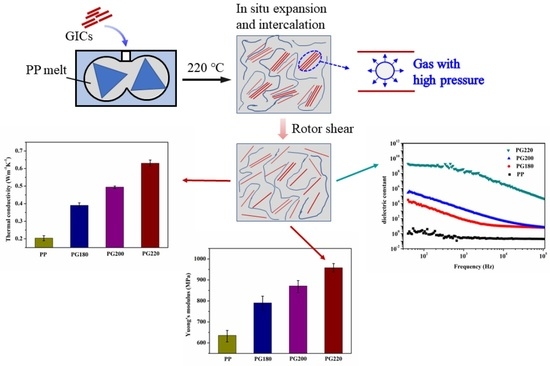
Highly Enhancing Electrical, Thermal, and Mechanical Properties of Polypropylene/Graphite Intercalation Compound Composites by In Situ Expansion during Melt Mixing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PP/GIC Composites
2.3. Characterization
3. Results and Discussion
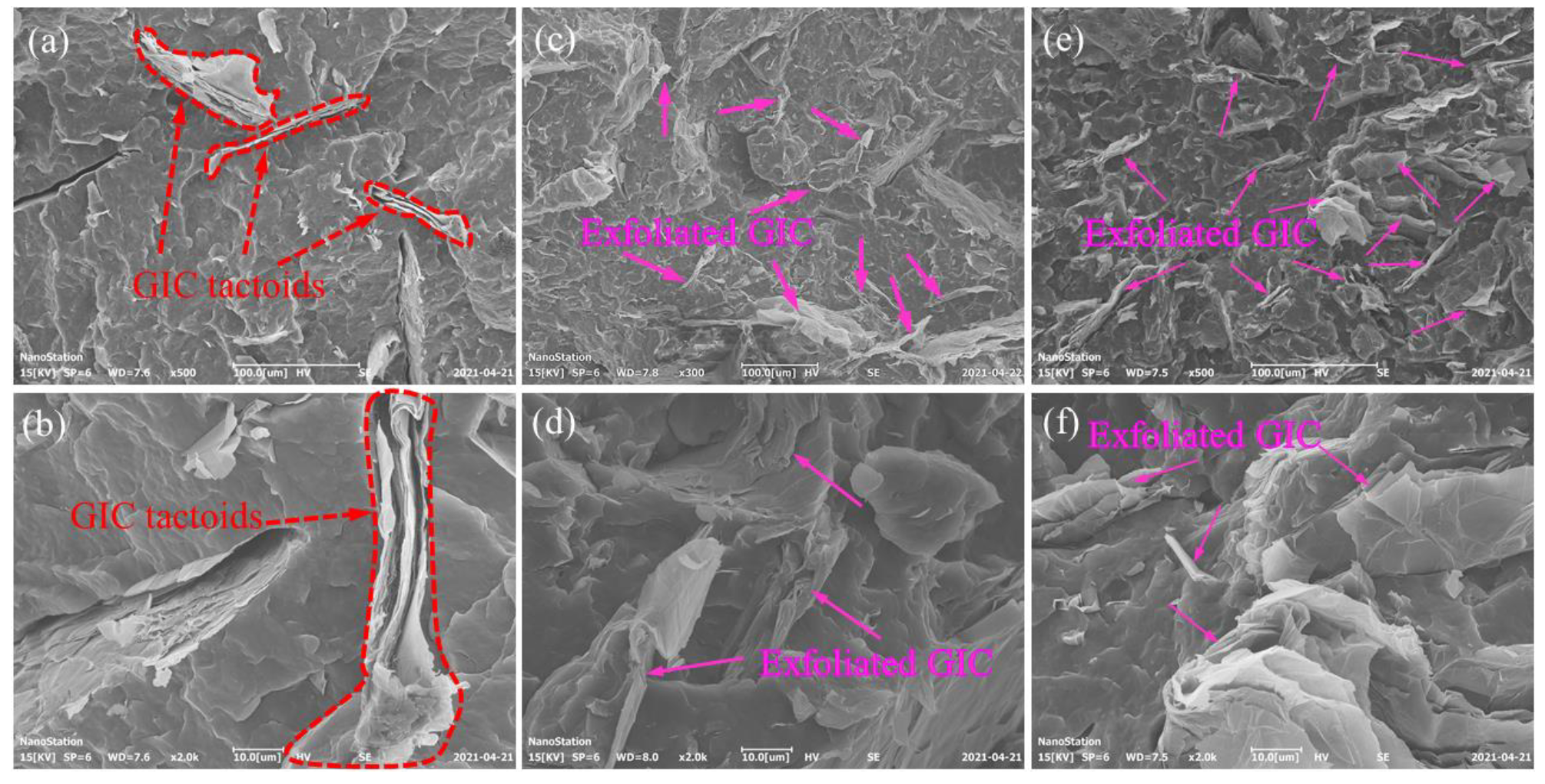
3.1. Exfoliation and Dispersion of GICs
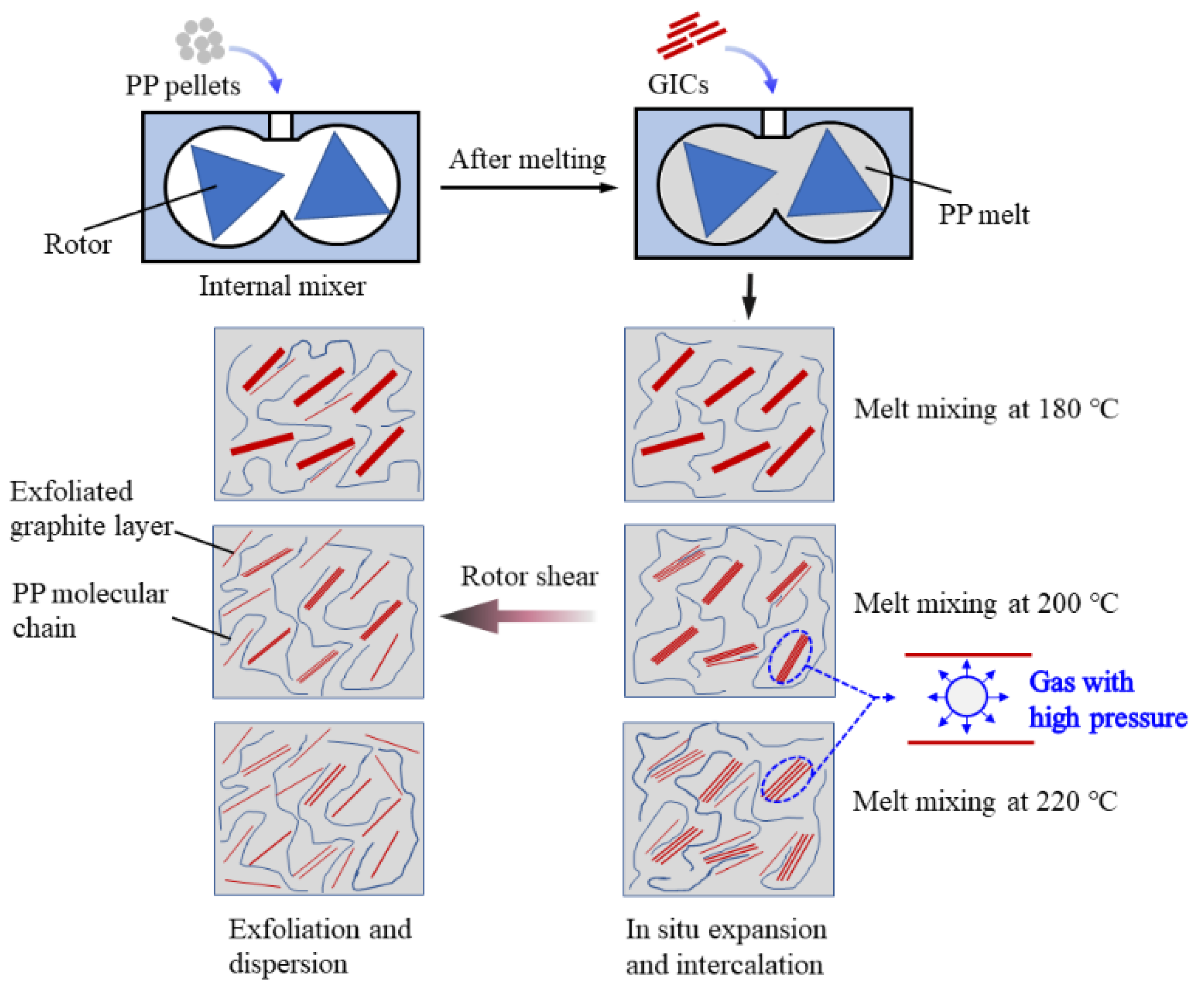
3.2. Mechanism for GIC Intercalation and Exfoliation during Melt Mixing
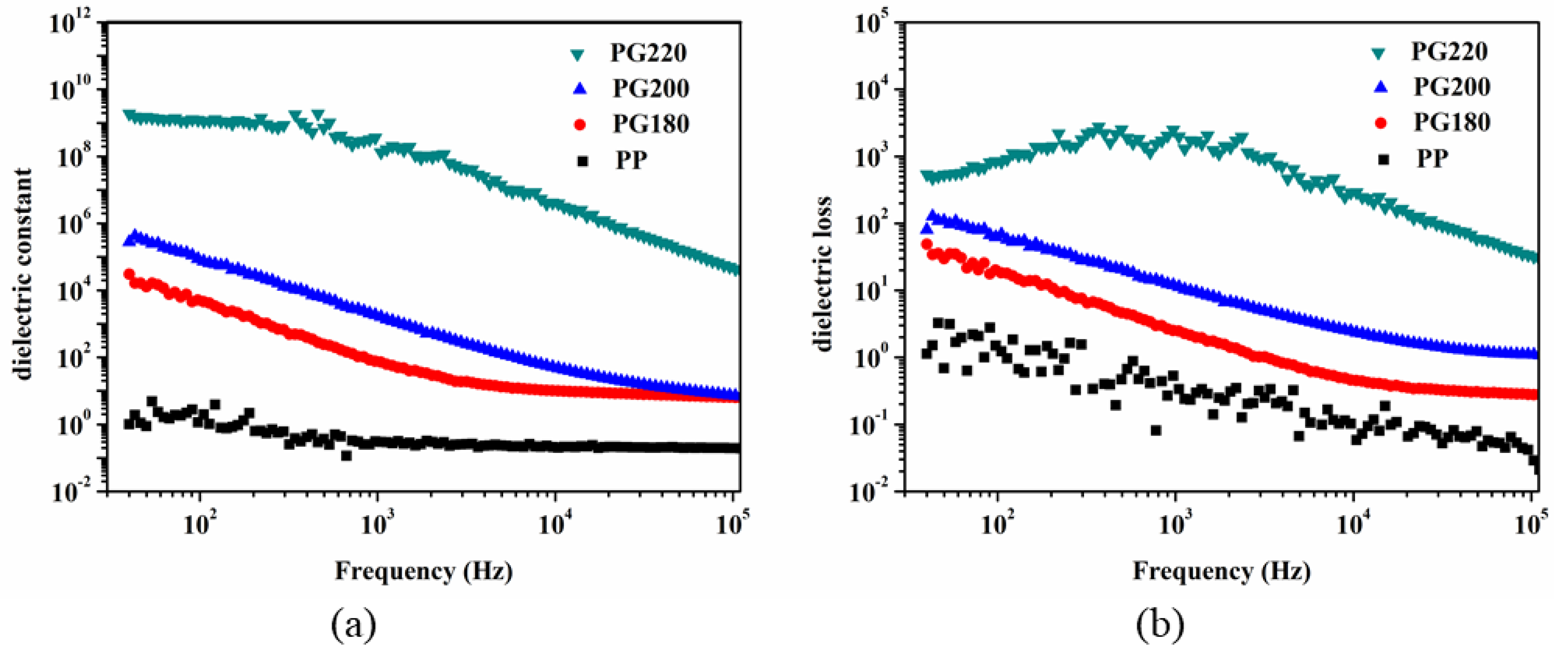
3.3. Dielectric Properties
3.4. Thermal Properties
3.5. Tensile Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, S.H.; Park, K.H.; Kim, B.H.; Choi, Y.W.; Jun, G.H.; Lee, D.J.; Kong, B.S.; Paik, K.W.; Jeon, S. Enhanced thermal conductivity of epoxy-graphene composites by using non-oxidized graphene flakes with non-covalent functionalization. Adv. Mater. 2013, 25, 732–737. [Google Scholar]
- Abolhasani, M.M.; Shirvanimoghaddam, K.; Naebe, M. PVDF/graphene composite nanofibers with enhanced piezoelectric performance for development of robust nanogenerators. Compos. Sci. Technol. 2017, 138, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Zheng, Z.; Bi, Y.; Lv, X.; Sun, S. Improving the electroactive phase, thermal and dielectric properties of PVDF/graphene oxide composites by using methyl methacrylate-co-glycidyl methacrylate copolymers as compatibilizer. J. Mater. Sci. 2019, 54, 3832–3846. [Google Scholar] [CrossRef]
- Chen, H.Y.; Ginzburg, V.V.; Yang, J.; Yang, Y.F.; Liu, W.; Huang, Y.; Du, L.B.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Li, Y.; Tang, J.; Huang, L.; Liu, J.; Wang, Y.; Ge, X.; Wang, L.; Li, G.; Sun, W. Effective exfoliation of expanded graphite in rigid poly(methyl methacrylate) and its dispersion and enhancement in poly(vinylidene fluoride). J. Nanosci. Nanotechnol. 2016, 16, 10021–10028. [Google Scholar] [CrossRef]
- Deng, S.; Zhu, Y.; Qi, X.; Yu, W.; Chen, F.; Fu, Q. Preparation of polyvinylidene fluoride/expanded graphite composites with enhanced thermal conductivity via ball milling treatment. RSC Adv. 2016, 6, 45578–45584. [Google Scholar] [CrossRef]
- Ho, Q.B.; Osazuwa, O.; Modler, R.; Daymond, M.; Gallerneault, M.T.; Kontopoulou, M. Exfoliation of graphite and expanded graphite by melt compounding to prepare reinforced, thermally and electrically conducting polyamide composites. Compos. Sci. Technol. 2019, 176, 111–120. [Google Scholar] [CrossRef]
- Cho, E.C.; Huang, J.H.; Li, C.P.; Chang-Jian, C.W.; Lee, K.C.; Hsiao, Y.S.; Huang, J.H. Graphene based thermoplastic composites and their application for LED thermal management. Carbon 2016, 102, 66–73. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, H.; Shen, Z.; Guo, B.; Zhang, L.; Jia, D. Grafting of polyester onto graphene for electrically and thermally conductive composites. Macromolecules 2012, 45, 3444–3451. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Colonna, S.; Monticelli, O.; Gomez, J.; Novara, C.; Saracco, G.; Fina, A. Effect of morphology and defectiveness of graphene-related materials on the electrical and thermal conductivity of their polymer nanocomposites. Polymer 2016, 102, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhao, C.; Li, Q.; Li, J.; Zhu, Y. A novel approach to align carbon nanotubes via water-assisted shear stretching. Compos. Sci. Technol. 2018, 164, 1–7. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: a review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef] [Green Version]
- Haghgoo, M.; Ansari, R.; Hassanzadeh-Aghdam, M.K. Prediction of electrical conductivity of carbon fiber-carbon nanotube-reinforced polymer hybrid composites. Compos. Part B Eng. 2019, 167, 728–735. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, J.J.; Chen, X.Q.; Weng, G.J. A Monte Carlo model with equipotential approximation and tunneling resistance for the electrical conductivity of carbon nanotube polymer composites. Carbon 2019, 146, 125–138. [Google Scholar] [CrossRef]
- Soares, B.G. Ionic liquid: A smart approach for developing conducting polymer composites A review. J. Mol. Liq. 2018, 262, 8–18. [Google Scholar] [CrossRef]
- Chen, G.; Weng, W.; Wu, D.; Wu, C. PMMA/graphite nanosheets composite and its conducting properties. Eur. Polym. J. 2003, 39, 2329–2335. [Google Scholar] [CrossRef]
- Yu, A.P.; Ramesh, P.; Itkis, M.E.; Bekyarova, E.; Haddon, R.C. Graphite nanoplatelet-epoxy composite thermal interface materials. J. Phys. Chem. C 2007, 111, 7565–7569. [Google Scholar] [CrossRef]
- Tang, L.C.; Wan, Y.J.; Yan, D.; Pei, Y.B.; Zhao, L.; Li, Y.B.; Wu, L.B.; Jiang, J.X.; Lai, G.Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Liu, Y.J.; Feng, C. An attempt towards fabricating reduced graphene oxide composites with traditional polymer processing techniques by adding chemical reduction agents. Compos. Sci. Technol. 2017, 140, 16–22. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Zhao, M.J.; Xia, Z.L.; Duan, H.J.; Zhao, G.Z.; Liu, Y.Q. Facile preparation of polyamide 6/exfoliated graphite nanoplate composites via ultrasound-assisted processing. Polym. Eng. Sci. 2018, 58, 1739–1745. [Google Scholar] [CrossRef]
- Tong, J.; Li, W.; Tan, L.C.; Chen, H.C. Fabrication of well dispersed poly(vinylidene fluoride)/expanded graphite/ionic liquid composites with improved properties by water-assisted mixing extrusion. Compos. Sci. Technol. 2020, 185, 107904. [Google Scholar] [CrossRef]
- Tong, J.; Li, W.; Chen, H.C.; Tan, L.C. Improving properties of poly(vinylidene fluoride) by adding expanded graphite without surface modification via water-assisted mixing extrusion. Macromol. Mater. Eng. 2020, 305, 2000270. [Google Scholar] [CrossRef]
- Ma, G.Q.; Sun, G.K.; Ma, Z.; Li, J.Q.; Sheng, J. In-line plasma induced graft-copolymerization of pentaerythritol triacrylate onto polypropylene. Chin. J. Polym. Sci. 2018, 36, 979–983. [Google Scholar] [CrossRef]
- Sefadi, J.S.; Luyt, A.S.; Pionteck, J.; Gohs, U. Effect of surfactant and radiation treatment on the morphology and properties of PP/EG composites. J. Mater. Sci. 2015, 50, 6021–6031. [Google Scholar] [CrossRef]
- Dini, M.; Mousavand, T.; Carreau, P.; Kamal, M.; Ton-That, M. Effect of water assisted extrusion and solid-state polymerization on the microstructure of PET/clay nanocomposites. Polym. Eng. Sci. 2014, 54, 1723–1736. [Google Scholar] [CrossRef]
- Wu, M.; Huang, H.X.; Tong, J.; Ke, D.Y. Enhancing thermal conductivity and mechanical properties of poly (methyl methacrylate) via adding expanded graphite and injecting water. Compos. Part A 2017, 102, 228–235. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Q.; Wen, B. Highly thermally conductive and superior electrical insulation polymer composites via in situ thermal expansion of expanded graphite and in situ oxidation of aluminum nanoflakes. ACS Appl. Mater. Inter. 2021, 13, 1511–1523. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, T.; Huang, T.; Zhang, N.; Wang, Y.; Yang, J. Fabrication of high-k poly(vinylidenefluoride)/nylon 6/carbon nanotube nanocomposites through selective localization of carbon nanotubes in blends. Polym. Int. 2017, 66, 604–611. [Google Scholar] [CrossRef]
- Jiao, Y.; Yuan, L.; Liang, G.; Gu, A. Facile preparation and origin of high-k carbon nanotube/poly(etherimide)/bismaleimide composites through controlling the location and distribution of carbon nanotubes. J. Phys. Chem. C 2014, 118, 24091–24101. [Google Scholar] [CrossRef]
- Yuan, J.K.; Li, W.L.; Yao, S.H.; Lin, Y.Q.; Sylvestre, A.; Bai, J. High dielectric permittivity and low percolation threshold in polymer composites based on SiC-carbon nanotubes micro/nano hybrid. Appl. Phys. Lett. 2011, 98, 032901. [Google Scholar] [CrossRef]
- Min, C.; Yu, D.; Cao, J.; Wang, G.; Feng, L. A graphite nanoplatelet/epoxy composite with high dielectric constant and high thermal conductivity. Carbon 2013, 55, 116–125. [Google Scholar] [CrossRef]
- Dang, Z.M.; Nan, C.W.; Xie, D.; Zhang, Y.H.; Tjong, S.C. Dielectric behavior and dependence of percolation threshold on the conductivity of fillers in polymer-semiconductor composites, Appl. Phys. Lett. 2004, 85, 97–99. [Google Scholar]
- Li, Y.C.; Tjong, S.C.; Li, R.K.Y. Electrical conductivity and dielectric response of poly(vinylidene fluoride)- graphite nanoplatelet composites. Synth. Met. 2010, 160, 1912–1919. [Google Scholar] [CrossRef]
- Sang, B.; Li, Z.W.; Li, X.H.; Yu, L.G.; Zhang, Z.J. Graphene-based flame retardants: a review. J. Mater. Sci. 2016, 51, 8271–8295. [Google Scholar] [CrossRef]
- Bian, J.; Wang, Z.J.; Lin, H.L.; Zhou, X.; Xiao, W.Q.; Zhao, X.W. Thermal and mechanical properties of polypropylene nanocomposites reinforced with nano-SiO2 functionalized graphene oxide, Compos. Part A Appl. Sci. Manuf. 2017, 97, 120–127. [Google Scholar] [CrossRef]
- Pham, V.H.; Dang, T.T.; Seung Hyun, H.; Kim, E.J.; Chung, J.S. Highly conductive poly(methyl methacrylate) (PMMA)-reduced graphene oxide composite prepared by self-assembly of PMMA latex and graphene oxide through electrostatic interaction. ACS Appl. Mater. Inter. 2012, 4, 2630–2636. [Google Scholar] [CrossRef]
- Krupa, I.; Cecen, V.; Boudenneá, A.; Križanov, Z.; Vávra, I.; Srnánek, R.; Radnóczi, G. Mechanical properties and morphology of composites based on the EVA copolymer filled with expanded graphite. Polym. Plast. Technol. Eng. 2012, 51, 1388–1393. [Google Scholar] [CrossRef]
- Kalaitzidon, K.; Fukushima, H.; Drzal, L.T. A new compounding method for exfoliated graphite-polypropylene nanocomposites with enhanced flexural properties lower percolation threshold. Compos. Sci. Technol. 2007, 67, 2045–2051. [Google Scholar] [CrossRef]

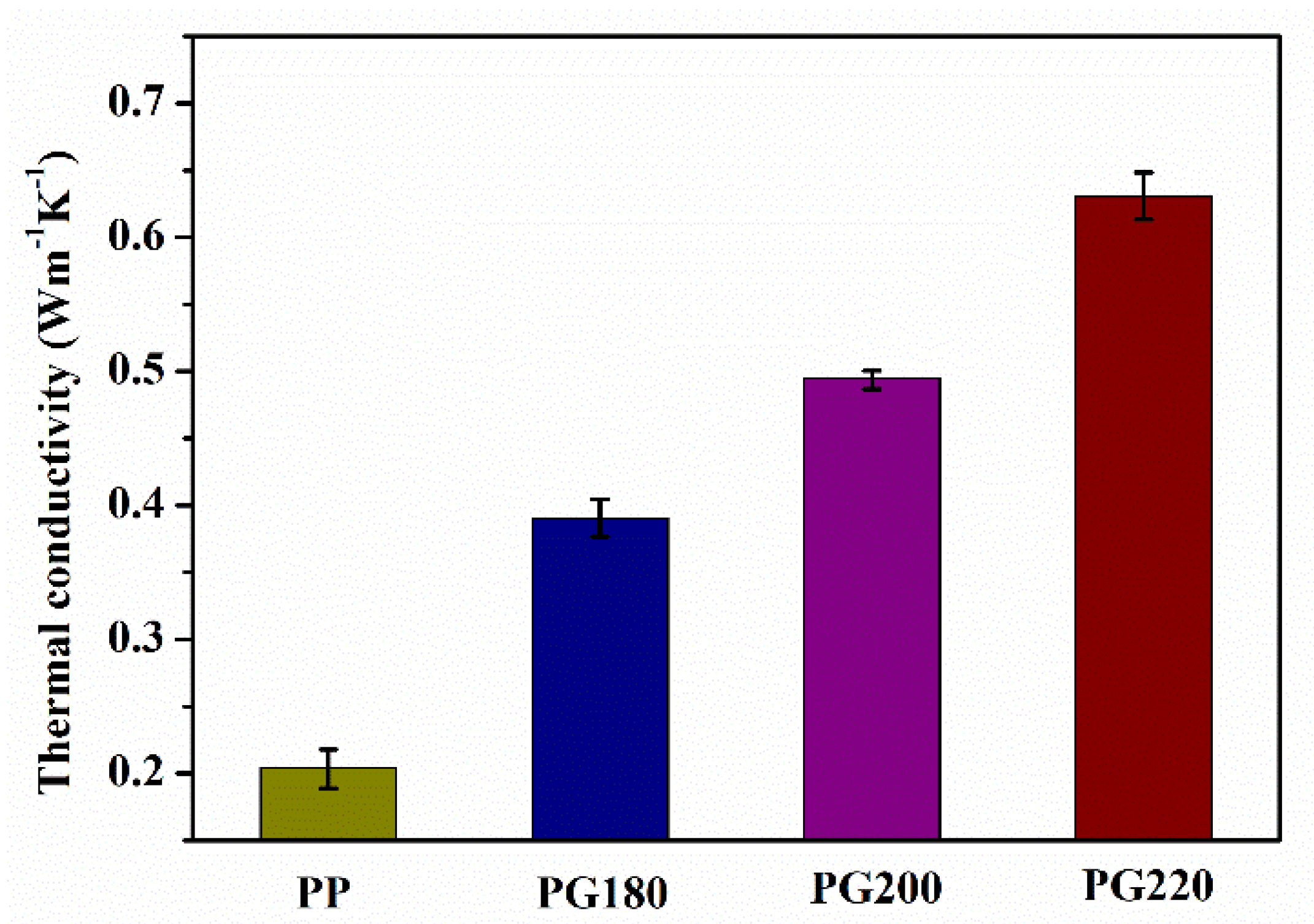

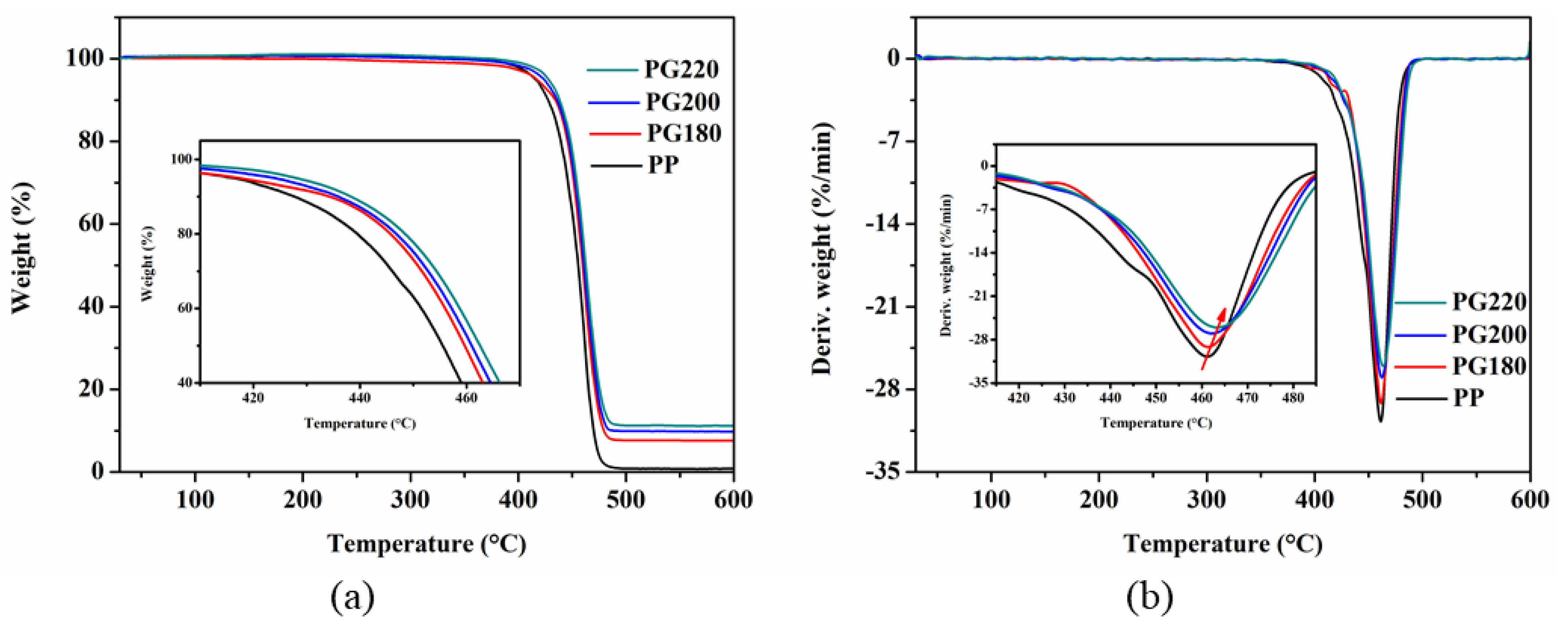
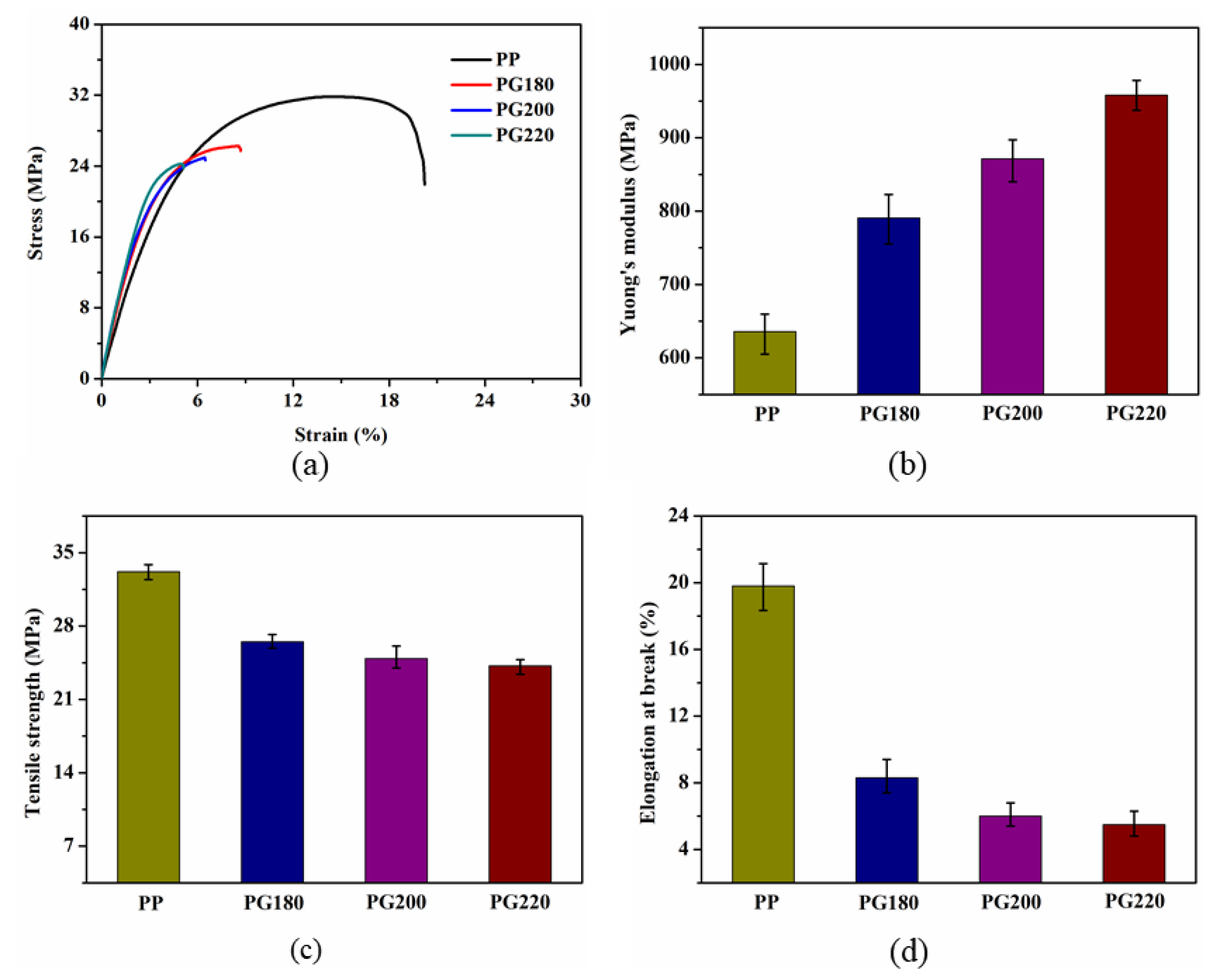
| Sample | Tc (°C) | ΔHm (J/g) | Tm (°C) | Xc (%) |
|---|---|---|---|---|
| PP | 115.4 | 106.8 | 164.0 | 51.6 |
| PG180 | 123.0 | 114.1 | 164.5 | 61.2 |
| PG200 | 124.5 | 101.2 | 164.8 | 54.3 |
| PG220 | 125.6 | 96.7 | 165.6 | 51.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Tong, J.; Li, W.; Zhang, H.; Hu, M.; Chen, H.; He, H. Highly Enhancing Electrical, Thermal, and Mechanical Properties of Polypropylene/Graphite Intercalation Compound Composites by In Situ Expansion during Melt Mixing. Polymers 2021, 13, 3095. https://doi.org/10.3390/polym13183095
Wang Z, Tong J, Li W, Zhang H, Hu M, Chen H, He H. Highly Enhancing Electrical, Thermal, and Mechanical Properties of Polypropylene/Graphite Intercalation Compound Composites by In Situ Expansion during Melt Mixing. Polymers. 2021; 13(18):3095. https://doi.org/10.3390/polym13183095
Chicago/Turabian StyleWang, Zhifeng, Jun Tong, Wei Li, Haichen Zhang, Manfeng Hu, Haichu Chen, and Hui He. 2021. "Highly Enhancing Electrical, Thermal, and Mechanical Properties of Polypropylene/Graphite Intercalation Compound Composites by In Situ Expansion during Melt Mixing" Polymers 13, no. 18: 3095. https://doi.org/10.3390/polym13183095
APA StyleWang, Z., Tong, J., Li, W., Zhang, H., Hu, M., Chen, H., & He, H. (2021). Highly Enhancing Electrical, Thermal, and Mechanical Properties of Polypropylene/Graphite Intercalation Compound Composites by In Situ Expansion during Melt Mixing. Polymers, 13(18), 3095. https://doi.org/10.3390/polym13183095