Fabrication of Diaminodiphenylmethane Modified Ammonium Polyphosphate to Remarkably Reduce the Fire Hazard of Epoxy Resins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of DDP Flame Retardant
2.3. Preparation of Flame-Retarded EP Blends
2.4. Characterization and Measurements
3. Results
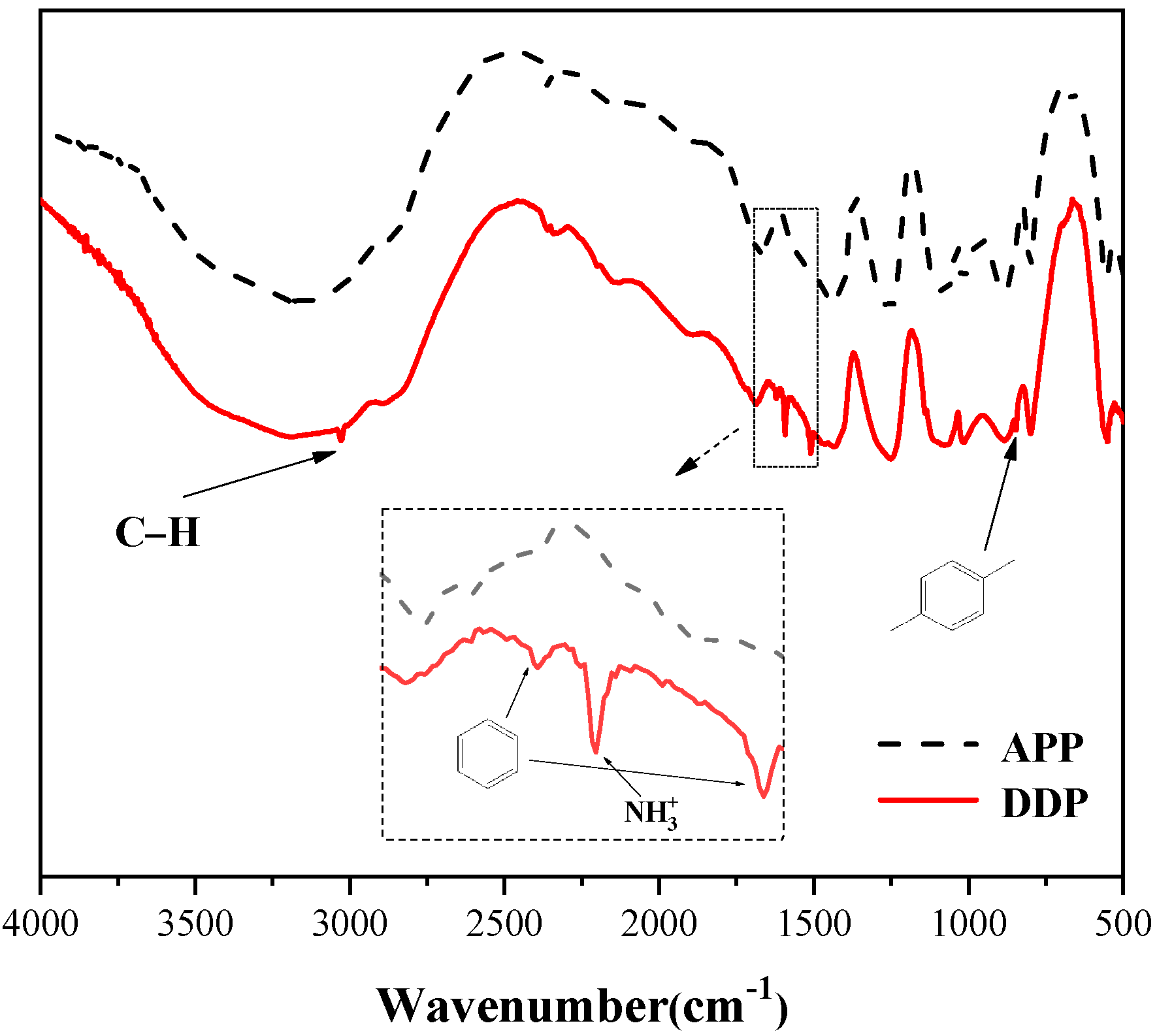
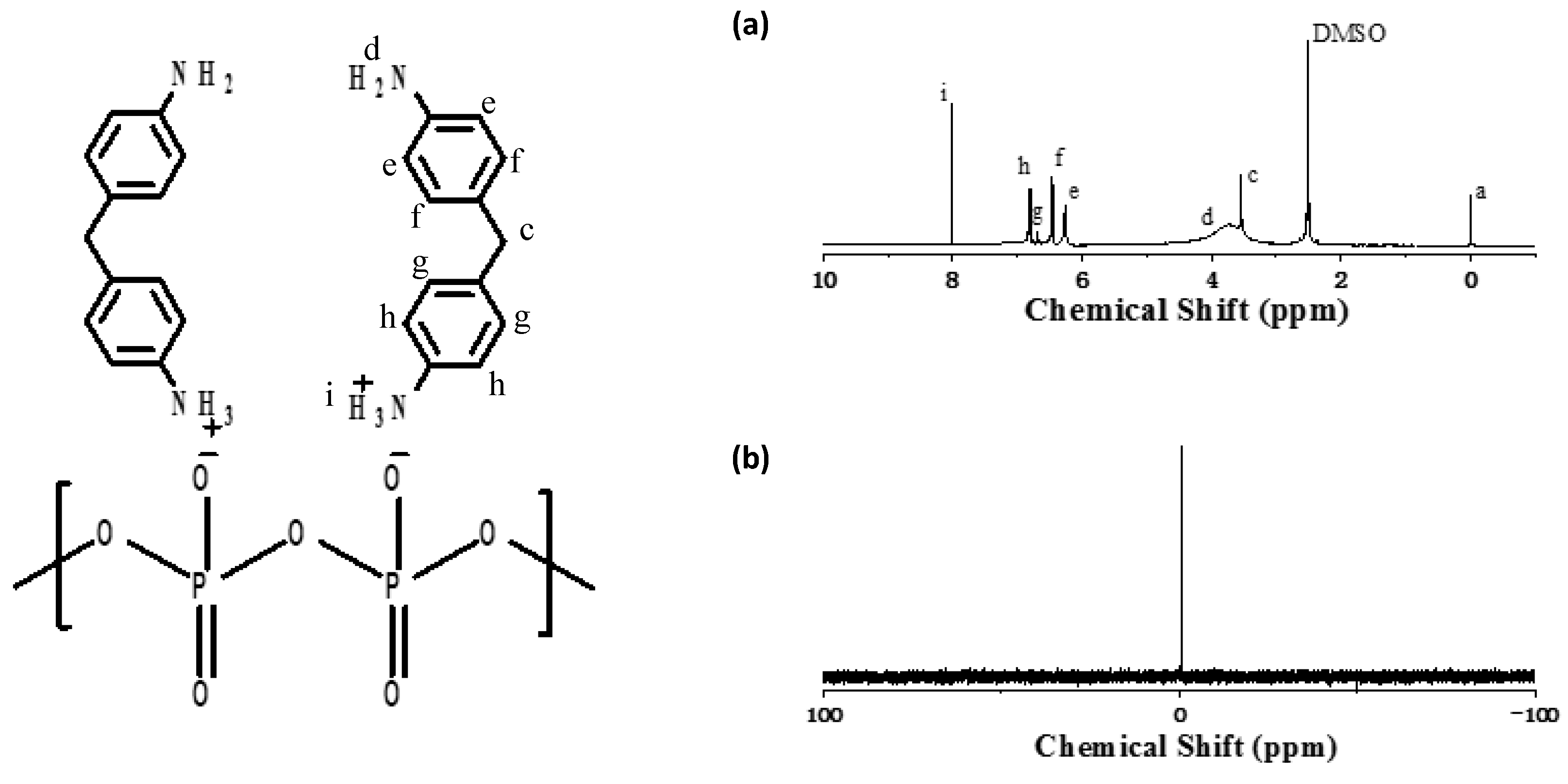
3.1. Preparation of DDP
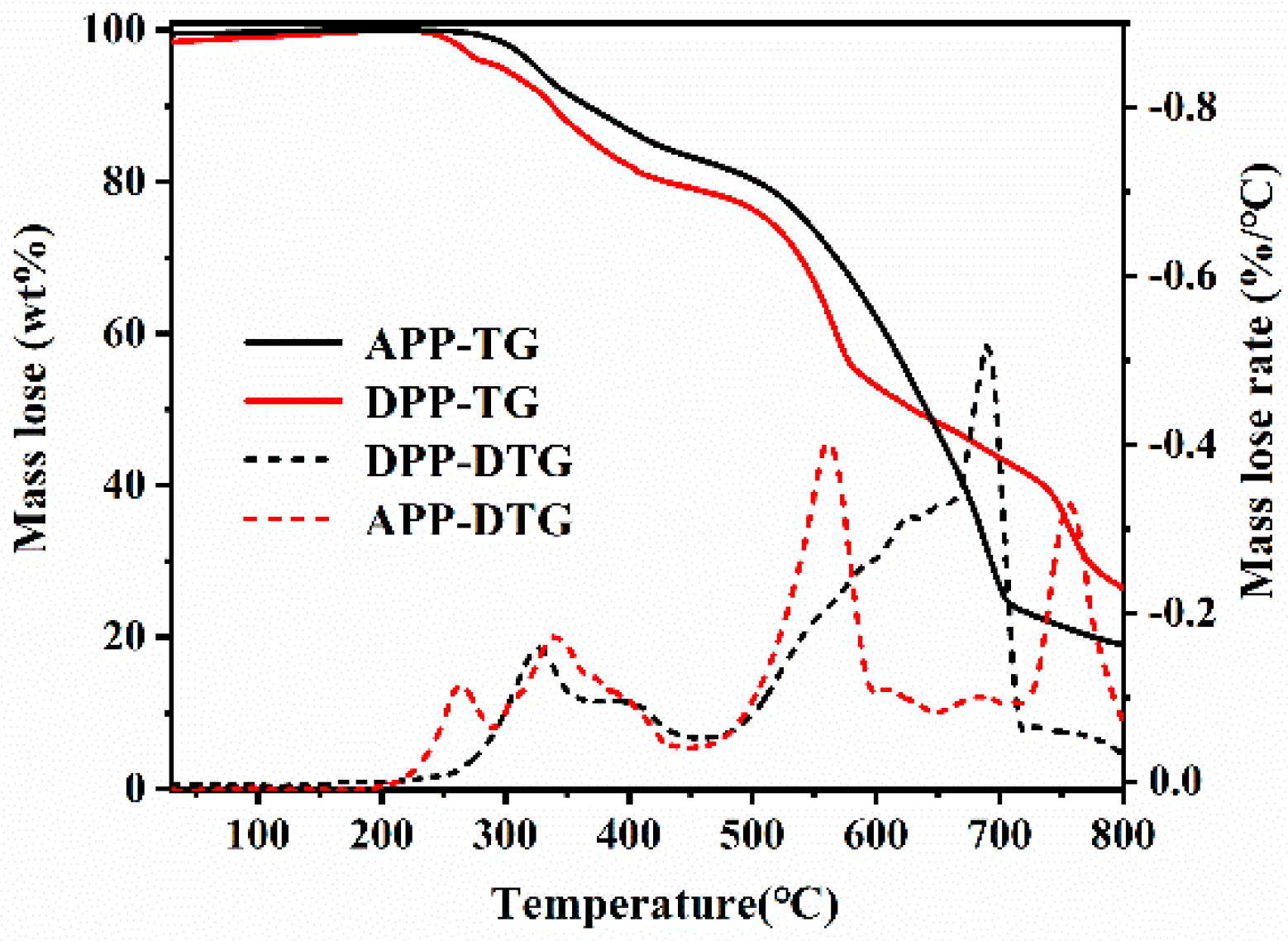
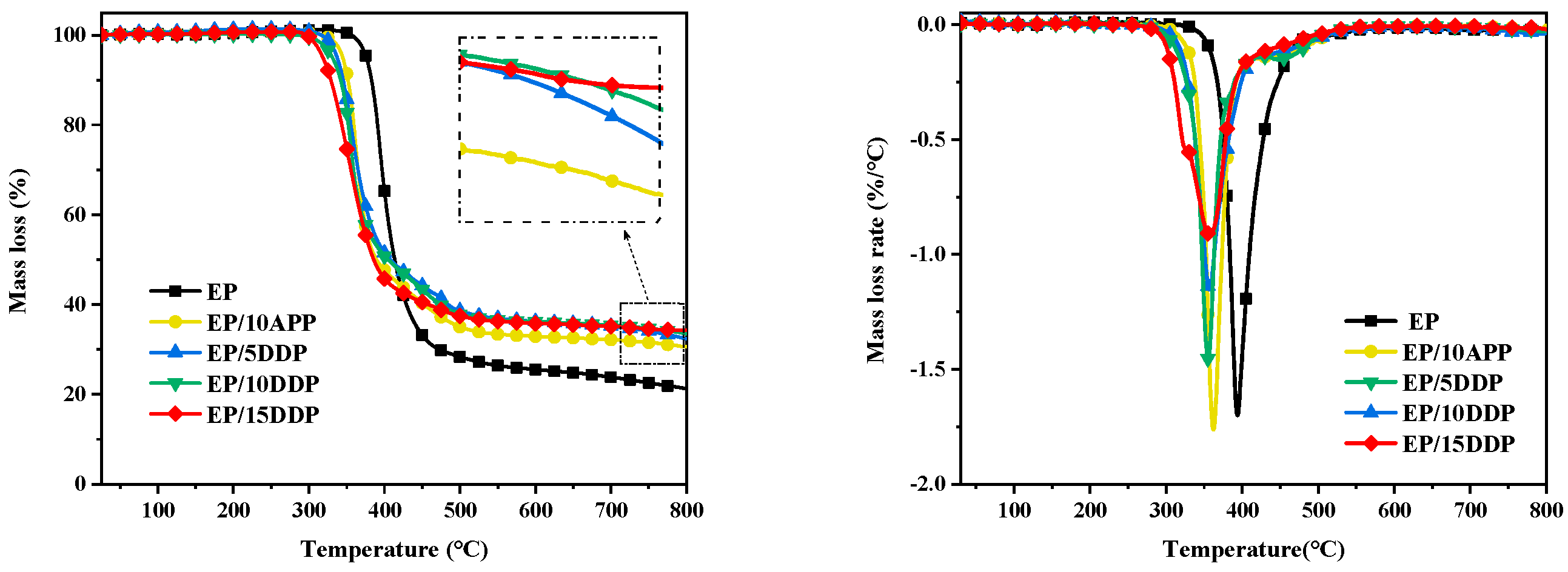
3.2. Thermal Behavior Analysis
3.3. DSC Analysis
3.4. LOI and UL94 Tests
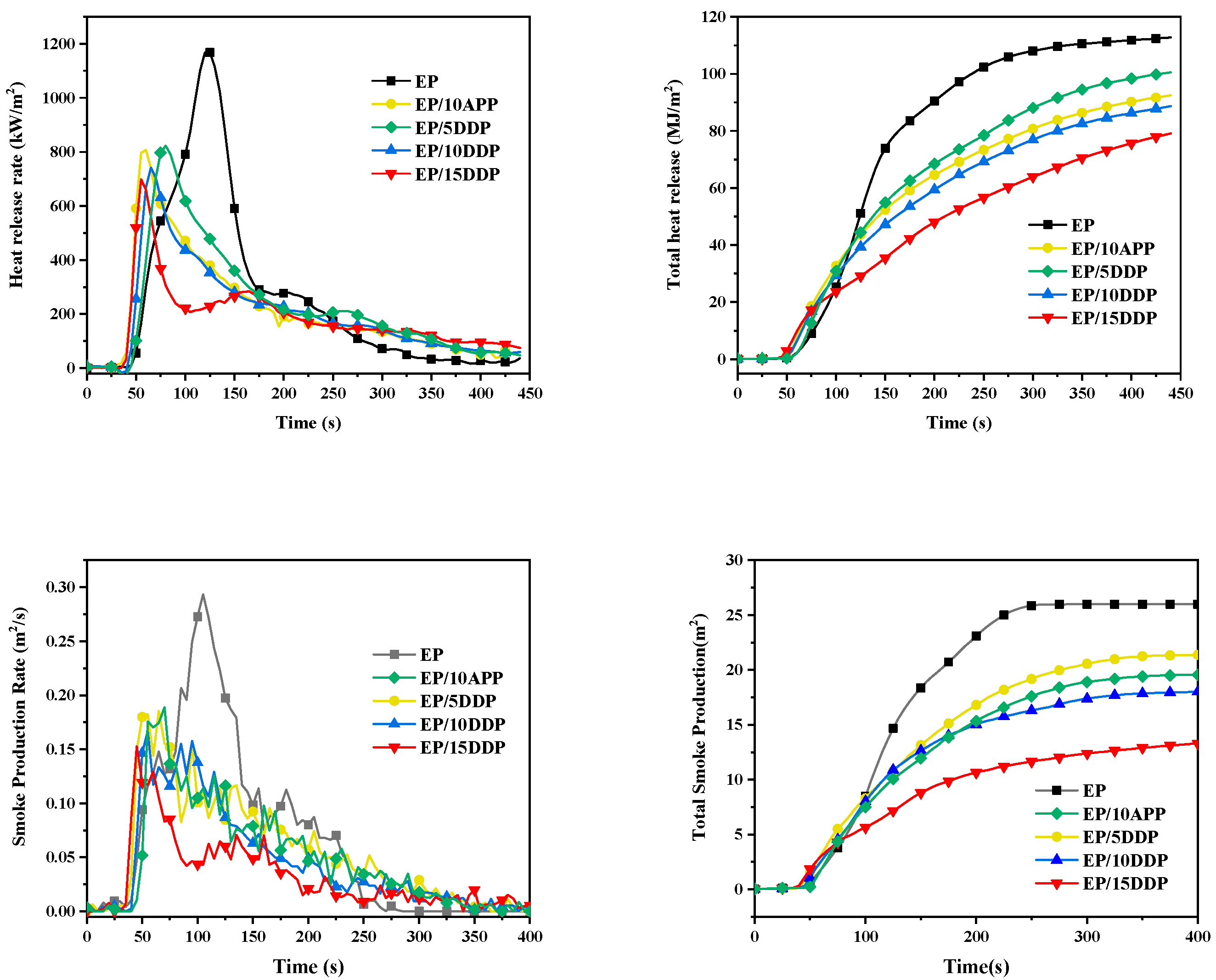
3.5. Cone Calorimeter Test
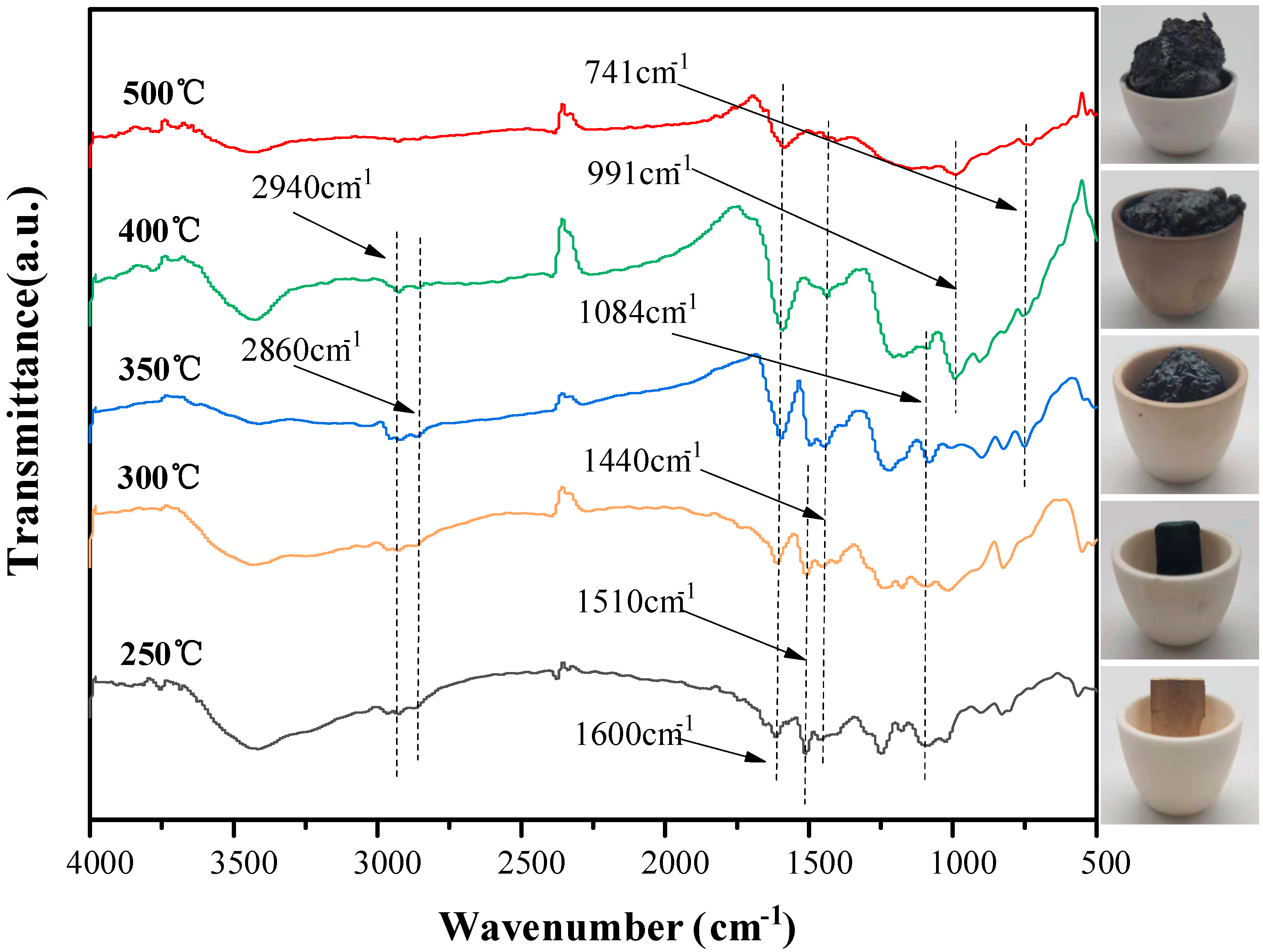
3.6. Char Residue Analysis
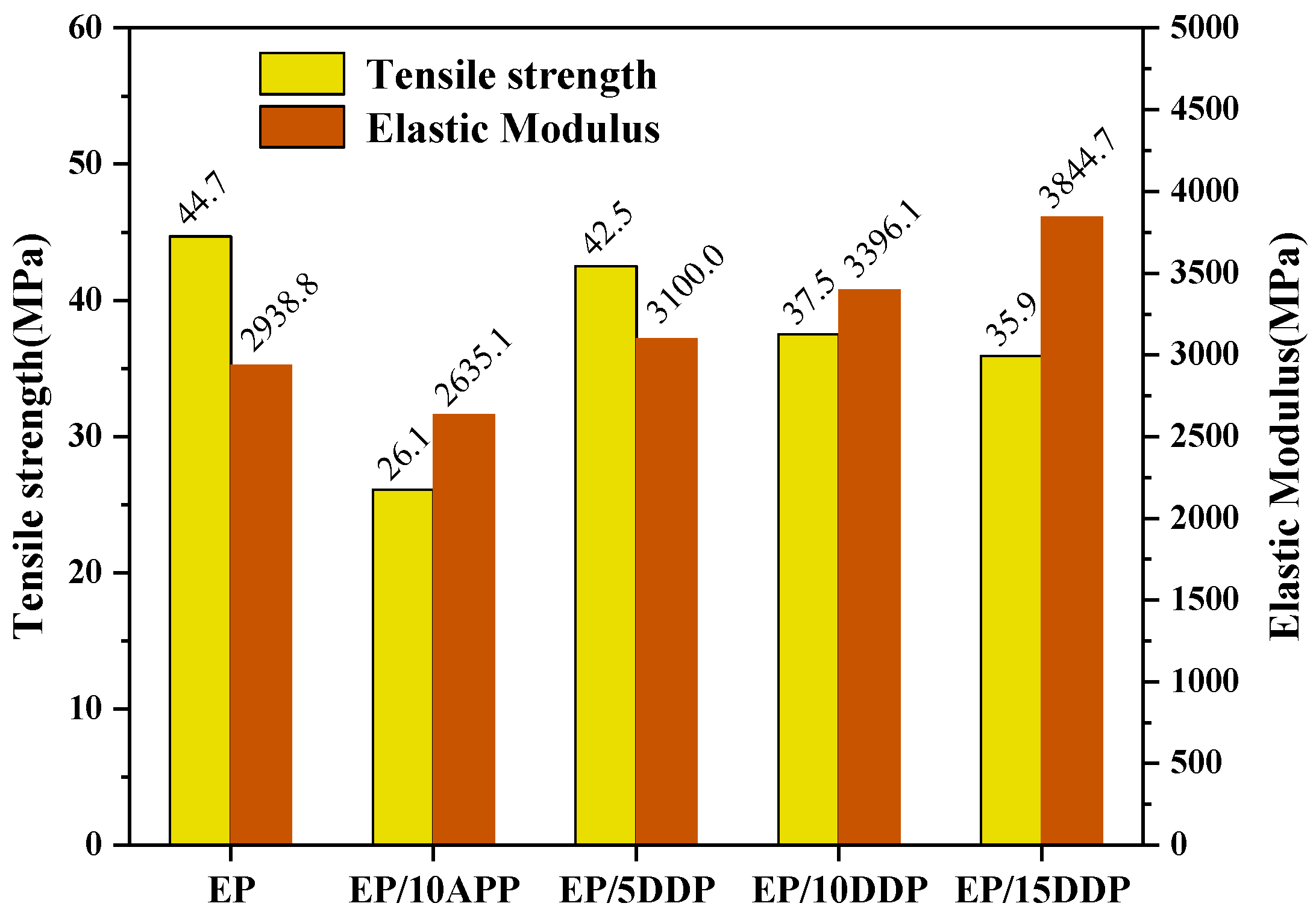
3.7. Tensile Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, D.; Xu, Y.J.; Long, J.W.; Shi, X.H. Epoxy resin flame-retarded via a novel melamine-organophosphinic acid salt: Thermal stability, flame retardance and pyrolysis behavior. J. Anal. Appl. Pyrolysis 2017, 128, 54–63. [Google Scholar] [CrossRef]
- Huo, S.; Song, P.; Yu, B.; Ran, S.; Chevali, V.S.; Liu, L.; Fang, Z.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Ahsan, I.M.; Asim, I.M.; Michele, F. Fire Retardancy of Aluminum Hydroxide Reinforced Flame Retardant Modified Epoxy Resin Composite. Russ. J. Appl. Chem. 2018, 91, 680–686. [Google Scholar]
- Howell, B.A.; Lienhart, G.W. Phosphorus esters of 1-dopyl-1,2-(4-hydroxyphenyl)ethene as effective flame retardants for polymeric materials. Fire Mater. 2020, 44, 1127–1134. [Google Scholar] [CrossRef]
- Howell, B.A.; Sun, W. Biobased flame retardants from tartaric acid and derivatives. Polym. Degrad. Stab. 2018, 157, 199–211. [Google Scholar] [CrossRef]
- Yu, G.; Bu, Q.; Cao, Z.; Du, X.; Xia, J.; Wu, M.; Huang, J. Brominated flame retardants (BFRs): A review on environmental contamination in China. Chemosphere 2016, 150, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Yu, J.; Zhang, M.; Qin, S.; Wang, T.; Huang, W.; Long, L. Flame-retardant effect of a phenethyl-bridged DOPO derivative and layered double hydroxides for epoxy resin. RSC Adv. 2017, 7, 46236–46245. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, W.; Zeng, G.; Du, J.; Zhang, W.; Yang, R. The Effect of Different Smoke Suppressants with APP for Enhancing the Flame Retardancy and Smoke Suppression on Vinyl Ester Resin. Polym. Eng. Sci. 2020, 60, 314–322. [Google Scholar] [CrossRef]
- Bar, M.; Alagirusamy, R.; Das, A. Flame Retardant Polymer Composites. Fibers Polym. 2014, 16, 705–717. [Google Scholar] [CrossRef]
- Shao, Z.B.; Deng, C.; Tan, Y.; Yu, L.; Chen, M.; Chen, L.; Wang, Y. Ammonium polyphosphate chemically-modified with ethanolamine as an efficient intumescent flame retardant for polypropylene. J. Mater. Chem. A 2014, 2, 13955–13965. [Google Scholar] [CrossRef]
- Chen, X.L.; Wang, K.; Gu, Y.X.; Jiao, C.; Liang, H.; Li, S. Influence of nickel citrate in flame retardant thermoplastic polyurethane elastomer composites based on ammonium polyphosphate. Express Polym. Lett. 2021, 15, 445–458. [Google Scholar] [CrossRef]
- Shi, C.; Qian, X.; Jing, J.; Che, H. Influence of β-FeOOH nanorods and ammonium polyphosphate on reducing the fire hazard of epoxy resins composites. J. Ther. Anal. Calorim. 2021. [Google Scholar] [CrossRef]
- Jun, Y.; Xu, P.; Zhang, P.; Fan, H. Surface-modified ammonium polyphosphate for flame-retardant and reinforced polyurethane composites. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127092. [Google Scholar]
- Shao, Z.B.; Cong, D.; Yi, T.; Chen, M.; Chen, L.; Wang, Y. Flame retardation of polypropylene via a novel intumescent flame retardant: Ethylenediamine-modified ammonium polyphosphate. Polym. Degrad. Stab. 2014, 106, 88–96. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Deng, C.; Tan, Y.; Chen, M.; Chen, L.; Wang, Y. A strategy to construct multifunctional ammonium polyphosphate for epoxy resin with simultaneously high fire safety and mechanical properties. Compos. Part A 2021, 149, 106529. [Google Scholar] [CrossRef]
- Sui, Y.; Dai, X.; Li, P.; Zhang, C. Superior radical scavenging and catalytic carbonization capacities of bioderived assembly modified ammonium polyphosphate as a mono-component intumescent flame retardant for epoxy resin. Eur. Polym. J. 2021, 156, 110601. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Zhang, J.; Xie, Y.; Zhang, G. Effects of innovative aromatic phosphorus containing flame-retardant polyols on rigid polyurethane foams. Chem. Papers 2021. [Google Scholar] [CrossRef]
- Shao, Z.B.; Cong, D.; Tan, Y.; Chen, M.; Chen, L.; Wang, Y. An Efficient Mono-Component Polymeric Intumescent Flame Retardant for Polypropylene: Preparation and Application. ACS Appl. Mater. Interfaces 2014, 6, 7363. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Li, D.F.; Fu, T.; He, L.; Wang, X.; Wang, Y. Biomimetic construction peanut-leaf structure on ammonium polyphosphate surface: Improving its compatibility with poly(lactic acid) and flame-retardant efficiency simultaneously. Chem. Eng. J. 2021, 412, 128737. [Google Scholar] [CrossRef]
- Tan, Y.; Shao, Z.B.; Yu, L.X.; Xu, Y.; Rao, W.; Chen, L.; Wang, Y. Polyethyleneimine modified ammonium polyphosphate toward polyamine-hardener for epoxy resin: Thermal stability, flame retardance and smoke suppression. Polym. Degrad. Stab. 2016, 131, 62–70. [Google Scholar] [CrossRef]
- Si, M.; Feng, J.; Hao, J.; Xu, L.; Du, J. Synergistic flame retardant effects and mechanisms of nano-Sb2O3 in combination with aluminum phosphinate in poly(ethylene terephthalate). Polym. Degrad. Stab. 2014, 100, 70–78. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.; Li, J. Regulating Effect of Exfoliated Clay on Intumescent Char Structure and Flame Retardancy of Polypropylene Composites. Ind. Eng. Chem. Res. 2016, 20, 5892–5901. [Google Scholar] [CrossRef]
- Zhang, C.; Miao, P.; Sun, L. Effect of phosphorus-containing flame retardants on flame retardancy and thermal stability of tetrafunctional epoxy resin. Polym. Adv. Technol. 2016, 26, 1531–1536. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Ma, S.; Yuan, W.; Li, Q.; Feng, J.; Zhu, J. Vanillin-derived phosphorus-containing compounds and ammonium polyphosphate as green fire-resistant systems for epoxy resins with balanced properties. Polym. Adv. Technol. 2019, 30, 264–278. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Deng, N.; Chu, Z. Synergistic effects of mono-component intumescent flame retardant grafted with carbon black on flame retardancy and smoke suppression properties of epoxy resins. J. Therm. Anal. Calorim. 2019, 138, 915–927. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Wang, X. Synergistic effects of organically modified montmorillonite on the flame-retardant and smoke suppression properties of transparent intumescent fire-retardant coatings. Prog Org. Coat. 2018, 122, 107–118. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Wang, X. Influence of nano-silica on the flame retardancy and smoke suppression properties of transparent intumescent fire-retardant coatings. Prog Org. Coat. 2017, 112, 319–329. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Zhang, L.; Wang, H. Synergistic effect of expandable graphite and intumescent flame retardants on the flame retardancy and thermal stability of polypropylene. J. Mater. Sci. 2016, 51, 5857–5871. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, Z.; Yan, L. Enhancing the flame-retardant and smoke suppression properties of transparent intumescent fire-retardant coatings by introducing boric acid as synergistic agent. J. Anal. Calorim. 2018, 133, 1241–1252. [Google Scholar] [CrossRef]
- Nguyen, C.; Kim, J. Thermal stabilities and flame retardancies of nitrogen phosphorus flame retardants based on bisphosphoramidates. Polym. Degrad. Stab. 2008, 93, 1037–1043. [Google Scholar] [CrossRef]




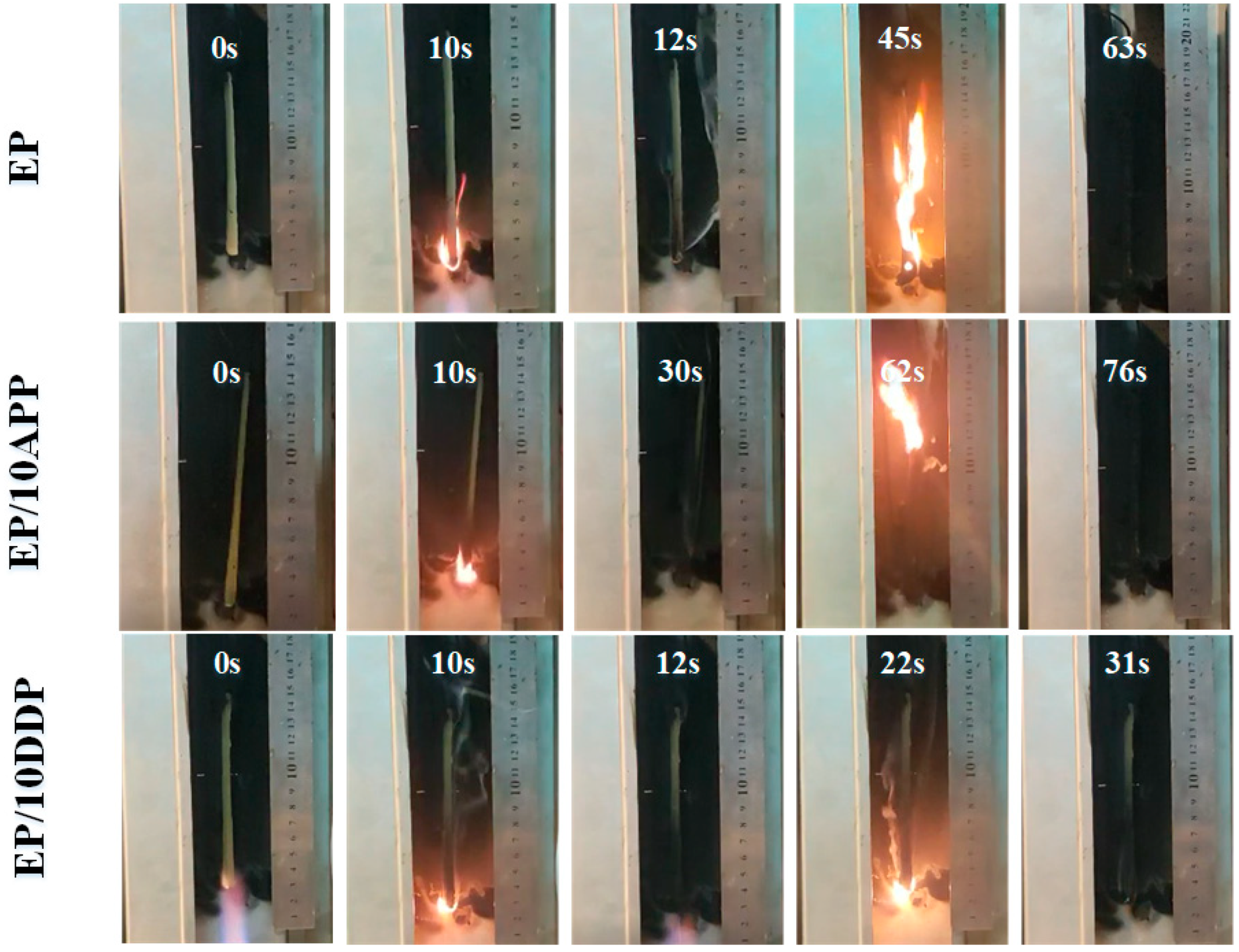


| Samples | EP (wt%) | DDM (wt%) | APP (wt%) | DDP (wt%) | LOI (%) | UL 94 Rating |
|---|---|---|---|---|---|---|
| EP | 80 | 20 | 0 | 0 | 26.3 | No rating |
| EP/5APP | 76 | 19 | 5 | 0 | 26.8 | No rating |
| EP/7.5APP | 74 | 18.5 | 7.5 | 0 | 28.6 | No rating |
| EP/10APP | 72 | 18 | 10 | 0 | 30.6 | No rating |
| EP/12.5APP | 70 | 17.5 | 12.5 | 0 | 33.8 | V-1 |
| EP/15APP | 68 | 17 | 15 | 0 | 34.4 | V-1 |
| EP/5DDP | 76 | 19 | 0 | 5 | 27.8 | No rating |
| EP/7.5DDP | 74 | 18.5 | 0 | 7.5 | 29.3 | No rating |
| EP/10DDP | 72 | 18 | 0 | 10 | 34.1 | V-1 |
| EP/12.5DDP | 70 | 17.5 | 0 | 12.5 | 35.4 | V-1 |
| EP/15DDP | 68 | 17 | 0 | 15 | 37.1 | V-0 |
| Samples | T5% (°C) | Tmax (°C) | PMLR (%/min) | Wexp (%) | Wcal | ΔW |
|---|---|---|---|---|---|---|
| EP | 375.8 | 391.8 | 1.68 | 21.3 | -- | -- |
| EP/10APP | 344.7 | 361.2 | 1.72 | 30.6 | 21.98 | 8.62 |
| EP/5DDP | 337.3 | 356.7 | 1.45 | 32.4 | 22.47 | 9.93 |
| EP/10DDP | 331.0 | 353.8 | 1.14 | 33.5 | 23.63 | 9.87 |
| EP/15DDP | 317.3 | 355.7 | 0.91 | 34.2 | 24.80 | 9.40 |
| Samples | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | PSPR (m2/s) | TSR (m2/m2) | Residue (%) |
|---|---|---|---|---|---|---|
| EP | 39 | 1186.7 | 111.8 | 0.293 | 1460.7 | 6.8 |
| EP/10APP | 29 | 866.1 | 98.6 | 0.189 | 1202.2 | 23.3 |
| EP/5DDP | 26 | 826.9 | 90.2 | 0.185 | 1102.6 | 24.9 |
| EP/10DDP | 34 | 753.4 | 86.3 | 0.167 | 1016.6 | 28.8 |
| EP/15DDP | 33 | 702.4 | 75.7 | 0.153 | 759.0 | 34.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liao, J.; Yan, L.; Liu, H. Fabrication of Diaminodiphenylmethane Modified Ammonium Polyphosphate to Remarkably Reduce the Fire Hazard of Epoxy Resins. Polymers 2021, 13, 3221. https://doi.org/10.3390/polym13193221
Wang F, Liao J, Yan L, Liu H. Fabrication of Diaminodiphenylmethane Modified Ammonium Polyphosphate to Remarkably Reduce the Fire Hazard of Epoxy Resins. Polymers. 2021; 13(19):3221. https://doi.org/10.3390/polym13193221
Chicago/Turabian StyleWang, Feiyue, Jiahao Liao, Long Yan, and Hui Liu. 2021. "Fabrication of Diaminodiphenylmethane Modified Ammonium Polyphosphate to Remarkably Reduce the Fire Hazard of Epoxy Resins" Polymers 13, no. 19: 3221. https://doi.org/10.3390/polym13193221
APA StyleWang, F., Liao, J., Yan, L., & Liu, H. (2021). Fabrication of Diaminodiphenylmethane Modified Ammonium Polyphosphate to Remarkably Reduce the Fire Hazard of Epoxy Resins. Polymers, 13(19), 3221. https://doi.org/10.3390/polym13193221








