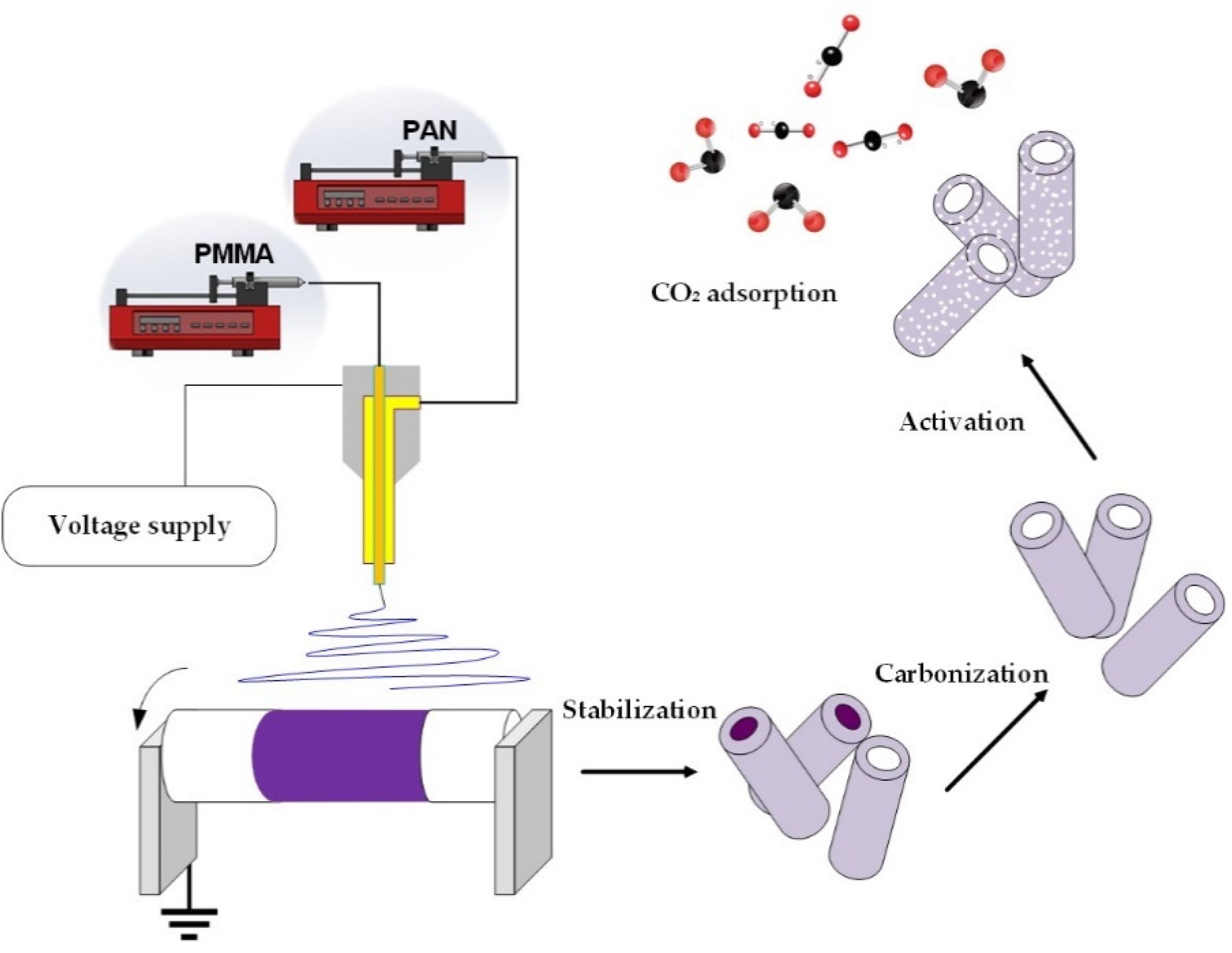
2.1. Fabrication of Hollow Electrospun Nanofibers
2.2. Characterizations
2.3. CO2 Adsorption Experiments
3. Results and Discussion
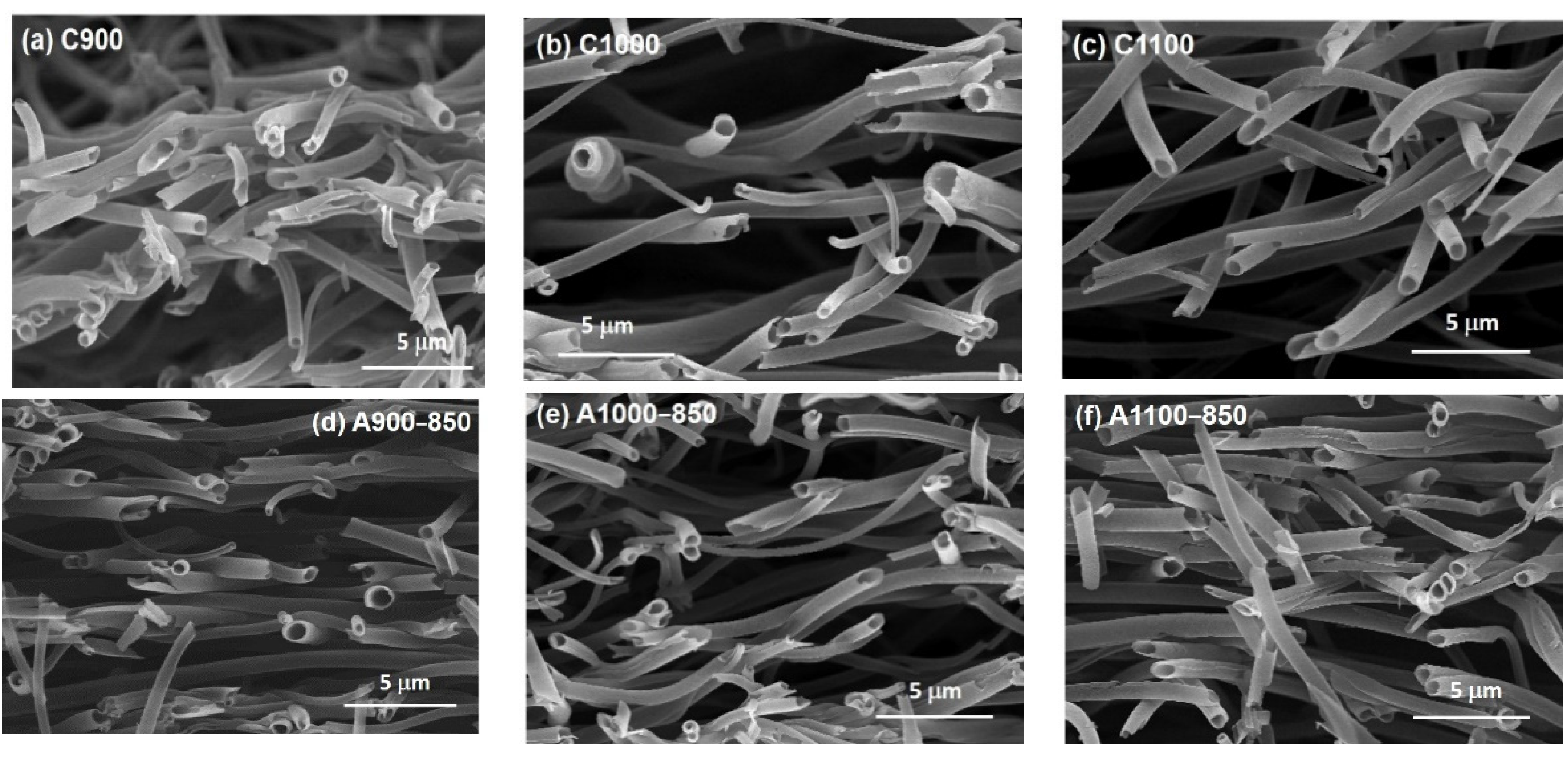
3.1. Field Emission Scanning Electron Microscopy (FESEM) Images
3.2. Elemental Analysis (EA)
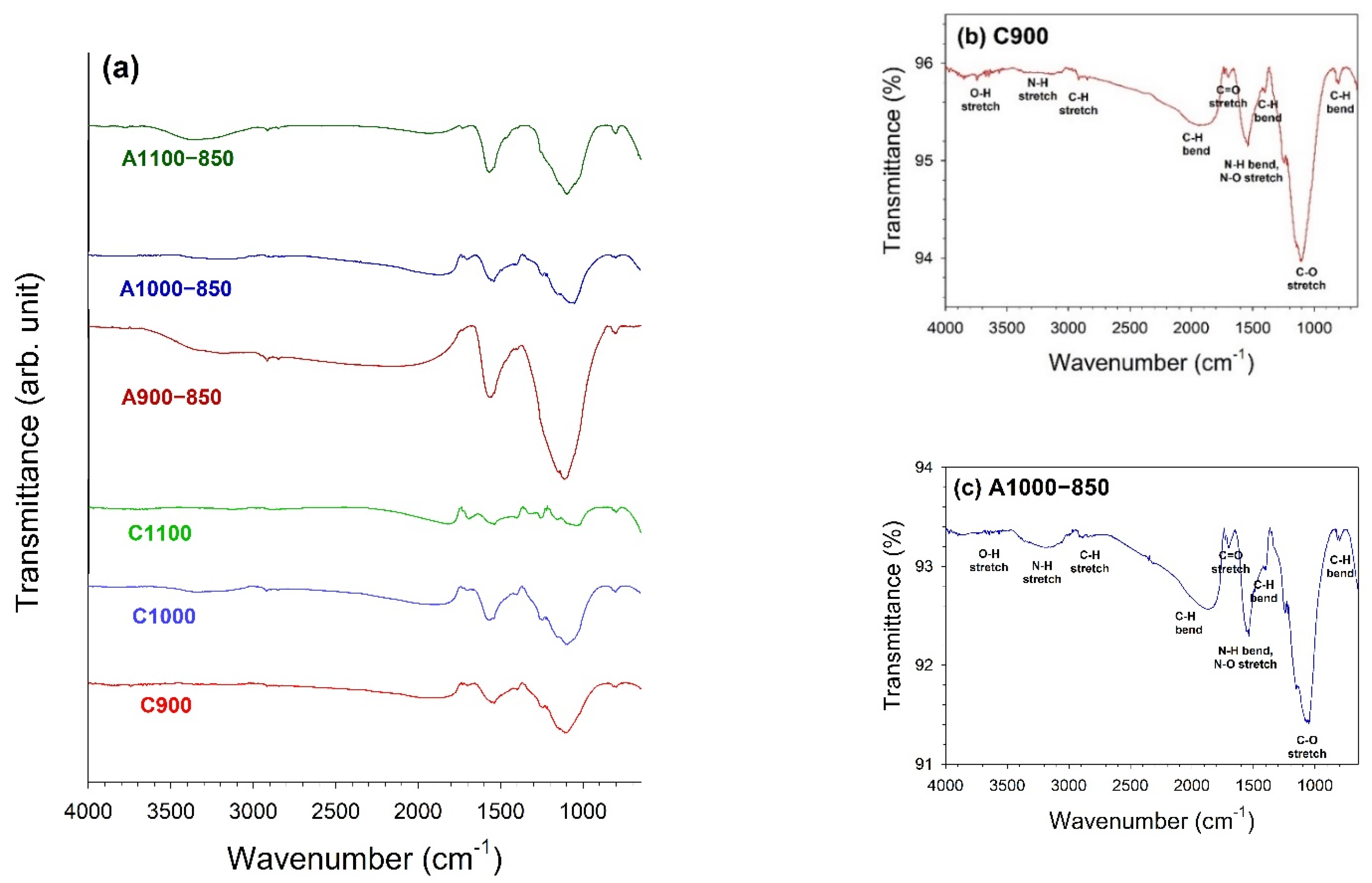
3.3. Fourier Transform Infrared (FTIR) Spectroscopy
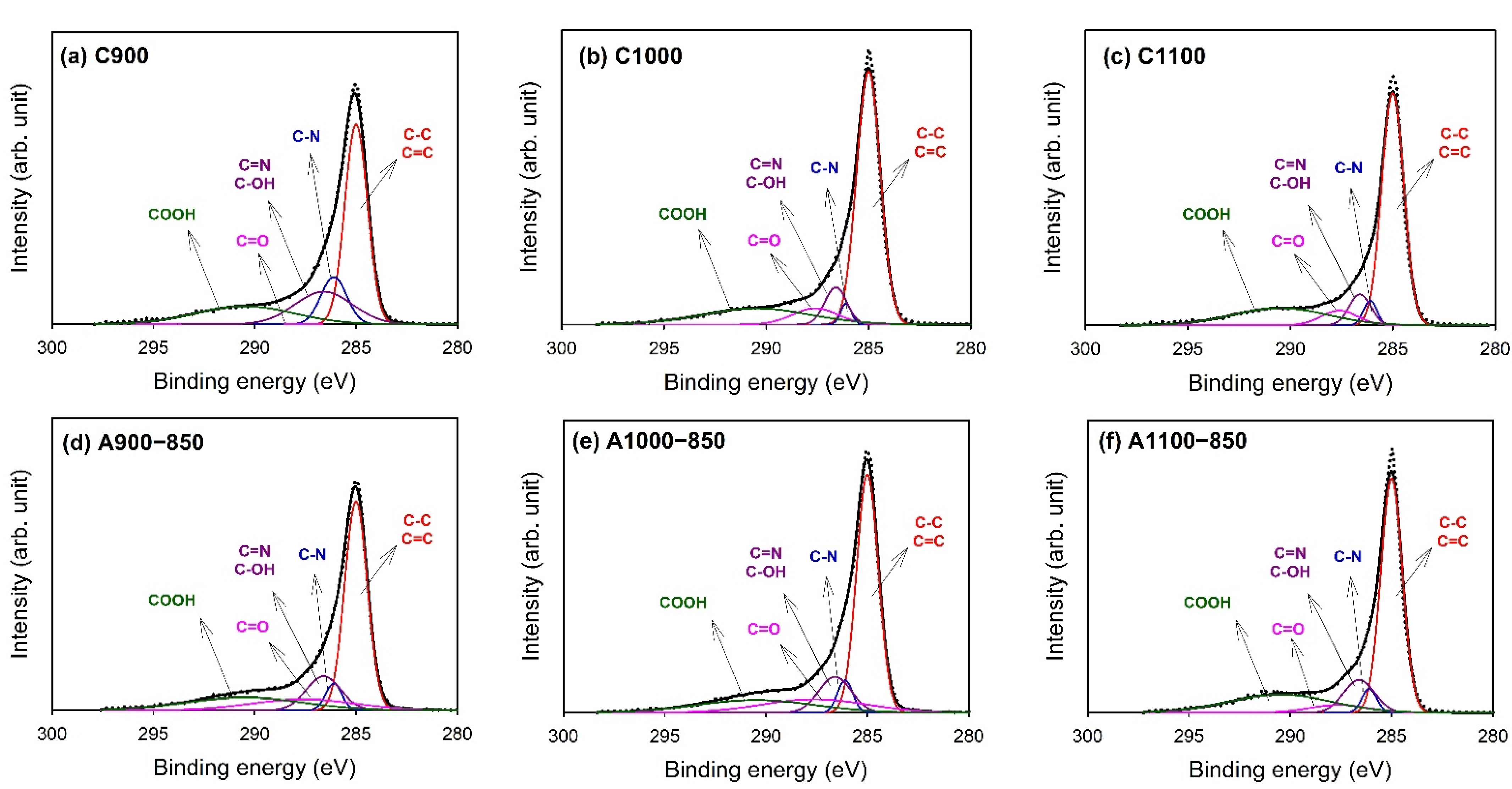
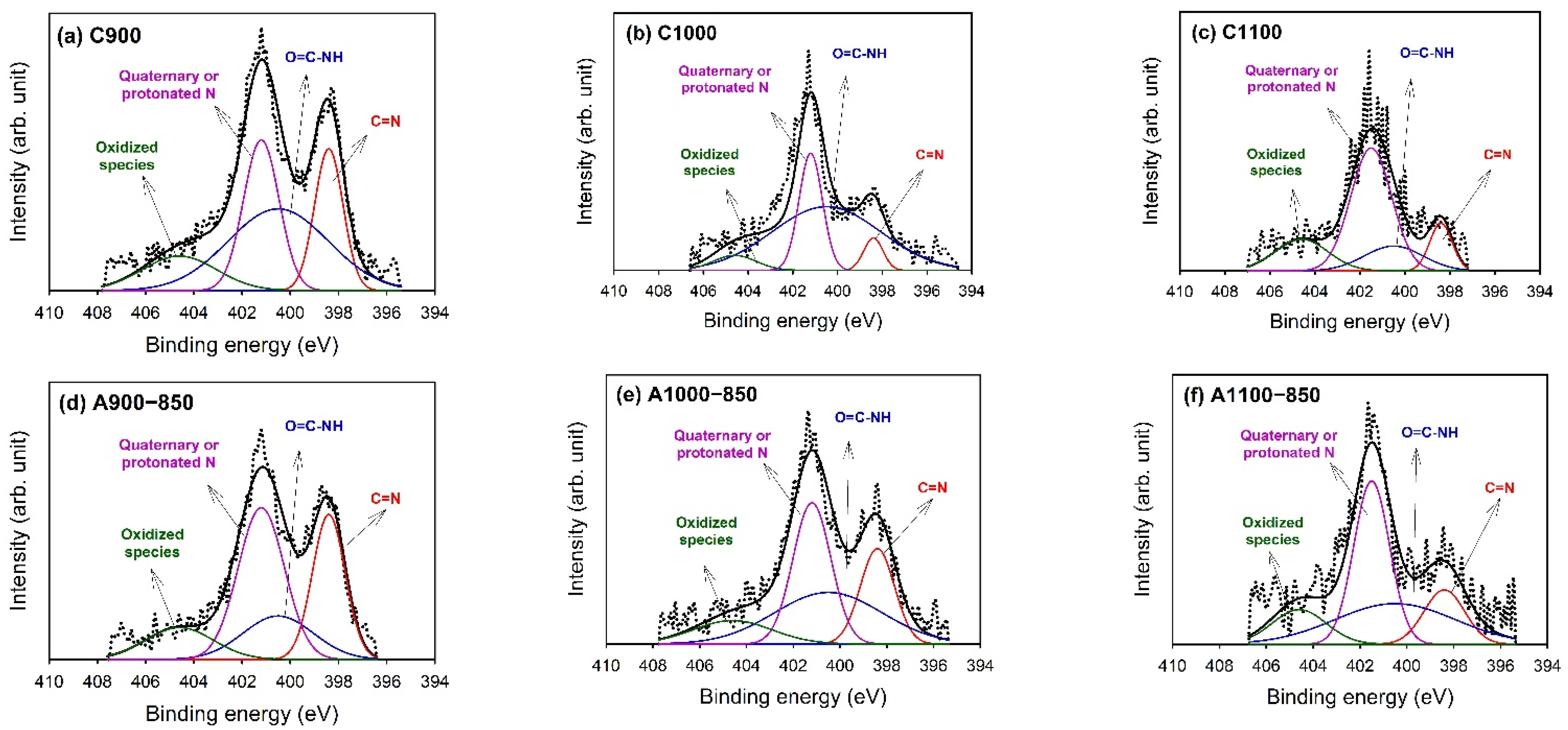
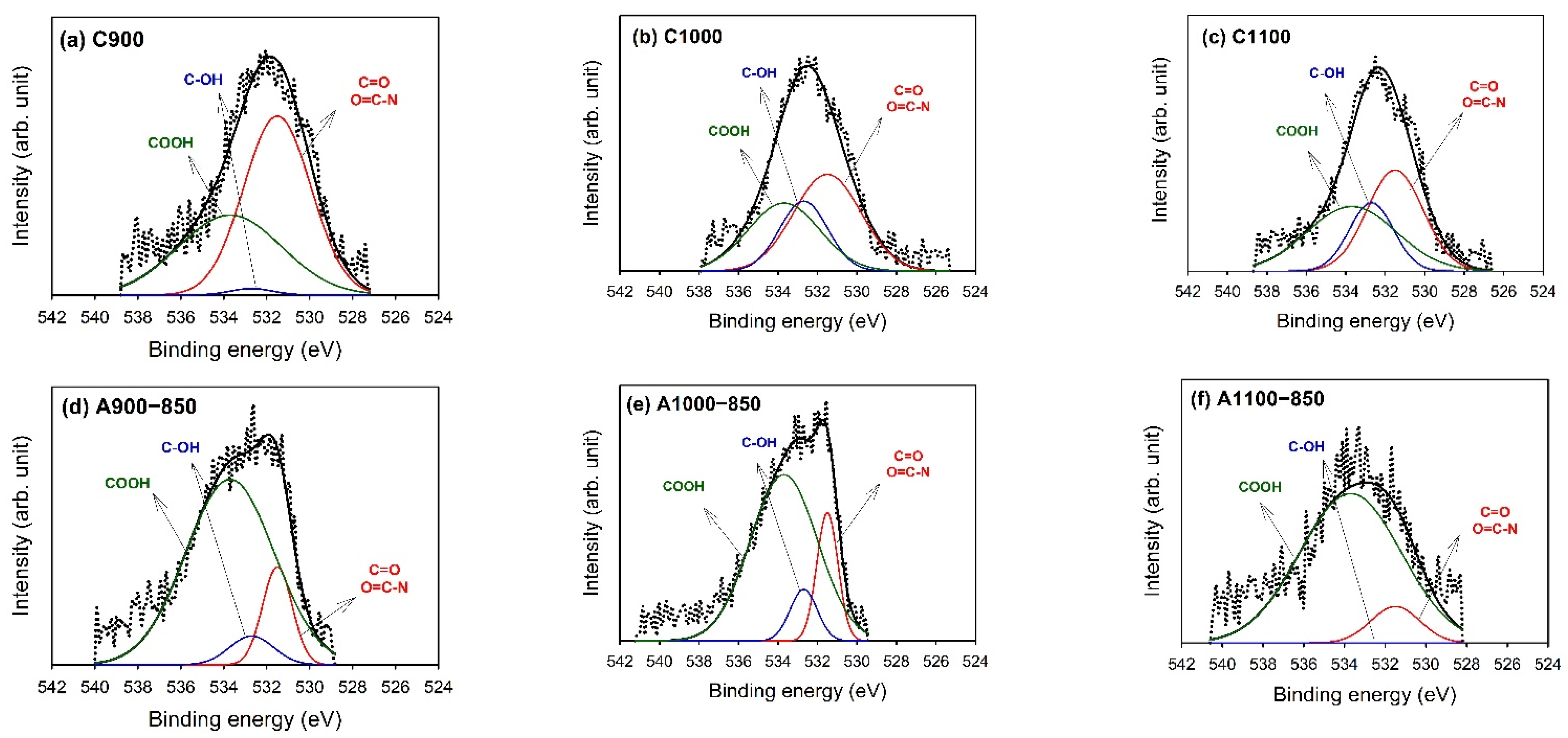
3.4. X-ray Photoelectron Spectroscopy (XPS)
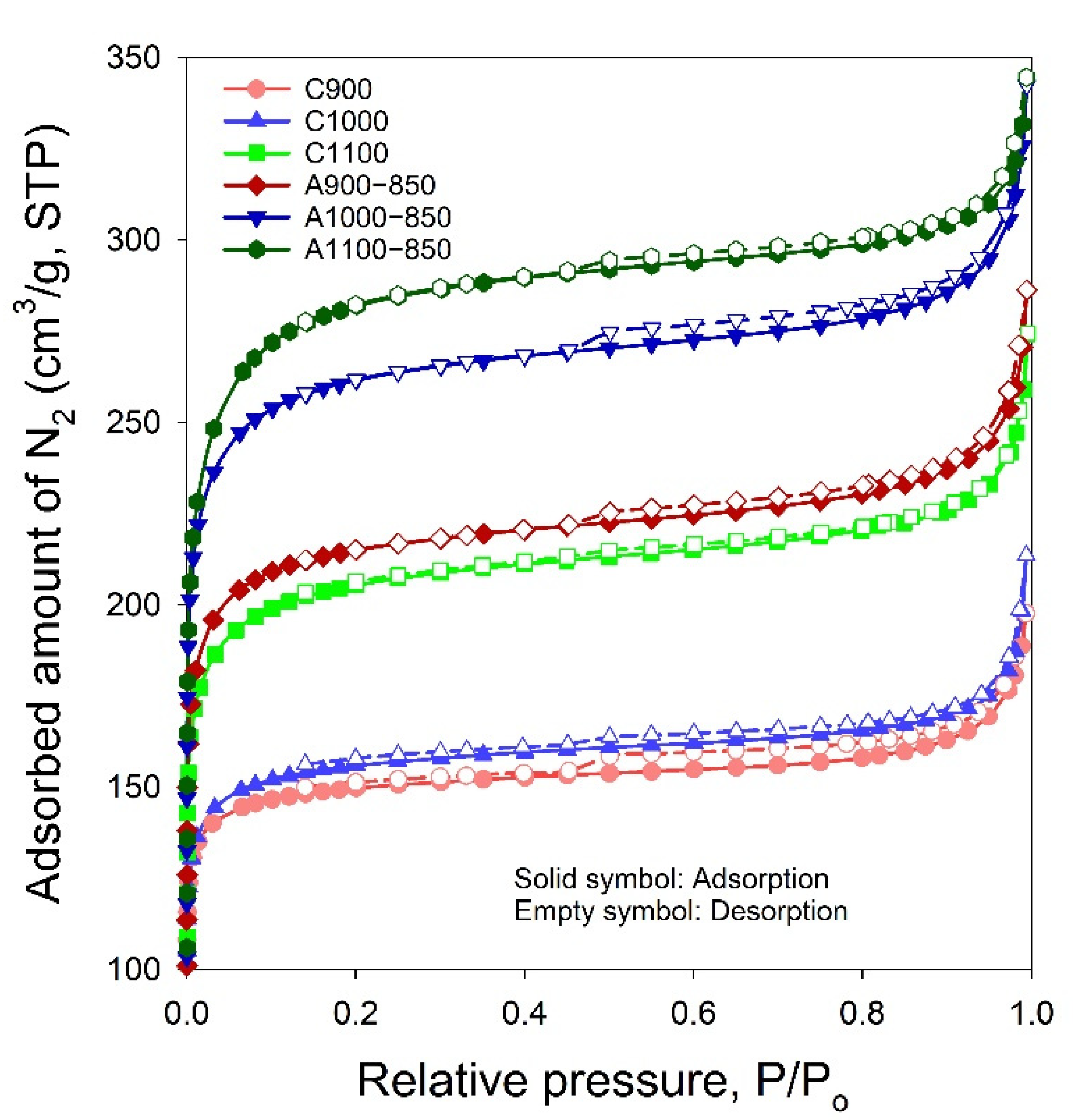
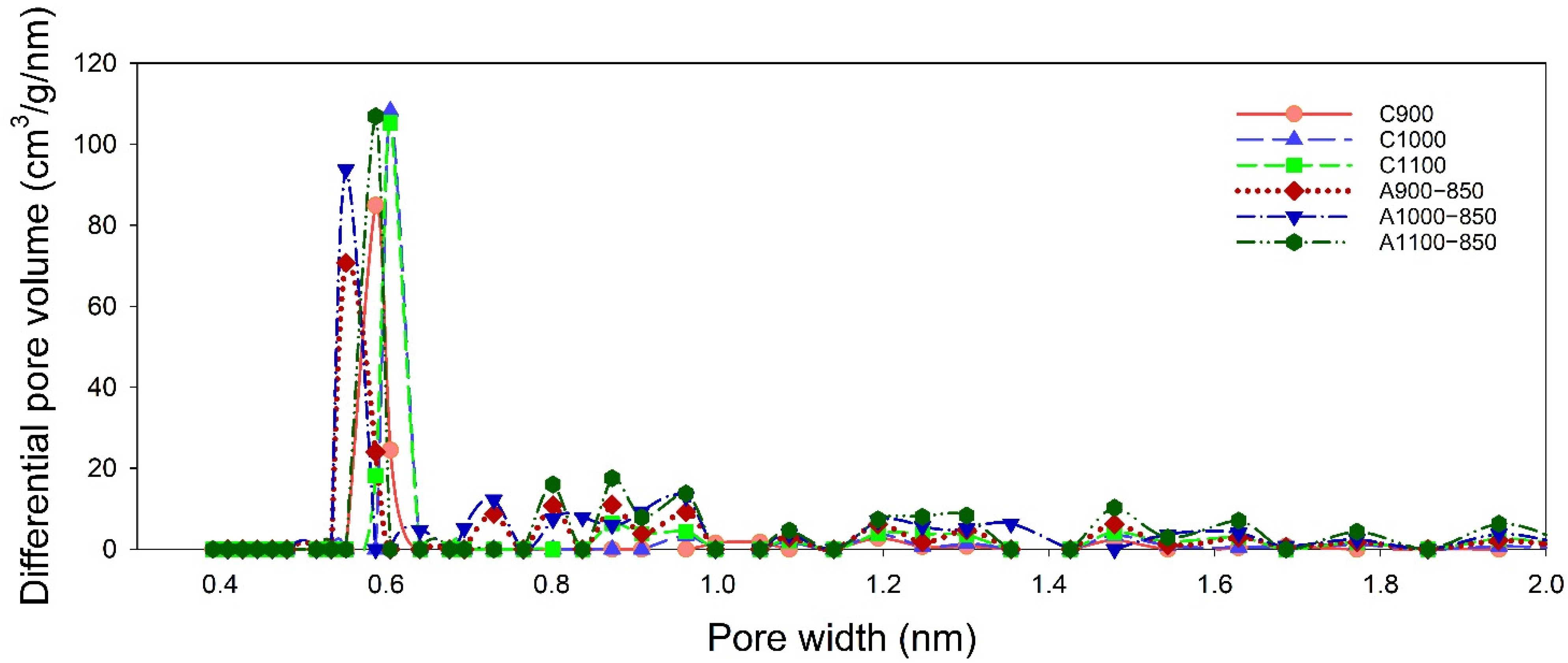
3.5. N2 Adsorption–Desorption Isotherms
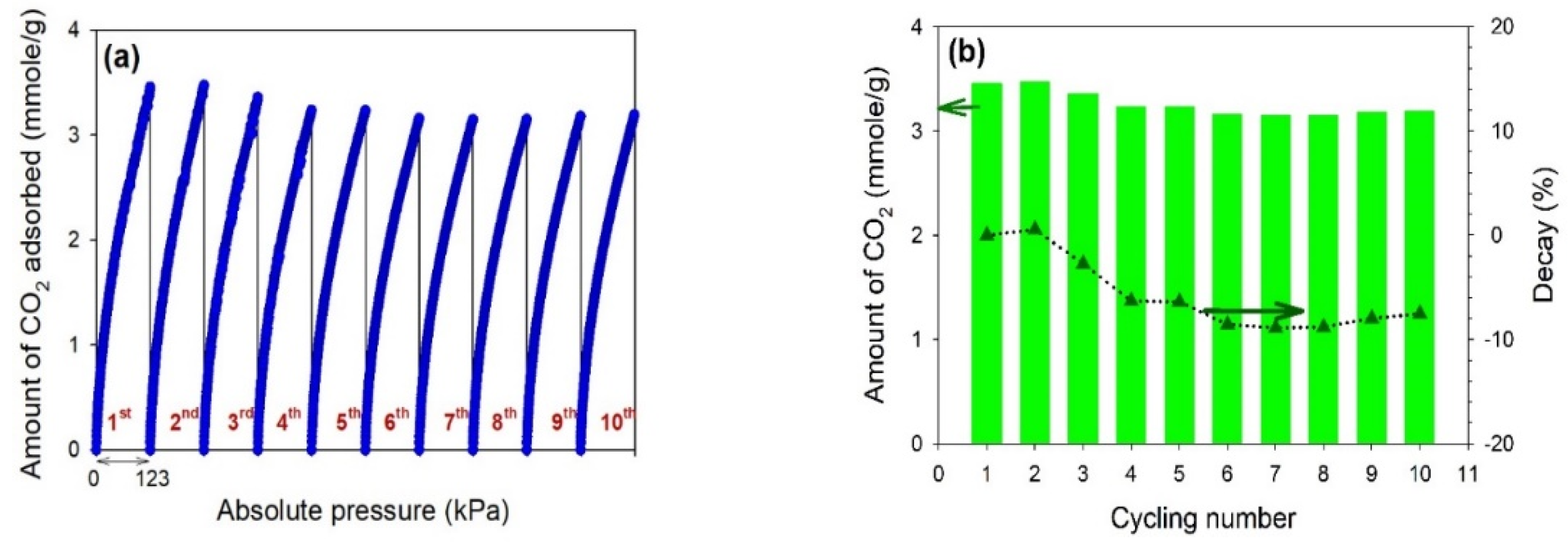
3.6. CO2 Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khandaker, T.; Hossain, M.S.; Dhar, P.K.; Rahman, M.S.; Hossain, M.A.; Ahmed, M.B. Efficacies of carbon-based adsorbents for carbon dioxide capture. Processes 2020, 8, 654. [Google Scholar] [CrossRef]
- Sharma, H.; Dhir, A. Capture of carbon dioxide using solid carbonaceous and non-carbonaceous adsorbents: A review. Environ. Chem. Lett. 2021, 19, 851–873. [Google Scholar] [CrossRef]
- Sharma, A.; Jindal, J.; Mittal, A.; Kumari, K.; Maken, S.; Kumar, N. Carbon materials as CO2 adsorbents: A review. Environ. Chem. Lett. 2021, 19, 875–910. [Google Scholar] [CrossRef]
- de Souza, K.C.; Wickramaratne, N.P.; Ello, A.S.; Costa, M.J.F.; da Costa, C.E.F.; Jaroniec, M. Enhancement of CO2 adsorption on phenolic resin-based mesoporous carbons by KOH activation. Carbon 2013, 65, 334–340. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Importance of small micropores in CO2 capture by phenolic resin-based activated carbon spheres. J. Mater. Chem. A 2013, 1, 112–116. [Google Scholar] [CrossRef]
- Presser, V.; McDonough, J.; Yeon, S.H.; Gogotsi, Y. Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energ. Environ. Sci. 2011, 4, 3059–3066. [Google Scholar] [CrossRef]
- Pevida, C.; Drage, T.C.; Snape, C.E. Silica-templated melamine–formaldehyde resin derived adsorbents for CO2 capture. Carbon 2008, 46, 1464–1474. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Tang, Z.; Zhang, Y. CO2 capture by activated and impregnated anthracites. Fuel Process. Technol. 2005, 86, 1487–1502. [Google Scholar] [CrossRef]
- Ondarçuhu, T.; Joachim, C. Drawing a single nanofiber over hundreds of microns. Eur. Phys. Lett. 1998, 42, 215–220. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Nasouri, K.; Shoushtari, A.M.; Kaflou, A. Investigation of polyacrylonitrile electrospun nanofibres morphology as a function of polymer concentration, viscosity and Berry number. Micro Nano Lett. 2012, 7, 423–426. [Google Scholar] [CrossRef]
- Xing, X.; Wang, Y.; Li, B. Nanofiber drawing and nanodevice assembly in poly(trimethylene terephthalate). Opt. Express 2008, 16, 10815–10822. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Li, J.; Zhang, X. Influence of thermal decomposition on morphologies and magnetic properties of iron nanofibres via electrospinning. Micro Nano Lett. 2011, 6, 967–970. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.H.; Li, H.J.; Zhai, J.; Song, Y.; Jiang, L.; Zhu, D. Super hydrophobic surface of aligned polyacrylonitrile nanoflbers. Angew. Chem. Int. Ed. 2002, 41, 1221–1223. [Google Scholar] [CrossRef]
- Martín, J.; Maiz, J.; Sacristan, J.; Mijangos, C. Tailored polymer-based nanorods and nanotubes by “template synthesis”: From preparation to applications. Polymer 2012, 53, 1149–1166. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.X.; Zhang, R. Synthetic nano-scale fibrous extracellular matrix. J. Biomed. Mater. Res. 1999, 46, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Ding, J.; Qiao, L.; Guo, A.; Dymov, B.P.; Gleeson, J.T.; Hashimoto, T.; Saijo, K. Polystyrene-block-poly(2-cinnamoylethyl methacrylate) nanofibers preparation, characterization, and liquid crystalline properties. Chem. Eur. J. 1999, 5, 2740–2749. [Google Scholar] [CrossRef]
- Wang, J.; Ouyang, Z.; Ren, Z.; Li, J.; Zhang, P.; Wei, G.; Su, Z. Self-assembled peptide nanofibers on graphene oxide as a novel nanohybrid for biomimetic mineralization of hydroxyapatite. Carbon 2015, 89, 20–30. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrostat. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Zhang, L.; Aboagye, A.; Kelkar, A.; Lai, C.; Fong, H. A review: Carbon nanofibers from electrospun polyacrylonitrile and their applications. J. Mater. Sci. 2014, 49, 463–480. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Ognibene, G.; Gangemi, C.M.A.; Spitaleri, L.; Gulino, A.; Purrello, R.; Cicala, G.; Fragalà, M.E. Role of the surface composition of the polyethersulfone–TiiP–H2T4 fibers on lead removal: From electrostatic to coordinative binding. J. Mater. Sci. 2019, 54, 8023–8033. [Google Scholar] [CrossRef]
- Gangemi, C.M.A.; Iudici, M.; Spitaleri, L.; Randazzo, R.; Gaeta, M.; D’Urso, A.; Gulino, A.; Purrello, R.; Fragalà, M.E. Polyethersulfone mats functionalized with porphyrin for removal of para-nitroaniline from aqueous solution. Molecules 2019, 24, 3344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, H.; Lin, T. Fiber generators in needleless electrospinning. J. Nanomater. 2012, 725950. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef] [Green Version]
- Ahmadian, A.; Shafiee, A.; Aliahmad, N.; Agarwal, M. Overview of nano-fiber mats fabrication via electrospinning and morphology analysis. Textiles 2021, 1, 206–226. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Koski, A.; Yim, K.; Shivkumar, S. Effect of molecular weight on fibrous PVA produced by electrospinning. Mater. Lett. 2004, 58, 493–497. [Google Scholar] [CrossRef]
- Gupta, P.; Elkins, C.; Long, T.E.; Wilkes, G.L. Electrospinning of linear homopolymers of poly(methyl methacrylate): Exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent. Polymer 2005, 46, 4799–4810. [Google Scholar] [CrossRef]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer–polymer interaction limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Hsu, C.M.; Shivkumar, S. Nano-sized beads and porous fiber constructs of poly(1‘-caprolactone) produced by electrospinning. J. Mater. Sci. 2004, 39, 3003–3013. [Google Scholar] [CrossRef]
- Sun, Z.; Zussman, E.; Yarin, A.L.; Wendorff, J.H.; Greiner, A. Compound core-shell polymer nanofibers by co-electrospinning. Adv. Mater. 2003, 15, 1929–1932. [Google Scholar] [CrossRef]
- Lin, T.; Wang, H.; Wang, X. Self-crimping bicomponent nanofibers electrospun from polyacrylonitrile and elastomeric polyurethane. Adv. Mater. 2005, 17, 2699–2703. [Google Scholar] [CrossRef] [Green Version]
- Gbewonyo, S.; Carpenter, A.W.; Gause, C.B.; Mucha, N.R.; Zhang, L. Low thermal conductivity carbon fibrous composite nanomaterial enabled by multi-scale porous structure. Mater. Des. 2017, 134, 218–225. [Google Scholar] [CrossRef]
- Zussman, E.; Yarin, A.L.; Bazilevsky, A.V.; Avrahami, R.; Feldman, M. Electrospun polyacrylonitrile/poly(methyl methacrylate)-derived turbostratic carbon micro-/nanotubes. Adv. Mater. 2006, 18, 348–353. [Google Scholar] [CrossRef]
- Xie, W.; Khan, S.; Rojas, O.J.; Parsons, G.N. Control of micro- and mesopores in carbon nanofibers and hollow carbon nanofibers derived from cellulose diacetate via vapor phase infiltration of diethyl zinc. ACS Sustain. Chem. Eng. 2018, 6, 13844–13853. [Google Scholar] [CrossRef]
- Zhang, L.; Hsieh, Y.L. Carbon nanofibers with nanoporosity and hollow channels from binary polyacrylonitrile systems. Eur. Polym. J. 2009, 45, 47–56. [Google Scholar] [CrossRef]
- Kaerkitcha, N.; Chuangchote, S.; Sagawa, T. Control of physical properties of carbon nanofibers obtained from coaxial electrospinning of PMMA and PAN with adjustable inner/outer nozzle-ends. Nanoscale Res. Lett. 2016, 11, 186. [Google Scholar] [CrossRef] [Green Version]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Wei, S.; Guo, Z. Coaxial electrospun nanostructures and their applications. J. Mater. Chem. A 2013, 1, 11513–11528. [Google Scholar] [CrossRef]
- El-Deen, A.G.; Barakat, N.A.M.; Khalild, K.A.; Kim, H.Y. Hollow carbon nanofibers as an effective electrode for brackish water desalination using the capacitive deionization process. New J. Chem. 2014, 38, 198–205. [Google Scholar] [CrossRef]
- Park, S.H.; Jung, H.R.; Lee, W.J. Hollow activated carbon nanofibers prepared by electrospinning as counter electrodes for dye-sensitized solar cells. Electrochim. Acta 2013, 102, 423–428. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, M.; Li, X.; Liu, Y.; Pan, H. Preparation of mesohollow and microporous carbon nanofiber and its application in cathode material for lithium–sulfur batteries. J. Alloys Compd. 2014, 608, 220–228. [Google Scholar] [CrossRef]
- Peng, Y.T.; Lo, C.T. Electrospun porous carbon nanofibers as lithium ion battery anodes. J. Solid State Electrochem. 2015, 19, 3401–3410. [Google Scholar] [CrossRef]
- Li, L.; Peng, S.; Lee, J.K.Y.; Ji, D.; Srinivasan, M.; Ramakrishna, S. Electrospun hollow nanofibers for advanced secondary batteries. Nano Energy 2017, 39, 111–139. [Google Scholar] [CrossRef]
- Zhang, T.; Qu, H.; Sun, K. Development of polydopamine coated electrospun PAN/PMMA nanofibrous membrane as composite separator for Lithium-ion batteries. Mater. Lett. 2019, 245, 10–13. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, J.; Zhang, L.; Qiu, Y. Recent advances in energy materials by electrospinning. Renew. Sustain. Energy Rev. 2018, 81, 1825–1858. [Google Scholar] [CrossRef]
- Zhao, W.; Yamamoto, Y.; Tagawa, S. Regulation of the thermal reactions of polyacrylonitrile by c-irradiation. Chem. Mater. 1999, 11, 1030–1034. [Google Scholar] [CrossRef]
- Qin, X.H. Structure and property of electrospinning PAN nanofibers by different preoxidation temperature. J. Therm. Anal. Calorim. 2010, 99, 571–575. [Google Scholar] [CrossRef]
- Othman, F.E.C.; Yusof, N.; Samitsu, S.; Abdullah, N.; Hamid, M.F.; Nagai, K.; Abidin, M.N.Z.; Azali, M.A.; Ismail, A.F.; Jaafar, J.; et al. Activated carbon nanofibers incorporated metal oxides for CO2 adsorption: Effects of different type of metal oxides. J. CO2 Util. 2021, 45, 101434. [Google Scholar] [CrossRef]
- Ji, M.; Wang, C.; Bai, Y.; Yu, M.; Wang, Y. Structural evolution of polyacrylonitrile precursor fibers during preoxidation and carbonization. Polym. Bull. 2007, 59, 527–536. [Google Scholar] [CrossRef]
- Suzuki, M. Adsorption Engineering; Elsevier Science: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Lee, J.S.; Kim, J.H.; Kim, J.T.; Suh, J.K.; Lee, J.M.; Lee, C.H. Adsorption equilibria of CO2 on zeolite 13X and zeolite X/activated carbon composite. J. Chem. Eng. Data 2002, 47, 1237–1242. [Google Scholar] [CrossRef]
- Lee, B.S.; Park, K.M.; Yu, W.R.; Youk, J.H. An effective method for manufacturing hollow carbon nanofibers and microstructural analysis. Macromol. Res. 2012, 20, 605–613. [Google Scholar] [CrossRef]
- Peterson, J.D.; Vyazovkin, S.; Wight, C.A. Kinetic study of stabilizing effect of oxygen on thermal degradation of poly(methyl methacrylate). J. Phys. Chem. B 1999, 103, 8087. [Google Scholar] [CrossRef]
- Wangxi, Z.; Jie, L.; Gang, W. Evolution of structure and properties of PAN precursors during their conversion to carbon fibers. Carbon 2003, 41, 2805–2812. [Google Scholar] [CrossRef]
- Yoon, C.M.; Long, D.; Jang, S.M.; Qiao, W.; Ling, L.; Miyawaki, J.; Rhee, C.K.; Mochida, I.; Yoon, S.H. Electrochemical surface oxidation of carbon nanofibers. Carbon 2011, 49, 96–105. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Sundaram, M.M.; Henry, D.J.; Gao, X. Alginate biopolymer effect on the electrodeposition of manganese dioxide on electrodes for supercapacitors. ACS Appl. Energy Mater. 2021, 4, 7040–7051. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Shalagina, A.E.; Podyacheva, O.Y.; Ischenko, A.V.; Kibis, L.S.; Boronin, A.I.; Chesalov, Y.A.; Kochubey, D.I.; Romanenko, A.I.; Anikeeva, O.B.; et al. Structure and electrical conductivity of nitrogen-doped carbon nanofibers. Carbon 2009, 47, 1922–1929. [Google Scholar] [CrossRef]
- Sundaram, M.M.; Appadoo, D. Traditional salt-in-water electrolyte vs. water-in-salt electrolyte with binary metal oxide for symmetric supercapacitors: Capacitive vs. faradaic. Dalton Trans. 2020, 49, 11743–11755. [Google Scholar] [CrossRef]
- Tuccitto, N.; Riela, L.; Zammataro, A.; Spitaleri, L.; Li-Destri, G.; Sfuncia, G.; Nicotra, G.; Pappalardo, A.; Capizzi, G.; Sfrazzetto, G.T. Functionalized carbon nanoparticle-based sensors for chemical warfare agents. ACS Appl. Nano Mater. 2020, 3, 8182–8191. [Google Scholar] [CrossRef]
- Giofrè, S.V.; Tiecco, M.; Celesti, C.; Patanè, S.; Triolo, C.; Gulino, A.; Spitaleri, L.; Scalese, S.; Scuderi, M.; Iannazzo, D. Eco-friendly 1,3-dipolar cycloaddition reactions on graphene quantum dots in natural deep eutectic solvent. Nanomaterials 2020, 10, 2549. [Google Scholar] [CrossRef]
- Graf, N.; Yegen, E.; Gross, T.; Lippitz, A.; Weigel, W.; Krakert, S.; Terfort, A.; Unger, W.E.S. XPS and NEXAFS studies of aliphatic and aromatic amine species on functionalized surfaces. Surf. Sci. 2009, 603, 2849–2860. [Google Scholar] [CrossRef]
- Pamula, E.; Rouxhet, P.G. Bulk and surface chemical functionalities of Type III PAN-based carbon fibers. Carbon 2003, 41, 1905–1915. [Google Scholar] [CrossRef]
- Gulino, A. Structural and electronic characterization of self-assembled molecular nanoarchitectures by X-ray photoelectron spectroscopy. Anal. Bioanal. Chem. 2013, 405, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Contino, A.; Maccarrone, G.; Fragalà, M.E.; Spitaleri, L.; Gulino, A. Conjugated gold–porphyrin monolayers assembled on inorganic surfaces. Chem. Eur. J. 2017, 23, 14937–14943. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Minakshi, M.; Whale, J.; Jean-Fulcrand, A.; Garnweitner, G. Effect of the anionic counterpart: Molybdate vs. tungstate in energy storage for pseudo-capacitor applications. Nanomaterials 2021, 11, 580. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Falco, C.; Titirici, M.M.; Fuertes, A.B. High-performance CO2 sorbents from algae. RSC Adv. 2012, 2, 12792–12797. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Zhang, L.; Zhang, G.; Shu, Z.; Shi, J. Chitosan derived nitrogen-doped microporous carbons for high performance CO2 capture. Carbon 2013, 61, 423–430. [Google Scholar] [CrossRef]
- Hao, G.P.; Li, W.C.; Qian, D.; Lu, A.H. Rapid synthesis of nitrogen-doped porous carbon monolith for CO2 capture. Adv. Mater. 2010, 22, 853–857. [Google Scholar] [CrossRef]
- Toth, J. Isotherm equations for monolayer adsorption of gases on heterogeneous solid surfaces. In Fundamentals of Adsorption; Myers, A., Belfort, G., Eds.; Engineering Foundation: New York, NY, USA, 1984; pp. 657–665. [Google Scholar]
- To, J.W.F.; He, J.; Mei, J.; Haghpanah, R.; Chen, Z.; Kurosawa, T. Hierarchical N-doped carbon as CO2 adsorbent with high CO2 selectivity from rationally designed polypyrrole precursor. J. Am. Chem. Soc. 2016, 38, 1001–1009. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Huang, C.C.; Chin, W.T. Carbon dioxide adsorption on carbon nanofibers with different porous structures. Appl. Sci. 2021, 11, 7724. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Lee, S.T.; Leo, Y.J.; Tseng, T.L. Importance of pore structure and surface chemistry in carbon dioxide adsorption on electrospun carbon nanofiber. Sens. Mater. 2020, 32, 2277–2288. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Wu, C.Y.; Chen, Y.J. Effects of activation on the properties of electrospun carbon nanofibers and their adsorption performance for carbon dioxide. Sep. Purif. Technol. 2020, 233, 116040. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, X.F.; Li, L.; Jin, L.; Zhang, Y.G.; Song, S.L.; Liu, R.P. Nitrogen-enriched porous carbon fiber as CO2 adsorbent with superior CO2 selectivity by air activation. Energy Fuels 2019, 33, 12558–12567. [Google Scholar] [CrossRef]
- Heo, Y.J.; Zhang, Y.; Rhee, K.Y.; Park, S.J. Synthesis of PAN/PVDF nanofiber composites-based carbon adsorbents for CO2 capture. Compos. B 2019, 156, 95–99. [Google Scholar] [CrossRef]
- Jeong, D.; Jie, W.; Adelodun, A.A.; Kim, S.; Jo, Y. Electrospun melamine-blended activated carbon nanofibers for enhanced control of indoor CO2. J. Appl. Polym. Sci. 2019, 136, 47747. [Google Scholar] [CrossRef]

| Sample | Elemental Analysis (wt.%) | XPS Analysis (at.%) | |||||
|---|---|---|---|---|---|---|---|
| C | H | N | Others | C 1 s | N 1 s | O 1 s | |
| C900 | 73.4 | 2.2 | 8.0 | 16.5 | 89.6 | 7.2 | 3.3 |
| C1000 | 78.4 | 2.2 | 3.6 | 15.8 | 93.2 | 3.3 | 3.6 |
| C1100 | 65.8 | 3.6 | 1.5 | 29.1 | 95.3 | 1.7 | 3.0 |
| A900−850 | 68.6 | 2.8 | 6.8 | 21.8 | 91.4 | 5.4 | 3.2 |
| A1000−850 | 64.6 | 3.5 | 3.6 | 28.2 | 92.6 | 3.1 | 4.3 |
| A1100−850 | 70.4 | 2.9 | 2.8 | 23.9 | 96.6 | 2.0 | 1.4 |
| Binding Energy (eV) | Type | C900 | C1000 | C1100 | A900−850 | A1000−850 | A1100−850 |
|---|---|---|---|---|---|---|---|
| 285 | C-C/C=C | 43.2 | 58.0 | 58.8 | 48.2 | 49.4 | 56.0 |
| (48) | (62) | (62) | (53) | (53) | (58) | ||
| 286.1 | C–N | 11.6 | 2.2 | 3.8 | 4.4 | 4.6 | 3.8 |
| (13) | (2) | (4) | (5) | (5) | (4) | ||
| 286.6 | C=N/C–OH | 17.9 | 8.0 | 7.7 | 12.0 | 11.0 | 11.2 |
| (20) | (9) | (8) | (13) | (12) | (12) | ||
| 287.6 | C=O | − | 7.4 | 6.2 | 11.7 | 13.4 | 5.0 |
| (0) | (8) | (6) | (13) | (14) | (5) | ||
| 290.5 | -COOH | 16.9 | 17.6 | 18.9 | 15.2 | 14.2 | 20.6 |
| (19) | (19) | (20) | (17) | (15) | (21) |
| Binding Energy (eV) | Type | C900 | C1000 | C1100 | A900−850 | A1000−850 | A1100−850 |
|---|---|---|---|---|---|---|---|
| 398.4 | N=C | 1.4 | 0.2 | 0.2 | 1.5 | 0.6 | 0.3 |
| (20) | (6) | (12) | (28) | (20) | (15) | ||
| 400.5 | O=C–NH | 2.9 | 2.1 | 0.3 | 1.0 | 1.1 | 0.7 |
| (41) | (63) | (16) | (18) | (35) | (33) | ||
| 401.2 | Quaternary or protonated N | 1.91 | 0.83 | 0.93 | 2.24 | 1.04 | 0.79 |
| (27) | (25) | (55) | (41) | (33) | (39) | ||
| 404.6 | Oxidized species | 0.9 | 0.2 | 0.3 | 0.7 | 0.4 | 0.3 |
| (13) | (6) | (18) | (13) | (12) | (13) |
| Binding Energy (eV) | Type | C900 | C1000 | C1100 | A900−850 | A1000−850 | A1100−850 |
|---|---|---|---|---|---|---|---|
| 531.5 | C=O/O=C–N | 1.3 | 1.6 | 1.2 | 0.5 | 0.8 | 0.1 |
| (38) | (44) | (39) | (14) | (18) | (11) | ||
| 532.7 | C–OH | 0.3 | 0.8 | 0.6 | 0.2 | 0.4 | − |
| (9) | (23) | (21) | (6) | (9) | (0) | ||
| 533.7 | COOH | 1.7 | 1.2 | 1.2 | 2.5 | 3.1 | 1.3 |
| (53) | (33) | (40) | (80) | (73) | (89) |
| Sample | SSA α (m2/g) | Smi β (m2/g) | Vt γ (cm3/g) | Vma φ (cm3/g) | Vme η (cm3/g) | Vmi η (cm3/g) | V< 0.7 nm η (cm3/g) | dHK ξ (nm) | Smi/SSA | Vmi/Vt | V<0.7 nm/Vt | V<0.7 nm/Vmi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C900 | 502 | 434 | 0.3057 | 0.0192 | 0.0497 | 0.2368 | 0.2170 | 1.35 | 0.86 | 0.77 | 0.71 | 0.92 |
| C1000 | 524 | 441 | 0.3303 | 0.0379 | 0.0518 | 0.2406 | 0.2059 | 1.43 | 0.84 | 0.73 | 0.62 | 0.86 |
| C1100 | 696 | 558 | 0.4244 | 0.0458 | 0.0631 | 0.3155 | 0.2360 | 1.50 | 0.80 | 0.74 | 0.56 | 0.75 |
| A900−850 | 725 | 597 | 0.4429 | 0.0360 | 0.0695 | 0.3374 | 0.1979 | 1.49 | 0.82 | 0.76 | 0.45 | 0.59 |
| A1000−850 | 883 | 714 | 0.5297 | 0.0428 | 0.0783 | 0.4086 | 0.2185 | 1.55 | 0.81 | 0.77 | 0.41 | 0.53 |
| A1100−850 | 954 | 734 | 0.5331 | 0.0428 | 0.0563 | 0.4340 | 0.2148 | 1.62 | 0.77 | 0.81 | 0.40 | 0.49 |
| Sample | Temperature (°C) | KF (mmol/g/kPa1/n) | n | R2 |
|---|---|---|---|---|
| A900−850 | 25 | 0.1988 | 1.69 | 0.9994 |
| 40 | 0.1186 | 1.55 | 0.9997 | |
| 55 | 0.0700 | 1.43 | 0.9999 | |
| A1000−850 | 25 | 0.1333 | 1.49 | 0.9997 |
| 40 | 0.0791 | 1.38 | 0.9998 | |
| 55 | 0.0414 | 1.24 | 0.9999 | |
| A1100−850 | 25 | 0.1166 | 1.61 | 0.9998 |
| 40 | 0.0666 | 1.45 | 0.9998 | |
| 55 | 0.0385 | 1.31 | 0.9999 |
| Adsorbent | Precursor | SBET (m2/g) | Conc. of CO2 | Temp. (°C) | CO2 Uptake (mmol/g) | Reference |
|---|---|---|---|---|---|---|
| Hollow ACNF | PAN | 725 | 1 atm 0.15 atm | 25 | 3.03 0.99 | This study |
| Hollow ACNF | PAN | 884 | 1 atm 0.15 atm | 25 | 3.16 0.94 | [73] |
| ACNF | PAN | 362 | 1 bar | 25 | 2.37 | [50] |
| ACNF | PAN | 872 | 1 atm 0.15 atm | 25 | 3.47 1.01 | [74] |
| ACNF | PAN | 897 | 1 atm 0.15 atm | 25 | 3.17 1.00 | [75] |
| ACNF | PAN | 486 | 1 bar 0.15 bar | 25 | 2.25 1.09 | [76] |
| ACNF | PAN, polyvinylidene fluoride | 925 | 1 bar | 25 | 2.21 | [77] |
| ACNF | PAN | 412 | 1 bar | 25 | 0.92 | [78] |
| ACNF | PAN Melamine | 547 | 1 bar | 25 | 1.44 | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, Y.-C.; Chin, W.-T.; Huang, C.-C. The Application of Hollow Carbon Nanofibers Prepared by Electrospinning to Carbon Dioxide Capture. Polymers 2021, 13, 3275. https://doi.org/10.3390/polym13193275
Chiang Y-C, Chin W-T, Huang C-C. The Application of Hollow Carbon Nanofibers Prepared by Electrospinning to Carbon Dioxide Capture. Polymers. 2021; 13(19):3275. https://doi.org/10.3390/polym13193275
Chicago/Turabian StyleChiang, Yu-Chun, Wei-Ting Chin, and Chih-Cheng Huang. 2021. "The Application of Hollow Carbon Nanofibers Prepared by Electrospinning to Carbon Dioxide Capture" Polymers 13, no. 19: 3275. https://doi.org/10.3390/polym13193275
APA StyleChiang, Y.-C., Chin, W.-T., & Huang, C.-C. (2021). The Application of Hollow Carbon Nanofibers Prepared by Electrospinning to Carbon Dioxide Capture. Polymers, 13(19), 3275. https://doi.org/10.3390/polym13193275





