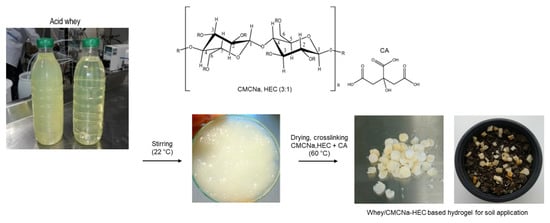
Biopolymer Hydrogel Based on Acid Whey and Cellulose Derivatives for Enhancement Water Retention Capacity of Soil and Slow Release of Fertilizers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Hydrogel
2.3. Hydrogel Characterization
2.3.1. Gel Fraction
2.3.2. Swelling Ratio (SR) under Various Conditions
2.3.3. Water-Holding Capacity (WHC) in Soil
2.3.4. Water-Retention (WR)
2.3.5. Reswelling Capacity (RSC) in Free Water and Soil
2.3.6. Biodegradability of the Hydrogel Using Soil Burial Method
2.3.7. Scanning Electron Microscopy
2.3.8. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.3.9. GC Analysis of Biodegradation in Soil
2.3.10. Loading of the Fertilizers
2.3.11. Release Studies in Soil Extract
3. Results and Discussion
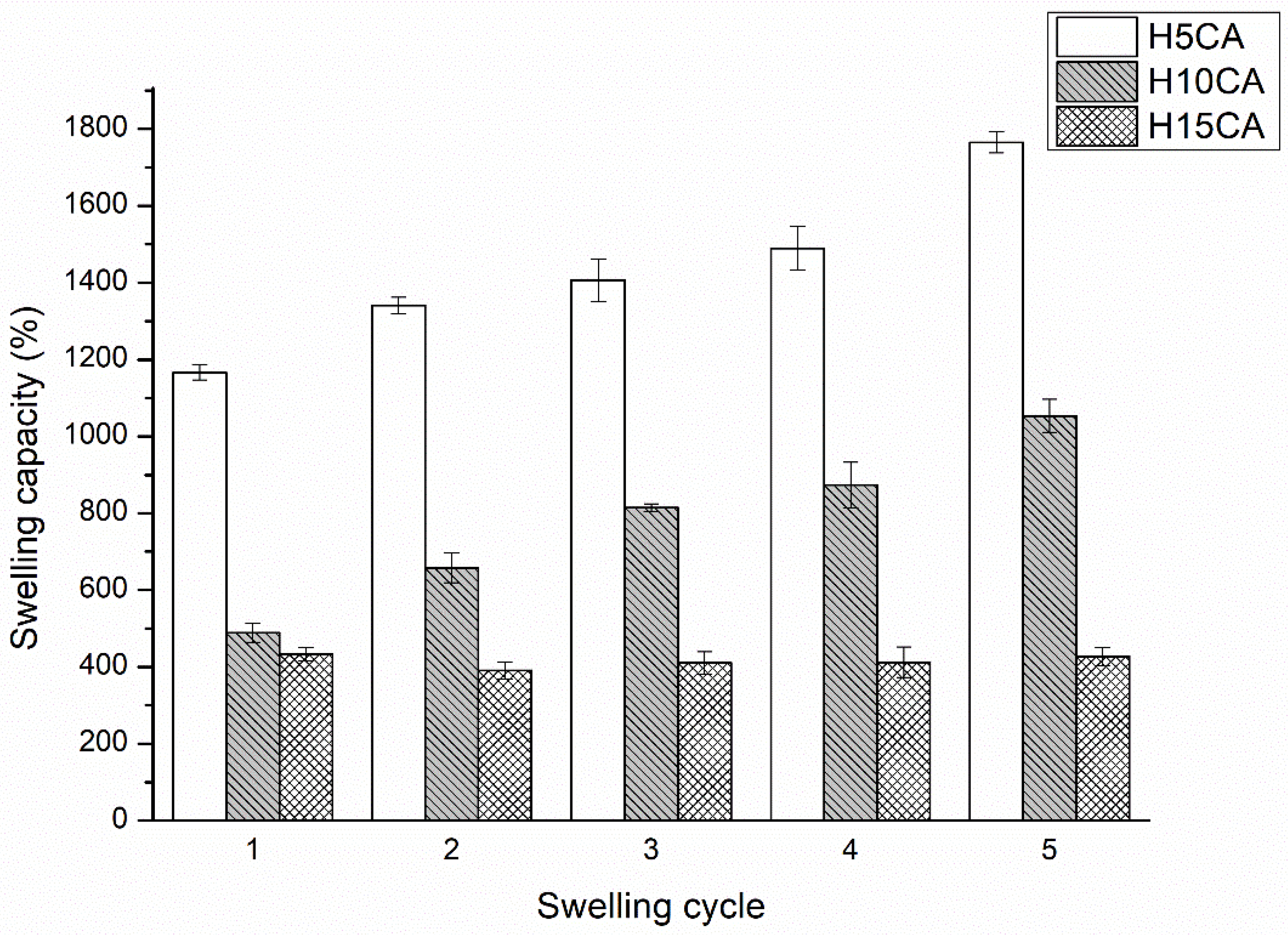
3.1. Swelling Properties
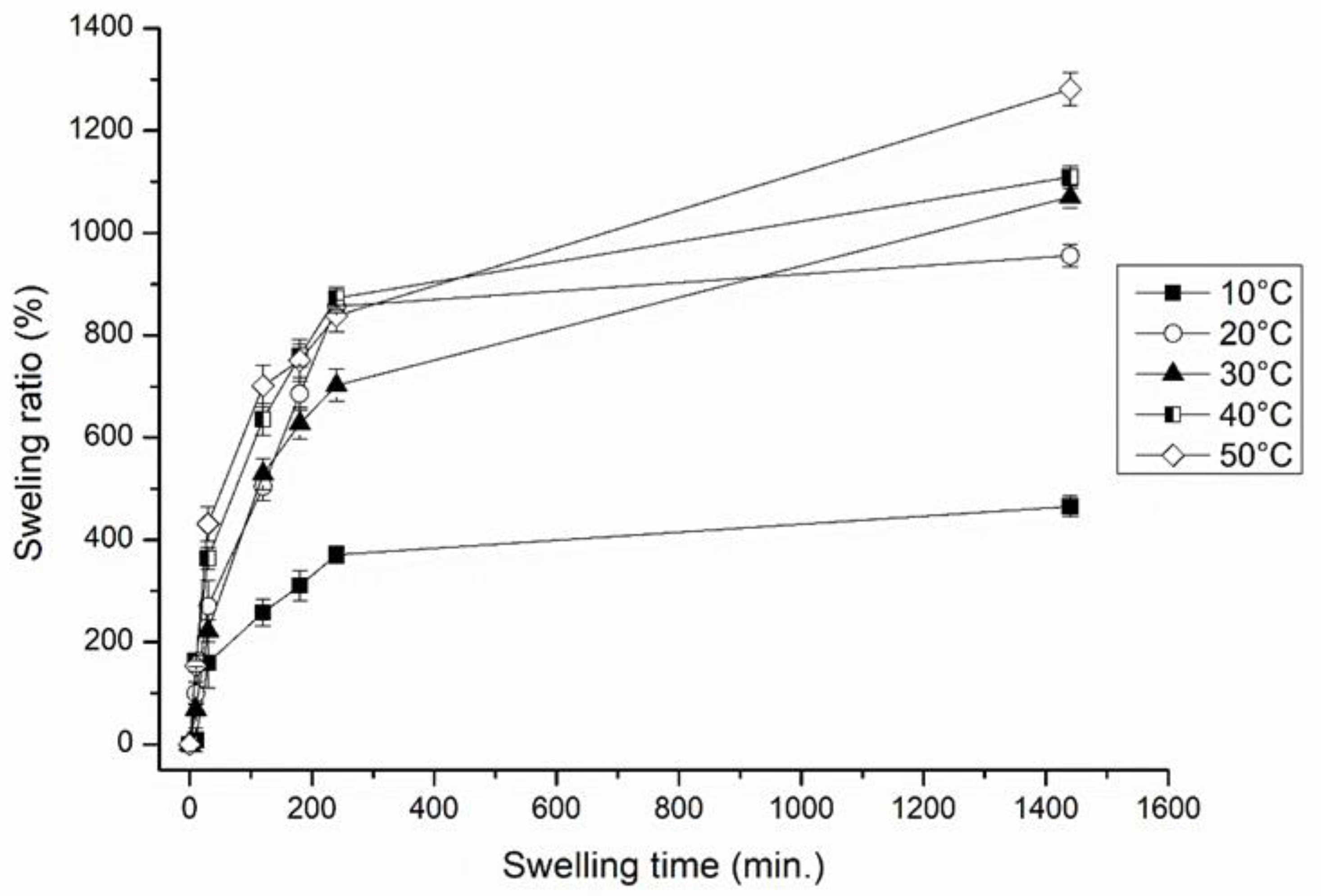
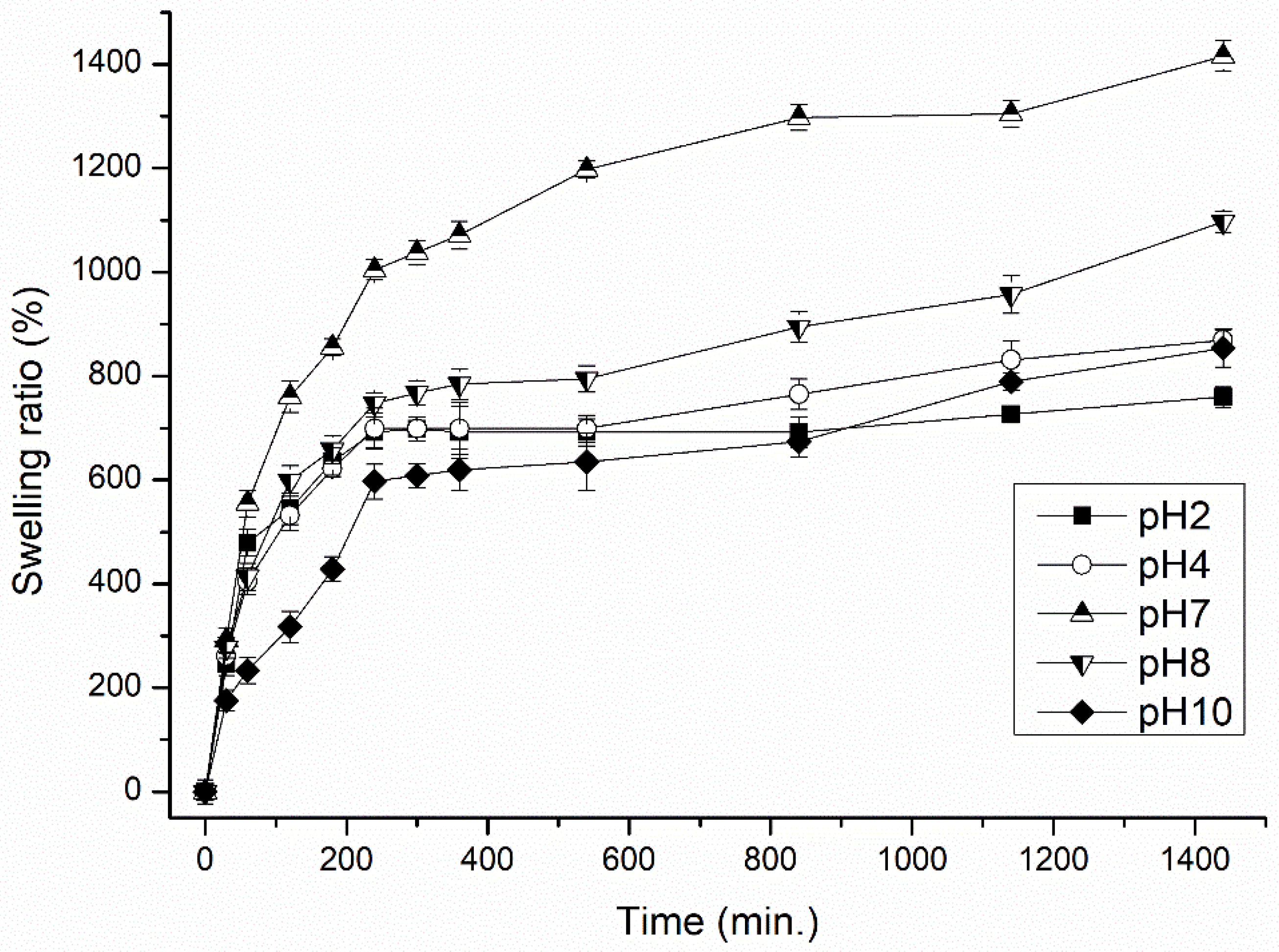
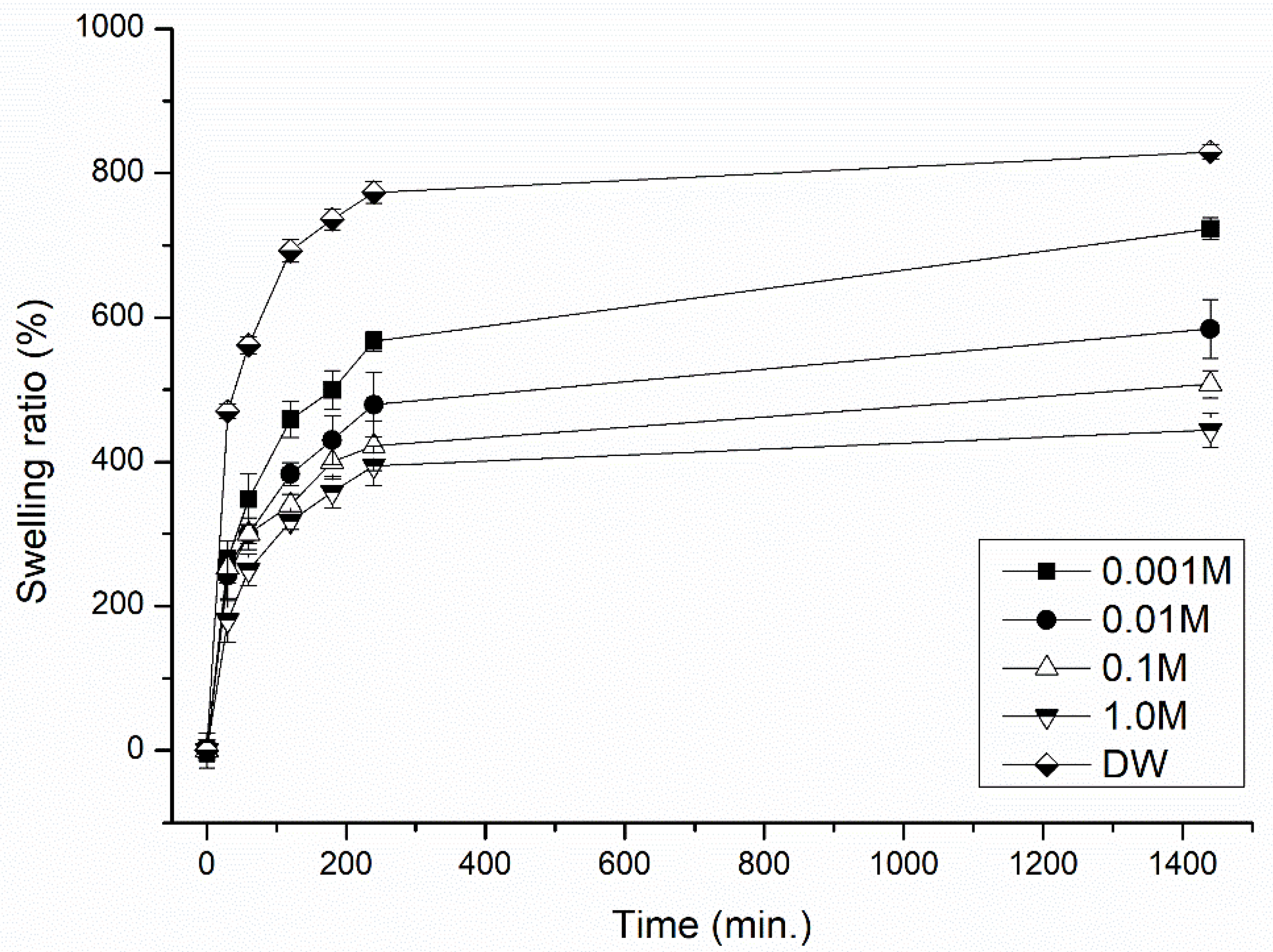
3.1.1. Effect of Temperature, pH and Ionic Strength (External Stimuli) of the Immersed Medium on the Degree of Swelling
3.1.2. Effect of Concentration of Citric Acid and Degree of Cross-Linking on the Swelling Properties of the Hydrogel
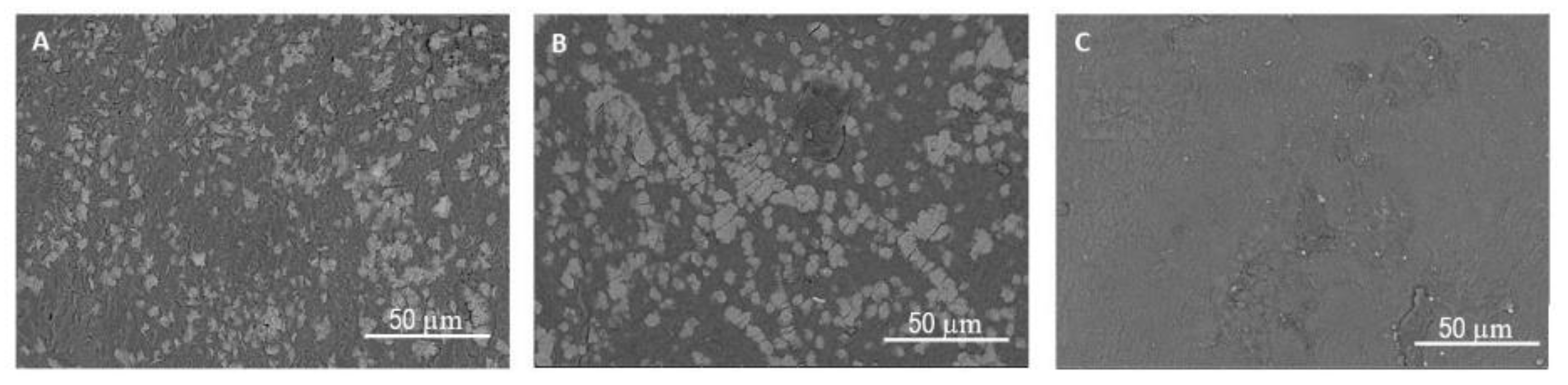
3.2. Hydrogel Morphology
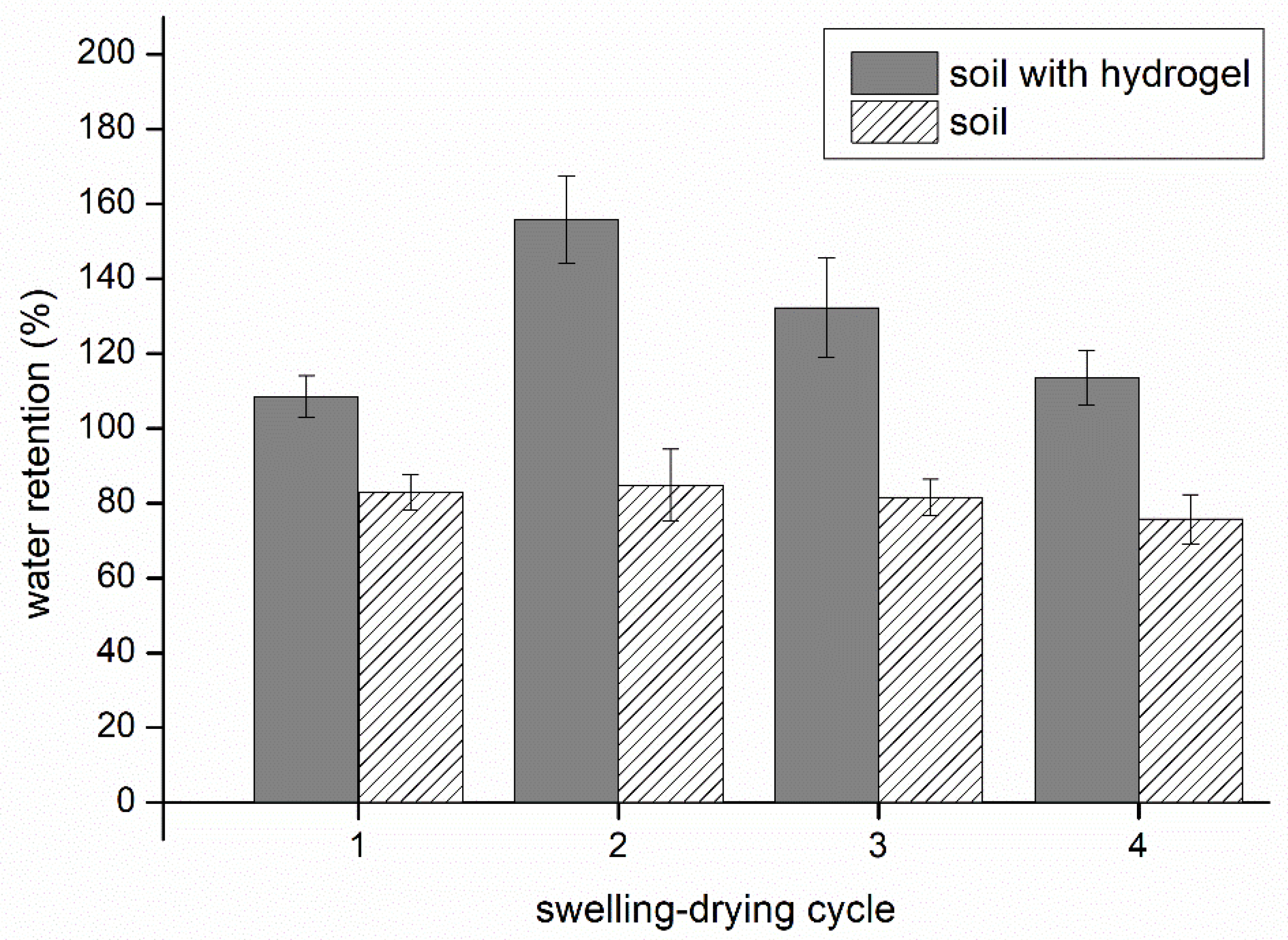
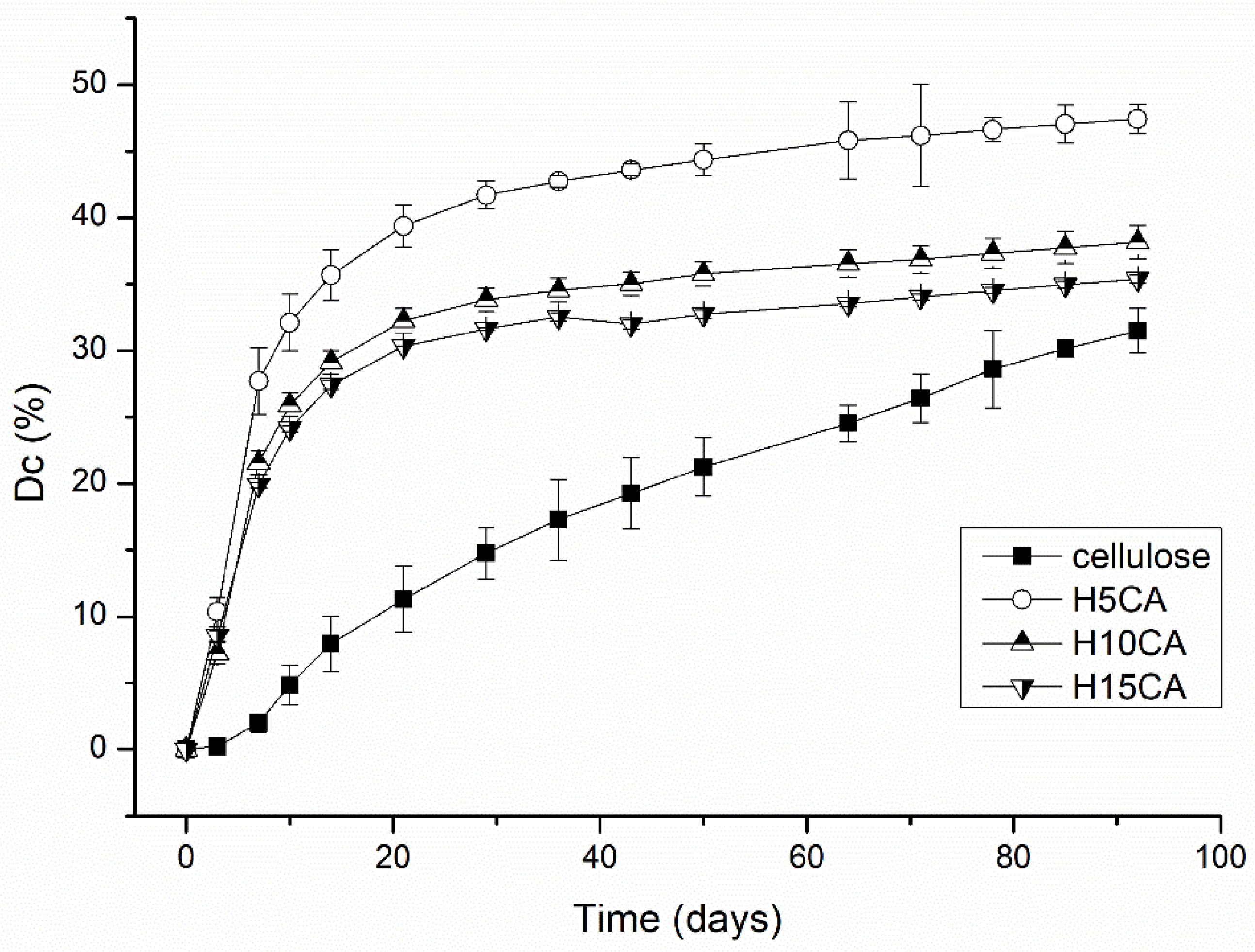
3.3. Water Retention in Soil
3.4. Water Re-Absorption in Soil
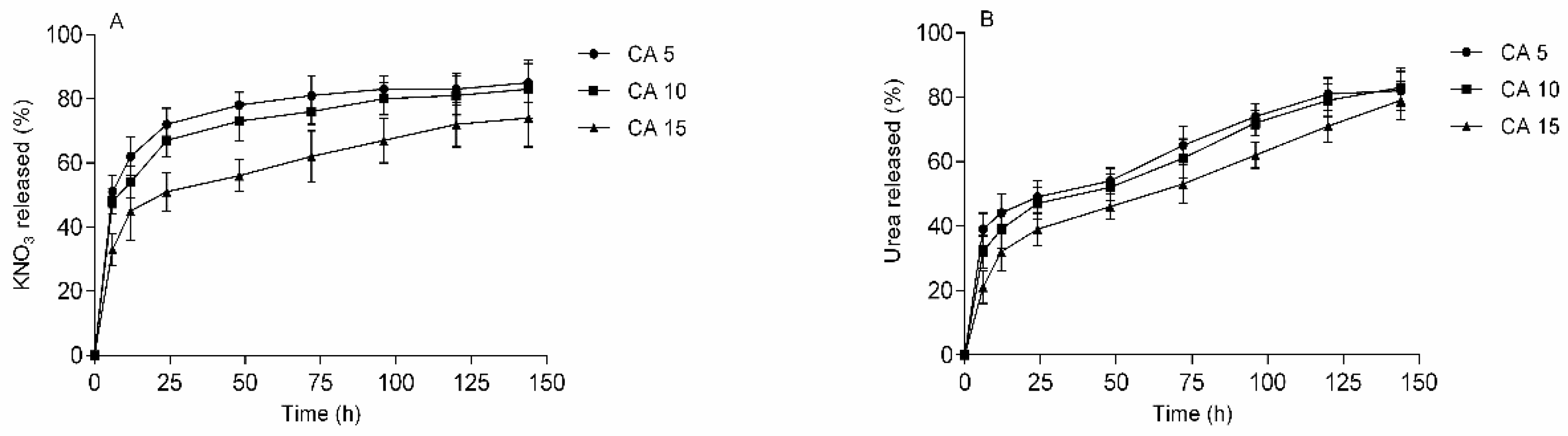
3.5. Release Study
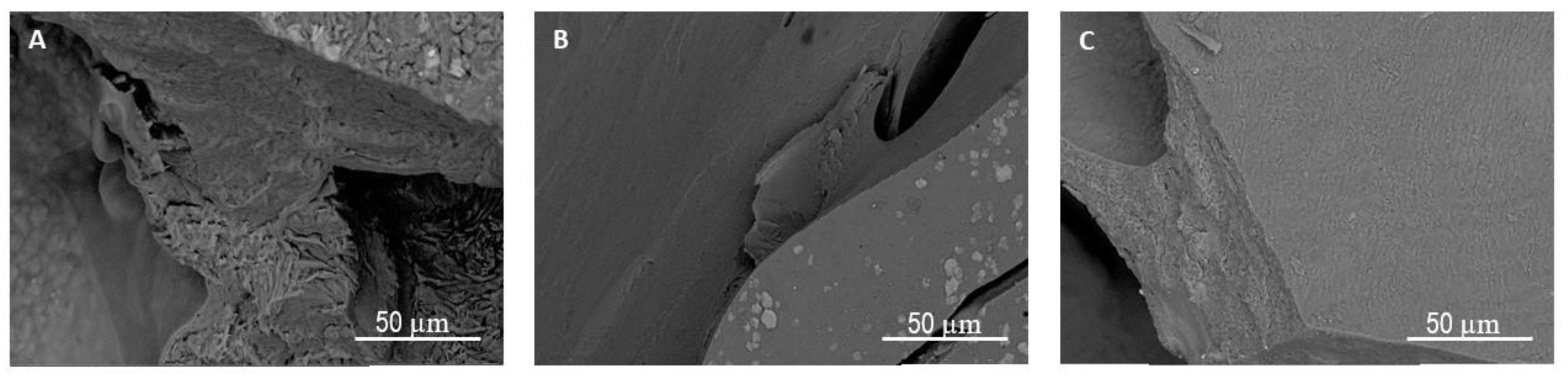
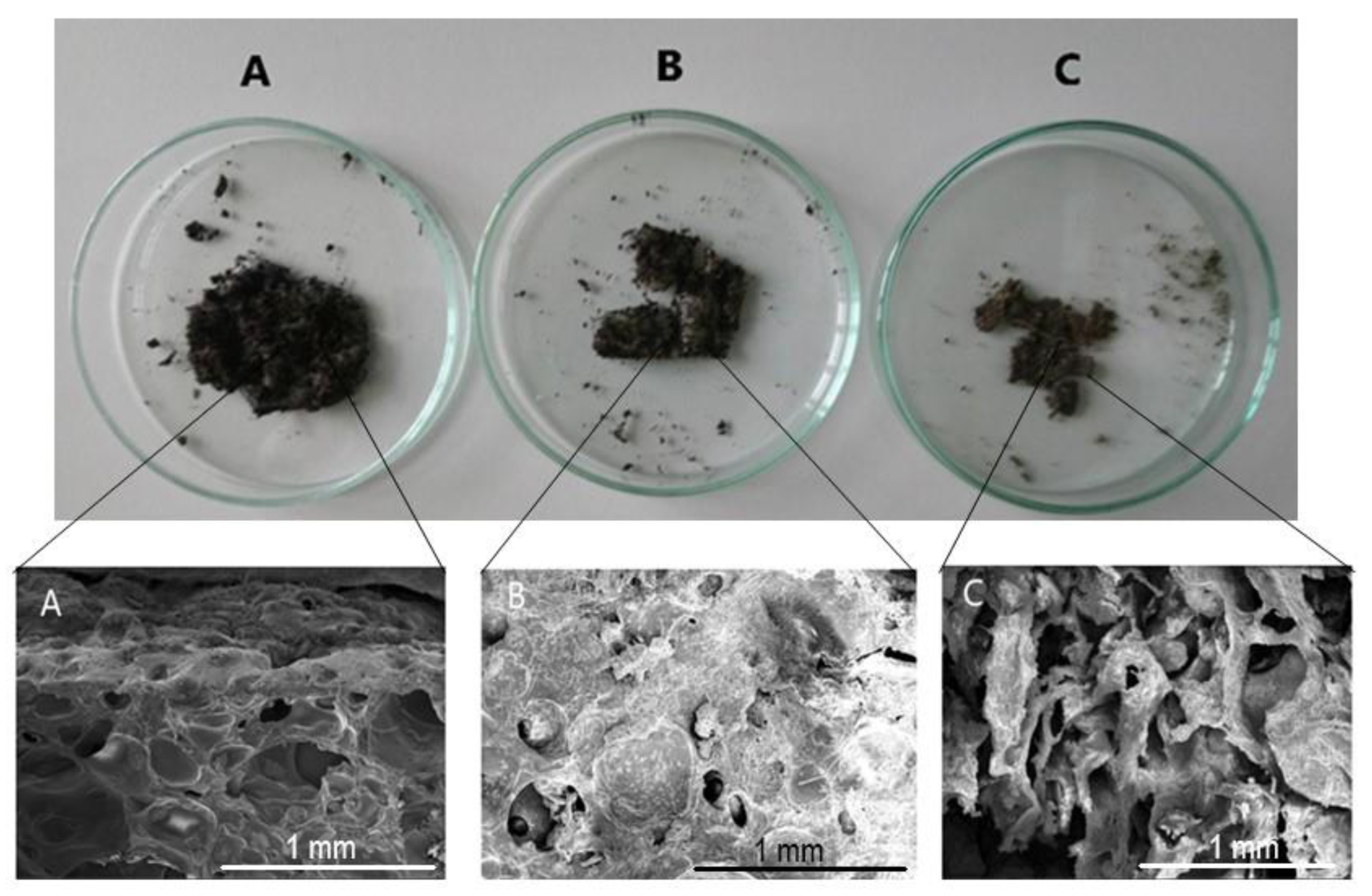
3.6. Biodegradation under Aerobic Conditions
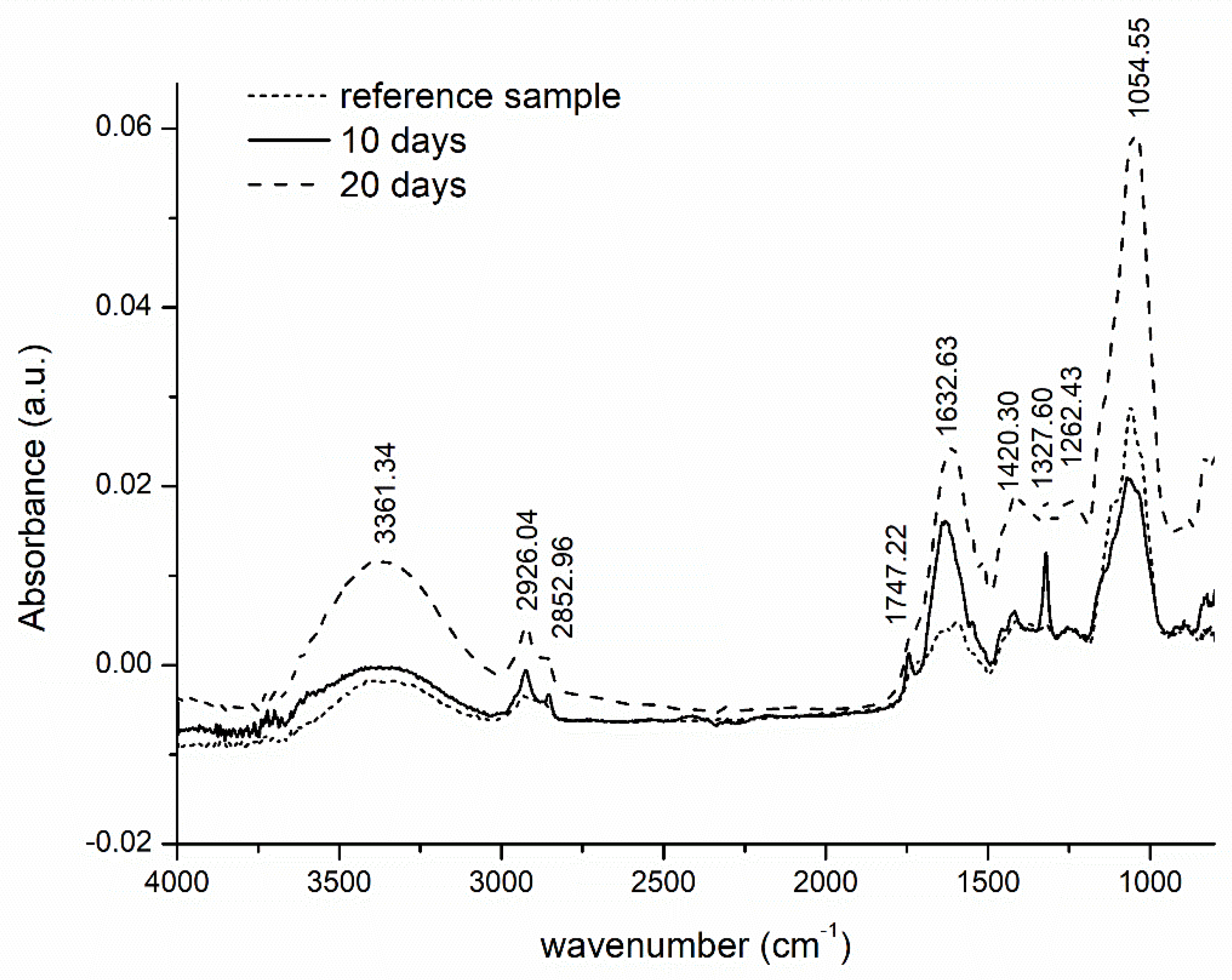
3.7. FTIR Evaluation of Structural Change in the Hydrogel during Biodegradation Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Calcagnile, P.; Sibillano, T.; Giannini, C.; Sannino, A.; Demitri, C. Biodegradable poly (lactic acid)/cellulose-based superabsorbent hydrogel composite material as water and fertilizer reservoir in agricultural applications. J. Appl. Polym. Sci. 2019, 136, 1–9. [Google Scholar] [CrossRef]
- Mohamady Ghobashy, M. Chapter 12-The application of natural polymer-based hydrogels for agriculture. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherland, 2020; pp. 329–356. ISBN 978-0-12-816421-1. [Google Scholar]
- Saruchi; Kumar, V.; Mittal, H.; Alhassan, S.M. Biodegradable hydrogels of tragacanth gum polysaccharide to improve water retention capacity of soil and environment-friendly controlled release of agrochemicals. Int. J. Biol. Macromol. 2019, 132, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Saruchi; Kaith, B.S.; Jindal, R.; Kumar, V. Biodegradation of Gum tragacanth acrylic acid based hydrogel and its impact on soil fertility. Polym. Degrad. Stab. 2015, 115, 24–31. [Google Scholar] [CrossRef]
- Isikci Koca, E.; Bozdag, G.; Cayli, G.; Kazan, D.; Cakir Hatir, P. Thermoresponsive hydrogels based on renewable resources. J. Appl. Polym. Sci. 2020, 137, 48861. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Agricultural waste-derived superabsorbent hydrogels: Preparation, performance, and socioeconomic impacts. J. Clean. Prod. 2020, 251, 119669. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, X.; Li, C.; Liu, H.; Cheng, Y.; Lu, J.; Zhang, K.; Wang, H. Super-swelling lignin-based biopolymer hydrogels for soil water retention from paper industry waste. Int. J. Biol. Macromol. 2019, 135, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Liang, H.; Sun, R.; Peng, P.; Jiang, Y.; She, D. Hydrogel synthesis based on lignin/sodium alginate and application in agriculture. Int. J. Biol. Macromol. 2020, 144, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Akay, A.; Sert, D. The effects of whey application on the soil biological properties and plant growth. Eurasian J. Soil Sci. 2020, 9, 349–355. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Synthesis and application of new temperature-responsive hydrogels based on carboxymethyl and hydroxyethyl cellulose derivatives for the functional finishing of cotton knitwear. Carbohydr. Polym. 2011, 85, 664–673. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of cellulose-based superabsorbent hydrogels as water reservoir in agriculture. Int. J. Polym. Sci. 2013, 2013. [Google Scholar] [CrossRef]
- Durpekova, S.; Filatova, K.; Cisar, J.; Ronzova, A.; Kutalkova, E.; Sedlarik, V. A Novel Hydrogel Based on Renewable Materials for Agricultural Application. Int. J. Polym. Sci. 2020, 2020, 8363418. [Google Scholar] [CrossRef]
- Sarmah, D.; Karak, N. Biodegradable superabsorbent hydrogel for water holding in soil and controlled-release fertilizer. J. Appl. Polym. Sci. 2020, 137, 48495. [Google Scholar] [CrossRef]
- Šerá, J.; Stloukal, P.; Jančová, P.; Verney, V.; Pekařová, S.; Koutný, M. Accelerated Biodegradation of Agriculture Film Based on Aromatic-Aliphatic Copolyester in Soil under Mesophilic Conditions. J. Agric. Food Chem. 2016, 64, 5653–5661. [Google Scholar] [CrossRef]
- Seki, Y.; Altinisik, A.; Demircioğlu, B.; Tetik, C. Carboxymethylcellulose (CMC)-hydroxyethylcellulose (HEC) based hydrogels: Synthesis and characterization. Cellulose 2014, 21, 1689–1698. [Google Scholar] [CrossRef]
- Maiti, M.; Kaith, B.S.; Jindal, R.; Jana, A.K. Synthesis and characterization of corn starch based green composites reinforced with Saccharum spontaneum L graft copolymers prepared under micro-wave and their effect on thermal, physio-chemical and mechanical properties. Polym. Degrad. Stab. 2010, 95, 1694–1703. [Google Scholar] [CrossRef]
- Erizal, E. Synthesis of poly (acrylamide-co-acrylic acid)-starch based superabsorbent hydrogels by gamma radiation: Study its swelling behavior. Indones. J. Chem. 2012, 12, 113–118. [Google Scholar] [CrossRef][Green Version]
- de Stéfano, J.C.Q.; Abundis-Correa, V.; Herrera-Flores, S.D.; Alvarez, A.J. PH-sensitive starch-based hydrogels: Synthesis and effect of molecular components on drug release behavior. Polymers 2020, 12, 1974. [Google Scholar] [CrossRef]
- Vaghani, S.S.; Patel, M.M.; Satish, C.S. Synthesis and characterization of pH-sensitive hydrogel composed of carboxymethyl chitosan for colon targeted delivery of ornidazole. Carbohydr. Res. 2012, 347, 76–82. [Google Scholar] [CrossRef]
- Michalik, R.; Wandzik, I. A Mini-Review on Chitosan-Based Hydrogels with Potential for Sustainable Agricultural Applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Demitri, C.; Madaghiele, M.; Grazia Raucci, M.; Sannino, A.; Ambrosio, L. Investigating the Structure-Related Properties of Cellulose-Based Superabsorbent Hydrogels. In Hydrogels-Smart Materials for Biomedical Applications; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Saeed, A.M. Temperature effect on swelling properties of commercial polyacrylic acid hydrogel beads. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 1614–1627. [Google Scholar]
- Barbucci, R.; Magnani, A.; Consumi, M. Swelling Behavior of Carboxymethylcellulose Hydrogels in Relation to Cross-Linking, pH, and Charge Density. Macromolecules 2000, 33, 7475–7480. [Google Scholar] [CrossRef]
- Chang, C.; He, M.; Zhou, J.; Zhang, L. Swelling Behaviors of pH- and Salt-Responsive Cellulose-Based Hydrogels. Macromolecules 2011, 44, 1642–1648. [Google Scholar] [CrossRef]
- Gupta, N.V.; Shivakumar, H.G. Investigation of Swelling Behavior and Mechanical Properties of a pH-Sensitive Superporous Hydrogel Composite. Iran. J. Pharm. Res. IJPR 2012, 11, 481–493. [Google Scholar]
- Tulain, U.R.; Ahmad, M.; Rashid, A.; Malik, M.Z.; Iqbal, F.M. Fabrication of pH-Responsive Hydrogel and Its In Vitro and In Vivo Evaluation. Adv. Polym. Technol. 2018, 37, 290–304. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, N.C. Synthesis of Mussel Inspired Polydopamine Coated Halloysite Nanotubes Based Semi-IPN: An Approach to Fine Tuning in Drug Release and Mechanical Toughening. Macromol. Symp. 2018, 382, 1–8. [Google Scholar] [CrossRef]
- Akhter, J.; Mahmood, K.; Malik, K.A.; Mardan, A.; Ahmad, M.; Iqbal, M.M. Effects of hydrogel amendment on water storage of sandy loam and loam soils and seedling growth of barley, wheat and chickpea. Plant Soil Environ. 2004, 50, 463–469. [Google Scholar] [CrossRef]
- Abdallah, A.M. The effect of hydrogel particle size on water retention properties and availability under water stress. Int. Soil Water Conserv. Res. 2019, 7, 275–285. [Google Scholar] [CrossRef]
- Sultana, S.; Rahaman, S.; Hasnine, S.M. Effect of Salinity on Swelling Behaviors of Superwater Absorbent Hydrogel Prepared from Carboxymethyl cellulose / Acrylamide Blends by Gamma Radiation. Am. J. Appl. Ind. Chem. 2018, 2, 20–26. [Google Scholar] [CrossRef]
- Bukhari, S.M.H.; Khan, S.; Rehanullah, M.; Ranjha, N.M. Synthesis and Characterization of Chemically Cross-Linked Acrylic Acid/Gelatin Hydrogels: Effect of pH and Composition on Swelling and Drug Release. Int. J. Polym. Sci. 2015, 2015. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Wong, R.S.H.; Ashton, M.; Dodou, K. Effect of crosslinking agent concentration on the properties of unmedicated hydrogels. Pharmaceutics 2015, 7, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Islam, M.S.; Christopher, L.P. Sustainable Production of Cellulose-Based Hydrogels with Superb Absorbing Potential in Physiological Saline. ACS Omega 2019, 4, 9419–9426. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Kang, Y.; Wang, A. Synthesis, swelling and responsive properties of a new composite hydrogel based on hydroxyethyl cellulose and medicinal stone. Compos. Part. B Eng. 2011, 42, 809–818. [Google Scholar] [CrossRef]
- Su, C.; Liu, J.; Yang, Z.; Jiang, L.; Liu, X.; Shao, W. UV-mediated synthesis of carboxymethyl cellulose/poly-N-isopropylacrylamide composite hydrogels with triple stimuli-responsive swelling performances. Int. J. Biol. Macromol. 2020, 161, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Rabat, N.E.; Hashim, S.; Majid, R.A. Effect of Different Monomers on Water Retention Properties of Slow Release Fertilizer Hydrogel. Procedia Eng. 2016, 148, 201–207. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef]
- Chen, J.; Liu, M.; Liu, H.; Ma, L. Synthesis, swelling and drug release behavior of poly(N,N-diethylacrylamide-co-N-hydroxymethyl acrylamide) hydrogel. Mater. Sci. Eng. C 2009, 29, 2116–2123. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Giri, A. Water sorption behaviour of highly swelling (carboxy methylcellulose-g-polyacrylamide) hydrogels and release of potassium nitrate as agrochemical. Carbohydr. Polym. 2003, 53, 271–279. [Google Scholar] [CrossRef]
- Rose, R.S.; Richardson, K.H.; Latvanen, E.J.; Hanson, C.A.; Resmini, M.; Sanders, I.A. Microbial degradation of plastic in aqueous solutions demonstrated by CO2 evolution and quantification. Int. J. Mol. Sci. 2019, 21, 1176. [Google Scholar] [CrossRef]
- Fesseha, H.; Abebe, F. Degradation of plastic materials using microorganisms: A review. Public Health Open J. 2019, 4, 57–63. [Google Scholar] [CrossRef]
- Tosin, M.; Pischedda, A.; Degli-Innocenti, F. Biodegradation kinetics in soil of a multi-constituent biodegradable plastic. Polym. Degrad. Stab. 2019, 166, 213–218. [Google Scholar] [CrossRef]
- Mali, K.K.; Dhawale, S.C.; Dias, R.J.; Dhane, N.S.; Ghorpade, V.S. Citric acid crosslinked carboxymethyl cellulose-based composite hydrogel films for drug delivery. Indian J. Pharm. Sci. 2018, 80, 657–667. [Google Scholar] [CrossRef]
- Tavera-Quiroz, M.J.; Feria Díaz, J.; Pinotti, A. Characterization of Methylcellulose Based Hydrogels by Using Citric Acid as a Crosslinking Agent. Int. J. Appl. Eng. Res. 2018, 13, 13302–13307. [Google Scholar]


| Sample Designation | Cellulose Derivatives (% w/w) | Citric Acid (% w/w) |
|---|---|---|
| HCA5 | 3 CMC/HEC (3:1) | 5 |
| HCA10 | 3 CMC/HEC (3:1) | 10 |
| HCA15 | 3 CMC/HEC (3:1) | 15 |
| Samples | Fertilizer | n | R2 | Release Mechanism |
|---|---|---|---|---|
| HCA5 | urea | 0.92 | 0.98 | Non-Fickian |
| KNO3 | 0.95 | 0.99 | Case II diffusion | |
| HCA10 | urea | 0.66 | 0.97 | Non-Fickian |
| KNO3 | 0.81 | 0.99 | Non-Fickian | |
| HCA15 | urea | 0.69 | 0.99 | Non-Fickian |
| KNO3 | 0.73 | 0.98 | Non-Fickian |
| Sample Designation | Weight Loss (%) at Different Time Intervals | |||
|---|---|---|---|---|
| 5 Days | 10 Days | 15 Days | 20 Days | |
| H10CA | 55.4 | 67.5 | 85.5 | 95.2 |
| H15CA | 54.6 | 62.8 | 80.8 | 87.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durpekova, S.; Di Martino, A.; Dusankova, M.; Drohsler, P.; Sedlarik, V. Biopolymer Hydrogel Based on Acid Whey and Cellulose Derivatives for Enhancement Water Retention Capacity of Soil and Slow Release of Fertilizers. Polymers 2021, 13, 3274. https://doi.org/10.3390/polym13193274
Durpekova S, Di Martino A, Dusankova M, Drohsler P, Sedlarik V. Biopolymer Hydrogel Based on Acid Whey and Cellulose Derivatives for Enhancement Water Retention Capacity of Soil and Slow Release of Fertilizers. Polymers. 2021; 13(19):3274. https://doi.org/10.3390/polym13193274
Chicago/Turabian StyleDurpekova, Silvie, Antonio Di Martino, Miroslava Dusankova, Petra Drohsler, and Vladimir Sedlarik. 2021. "Biopolymer Hydrogel Based on Acid Whey and Cellulose Derivatives for Enhancement Water Retention Capacity of Soil and Slow Release of Fertilizers" Polymers 13, no. 19: 3274. https://doi.org/10.3390/polym13193274
APA StyleDurpekova, S., Di Martino, A., Dusankova, M., Drohsler, P., & Sedlarik, V. (2021). Biopolymer Hydrogel Based on Acid Whey and Cellulose Derivatives for Enhancement Water Retention Capacity of Soil and Slow Release of Fertilizers. Polymers, 13(19), 3274. https://doi.org/10.3390/polym13193274