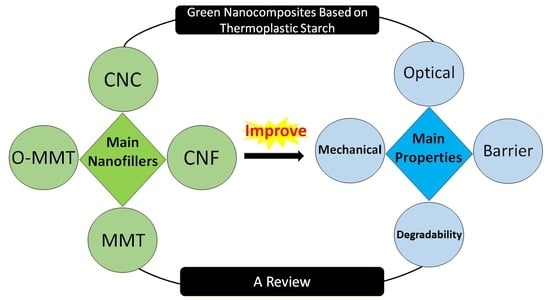
Green Nanocomposites Based on Thermoplastic Starch: A Review
Abstract
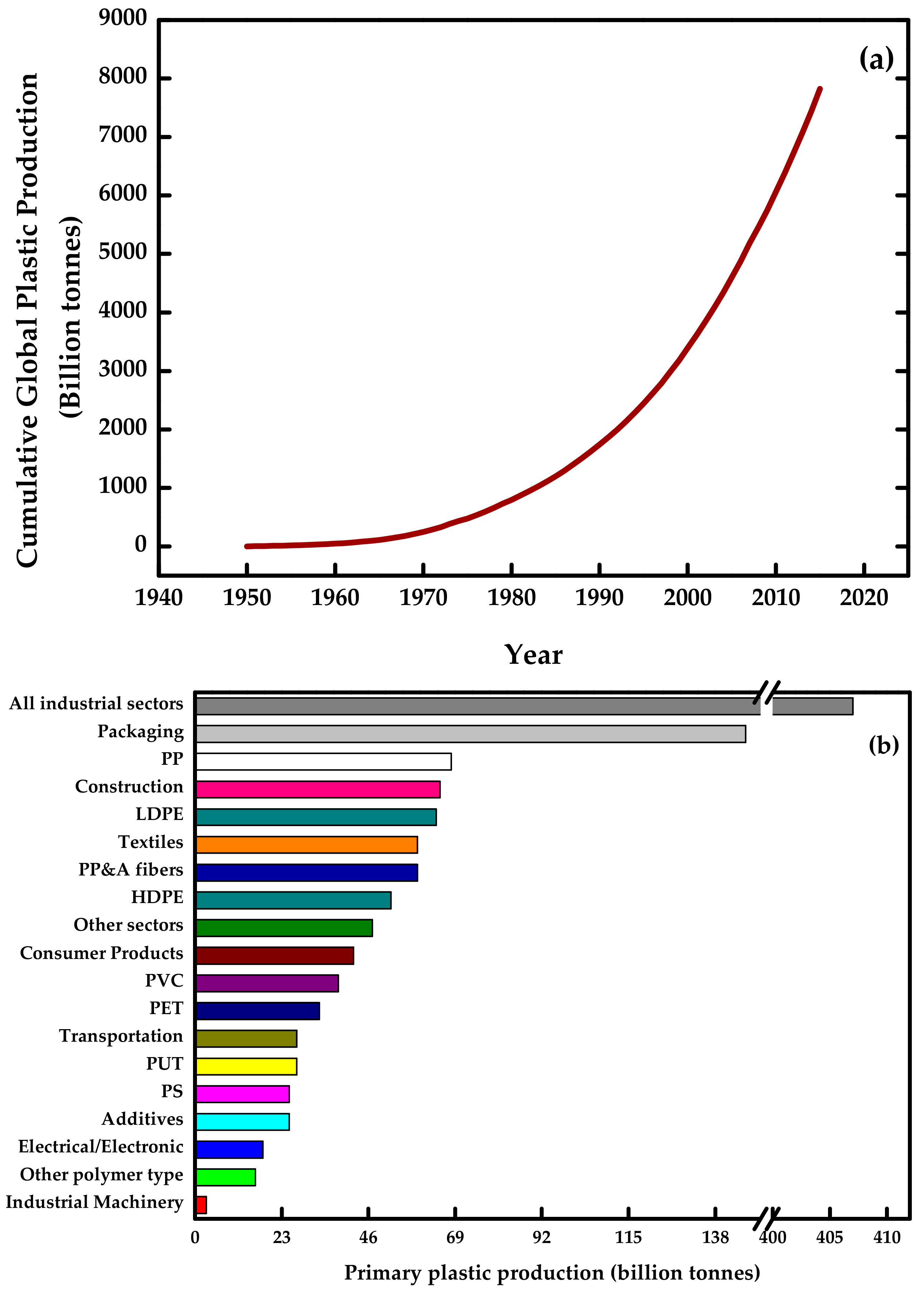
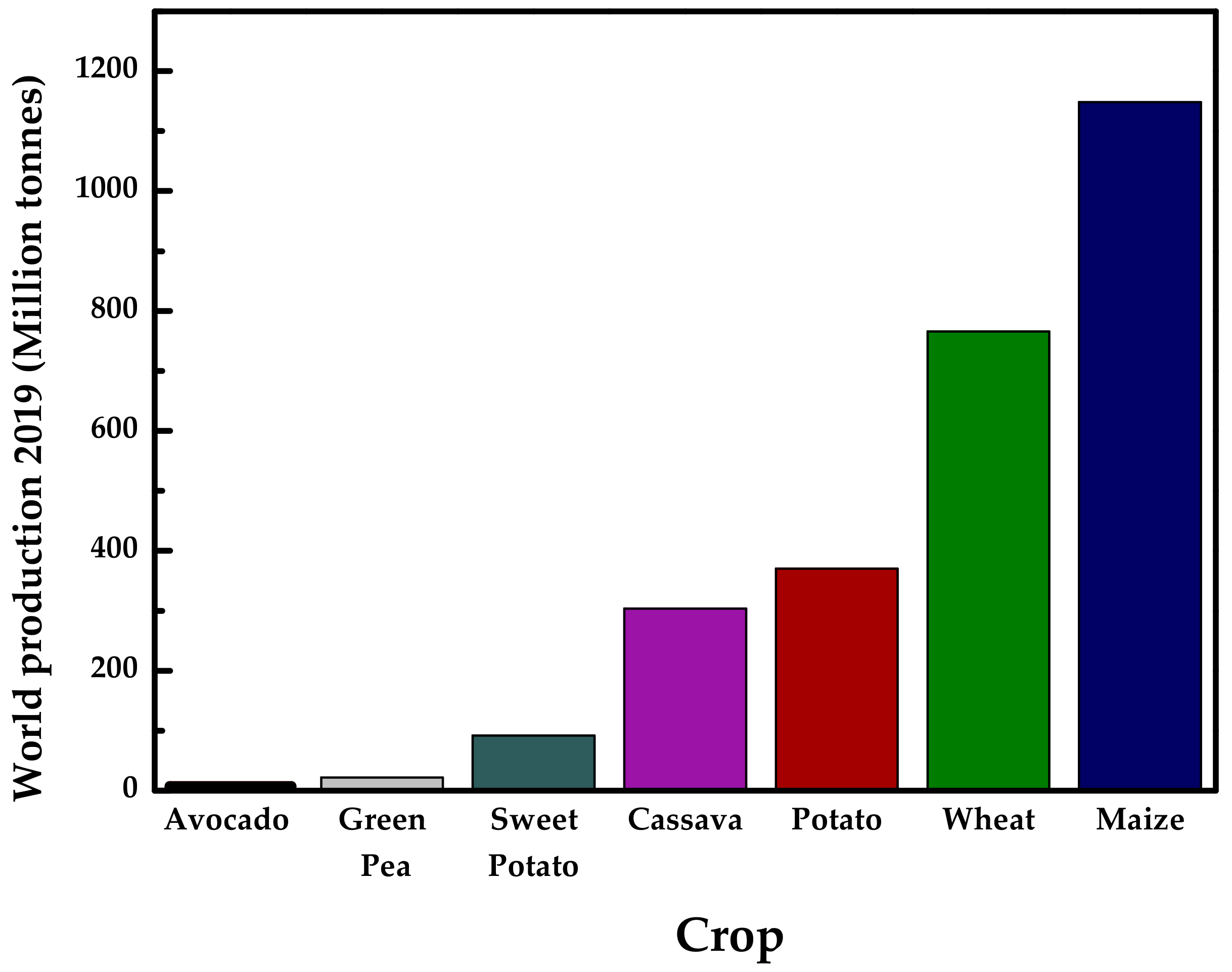
:1. Introduction
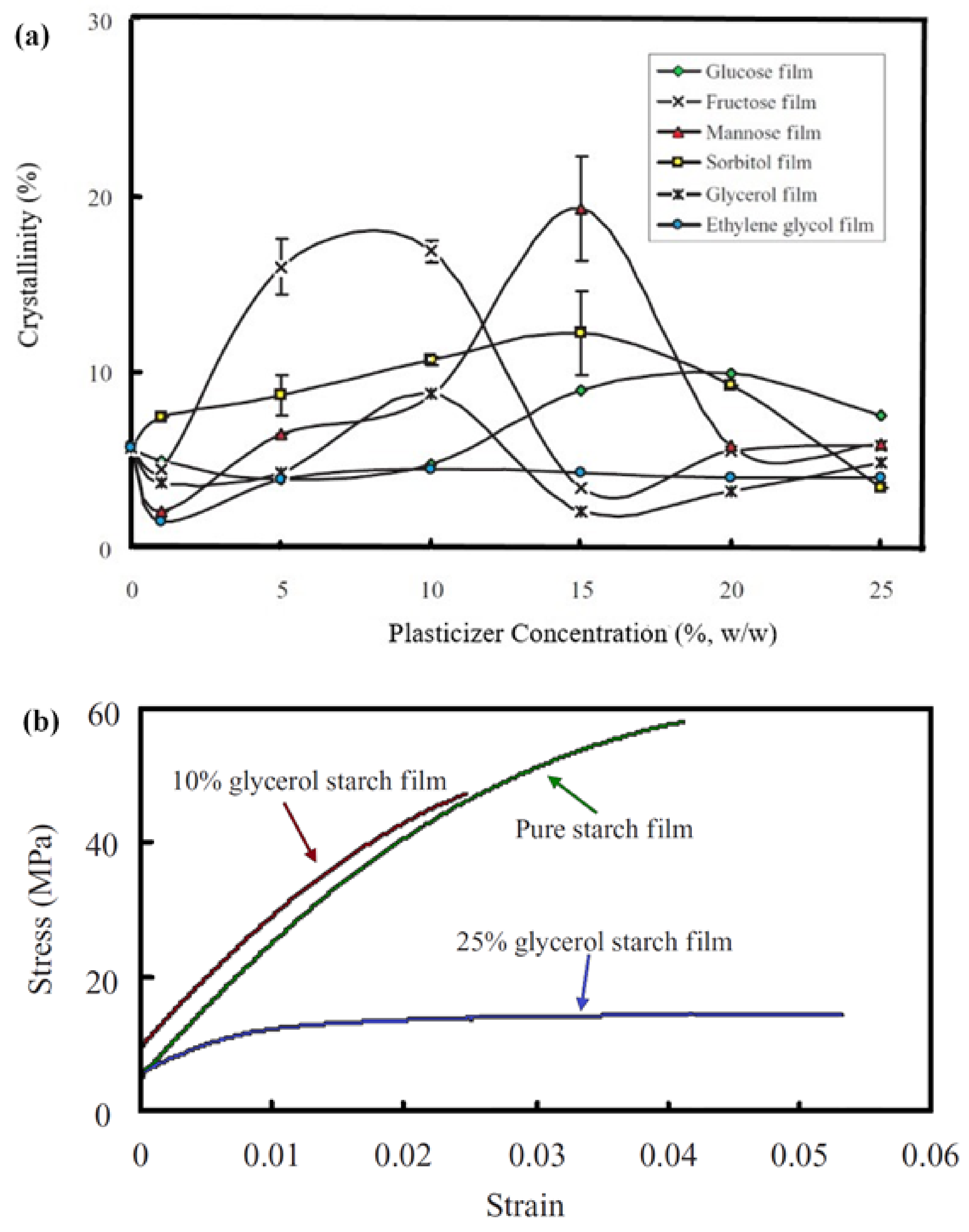
2. Thermoplastic Starch
3. Nanocomposites: General Trend in the Main Properties
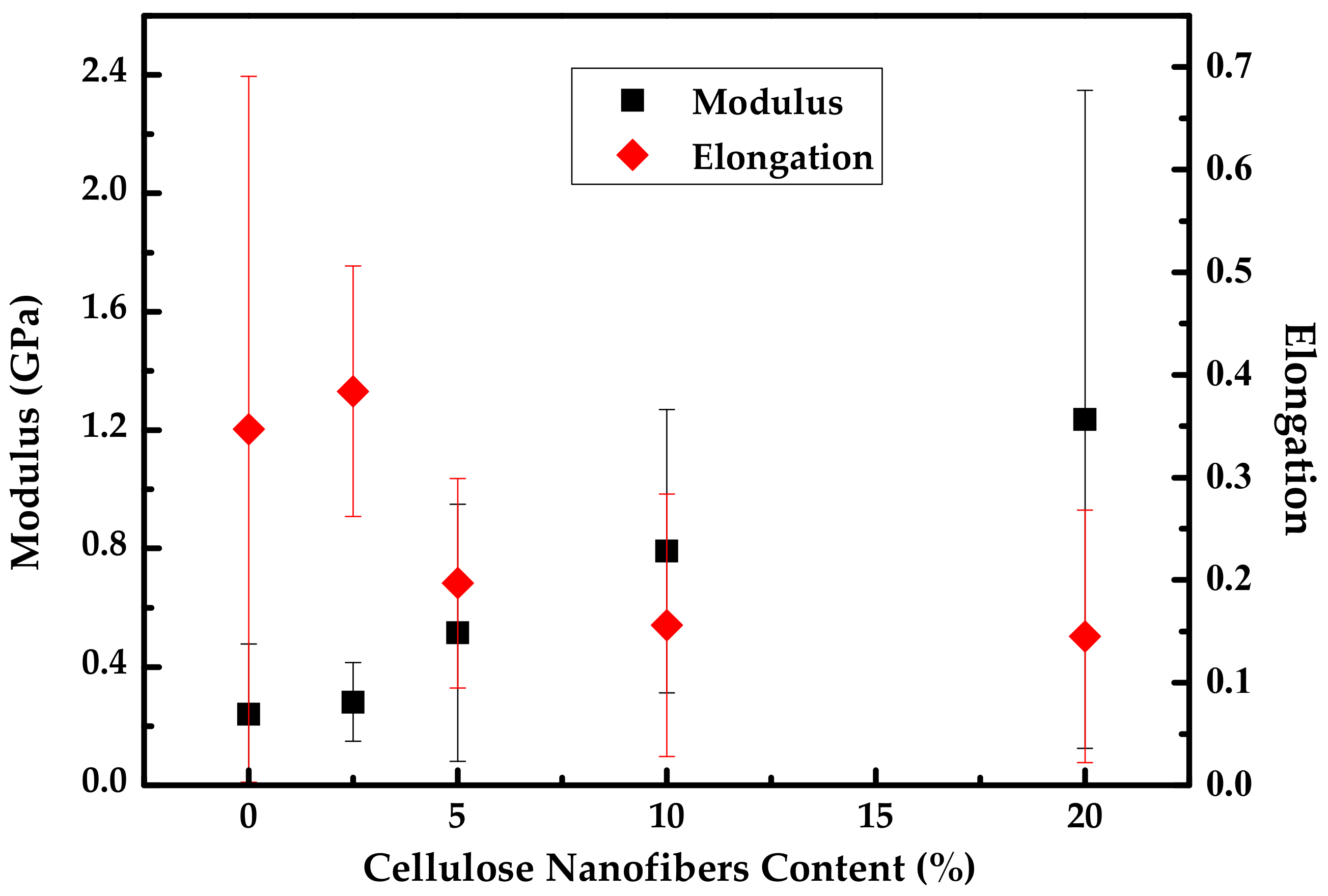
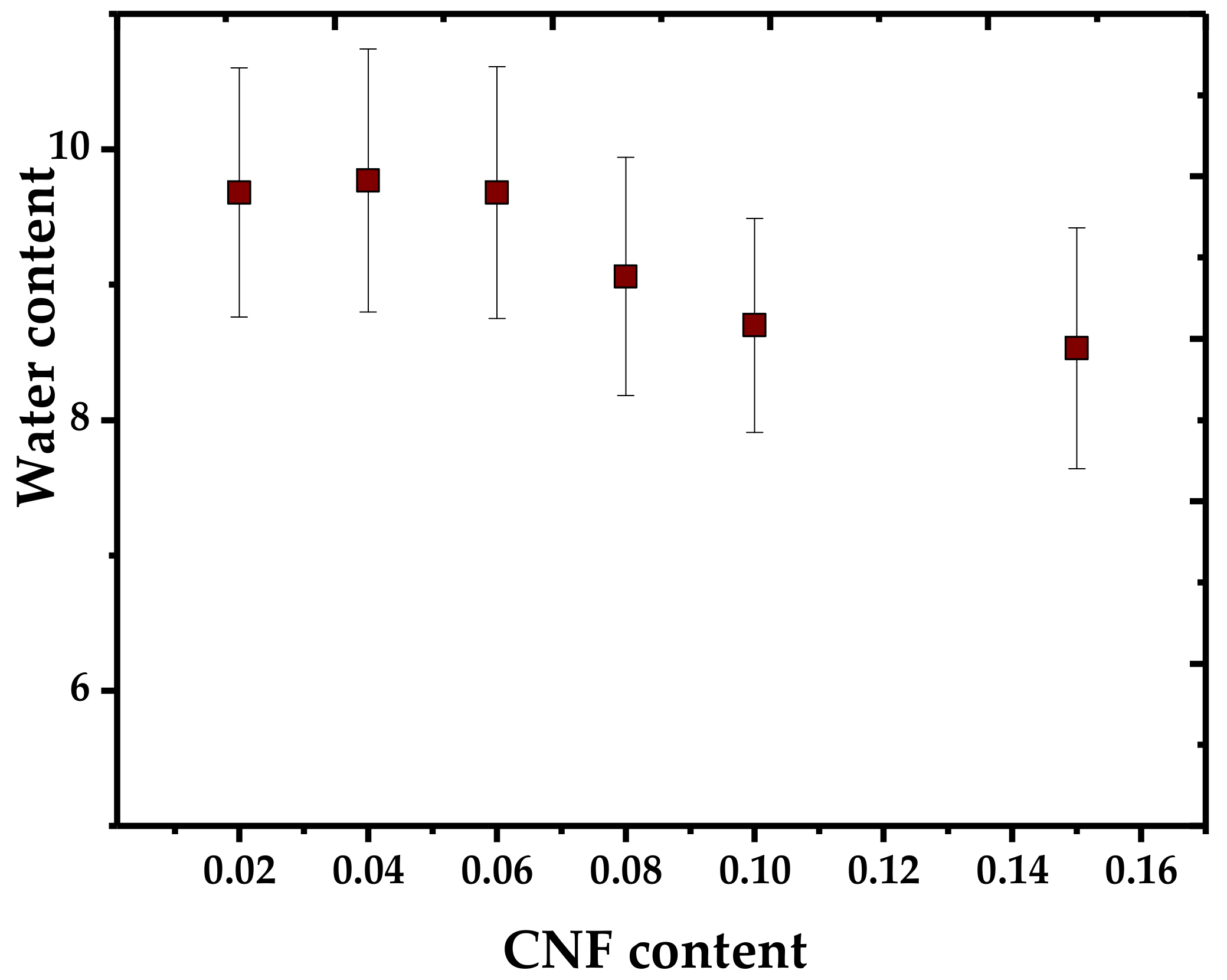
4. Cellulose Nanofibers (CNF)
5. Cellulose Nanocrystals (CNC)
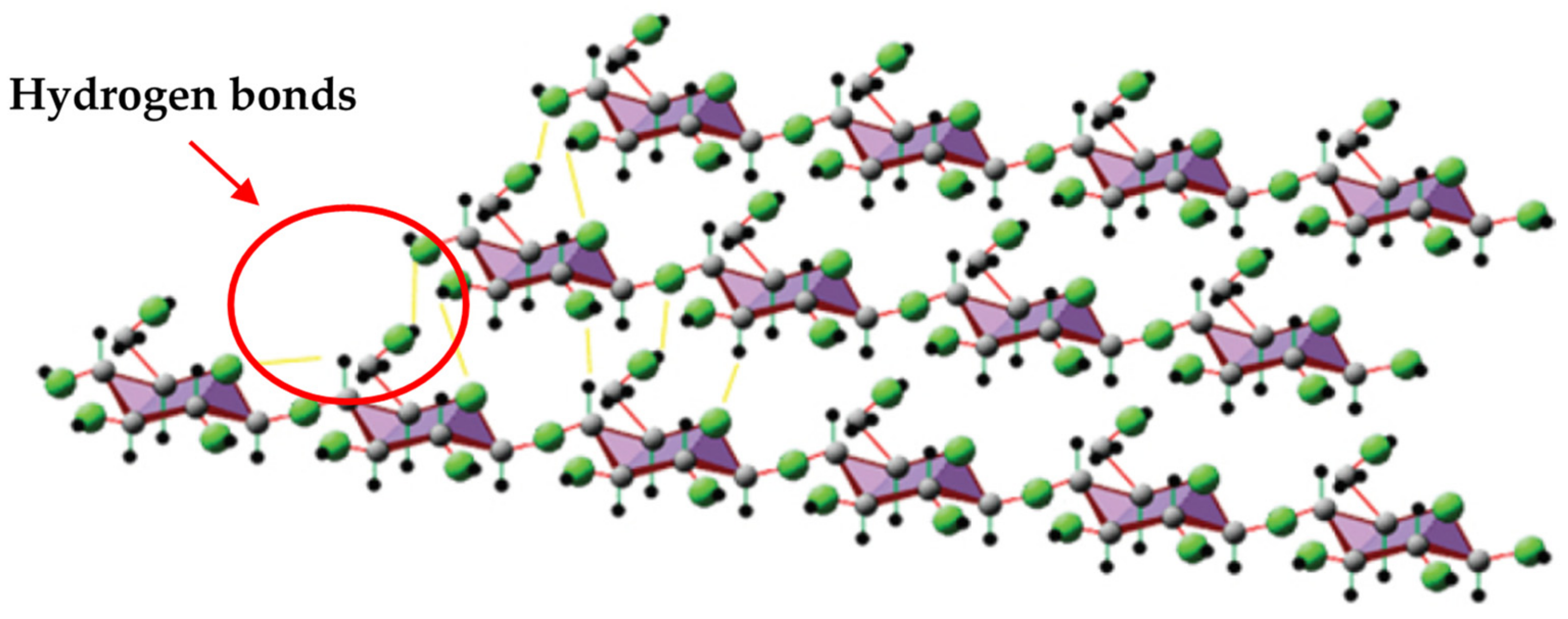
6. Natural Montmorillonite (MMT)
7. Organically Modified Montmorillonite (O-MMT)
8. Other Nanofillers
9. Analysis and Discussion for Packaging Purposes
10. Final Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Deepthi, M.V.; Sailaja, R.R.N.; Ananthapadmanabha, G.S.; Avadhani, G.S. Cross-linked Chitosan/Thermoplastic Starch Reinforced with Multiwalled Carbon Nanotubes Using Maleate Esters as Coupling Agent: Mechanical, Tribological and Thermal Characteristics. Polym.-Plast. Technol. Eng. 2014, 53, 1476–1486. [Google Scholar] [CrossRef]
- #BeatPlasticPollution This World Environment Day. Available online: https://www.unenvironment.org/interactive/beat-plastic-pollution/ (accessed on 20 October 2020).
- Tang, X.; Alavi, S. Recent advances in starch, polyvinyl alcohol based polymer blends, nanocomposites and their biodegradability. Carbohydr. Polym. 2011, 85, 7–16. [Google Scholar] [CrossRef]
- Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Effect of nanocellulose as a filler on biodegradable thermoplastic starch films from tuber, cereal and legume. Carbohydr. Polym. 2017, 157, 1094–1104. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [Green Version]
- Karger-Kocsis, J.; Kmetty, Á.; Lendvai, L.; Drakopoulos, S.X.; Bárány, T. Water-Assisted Production of Thermoplastic Nanocomposites: A Review. Materials 2015, 8, 72–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Pacheco, E.; Canto-Pinto, J.C.; Moo-Huchin, V.M.; Estrada-Mota, I.A.; Estrada-León, R.J.; Chel-Guerrero, L. Thermoplastic Starch (TPS)-Cellulosic Fibers Composites: Mechanical Properties and Water Vapor Barrier: A Review. In Composites from Renewable and Sustainable Materials; Intech Open: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Khan, B.; Bilal Khan Niazi, M.; Samin, G.; Jahan, Z. Thermoplastic Starch: A Possible Biodegradable Food Packaging Material—A Review. J. Food Process. Eng. 2017, 40, e12447. [Google Scholar] [CrossRef]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Hammache, Y.; Serier, A.; Chaoui, S. The effect of thermoplastic starch on the properties of polypropylene/high density polyethylene blend reinforced by nano-clay. Mater. Res. Express 2020, 7, 025308. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Geethamma, V.; Gopi, S.; Thomas, M.G.; Kunaver, M.; Huskić, M.; Kalarikkal, N.; Volova, T.; Rouxel, D.; Thomas, S. Thermal, biodegradation and theoretical perspectives on nanoscale confinement in starch/cellulose nanocomposite modified via green crosslinker. Int. J. Biol. Macromol. 2019, 134, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Valle, U.; Jose, H.; Pedro, J.; Valle, U. Influencia del tiempo de almacenamiento en las propiedades estructurales de un almidón termoplástico de yuca (TPS). Ing. Compet. 2009, 11, 1–27. [Google Scholar]
- Shayan, M.; Azizi, H.; Ghasemi, I.; Karrabi, M. Influence of modified starch and nanoclay particles on crystallization and thermal degradation properties of cross-linked poly(lactic acid). J. Polym. Res. 2019, 26, 238. [Google Scholar] [CrossRef]
- Solati, M.; Saeidi, A.; Ghasemi, I. The effect of graphene nanoplatelets on dynamic properties, crystallization, and morphology of a biodegradable blend of poly(lactic acid)/thermoplastic starch. Iran. Polym. J. 2019, 28, 649–658. [Google Scholar] [CrossRef]
- Shayan, M.; Azizi, H.; Ghasemi, I.; Karrabi, M. Effect of modified starch and nanoclay particles on biodegradability and mechanical properties of cross-linked poly lactic acid. Carbohydr. Polym. 2015, 124, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Manepalli, P.H.; Alavi, S. Mathematical modeling of mechanical and barrier properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/thermoplastic starch based nanocomposites. J. Food Eng. 2019, 261, 60–65. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Afkhami, A.; Rezaei, M.; Malmiri, H.J. Effect of chitosan incorporation on crystallinity, mechanical and rheological properties, and photodegradability of PE/TPS blends. J. Thermoplast. Compos. Mater. 2021, 34, 780–800. [Google Scholar] [CrossRef]
- Ahmadi, M.; Behzad, T.; Bagheri, R. Reinforcement effect of poly (methyl methacrylate)-g-cellulose nanofibers on LDPE/thermoplastic starch composites: Preparation and characterization. Iran. Polym. J. 2017, 26, 733–742. [Google Scholar] [CrossRef]
- Sabetzadeh, M.; Bagheri, R.; Masoomi, M. Effect of nanoclay on the properties of low density polyethylene/linear low density polyethylene/thermoplastic starch blend films. Carbohydr. Polym. 2016, 141, 75–81. [Google Scholar] [CrossRef]
- López, O.V.; Castillo, L.A.; Barbosa, S.E.; Villar, M.A.; Alejandra García, M.A. Processing-properties-applications relationship of nanocomposites based on thermoplastic corn starch and talc. Polym. Compos. 2018, 39, 1331–1338. [Google Scholar] [CrossRef]
- Inceoglu, F.; Menceloglu, Y.Z. Transparent low-density polyethylene/starch nanocomposite films. J. Appl. Polym. Sci. 2013, 129, 1907–1914. [Google Scholar] [CrossRef]
- Sharif, A.; Aalaie, J.; Shariatpanahi, H.; Hosseinkhanli, H.; Khoshniyat, A. Study on the structure and properties of nanocomposites based on high-density polyethylene/starch blends. J. Polym. Res. 2011, 18, 1955–1969. [Google Scholar] [CrossRef]
- Kumanayaka, T.; Parthasarathy, R.; Jollands, M. Accelerating effect of montmorillonite on oxidative degradation of polyethylene nanocomposites. Polym. Degrad. Stab. 2010, 95, 672–676. [Google Scholar] [CrossRef]
- Chiu, F.-C.; Lai, S.-M.; Ti, K.-T. Characterization and comparison of metallocene-catalyzed polyethylene/thermoplastic starch blends and nanocomposites. Polym. Test. 2009, 28, 243–250. [Google Scholar] [CrossRef]
- Mahdieh, Z.; Bagheri, R.; Eslami, M.; Amiri, M.; Shokrgozar, M.A.; Mehrjoo, M. Thermoplastic starch/ethylene vinyl alcohol/forsterite nanocomposite as a candidate material for bone tissue engineering. Mater. Sci. Eng. C 2016, 69, 301–310. [Google Scholar] [CrossRef]
- Sessini, V.; Raquez, J.-M.; Re, G.L.; Mincheva, R.; Kenny, J.M.; Dubois, P.; Peponi, L. Multiresponsive Shape Memory Blends and Nanocomposites Based on Starch. ACS Appl. Mater. Interfaces 2016, 8, 19197–19201. [Google Scholar] [CrossRef] [PubMed]
- Guarás, M.P.; Alvarez, V.A.; Ludueña, L.N. Biodegradable nanocomposites based on starch/polycaprolactone/compatibilizer ternary blends reinforced with natural and organo-modified montmorillonite. J. Appl. Polym. Sci. 2016, 133, 6–11. [Google Scholar] [CrossRef]
- Majdzadeh-Ardakani, K.; Nazari, B. Improving the mechanical properties of thermoplastic starch/poly(vinyl alcohol)/clay nanocomposites. Compos. Sci. Technol. 2010, 70, 1557–1563. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Averous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Tessier, R.; Lafranche, E.; Krawczak, P. Development of novel melt-compounded starch-grafted polypropylene/polypropylene-grafted maleic anhydride/organoclay ternary hybrids. Express Polym. Lett. 2012, 6, 937–952. [Google Scholar] [CrossRef] [Green Version]
- Fourati, Y.; Magnin, A.; Putaux, J.-L.; Boufi, S. One-step processing of plasticized starch/cellulose nanofibrils nanocomposites via twin-screw extrusion of starch and cellulose fibers. Carbohydr. Polym. 2020, 229, 115554. [Google Scholar] [CrossRef] [PubMed]
- Nessi, V.; Falourd, X.; Maigret, J.-E.; Cahier, K.; D’Orlando, A.; Descamps, N.; Gaucher, V.; Chevigny, C.; Lourdin, D. Cellulose nanocrystals-starch nanocomposites produced by extrusion: Structure and behavior in physiological conditions. Carbohydr. Polym. 2019, 225, 115123. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, M.; Simão, R.A. Preparation and characterization of starch composites with cellulose nanofibers obtained by plasma treatment and ultrasonication. Plasma Process. Polym. 2019, 16, e1800167. [Google Scholar] [CrossRef]
- Park, C.W.; Han, S.Y.; Seo, P.N.; Youe, W.J.; Kim, Y.S.; Choi, S.K.; Kim, N.H.; Lee, S.H. Property comparison of thermoplastic starch reinforced by cellulose nanofibrils with different chemical compositions. BioResources 2019, 14, 1564–1578. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Gopi, S.; Geethamma, V.G.; Kalarikkal, N.; Thomas, S. Cellulose Nanofiber vs Nanocrystals from Pineapple Leaf Fiber: A Comparative Studies on Reinforcing Efficiency on Starch Nanocomposites. Macromol. Symp. 2018, 380, 1–7. [Google Scholar] [CrossRef]
- González, K.; Retegi, A.; González, A.; Eceiza, A.; Gabilondo, N. Starch and cellulose nanocrystals together into thermoplastic starch bionanocomposites. Carbohydr. Polym. 2015, 117, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Angellier, H.; Molina-Boisseau, S.; Dole, A.P.; Dufresne, A. Thermoplastic Starch-Waxy Maize Starch Nanocrystals Nanocomposites. Biomacromolecules 2006, 7, 531–539. [Google Scholar] [CrossRef]
- González, K.; Iturriaga, L.; González, A.; Eceiza, A.; Gabilondo, N. Improving mechanical and barrier properties of thermoplastic starch and polysaccharide nanocrystals nanocomposites. Eur. Polym. J. 2020, 123, 109415. [Google Scholar] [CrossRef]
- Ren, L.; Fu, Y.; Chang, Y.; Jiang, M.; Tong, J.; Zhou, J. Performance improvement of starch films reinforced with starch nanocrystals (SNCs) modified by cross-linking. Starch Stärke 2017, 69, 1600025. [Google Scholar] [CrossRef]
- Garcia, N.L.; Ribba, L.; Dufresne, A.; Aranguren, M.I.; Goyanes, S. Effect of glycerol on the morphology of nanocomposites made from thermoplastic starch and starch nanocrystals. Carbohydr. Polym. 2011, 84, 203–210. [Google Scholar] [CrossRef]
- Azevedo, V.M.; Carvalho, R.A.; Borges, S.; Claro, P.I.C.; Hasegawa, F.K.; Yoshida, M.I.; Marconcini, J.M. Thermoplastic starch/whey protein isolate/rosemary essential oil nanocomposites obtained by extrusion process: Antioxidant polymers. J. Appl. Polym. Sci. 2019, 136, 1–12. [Google Scholar] [CrossRef]
- Vaezi, K.; Asadpour, G.; Sharifi, H. Effect of ZnO nanoparticles on the mechanical, barrier and optical properties of thermoplastic cationic starch/montmorillonite biodegradable films. Int. J. Biol. Macromol. 2019, 124, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Sessini, V.; Arrieta, M.P.; Raquez, J.-M.; Dubois, P.; Kenny, J.M.; Peponi, L. Thermal and composting degradation of EVA/Thermoplastic starch blends and their nanocomposites. Polym. Degrad. Stab. 2019, 159, 184–198. [Google Scholar] [CrossRef]
- Ren, J.; Dang, K.M.; Pollet, E.; Avérous, L. Preparation and Characterization of Thermoplastic Potato Starch/Halloysite Nano-Biocomposites: Effect of Plasticizer Nature and Nanoclay Content. Polymers 2018, 10, 808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.; Figueroa, N.E.; Sanfuentes, E.A. Thermoplastic starch/clay nanocomposites loaded with essential oil constituents as packaging for strawberries − In vivo antimicrobial synergy over Botrytis cinerea. Postharvest Biol. Technol. 2017, 129, 29–36. [Google Scholar] [CrossRef]
- Mohan, T.; Kanny, K. Thermoforming studies of corn starch-derived biopolymer film filled with nanoclays. J. Plast. Film Sheeting 2016, 32, 163–188. [Google Scholar] [CrossRef]
- Kelnar, I.; Kaprálková, L.; Brozova, L.; Hromádková, J.; Kotek, J. Effect of chitosan on the behaviour of the wheat B-starch nanocomposite. Ind. Crop. Prod. 2013, 46, 186–190. [Google Scholar] [CrossRef]
- Fabra, M.J.; López-Rubio, A.; Ambrosio-Martín, J.; Lagaron, J.M. Improving the barrier properties of thermoplastic corn starch-based films containing bacterial cellulose nanowhiskers by means of PHA electrospun coatings of interest in food packaging. Food Hydrocoll. 2016, 61, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Aouada, F.A.; Mattoso, L.H.; Longo, E. Enhanced bulk and superficial hydrophobicities of starch-based bionanocomposites by addition of clay. Ind. Crop. Prod. 2013, 50, 449–455. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Sreekala, M.; Kunaver, M.; Huskić, M.; Thomas, S. Morphology, transport characteristics and viscoelastic polymer chain confinement in nanocomposites based on thermoplastic potato starch and cellulose nanofibers from pineapple leaf. Carbohydr. Polym. 2017, 169, 176–188. [Google Scholar] [CrossRef]
- Ren, P.; Shen, T.; Wang, F.; Wang, X.; Zhang, Z. Study on Biodegradable Starch/OMMT Nanocomposites for Packaging Applications. J. Polym. Environ. 2009, 17, 203–207. [Google Scholar] [CrossRef]
- Schmitt, H.; Creton, N.; Prashantha, K.; Soulestin, J.; Lacrampe, M.; Krawczak, P. Melt-blended halloysite nanotubes/wheat starch nanocomposites as drug delivery system. Polym. Eng. Sci. 2015, 55, 573–580. [Google Scholar] [CrossRef]
- Ning, W.; Xingxiang, Z.; Xuechen, W.; Haihui, L. Ionic liquids modified montmorillonite/thermoplastic starch nanocomposites as ionic conducting biopolymer. Macromol. Res. 2009, 17, 285–288. [Google Scholar] [CrossRef]
- Janssen, L.; Moscicki, L. Thermoplastic Starch: A Green Material for Various Industries; Janssen, L., Moscicki, L., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; ISBN 978-3-527-32528-3. [Google Scholar]
- Dufresne, A.; Castaño, J. Polysaccharide nanomaterial reinforced starch nanocomposites: A review. Starch Stärke 2017, 69, 1500307. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Ashogbon, A.O.; Kumar, M. Recent advances in thermoplastic starches for food packaging: A review. Food Packag. Shelf Life 2021, 30, 100743. [Google Scholar] [CrossRef]
- Castillo, L.A.; López, O.V.; Ninago, M.D.; Versino, F.; Barbosa, S.E.; García, M.A.; Villar, M.A. Effect of Mineral and Organic Fillers on Processing, Structure, and Final Properties of Starch. In Composites and Nanocomposites Based on Starches; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128122570. [Google Scholar]
- Osorio, M.A.; Restrepo, D.; Velásquez-Cock, J.A.; Zuluaga, R.O.; Montoya, U.; Rojas, O.; Gañán, P.F.; Marín, D.; Castro, C.I. Synthesis of Thermoplastic Starch-Bacterial Cellulose Nanocomposites via in situ Fermentation. J. Braz. Chem. Soc. 2014, 25, 1607–1613. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; Liu, Q. Thermoplastic Starch Processing and Characteristics—A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1353–1370. [Google Scholar] [CrossRef]
- Morán, J.I.; Vazquez, A.; Cyras, V.P. Bio-nanocomposites based on derivatized potato starch and cellulose, preparation and characterization. J. Mater. Sci. 2013, 48, 7196–7203. [Google Scholar] [CrossRef]
- Cyras, V.P.; Manfredi, L.B.; Ton-That, M.-T.; Vázquez, A. Physical and mechanical properties of thermoplastic starch/montmorillonite nanocomposite films. Carbohydr. Polym. 2008, 73, 55–63. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Biocomposites from wheat straw nanofibers: Morphology, thermal and mechanical properties. Compos. Sci. Technol. 2008, 68, 557–565. [Google Scholar] [CrossRef]
- Perotti, G.; Kijchavengkul, T.; Auras, R.; Constantino, V. Nanocomposites Based on Cassava Starch and Chitosan-Modified Clay: Physico-Mechanical Properties and Biodegradability in Simulated Compost Soil. J. Braz. Chem. Soc. 2017, 28, 649–658. [Google Scholar] [CrossRef]
- Olivato, J.; Marini, J.; Yamashita, F.; Pollet, E.; Grossmann, M.; Avérous, L. Sepiolite as a promising nanoclay for nano-biocomposites based on starch and biodegradable polyester. Mater. Sci. Eng. C 2017, 70, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, F.; Phang, S.W.; Sin, L.T. Mechanical behaviour of thermoplastic starch/montmorillonite/alumina trihydrate nanocomposites. J. Eng. Sci. Technol. 2016, 11, 1344–1359. [Google Scholar]
- DeLeo, C.; Pinotti, C.A.; do Gonçalves, M.C.; Velankar, S. Preparation and Characterization of Clay Nanocomposites of Plasticized Starch and Polypropylene Polymer Blends. J. Polym. Environ. 2011, 19, 689–697. [Google Scholar] [CrossRef]
- Schlemmer, D.; Angélica, R.S.; Sales, M.J.A. Morphological and thermomechanical characterization of thermoplastic starch/montmorillonite nanocomposites. Compos. Struct. 2010, 92, 2066–2070. [Google Scholar] [CrossRef]
- Teixeira, E.D.M.; Pasquini, D.; Curvelo, A.A.; Corradini, E.; Belgacem, M.N.; Dufresne, A. Cassava bagasse cellulose nanofibrils reinforced thermoplastic cassava starch. Carbohydr. Polym. 2009, 78, 422–431. [Google Scholar] [CrossRef]
- De Takahashi, G.C.S.; Barbosa, H.D.; De Bergamasco, R.C.; Madrona, G.S.; Tonon, L.A.C.; Yamashita, F.; Scapim, M.R.D.S. Development and active biodegradable film evaluation incorporated with oregano essential oil and nanoclay. Chem. Eng. Trans. 2017, 57, 403–408. [Google Scholar] [CrossRef]
- Cucinelli Neto, R.P.; da Rocha Rodrigues, E.J.; Bruno Tavares, M.I. Proton NMR relaxometry as probe of gelatinization, plasticization and montmorillonite-loading effects on starch-based materials. Carbohydr. Polym. 2018, 182, 123–131. [Google Scholar] [CrossRef]
- Karimi, S.; Abdulkhani, A.; Tahir, P.M.; Dufresne, A. Effect of cellulosic fiber scale on linear and non-linear mechanical performance of starch-based composites. Int. J. Biol. Macromol. 2016, 91, 1040–1044. [Google Scholar] [CrossRef]
- Kaushik, A.; Kumra, J. Morphology, thermal and barrier properties of green nanocomposites based on TPS and cellulose nanocrystals. J. Elastomers Plast. 2014, 46, 284–299. [Google Scholar] [CrossRef]
- Viguié, J.; Molina-Boisseau, S.; Dufresne, A. Processing and Characterization of Waxy Maize Starch Films Plasticized by Sorbitol and Reinforced with Starch Nanocrystals. Macromol. Biosci. 2007, 7, 1206–1216. [Google Scholar] [CrossRef]
- Lendvai, L.; Apostolov, A.; Karger-Kocsis, J. Characterization of layered silicate-reinforced blends of thermoplastic starch (TPS) and poly(butylene adipate-co-terephthalate). Carbohydr. Polym. 2017, 173, 566–572. [Google Scholar] [CrossRef]
- Rani, A.; Monga, S.; Bansal, M.; Sharma, A. Bionanocomposites reinforced with cellulose nanofibers derived from sugarcane bagasse. Polym. Compos. 2018, 39, E55–E64. [Google Scholar] [CrossRef]
- Sessini, V.; Raquez, J.-M.; Lourdin, D.; Maigret, J.-E.; Kenny, J.M.; Dubois, P.; Peponi, L. Humidity-Activated Shape Memory Effects on Thermoplastic Starch/EVA Blends and Their Compatibilized Nanocomposites. Macromol. Chem. Phys. 2017, 218, 1700388. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Chen, Y.; Chang, P.R.; Stumborg, M.; Huneault, M.A. Green composites reinforced with hemp nanocrystals in plasticized starch. J. Appl. Polym. Sci. 2008, 109, 3804–3810. [Google Scholar] [CrossRef] [Green Version]
- Raquez, J.-M.; Nabar, Y.; Narayan, R.; Dubois, P. Preparation and characterization of maleated thermoplastic starch-based nanocomposites. J. Appl. Polym. Sci. 2011, 122, 639–647. [Google Scholar] [CrossRef]
- Pitiphatharaworachot, S.; Chitbanyong, K.; Sungkaew, S.; Pisutpiched, S.; Khantayanuwong, S.; Puangsin, B. Starch nanocomposites reinforced with TEMPO-oxidized cellulose nanofibrils derived from bamboo holocellulose. BioResources 2019, 14, 2784–2797. [Google Scholar] [CrossRef]
- Qiao, X.; Jiang, W.; Sun, K. Reinforced Thermoplastic Acetylated Starch with Layered Silicates. Starch Stärke 2005, 57, 581–586. [Google Scholar] [CrossRef]
- García, N.L.; Famá, L.; D’Accorso, N.B.; Goyanes, S. Biodegradable Starch Nanocomposites. In Eco-Friendly Polymer Nanocomposites: Processing and Properties; Springer: Berlin/Heidelberg, Germany, 2015; pp. 17–77. ISBN 978-81-322-2469-3. [Google Scholar]
- Zicans, J.; Meri, R.M.; Kalnins, M.; Maksimovs, R.; Jansons, J. Modeling and experimental investigations of elastic and creep properties of thermoplastic polymer nanocomposites. ZAMM 2015, 95, 1110–1198. [Google Scholar] [CrossRef]
- Teacă, C.-A.; Bodîrlău, R. Multicomponent Polymer Composite/Nanocomposite Systems Using Polymer Matrices from Sustainable Renewable Sources. In Eco-Friendly Polymer Nanocomposites: Processing and Properties; Springer: Berlin/Heidelberg, Germany, 2015; pp. 469–494. ISBN 978-81-322-2469-3. [Google Scholar]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Garrido-Miranda, K.A.; Pereira, E.D. Release of essential oil constituent from thermoplastic starch/layered silicate bionanocomposite film as a potential active packaging material. Eur. Polym. J. 2018, 109, 64–71. [Google Scholar] [CrossRef]
- Arroyo, O.; Huneault, M.; Favis, B.; Bureau, M. Processing and properties of PLA/thermoplastic starch/montmorillonite nanocomposites. Polym. Compos. 2010, 31, 114–127. [Google Scholar] [CrossRef]
- Javanbakht, S.; Namazi, H. Solid state photoluminescence thermoplastic starch film containing graphene quantum dots. Carbohydr. Polym. 2017, 176, 220–226. [Google Scholar] [CrossRef]
- Sarsari, N.A.; Pourmousa, S.; Tajdini, A. Physical and Mechanical Properties of Walnut Shell Flour-Filled Thermoplastic Starch Composites. Bioresources 2016, 11, 6968–6983. [Google Scholar] [CrossRef] [Green Version]
- Mohseni, M.S.; Khalilzadeh, M.; Mohseni, M.; Hargalani, F.Z.; Getso, M.I.; Raissi, V.; Raiesi, O. Green synthesis of Ag nanoparticles from pomegranate seeds extract and synthesis of Ag-Starch nanocomposite and characterization of mechanical properties of the films. Biocatal. Agric. Biotechnol. 2020, 25, 101569. [Google Scholar] [CrossRef]
- Fu, D.; Netravali, A.N. Green composites based on avocado seed starch and nano- and micro-scale cellulose. Polym. Compos. 2020, 41, 4631–4648. [Google Scholar] [CrossRef]
- Lubis, M.; Harahap, M.B.; Hendra, M.; Ginting, S.; Sartika, M.; Azmi, H. Effect of Microcrystalline Cellulose (MCC) from Sugar Palm Fibres and Glycerol Addition on Mechanical Properties of Bioplastic from Avocado Seed Starch (Persea Americana Mill); Academic Fora: Kuala Lumpur, Malaysia, 2016; Volume 331, pp. 1–10. [Google Scholar]
- Lacerda, L.G.; Colman, T.A.D.; Bauab, T.; Da Silva Carvalho Filho, M.A.; Demiate, I.M.; De Vasconcelos, E.C.; Schnitzler, E. Thermal, structural and rheological properties of starch from avocado seeds (Persea americana, Miller) modified with standard sodium hypochlorite solutions. J. Therm. Anal. Calorim. 2014, 115, 1893–1899. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 20 October 2020).
- Ellis, R.P.; Cochrane, M.P.; Dale, M.F.B.; Duffus, C.M.; Lynn, A.; Morrison, I.M.; Prentice, R.D.M.; Swanston, J.S.; Tiller, S.A. Starch production and industrial use. J. Sci. Food Agric. 1998, 77, 289–311. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Nayak, S.K. Biodegradable PBAT/Starch Nanocomposites. Polym.-Plast. Technol. Eng. 2010, 49, 1406–1418. [Google Scholar] [CrossRef]
- Chung, Y.-L.; Lai, H.-M. Recent Progress in the Development of Starch-Layered Silicate Nanocomposites. In Handbook of Polymernanocomposites. Processing, Performance and Application; Springer: Berlin/Heidelberg, Germany, 2014; pp. 69–86. ISBN 9783642386497. [Google Scholar]
- Gallant, D.J.; Bouchet, B.; Baldwin, P.M. Microscopy of starch: Evidence of a new level of granule organization. Carbohydr. Polym. 1997, 32, 177–191. [Google Scholar] [CrossRef]
- Park, H.-M.; Lee, W.-K.; Park, C.-Y.; Cho, W.-J.; Ha, C.-S. Environmentally friendly polymer hybrids Part I Mechanical, thermal, and barrier properties of thermoplastic starch/clay nanocomposites. J. Mater. Sci. 2003, 38, 909–915. [Google Scholar] [CrossRef]
- Asrofi, M.; Abral, H.; Kasim, A.; Pratoto, A.; Mahardika, M.; Hafizulhaq, F. Mechanical Properties of a Water Hyacinth Nanofiber Cellulose Reinforced Thermoplastic Starch Bionanocomposite: Effect of Ultrasonic Vibration during Processing. Fibers 2018, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-M.; Li, X.; Jin, C.-Z.; Park, C.-Y.; Cho, W.-J.; Ha, C.-S. Preparation and Properties of Biodegradable Thermoplastic Starch/Clay Hybrids. Macromol. Mater. Eng. 2002, 287, 553–558. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Nabar, Y.; Narayan, R.; Dubois, P. New Developments in Biodegradable Starch-based Nanocomposites. Int. Polym. Process. 2007, 22, 463–470. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J. Crystallization of High-Amylose Starch by the Addition of Plasticizers at Low and Intermediate Concentrations. J. Food Sci. 2010, 75, N8–N16. [Google Scholar] [CrossRef]
- Carvalho, A.J. Starch: Major Sources, Properties and Applications as Thermoplastic Materials. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 321–342. [Google Scholar] [CrossRef]
- Hietala, M.; Mathew, A.P.; Oksman, K. Bionanocomposites of thermoplastic starch and cellulose nanofibers manufactured using twin-screw extrusion. Eur. Polym. J. 2013, 49, 950–956. [Google Scholar] [CrossRef]
- Van Soest, J.J.G.; Borger, D.B. Structure and properties of compression-molded thermoplastic starch materials from normal and high-amylose maize starches. J. Appl. Polym. Sci. 1997, 64, 631–644. [Google Scholar] [CrossRef]
- Osman, A.F.; Ashafee, A.M.T.; Adnan, S.A.; Alakrach, A. Influence of Hybrid Cellulose/Bentonite Fillers on Structure, Ambient, and Low Temperature Tensile Properties of Thermoplastic Starch Composites. Polym. Eng. Sci. 2020, 60, 810–822. [Google Scholar] [CrossRef]
- Aouada, F.A.; Mattoso, L.H.; Longo, E. New strategies in the preparation of exfoliated thermoplastic starch-montmorillonite nanocomposites. Ind. Crop. Prod. 2011, 34, 1502–1508. [Google Scholar] [CrossRef]
- Tunjano, V.; Salcedo, F.; Jiménez, I.; Medina, J.; Alvarez, O.; Prieto, E. Estudio de las propiedades térmicas y mecánicas del almidón termoplástico (TPS) reforzado con nanoarcilla. Rev. Latinoam. Metal. Mater. 2009, 1, 29–36. [Google Scholar]
- Brody, A.L.; Bugusu, B.; Han, J.H.; Sand, C.K.; McHugh, T.H. Innovative food packaging solutions. J. Food Sci. 2008, 73, 107–116. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R: Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Mehta, A.; Bhardwaj, K.K.; Gupta, R. Biodegradable Polymers for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9781536122527. [Google Scholar]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sarazin, P.; Orts, W.J.; Imam, S.H.; Favis, B.D. Biodegradation of Thermoplastic Starch and its Blends with Poly(lactic acid) and Polyethylene: Influence of Morphology. Macromol. Chem. Phys. 2011, 212, 1147–1154. [Google Scholar] [CrossRef]
- Savadekar, N.; Mhaske, S. Synthesis of nano cellulose fibers and effect on thermoplastics starch based films. Carbohydr. Polym. 2012, 89, 146–151. [Google Scholar] [CrossRef]
- Babaee, M.; Jonoobi, M.; Hamzeh, Y.; Ashori, A. Biodegradability and mechanical properties of reinforced starch nanocomposites using cellulose nanofibers. Carbohydr. Polym. 2015, 132, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Chen, Y.; Chang, P.R.; Huneault, M.A. Preparation and properties of plasticized starch/multiwalled carbon nanotubes composites. J. Appl. Polym. Sci. 2007, 106, 1431–1437. [Google Scholar] [CrossRef] [Green Version]
- Ramezani, H.; Behzad, T.; Bagheri, R. Synergistic effect of graphene oxide nanoplatelets and cellulose nanofibers on mechanical, thermal, and barrier properties of thermoplastic starch. Polym. Adv. Technol. 2020, 31, 553–565. [Google Scholar] [CrossRef]
- Zhang, C.-W.; Nair, S.S.; Chen, H.; Yan, N.; Farnood, R.; Li, F.-Y. Thermally stable, enhanced water barrier, high strength starch bio-composite reinforced with lignin containing cellulose nanofibrils. Carbohydr. Polym. 2020, 230, 115626. [Google Scholar] [CrossRef]
- Lisdayana, N.; Fahma, F.; Sunarti, T.C.; Iriani, E.S. Thermoplastic Starch–PVA Nanocomposite Films Reinforced with Nanocellulose from Oil Palm Empty Fruit Bunches (OPEFBs): Effect of Starch Type. J. Nat. Fibers 2020, 17, 1069–1080. [Google Scholar] [CrossRef]
- Kaushik, A.; Kaur, R. Thermoplastic starch nanocomposites reinforced with cellulose nanocrystals: Effect of plasticizer on properties. Compos. Interfaces 2016, 23, 701–717. [Google Scholar] [CrossRef]
- Kaushik, A.; Singh, M.; Verma, G. Green nanocomposites based on thermoplastic starch and steam exploded cellulose nanofibrils from wheat straw. Carbohydr. Polym. 2010, 82, 337–345. [Google Scholar] [CrossRef]
- Nasri-Nasrabadi, B.; Mehrasa, M.; Rafienia, M.; Bonakdar, S.; Behzad, T.; Gavanji, S. Porous starch/cellulose nanofibers composite prepared by salt leaching technique for tissue engineering. Carbohydr. Polym. 2014, 108, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Granda, L.A.; Oliver-Ortega, H.; Fabra, M.J.; Tarrés, Q.; Pèlach, M.À.; Lagarón, J.M.; Méndez, J.A. Improved Process to Obtain Nanofibrillated Cellulose (CNF) Reinforced Starch Films with Upgraded Mechanical Properties and Barrier Character. Polymers 2020, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Metzger, C.; Briesen, H. Thermoplastic Starch Nanocomposites Reinforced with Cellulose Nanocrystal Suspensions Containing Residual Salt from Neutralization. Macromol. Mater. Eng. 2021, 306, 2100161. [Google Scholar] [CrossRef]
- Gray, N.; Hamzeh, Y.; Kaboorani, A.; Abdulkhani, A. Influence of cellulose nanocrystal on strength and properties of low density polyethylene and thermoplastic starch composites. Ind. Crop. Prod. 2018, 115, 298–305. [Google Scholar] [CrossRef]
- Chen, D.; Lawton, D.; Thompson, M.; Liu, Q. Biocomposites reinforced with cellulose nanocrystals derived from potato peel waste. Carbohydr. Polym. 2012, 90, 709–716. [Google Scholar] [CrossRef] [Green Version]
- Vaezi, K.; Asadpour, G.; Sharifi, S.H. Bio nanocomposites based on cationic starch reinforced with montmorillonite and cellulose nanocrystals: Fundamental properties and biodegradability study. Int. J. Biol. Macromol. 2020, 146, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Wang, N. Production of Thermoplastic Starch/MMT-Sorbitol Nanocomposites by Dual-Melt Extrusion Processing. Macromol. Mater. Eng. 2007, 292, 723–728. [Google Scholar] [CrossRef]
- Chivrac, F.; Pollet, E.; Schmutz, M.; Avérous, L. New Approach to Elaborate Exfoliated Starch-Based Nanobiocomposites. Biomacromolecules 2008, 9, 896–900. [Google Scholar] [CrossRef]
- Müller, C.; Laurindo, J.B.; Yamashita, F. Composites of thermoplastic starch and nanoclays produced by extrusion and thermopressing. Carbohydr. Polym. 2012, 89, 504–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, S.C.; Salcedo, F. Gelatinization and retrogradation phenomena in starch/montmorillonite nanocomposites plasticized with different glycerol/water ratios. Carbohydr. Polym. 2016, 151, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Boonprasith, P.; Wootthikanokkhan, J.; Nimitsiriwat, N. Mechanical, thermal, and barrier properties of nanocomposites based on poly(butylene succinate)/thermoplastic starch blends containing different types of clay. J. Appl. Polym. Sci. 2013, 130, 1114–1123. [Google Scholar] [CrossRef]
- Katerinopoulou, K.; Giannakas, A.; Grigoriadi, K.; Barkoula, N.M.; Ladavos, A. Preparation and characterization of acetylated corn starch–(PVOH)/clay nanocomposite films. Carbohydr. Polym. 2014, 102, 216–222. [Google Scholar] [CrossRef]
- Nistor, M.-T.; Vasile, C. TG/FTIR/MS study on the influence of nanoparticles content upon the thermal decomposition of starch/poly(vinyl alcohol) montmorillonite nanocomposites. Iran. Polym. J. 2013, 22, 519–536. [Google Scholar] [CrossRef]
- Dai, H.; Chang, P.R.; Geng, F.; Yu, J.; Ma, X. Preparation and Properties of Thermoplastic Starch/Montmorillonite Nanocomposite Using N-(2-Hydroxyethyl)formamide as a New Additive. J. Polym. Environ. 2009, 17, 225–232. [Google Scholar] [CrossRef]
- Paglicawan, M.A.; Basilia, B.A.; Navarro, M.T.V.; Emolaga, C.S. Influence of Nanoclay on the Properties of Thermoplastic Starch/Poly(lactic acid) Blends. J. Biobased Mater. Bioenergy 2013, 7, 102–107. [Google Scholar] [CrossRef]
- Dean, K.M.; Petinakis, E.; Goodall, L.; Miller, T.; Yu, L.; Wright, N. Nanostabilization of thermally processed high amylose hydroxylpropylated starch films. Carbohydr. Polym. 2011, 86, 652–658. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Liu, H.; Wang, N. Impact of Pre-Processing of Montmorillonite on the Properties of Melt-Extruded Thermoplastic Starch/Montmorillonite Nanocomposites. Starch Stärke 2009, 61, 489–494. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Han, N.; Bai, S. Effect of citric acid and processing on the performance of thermoplastic starch/montmorillonite nanocomposites. Carbohydr. Polym. 2009, 76, 68–73. [Google Scholar] [CrossRef]
- Dean, K.M.; Do, M.D.; Petinakis, E.; Yu, L. Key interactions in biodegradable thermoplastic starch/poly(vinyl alcohol)/montmorillonite micro- and nanocomposites. Compos. Sci. Technol. 2008, 68, 1453–1462. [Google Scholar] [CrossRef]
- Dean, K.; Yu, L.; Wu, D.Y. Preparation and characterization of melt-extruded thermoplastic starch/clay nanocomposites. Compos. Sci. Technol. 2007, 67, 413–421. [Google Scholar] [CrossRef]
- Huang, M.; Yu, J.; Ma, X. High mechanical performance MMT-urea and formamide-plasticized thermoplastic cornstarch biodegradable nanocomposites. Carbohydr. Polym. 2006, 63, 393–399. [Google Scholar] [CrossRef]
- De Souza, A.G.; dos Santos, N.M.A.; da Silva Torin, R.F.; dos Santos Rosa, D. Synergic antimicrobial properties of Carvacrol essential oil and montmorillonite in biodegradable starch films. Int. J. Biol. Macromol. 2020, 164, 1737–1747. [Google Scholar] [CrossRef]
- Majdzadeh-Ardakani, K.; Navarchian, A.H.; Sadeghi, F. Optimization of mechanical properties of thermoplastic starch/clay nanocomposites. Carbohydr. Polym. 2010, 79, 547–554. [Google Scholar] [CrossRef]
- Bocchini, S.; Battegazzore, D.; Frache, A. Poly (butylensuccinate co-adipate)-thermoplastic starch nanocomposite blends. Carbohydr. Polym. 2010, 82, 802–808. [Google Scholar] [CrossRef]
- Mansour, G.; Zoumaki, M.; Marinopoulou, A.; Tzetzis, D.; Prevezanos, M.; Raphaelides, S.N. Characterization and properties of non-granular thermoplastic starch—Clay biocomposite films. Carbohydr. Polym. 2020, 245, 116629. [Google Scholar] [CrossRef]
- Taghizadeh, M.T.; Abbasi, Z.; Nasrollahzade, Z. Study of enzymatic degradation and water absorption of nanocomposites starch/polyvinyl alcohol and sodium montmorillonite clay. J. Taiwan Inst. Chem. Eng. 2012, 43, 120–124. [Google Scholar] [CrossRef]
- Chen, M.; Chen, B.; Evans, J.R.G. Novel thermoplastic starch—Clay nanocomposite foams. Nanotechnology 2005, 16, 2334–2337. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Evans, J.R. Thermoplastic starch–clay nanocomposites and their characteristics. Carbohydr. Polym. 2005, 61, 455–463. [Google Scholar] [CrossRef]
- Bagdi, K.; Müller, P.; Pukánszky, B. Thermoplastic starch/layered silicate composites: Structure, interaction, properties. Compos. Interfaces 2006, 13, 1–17. [Google Scholar] [CrossRef]
- Ayana, B.; Suin, S.; Khatua, B. Highly exfoliated eco-friendly thermoplastic starch (TPS)/poly (lactic acid)(PLA)/clay nanocomposites using unmodified nanoclay. Carbohydr. Polym. 2014, 110, 430–439. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Hrymak, A.; Han, J.H. Characterization of Extruded Thermoplastic Starch Reinforced by Montmorillonite Nanoclay. J. Polym. Environ. 2013, 21, 122–131. [Google Scholar] [CrossRef]
- Derungs, I.; Rico, M.; López, J.; Barral, L.; Montero, B.; Bouza, R. Influence of the hydrophilicity of montmorillonite on structure and properties of thermoplastic wheat starch/montmorillonite bionanocomposites. Polym. Adv. Technol. 2021. [Google Scholar] [CrossRef]
- Mondragón, M.; Hernández, E.; Rivera-Armenta, J.; Rodríguez-González, F. Injection molded thermoplastic starch/natural rubber/clay nanocomposites: Morphology and mechanical properties. Carbohydr. Polym. 2009, 77, 80–86. [Google Scholar] [CrossRef]
- Huang, M.-F.; Yu, J.-G.; Ma, X.-F. Studies on the properties of Montmorillonite-reinforced thermoplastic starch composites. Polymer 2004, 45, 7017–7023. [Google Scholar] [CrossRef]
- Behera, A.K. Mechanical and biodegradation analysis of thermoplastic starch reinforced nano-biocomposites. IOP Conf. Ser. Mater. Sci. Eng. 2018, 410, 012001. [Google Scholar] [CrossRef] [Green Version]
- Hanifi, S.; Oromiehie, A.; Ahmadi, S.; Farhadnejad, H. (Corn Starch and Montmorillonite Nanocomposite)-Reinforced Polypropylene: Preparation, Properties, and Biodegradability. J. Vinyl Addit. Technol. 2014, 20, 16–23. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Najafi, F.S.A.; Shahabadi, I.; Garmabi, H. A response surface study on microstructure and mechanical properties of poly(lactic acid)/thermoplastic starch/nanoclay nanocomposites. J. Compos. Mater. 2016, 50, 269–278. [Google Scholar] [CrossRef]
- Magalhães, N.F.; De Andrade, C.T. Properties of melt-processed poly(hydroxybutyrate-co-hydroxyvalerate)/starch 1:1 blend nanocomposites. Polímeros 2013, 23, 366–372. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-X.; Yu, Z.-Z.; Xie, X.-L.; Naito, K.; Kagawa, Y. Preparation and crystalline morphology of biodegradable starch/clay nanocomposites. Polymer 2007, 48, 7193–7200. [Google Scholar] [CrossRef]
- Park, H.-M.; Kim, G.-H.; Ha, C.-S. Preparation and characterization of biodegradable aliphatic polyester/thermoplastic starch/organoclay ternary hybrid nanocomposites. Compos. Interfaces 2007, 14, 427–438. [Google Scholar] [CrossRef]
- Gao, W.; Liu, P.; Li, X.; Qiu, L.; Hou, H.; Cui, B. The co-plasticization effects of glycerol and small molecular sugars on starch-based nanocomposite films prepared by extrusion blowing. Int. J. Biol. Macromol. 2019, 133, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, B.; Behzad, T.; Salehinik, F.; Zamani, A.; Heidarian, P. Incorporation of Extracted Mucor indicus Fungus Chitin Nanofibers into Starch Biopolymer: Morphological, Physical, and Mechanical Evaluation. Starch-Stärke 2021, 73, 2000218. [Google Scholar] [CrossRef]
- Olivato, J.; Marini, J.; Pollet, E.; Yamashita, F.; Grossmann, M.; Avérous, L. Elaboration, morphology and properties of starch/polyester nano-biocomposites based on sepiolite clay. Carbohydr. Polym. 2015, 118, 250–256. [Google Scholar] [CrossRef]
- Ceballos, R.L.; von Bilderling, C.; Guz, L.; Bernal, C.; Famá, L. Effect of greenly synthetized silver nanoparticles on the properties of active starch films obtained by extrusion and compression molding. Carbohydr. Polym. 2021, 261, 117871. [Google Scholar] [CrossRef]
- Da Silva, G.L.P.; Morais, L.C.D.A.; Olivato, J.B.; Marini, J.; Ferrari, P.C. Antimicrobial dressing of silver sulfadiazine-loaded halloysite/cassava starch-based (bio)nanocomposites. J. Biomater. Appl. 2021, 35, 1096–1108. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R.; Pollet, E.; Avérous, L. Morphology and properties of thermoplastic starch blended with biodegradable polyester and filled with halloysite nanoclay. Carbohydr. Polym. 2020, 242, 116392. [Google Scholar] [CrossRef]
- Peres, A.M.; Orefice, R. Effect of incorporation of Halloysite nanotubes on the structure and properties of low-density polyethylene/thermoplastic starch blend. J. Polym. Res. 2020, 27, 1–10. [Google Scholar] [CrossRef]
- Aouada, F.; Mattoso, L.H.; Longo, E. A simple procedure for the preparation of laponite and thermoplastic starch nanocomposites: Structural, mechanical, and thermal characterizations. J. Thermoplast. Compos. Mater. 2013, 26, 109–124. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, L.; Chen, M.; Yu, J. Effect of carboxylate multi-walled carbon nanotubes on the performance of thermoplastic starch nanocomposites. Carbohydr. Polym. 2011, 83, 447–451. [Google Scholar] [CrossRef]
- Merino, D.; Gutiérrez, T.; Alvarez, V.A. Structural and Thermal Properties of Agricultural Mulch Films Based on Native and Oxidized Corn Starch Nanocomposites. Starch Stärke 2019, 71, 1–9. [Google Scholar] [CrossRef]
- Castillo, L.A.; López, O.V.; García, M.A.; Barbosa, S.E.; Villar, M.A. Crystalline morphology of thermoplastic starch/talc nanocomposites induced by thermal processing. Heliyon 2019, 5, e01877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, L.; López, O.; López, C.; Zaritzky, N.; García, M.A.; Barbosa, S.; Villar, M. Thermoplastic starch films reinforced with talc nanoparticles. Carbohydr. Polym. 2013, 95, 664–674. [Google Scholar] [CrossRef]
- Taherimehr, M.; Bagheri, R.; Taherimehr, M. In-vitro evaluation of thermoplastic starch/ beta-tricalcium phosphate nano-biocomposite in bone tissue engineering. Ceram. Int. 2021, 47, 15458–15463. [Google Scholar] [CrossRef]
- Trinh, B.M.; Chang, C.C.; Mekonnen, T.H. Facile fabrication of thermoplastic starch/poly (lactic acid) multilayer films with superior gas and moisture barrier properties. Polymer 2021, 223, 123679. [Google Scholar] [CrossRef]
- Yahia, R.; Owda, M.E.; Abou-Zeid, R.E.; Abdelhai, F.; Gad, E.S.; Saleh, A.K.; El-Gamil, H.Y. Synthesis and characterization of thermoplastic starch/ PVA /cardanol oil composites loaded with in-situ silver nanoparticles. J. Appl. Polym. Sci. 2021, 2021, 51511. [Google Scholar] [CrossRef]
- Ochigbo, S.S.; Luyt, A.S.; Mofokeng, J.P.; Antić, Ž.; Dramićanin, M.D.; Djoković, V. Dynamic mechanical and thermal properties of the composites of thermoplastic starch and lanthanum hydroxide nanoparticles. J. Appl. Polym. Sci. 2013, 127, 699–709. [Google Scholar] [CrossRef]
- Kwaśniewska, A.; Chocyk, D.; Gładyszewski, G.; Borc, J.; Świetlicki, M.; Gładyszewska, B. The Influence of Kaolin Clay on the Mechanical Properties and Structure of Thermoplastic Starch Films. Polymers 2020, 12, 73. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Zhang, M.; Qian, P.; Zhu, Q. Preparation and characterization of thermoplastic starch-kaolinite nanocomposite films. Polym. Compos. 2012, 33, 889–896. [Google Scholar] [CrossRef]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of Bioplastic Packaging Materials: An Overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.J.; Haugaard, V.; Festersen, R.; Bertelsen, G. Production and applications of biobased packaging materials for the food industry. Food Addit. Contam. 2002, 19 (Supp. S1), 172–177. [Google Scholar] [CrossRef] [PubMed]



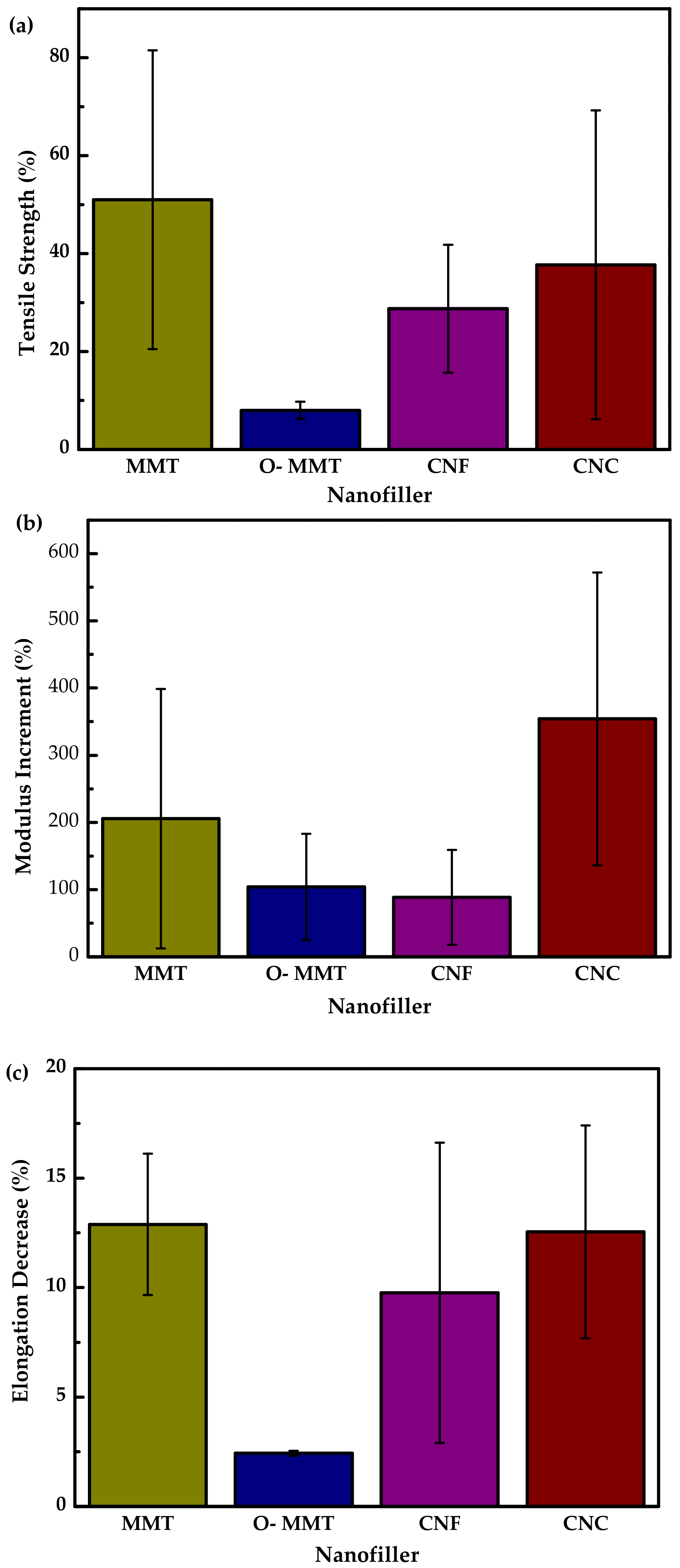
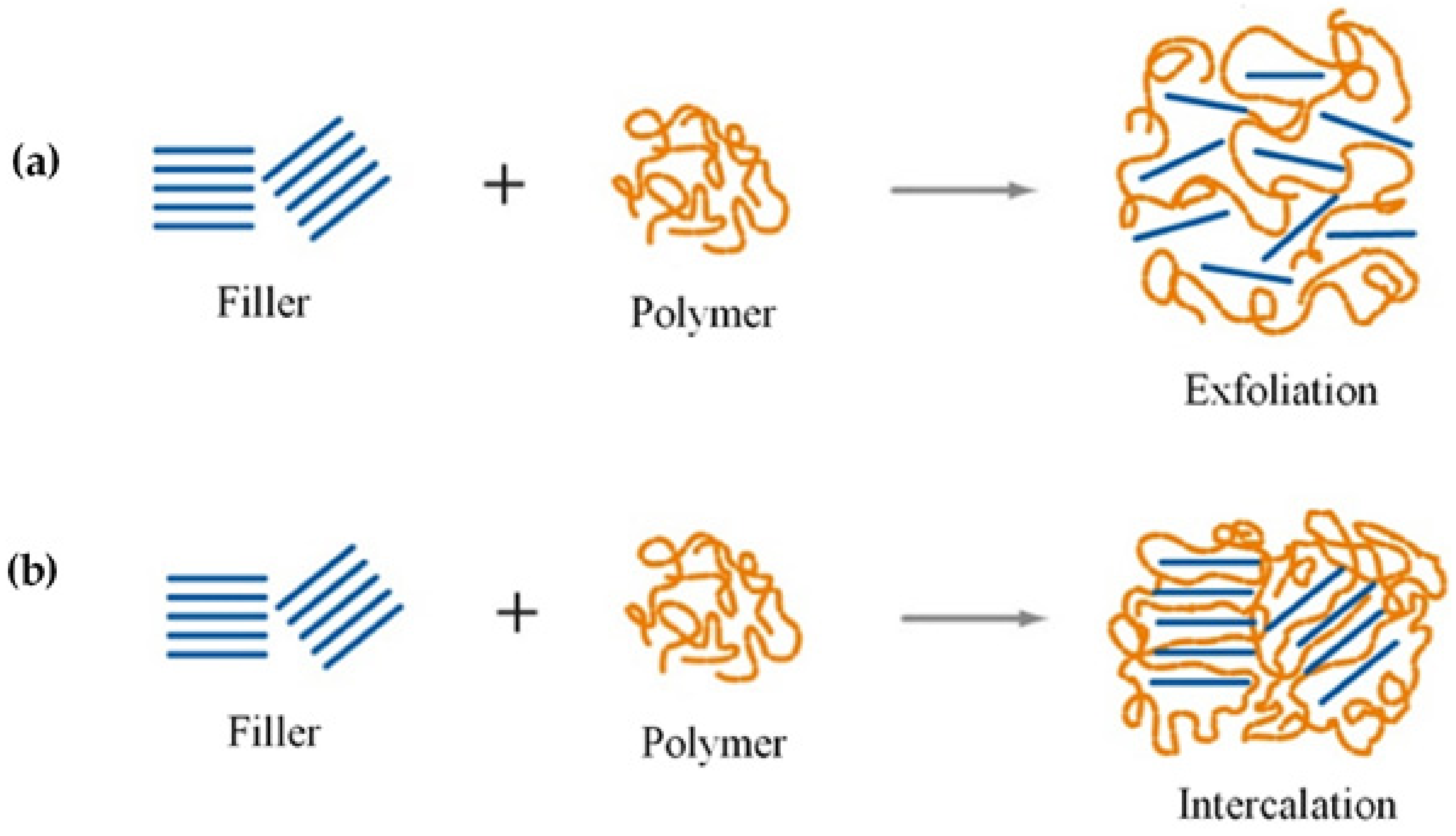
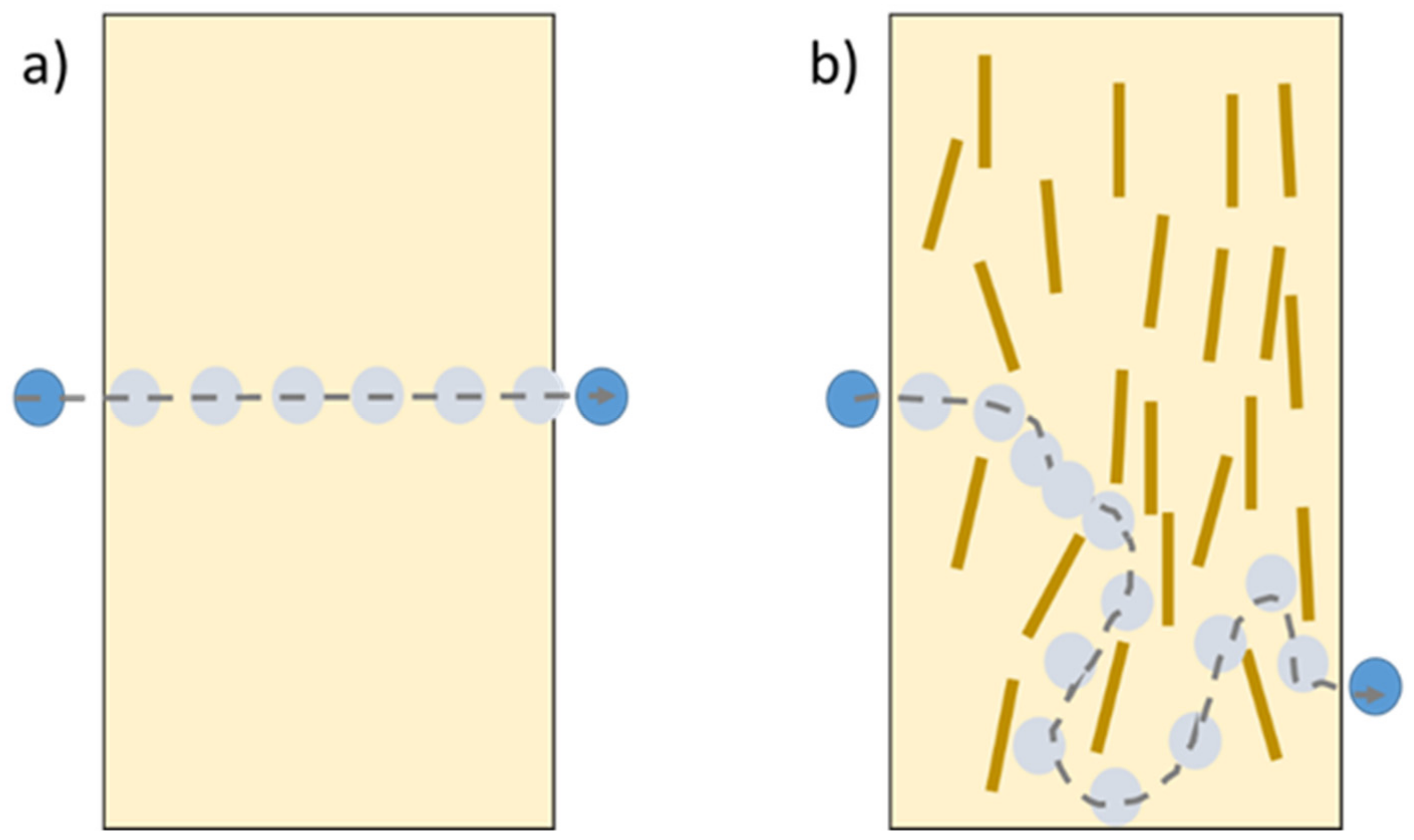
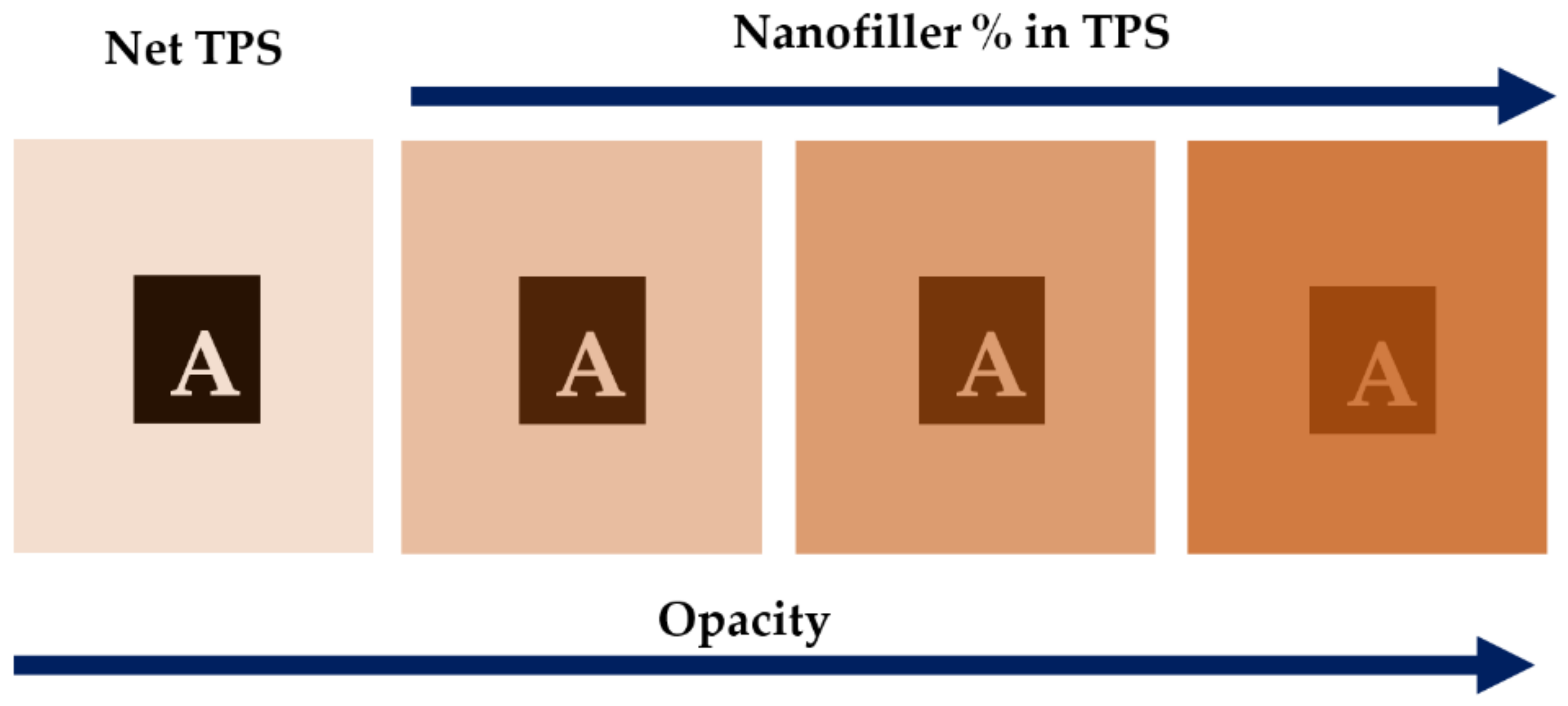

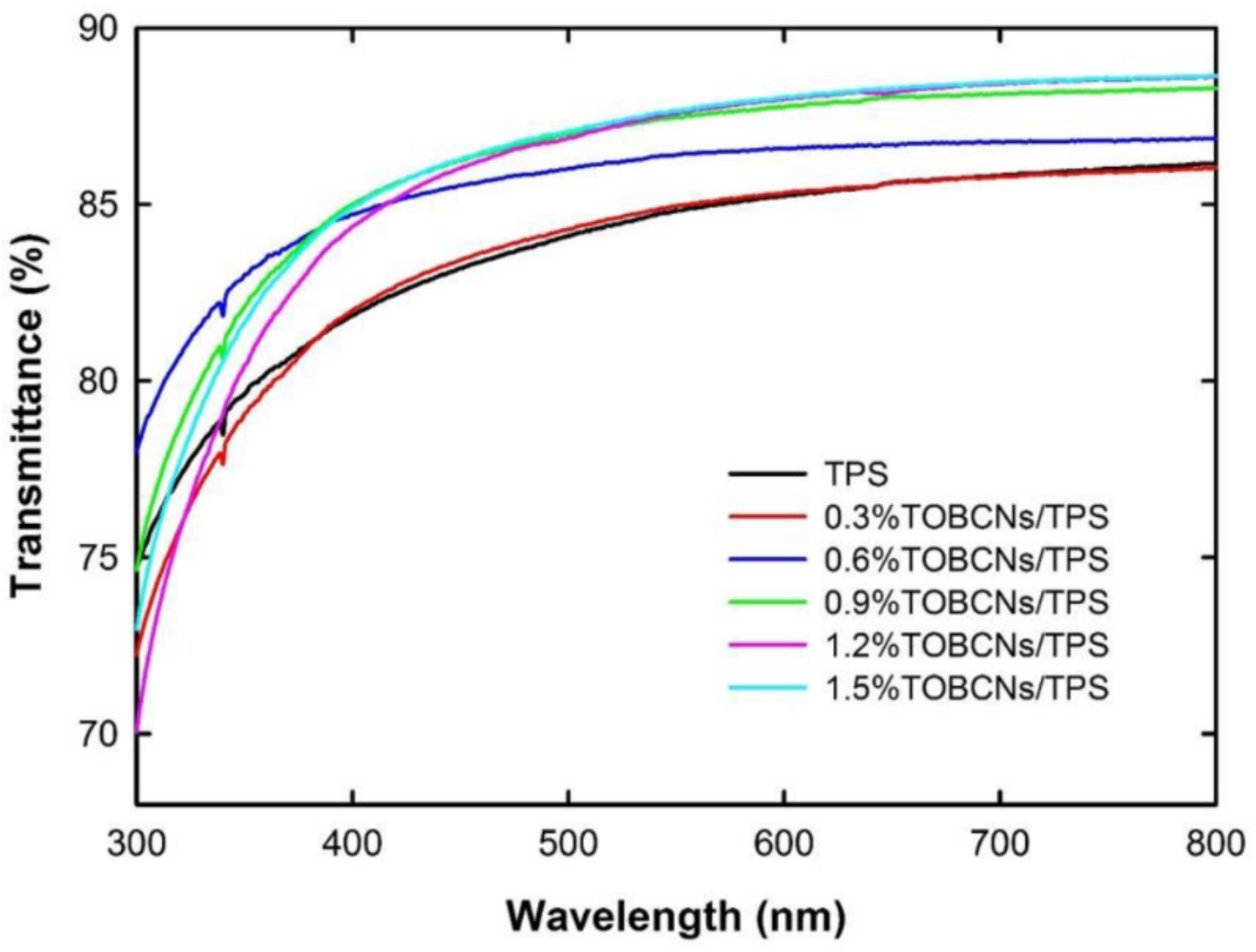
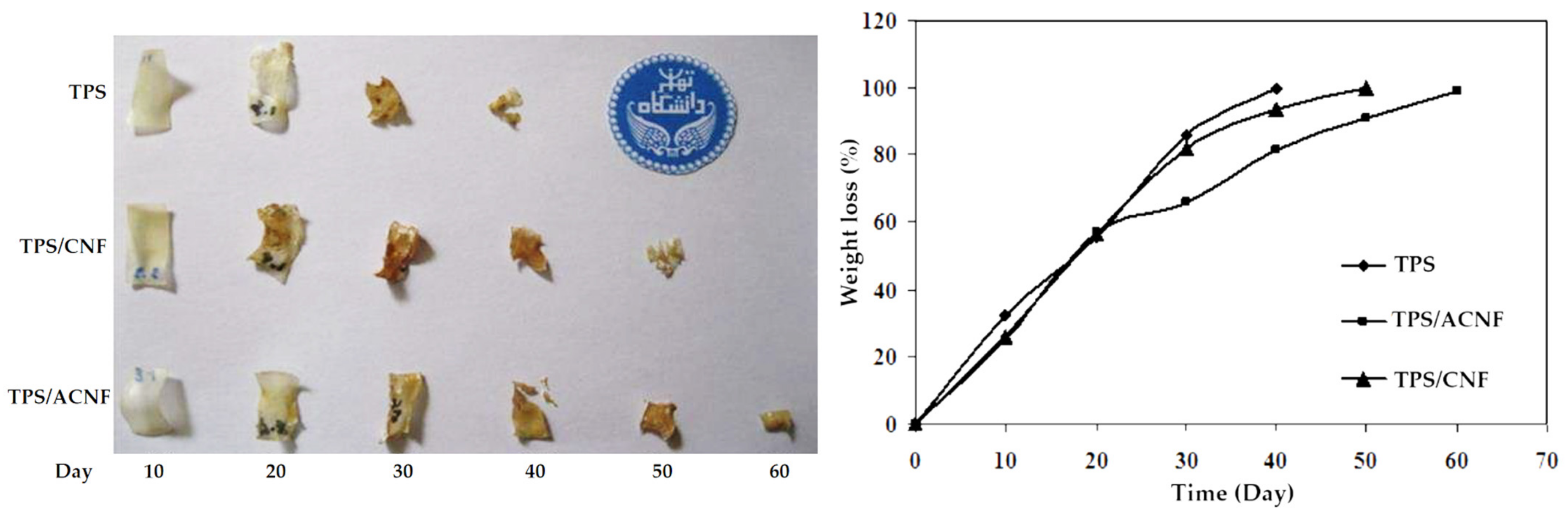

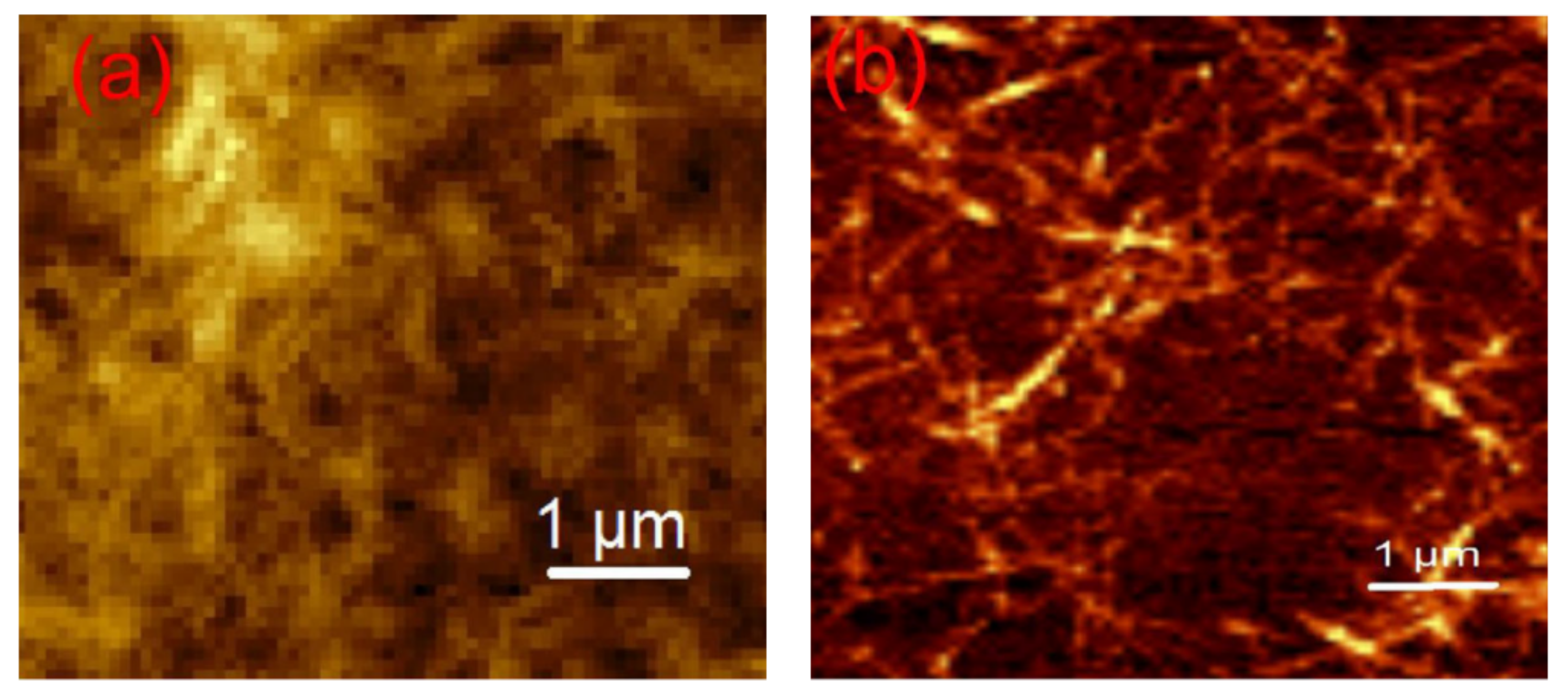
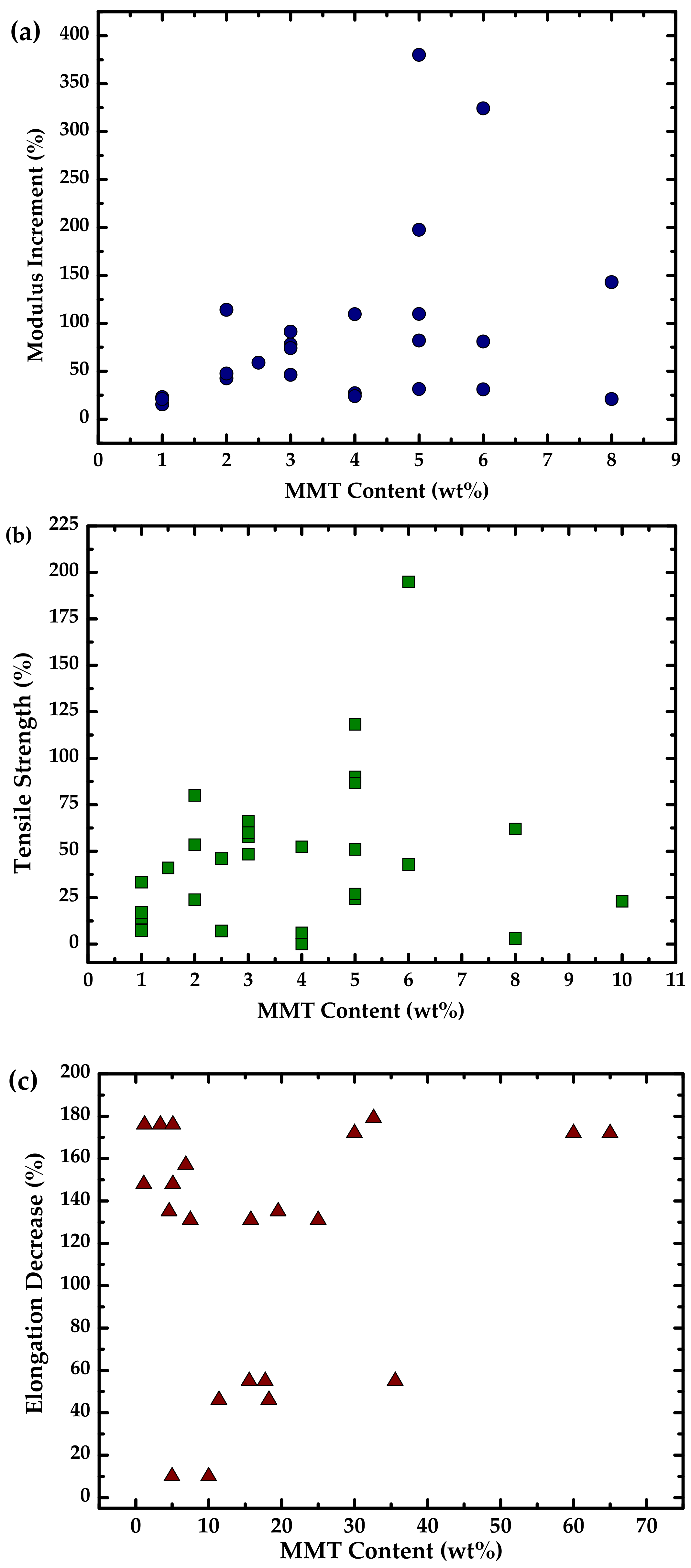
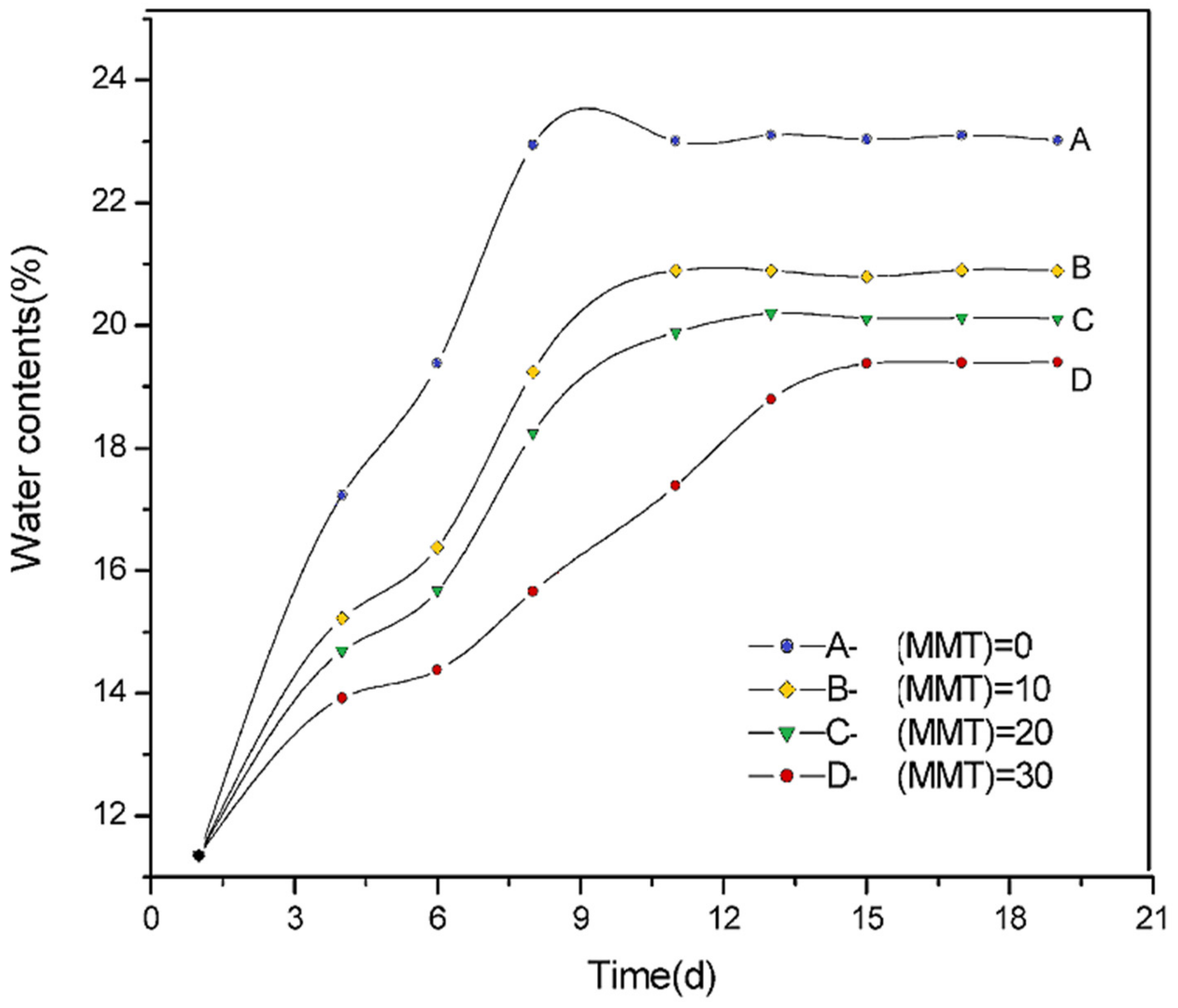

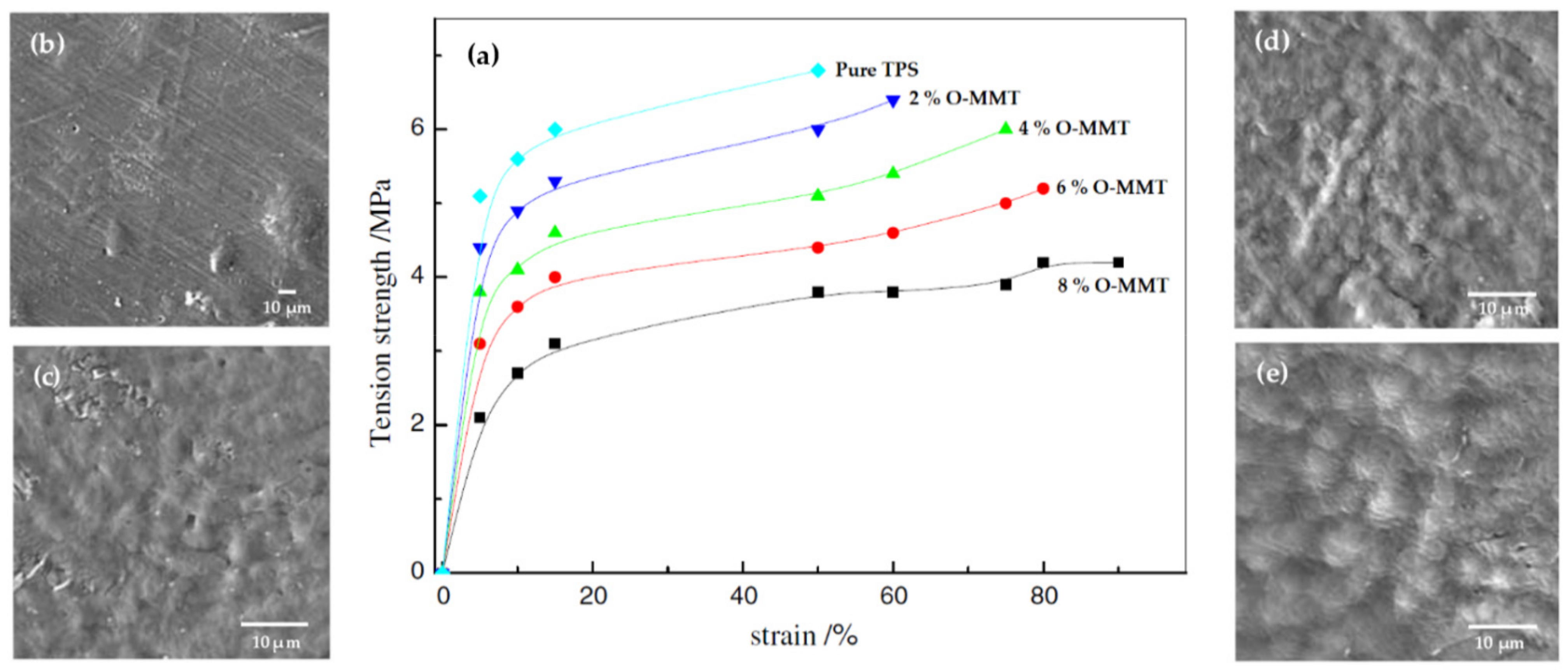
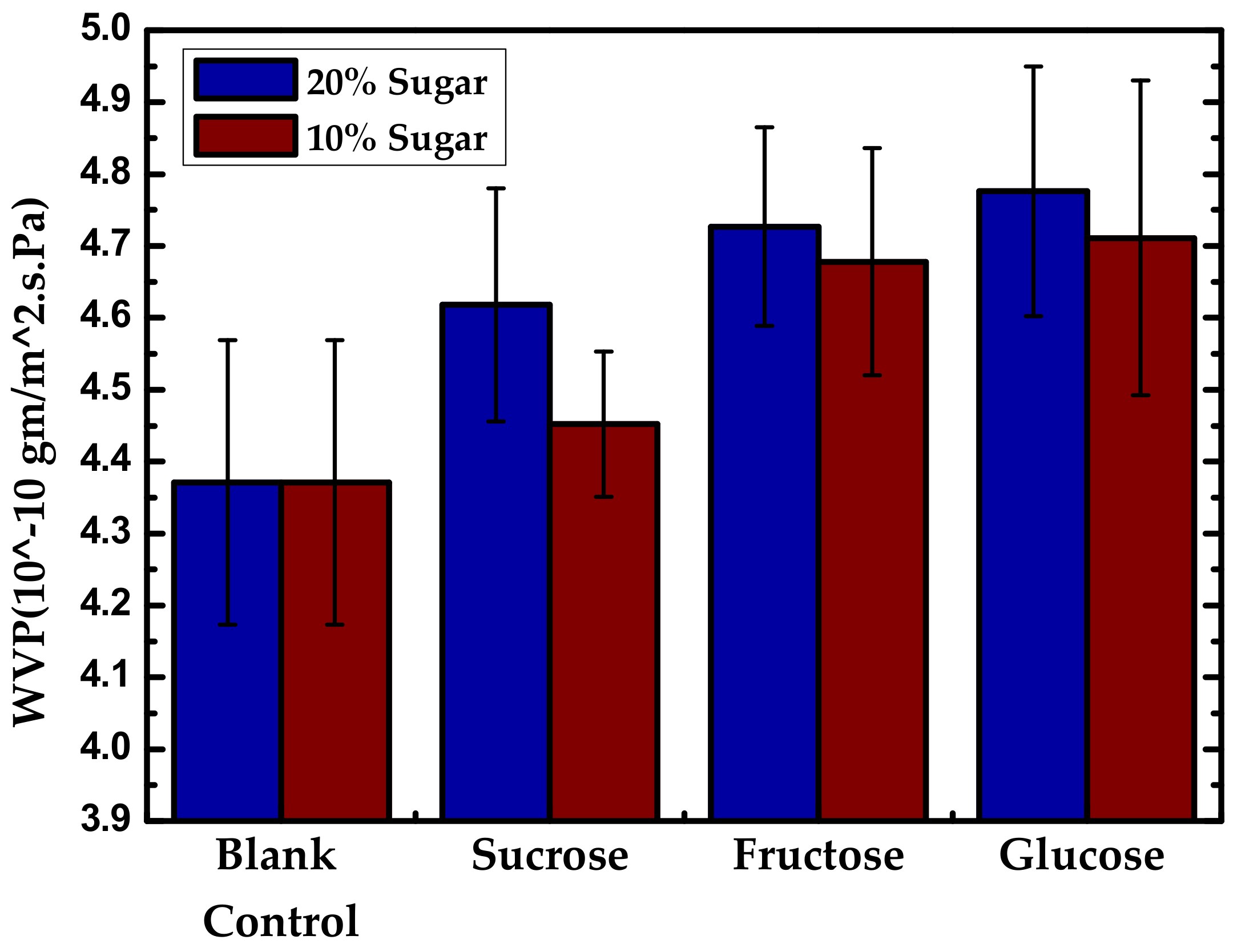
| Starch Source | Plasticizer | Nanofiller | Wt % | Reference |
|---|---|---|---|---|
| NI | Glycerol | Cotton nanofibers | 0.1–1 | [116] |
| Cassava | Glycerol/Sorbitol (1:1) | Cellulose cassava bagasse nanofibers | 5–20 | [70] |
| Corn | Glycerol | Cellulose nanofibers (eucalyptus pulp) | 2–15 | [33] |
| Glycerol | Cellulose nanofibers | 2–12 | [35] | |
| Glycerol | Cellulose nanofibers | 1–30 | [36] | |
| Glycerol | Cellulose nanofibers | 5 | [12] | |
| Glycerol | Cellulose nanofibers | 10 | [117] | |
| Glycerol | Cotton cellulose nanofibers | 0.5–5 | [118] | |
| Glycerol | Graphene oxide nanoplatelets, Cellulose nanofibers | 1–5, 5–15 | [119] | |
| Glycerol | Lignin cellulose nanofibers | 5–15 | [120] | |
| Corn, cassava, sago | Glycerol, formaldehyde | Oil palm empty fruit bunches cellulose nanofibers | 1–3 | [121] |
| Maize | Sorbitol | Sugarcane bagasse cellulose Nanofibers | 4–20 | [77] |
| Glycerol and sorbitol | Cotton nanofibers | 5–20 | [122] | |
| Glycerol | Cellulose nanofibers | 5–15 | [123] | |
| Merck-modified starch | Glycerol | Rice straw cellulose nanofibers | 5–15 | [124] |
| Potato | Glycerol | Cellulose nanofibers and nanocrystals | 1–3 | [37] |
| Water/Glycerol | Sisal cellulose nanofibers | 2.5–20 | [62] | |
| Glycerol | Wheat straw cellulose nanofibers | 2–10 | [64] | |
| Glycerol | Pineapple leaf cellulose nanofibers | 1–4 | [52] | |
| D-Sorbitol | Cellulose nanofibers | 5–20 | [106] | |
| Glycerol | Bleached eucalyptus pulp cellulose nanofibers | 0.18–0.36 | [125] | |
| Tapioca | Glycerol | Cellulose nanofibers | 0.3–1.5 | [81] |
| Waxy maize | Glycerol | Cellulosic nanofibers | 2–10 | [73] |
| Starch Source | Plasticizer | Nanofiller | Wt % | Reference |
|---|---|---|---|---|
| NI | Glycerol | Cellulose nanocrystals | 5 | [126] |
| Corn | Glycerol | Waxy corn starch nanocrystals Cellulose nanocrystals | 1–5 | [40] |
| Glycerol | Cellulose nanocrystal | 1–2 | [127] | |
| Glycerol | Starch nanocrystals | 1–2 | [41] | |
| Field pea | Glycerol, concentrated sulfuric acid, and sodium hypochlorite solution | Hemp cellulose nanocrystals | 5–30 | [79] |
| Maize | Glycerol | Waxy starch nanocrystals (WSNC)/Cellulose cellulose nanocrystals | 1–5 | [38] |
| Glycerol | Cotton cellulose nanocrystals | 4–8 | [74] | |
| Glycerol | Cellulose nanocrystals | 5–25 | [75] | |
| Glycerol | Waxy maize starch nanocrystals | 2.5 | [42] | |
| Potato | Glycerol | Cellulose nanocrystals | 1.5–10 | [34] |
| Glycerol | Cellulose nanofibers and nanocrystals | 1–3 | [37] | |
| Glycerol | Cellulose nanocrystals | 1–2 | [128] | |
| Glycerol | Waxy maize nanocrystals | 5–15 | [39] | |
| Potato, corn, pea | Glycerol | Cellulose nanocrystals | 2–5 | [5] |
| Starch Source | Plasticizer | Nanofiller | wt % | Reference |
|---|---|---|---|---|
| Acetylated cassava | Water | Montmorillonite | 1–10 | [69] |
| Cassava | Glycerol | Montmorillonite, alumina trihydrate | 26–37, 1–6 | [67] |
| Glycerol | Montmorillonite | 3–5 | [133] | |
| Glycerol | Montmorillonite, Cloisite 30B | 5 | [68] | |
| Glycerol | Na-montmorillonite (Closite® Na+) | 1–2 | [71] | |
| Glycerol | Montmorillonite | 2–4 | [134] | |
| Glycerol | Sodium montmorillonite, modified organo-montmorillonite | NI | [135] | |
| Cationic starch | Glycerol | Sodium montmorillonite, ZnO | 3–5, 0.5–1 | [44] |
| Cereal | Glycerol | Montmorillonite/Chitosan | 3–5/0.6–1 | [49] |
| Corn | Glycerol | Sodium montmorillonite | 1 | [43] |
| Glycerol/water | Montmorillonite clay | 3–4.5 | [86] | |
| Glycerol/water | Hydrophilic bentonite, sodium montmorillonite/Essential oils constituents | 0.5 | [47] | |
| Glycerol | Walnut shell flour/Montmorillonite (MMT) | 30–50/3–5 | [89] | |
| Glycerol | Montmorillonite | 0–5 | [83] | |
| Glycerol | Montmorillonite | 1–6 | [84] | |
| Glycerol | Sodium montmorillonite | 3–5 | [136] | |
| Glycerol | Montmorillonite | 1–5 | [137] | |
| Glycerol | Sodium montmorillonite | 2–5 | [138] | |
| Glycerol | Sodium montmorillonite | 2–8 | [138] | |
| Glycerol | Montmorillonite clay | 1–5 | [51] | |
| Glycerol/water | Montmorillonite clay | 1–9 | [139] | |
| Glycerol | Montmorillonite clay | 1–5 | [109] | |
| Water | Sodium montmorillonite clay | 5 | [140] | |
| Glycerol | Montmorillonite (natural and glycerol-activated) | 1–9 | [141] | |
| Glycerol | Natural montmorillonite | 2–6 | [97] | |
| Glycerol | Sodium montmorillonite | 1–9 | [142] | |
| Water, partially hydrolyzed polyvinyl alcohol | Natural montmorillonite | 1–5 | [143] | |
| Sorbitol, formamide | Sodium montmorillonite | 2–10 | [131] | |
| Water | Natural montmorillonite, fluorohectorite | 1–3.2 | [144] | |
| Citric acid, formamide, and ethanolamine | Sodium montmorillonite | 2–10 | [145] | |
| Glycerol | Montmorillonite | 0.03–0.1 (g) | [146] | |
| Corn, wheat, potato | Glycerol | Natural montmorillonite, Cloisite 30B | 3–15 | [147] |
| Granular Maize | Glycerol | Montmorillonite | 1–7 | [72] |
| Maize | Glycerol | Natural montmorillonite, Cloisite 30B | 5 | [148] |
| Glycerol | Montorillonite | 10–20 | [149] | |
| Merck starch | Glycerol | Natural montmorillonite | 1–5 | [150] |
| Pearl silver corn starch | Glycerol | Natural montmorillonite, Cloisite 30B | 1, 1–5 | [80] |
| Potato | Glycerol | Sodium montmorillonite | 2–5 | [63] |
| Urea | Montmorillonite | [151] | ||
| Glycerol | Montmorillonite, kaolinite, hectorite and treated hectorite | 6–22/5–18/5–20/5–19 | [152] | |
| Glycerol | Organically modified montmorillonite (Cloisite 30B), Natural montmorillonite Sodium montmorillonite | 2.5–10 | [153] | |
| Glycerol | Sodium montmorillonite | 2–5 | [63] | |
| Glycerol/water | Sodium montmorillonite | 1–1.5 | [154] | |
| Glycerol | Montmorillonite | 4–8 | [155] | |
| Glycerol/water | Cloisite 30B, Cloisite 10A, Cloisite 6A and Sodium montmorillonite | 5 | [102] | |
| Sweet potato | Carbamide and ethanolamine | Sodium montmorillonite | 2–8 | [148] |
| Tapioca (Acetylated) | Glycerol | Natural and organically modified montmorillonite | 5 | [150] |
| Wheat | Glycerol | Sodium montmorillonite, Aminododecanoic-acid-treated organophilic clays | Silicate content: 0.5–7 (vol) | [80] |
| Water/Glycerol | Montmorillonite | 2–5 | [87] | |
| Glycerol | Montmorillonite, Cloisite 30B, Cloisite 10A | 1–5 | [156] |
| Starch Source | Plasticizer | Nanofiller | wt % | Reference |
|---|---|---|---|---|
| NI | Glycerol | Cloisite 30B | 3 | [160] |
| Cassava | Glycerol | Montmorillonite, Cloisite 30B | 5 | [68] |
| Glycerol | Sodium montmorillonite, modified organo-montmorillonite | NI | [135] | |
| Corn | Glycerol | Cloisite 30B | 1–5 | [48] |
| Sorbitol | Closite 30B | 1–5 | [161] | |
| Glycerol | Montmorillonite clay | 1–5 | [51] | |
| Glycerol | Cloisite 30B | 2.5–10 | [162] | |
| Glycerol | Montmorillonite (natural and glycerol-activated) | 1–9 | [141] | |
| Glycerol | Pristine clay (p-clay), Cloisite 93A | 3 | [163] | |
| Corn, wheat, potato | Glycerol | Natural montmorillonite, Cloisite 30B | 3–15 | [147] |
| Maize | Glycerol/distilled water | Bentonite and organically modified montmorillonite | 40–50 | [76] |
| Glycerol | Natural montmorillonite, Cloisite 30B | 5 | [148] | |
| Pearl silver corn starch | Glycerol | Natural montmorillonite, Cloisite 30B | 1, 1–5 | [80] |
| Potato | Glycerol/water | Cloisite (organoclay) | 5 | [164] |
| Glycerol | Cloisite 30B, natural sodium montmorillonite | 2.5–10 | [100] | |
| Glycerol/water | Cloisite 30B, Cloisite 10A, Cloisite 6A and Sodium montmorillonite | 5 | [102] | |
| Tapioca (Acetylated) | Glycerol | Natural and organically modified montmorillonite | 5 | [82] |
| Wheat | Glycerol | Sodium montmorillonite, aminododecanoic-acid-treated organophilic clays | Silicate content: 0.5–7 (vol) | [153] |
| Glycerol | Montmorillonite, Cloisite 30B, Cloisite 10A | 1–5 | [156] |
| Starch Source | Plasticizer | Nanofiller | wt % | Reference |
|---|---|---|---|---|
| NI | Glycerol | Chitin nanofibers | 3–10 | [166] |
| Glycerol | Silver nanoparticles | 0.5–1 | [90] | |
| Cassava | Glycerol | Chitosan-modified Veegum® HS clay (smectite) | 2.5–5 | [65] |
| Glycerol | Sepiolite | 1–5 | [66] | |
| Glycerol | Sepiolite | 1–5 | [167] | |
| Glycerol | Silver nanoparticles | 0.006–0.15 | [168] | |
| Glycerol | Halloysite nanotubes | 2 | [169] | |
| Glycerol | Halloysite nanoclay | 1–5 | [170] | |
| Glycerol | Halloysite nanotubes | 2–8 | [171] | |
| Corn | Glycerol | Talc nanoparticles | 1–5 | [87] |
| Glycerol | Graphene quantum dots (GQD) | 0.05–0.5 | [88] | |
| Glycerol | Laponite | 1–5 | [172] | |
| Glycerol | Carboxylate multi-walled carbon nanotubes (CMWNTs) | 0.5–3 | [173] | |
| Glycerol | Bentonite, chitosan | 4 | [174] | |
| Glycerol | Talc nanoparticles | 0–5 | [175] | |
| Glycerol | Bacterial cellulose nanowhiskers (BCNW) | 2–20 | [50] | |
| Glycerol | Talc | 1–5 | [176] | |
| Glycerol | Bentonite, organically modified montmorillonite | 40–50 | [76] | |
| Glycerol | Beta-tricalcium phosphate nanoparticles | 3–10 | [177] | |
| Glycerol | Nanoclay: bentonite (H2Al2O6Si) | 1–5 | [178] | |
| Sorbitol | Cardanol oil, in situ silver nanoparticles | 0.2–0.6, 1–4 (mmol) | [179] | |
| Glycerol | Graphene oxide nanoplatelets, cellulose nanofibers | 1–5, 5–15 | [119] | |
| Maize | Glycerol | Lanthanum hydroxide nanoparticles | 1–3 | [180] |
| Ethyl vinyl acetate | Bentonite | [45] | ||
| Glycerol | Zirconium glycine-N,N-dimethylphosphonate (ZGDMP) | 0.2–1 | [77] | |
| Pea | Glycerol | Acid-treated multi-walled carbon nanotubes (MWCNTs) | 0.1–3 | [80] |
| Glycerol | Natural bentonite | 1 | [78] | |
| Polyethylene glycol and glycerol | Particles of AgNO3, Silver | 2.5–5, 0.5–1 | [90] | |
| Pomegranate | Glycerol | Halloysite nanoclay | 3–7 | [46] |
| Glycerol | Talc, bentonite | 1–5, 1–5 | [59] | |
| Potato | Glycerol | Bacterial cellulose (BC) nanoribbons | [60] | |
| Glycerol | Kaolin clay | 5–15 | [181] | |
| Glycerol | Multi-walled carbon nanotubes (MWCNT) | 0.25–10 | [2] | |
| Tapioca | Glycerol | Kaolinite | 10–60 | [182] |
| Glycerol, urea, ethanolamine | Halloysites nanotubes | 6 | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivadeneira-Velasco, K.E.; Utreras-Silva, C.A.; Díaz-Barrios, A.; Sommer-Márquez, A.E.; Tafur, J.P.; Michell, R.M. Green Nanocomposites Based on Thermoplastic Starch: A Review. Polymers 2021, 13, 3227. https://doi.org/10.3390/polym13193227
Rivadeneira-Velasco KE, Utreras-Silva CA, Díaz-Barrios A, Sommer-Márquez AE, Tafur JP, Michell RM. Green Nanocomposites Based on Thermoplastic Starch: A Review. Polymers. 2021; 13(19):3227. https://doi.org/10.3390/polym13193227
Chicago/Turabian StyleRivadeneira-Velasco, Katherine E., Christian A. Utreras-Silva, Antonio Díaz-Barrios, Alicia E. Sommer-Márquez, Juan P. Tafur, and Rose M. Michell. 2021. "Green Nanocomposites Based on Thermoplastic Starch: A Review" Polymers 13, no. 19: 3227. https://doi.org/10.3390/polym13193227
APA StyleRivadeneira-Velasco, K. E., Utreras-Silva, C. A., Díaz-Barrios, A., Sommer-Márquez, A. E., Tafur, J. P., & Michell, R. M. (2021). Green Nanocomposites Based on Thermoplastic Starch: A Review. Polymers, 13(19), 3227. https://doi.org/10.3390/polym13193227