The Effects of Chain-Extending Cross-Linkers on the Mechanical and Thermal Properties of Poly(butylene adipate terephthalate)/Poly(lactic acid) Blown Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tensile Properties
2.2. Tear Properties
2.3. Seal Strength
2.4. Dynamic Mechanical Analysis (DMA)
2.5. Melt Volume Rate (MVR)
2.6. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institute of Bioplastics and Biocomposites. Bioplastics Facts and Statistics. 2016. Available online: https://www.ifbb-hannover.de/files/IfBB/downloads/faltblaetter_broschueren/Biopolymers-Facts-Statistics_2016.pdf (accessed on 23 November 2020).
- Mantia, F.P.L.; Botta, L.; Mistretta, M.C.; Fiore, A.D.; Titone, V. Recycling of a Biodegradable Polymer Blend. Polymer 2020, 12, 2297. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W.; Wen, X.; Tang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263, 114469. [Google Scholar] [CrossRef]
- Narancic, T.; O’Connor, K. Plastic waste as a global challenge: Are biodegradable plastics the answer to the plastic waste problem? Microbiology 2018, 165, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable Poly(butylene succinate) and Poly(butylene adipate-co-terephthalate) Blends: Reactive Extrusion and Performance Evaluation. Polym. Environ. 2014, 22, 336–349. [Google Scholar] [CrossRef]
- Gu, S.Y.; Zhang, K.; Ren, J.; Zhan, H. Melt rheology of polylactide/poly(butylene adipate-co-terephthalate) blends. Carb. Polym. 2008, 74, 79–85. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)-PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Lackner, M.; Ivanič, F.; Kováčová, M.; Chodák, I. Mechanical properties and structure of mixtures of poly(butylene-adipate-co-terephthalate) (PBAT) with thermoplastic starch (TPS). Int. J. Biobased Plast. 2021, 3, 126–138. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Cividanes, L.S.; Gouveia, R.F.; Lona, L.M.F. An overview on properties and applications of poly(butylene adipate-co-terephthalate)–PBAT based composites. Polym. Eng. Sci. 2019, 59, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Ivanič, F.; Kováčová, M.; Chodák, I. The effect of plasticizer selection on properties of blends poly(butylene adipate-co-terephthalate) with thermoplastic starch. Eur. Polym. J. 2019, 116, 99–105. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactide) Stereocomplexes: Formation, Structure, Properties, Degradation, and Applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef]
- Ahmed, J.; Varshney, S.K. Polylactides—Chemistry, Properties and Green Packaging Technology: A Review. Int. J. Food Prop. 2011, 14, 37–58. [Google Scholar] [CrossRef]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N. Synthesis of Poly(Lactic Acid): A Review. J. Macromol. Sci. Part C 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Ljungberg, N.; Wesslén, B. The effects of plasticizers on the dynamic mechanical and thermal properties of poly(lactic acid). J. Appl. Polym. Sci. 2002, 86, 1227–1234. [Google Scholar] [CrossRef]
- Oliveira, M.; Santos, E.; Araújo, A.; Fechine, G.J.M.; Machado, A.V.; Botelho, G. The effects of plasticizers on the dynamic mechanical and thermal properties of poly(lactic acid). Polym. Test. 2016, 51, 109–116. [Google Scholar] [CrossRef]
- Li, X.; Ai, X.; Pan, H.; Yang, J.; Gao, G.; Zhang, H.; Yang, H.; Dong, L. The morphological, mechanical, rheological, and thermal properties of PLA/PBAT blown films with chain extender. Polym. Adv. Technol. 2018, 29, 1706–1717. [Google Scholar] [CrossRef]
- Chiu, H.T.; Huang, S.Y.; Chen, Y.F.; Kuo, M.T.; Chiang, T.Y.; Chang, C.Y.; Wang, Y.H. Heat Treatment Effects on the Mechanical Properties and Morphologies of Poly (Lactic Acid)/Poly (Butylene Adipate-co-terephthalate) Blends. Int. J. Polym. Sci. 2013, 2013, e951696. [Google Scholar] [CrossRef]
- Hongdilokkul, P.; Keeratipinit, K.; Chawthai, S.; Hararak, B.; Seadan, M.; Suttiruengwong, S. A study on properties of PLA/PBAT from blown film process. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, e012112. [Google Scholar] [CrossRef] [Green Version]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.; Ngouajio, M.; Fernandez, R.T. Biodegradation and hydrolysis rate of aliphatic aromatic polyester. Polym. Degrad. Stab. 2010, 95, 2641–2647. [Google Scholar] [CrossRef]
- Larranaga, A.; Lizundia, E. A review on the thermomechanical properties and biodegradation behaviour of polyesters. Eur. Polym. J. 2019, 121, 109296. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Olivato, J.B.; Müller, C.M.O.; Yamashita, F.; Grossmann, M.V.E.; Nobrega, M.M. Study of the Compatibilizer Effect in the Properties of Starch/Polyester Blends. Polímeros 2013, 23, 346–351. [Google Scholar] [CrossRef] [Green Version]
- Al-Itry, R.; Amnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degr. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Witt, U.; Müller, R.J.; Deckwer, W.-D. Biologisch abbaubare Polyester-Copolymere mit einstellbaren Anwendungseigenschaften auf Basis chemischer Massenprodukte. Chem. Ing. Tech. 1995, 67, 904–907. [Google Scholar] [CrossRef]
- Witt, U.; Müller, R.J.; Deckwer, W.-D. Biodegradation of Polyester Copolymers Containing Aromatic Compounds. J. Macr. Sci. A 1995, 32, 851–856. [Google Scholar] [CrossRef]
- Nunes, E.C.D.; Souza, A.G.; Coiado, R.D.S.; Moura, E.A.B.; Rosa, D.S. Evaluation of the poly (lactic acid) and calcium carbonate effects on the mechanical and morphological properties in PBAT blends and composites. Int. J. Innov. Sci. Eng. Technol. 2017, 4, 313–318. [Google Scholar]
- Shahlari, M.; Lee, S. Mechanical and morphological properties of poly(butylene adipate-co-terephthalate) and poly(lactic acid) blended with organically modified silicate layers. Polym. Eng. Sci. 2012, 52, 1420–1428. [Google Scholar] [CrossRef]
- Wang, B.; Jin, Y.; Kang, K.; Yang, N.; Weng, Y.; Huang, Z.; Men, S. Investigation on compatibility of PLA/PBAT blends modified by epoxy-terminated branched polymers through chemical micro-crosslinking. e-Polymer 2020, 20, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Zou, B.; Yan, Y.; Ma, P.; Chen, M. Effect of Chain-Extenders on the Properties and Hydrolytic Degradation Behavior of the Poly(lactide)/Poly(butylene adipate-co-terephthalate) Blends. Int. J. Mol. Sci. 2013, 14, 20189–20203. [Google Scholar] [CrossRef] [Green Version]
- Arruda, L.C.; Megaton, M.; Bretas, R.E.S.; Ueki, M.N. Influence of chain extender on mechanical, thermal and morphological properties of blown films of PLA/PBAT blends. Polym. Test. 2015, 43, 27–37. [Google Scholar] [CrossRef]
- Pan, H.; Li, Z.; Yang, J.; Li, X.; Ai, X.; Hao, Y.; Zhang, H.; Dong, L. The effect of MDI on the structure and mechanical properties of poly(lactic acid) and poly(butylene adipate-co-butylene terephthalate) blends. RSC Adv. 2018, 8, 4610–4623. [Google Scholar] [CrossRef] [Green Version]
- BIO-FED Website. TDPG of M·VERA® B5029. Available online: https://bio-fed.com/fileadmin/bio-fed/PDFs/BIO-FED_TDPG_MVERA_B5029_B0155_2019-10-11.pdf (accessed on 25 October 2019).
- SONGWON Website. SONGNOXTM Product Descriptions. Available online: https://www.songwon.com/products/songnox-1680 (accessed on 25 October 2019).
- SpecialChem Webiste. Technical Datasheet of 1,3-Phenylene-bis-oxazoline. Available online: https://polymer-additives.specialchem.com/product/a-evonik-1-3-phenylene-bis-oxazoline (accessed on 25 October 2019).
- Lanxess website. Technical Datasheet of Stabaxol® P110. Available online: https://add.lanxess.com/fileadmin/product-import/stabaxol_p_110_en_rcr.pdf (accessed on 20 November 2020).
- Nisshinbo Chem Webiste. Hydrolysis Stabilizer for Polyesters Including Biodegradable Resin. Available online: https://www.nisshinbo-chem.co.jp/english/products/carbodilite/poly.html (accessed on 25 October 2019).
- Frank-PTI Website. Documentation for the Determination of Elmendorfer Tear Tester. Available online: https://www.frankpti.com/content/documents/en/81.pdf (accessed on 6 March 2020).
- Rigolin, T.R.; Costa, L.C.; Chinellato, M.A.; Muñoz, P.A.R.; Bettini, S.H.P. Chemical modification of poly(lactic acid) and its use as matrix in poly(lactic acid) poly(butylene adipate-co-terephthalate) blends. Polym. Test. 2017, 63, 542–549. [Google Scholar] [CrossRef]
- Möginger, B.; Fritz, U. Thermal properties of strained thermoplastic polymers. Polym. Int. 1991, 26, 121–128. [Google Scholar] [CrossRef]
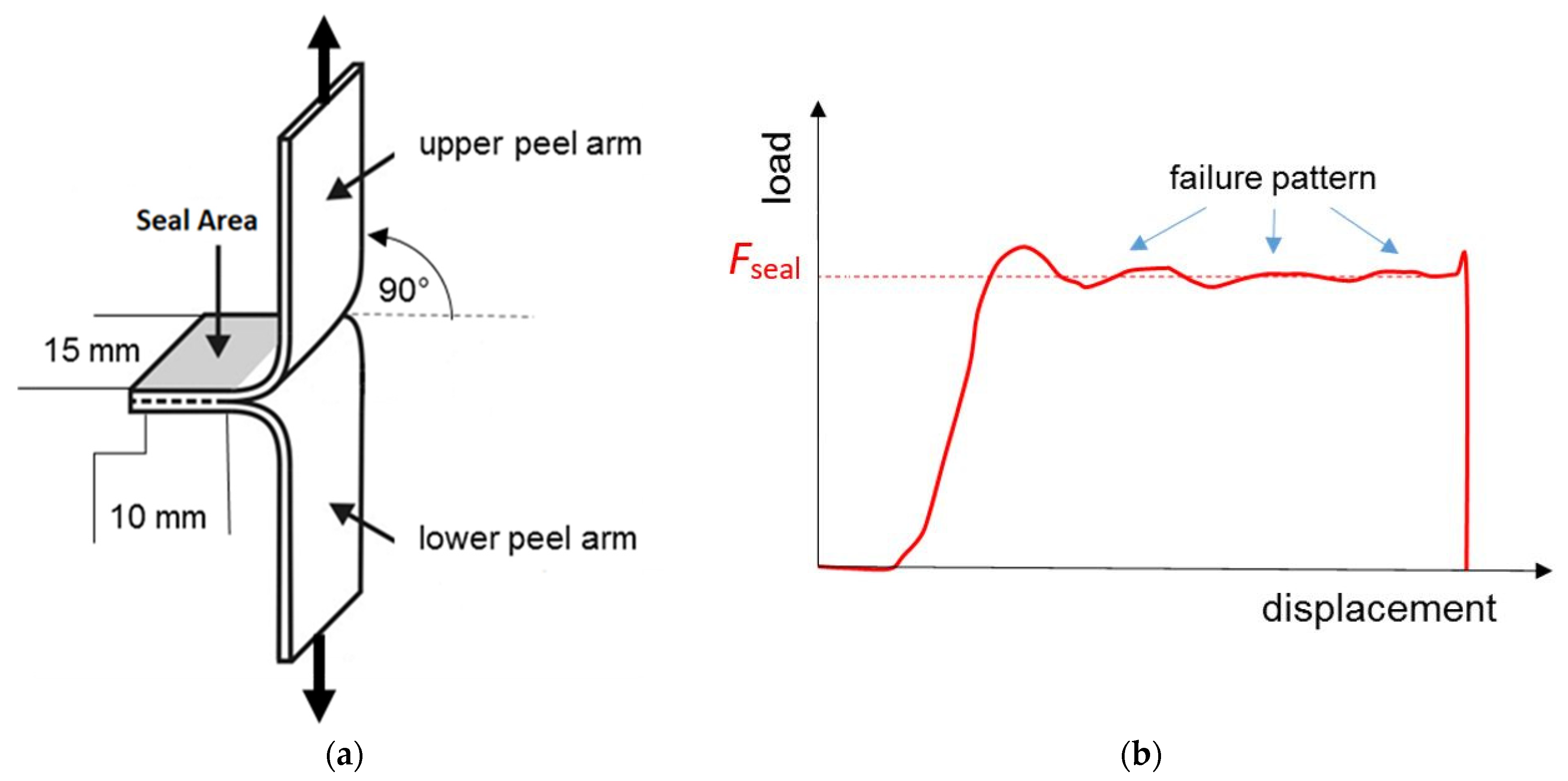
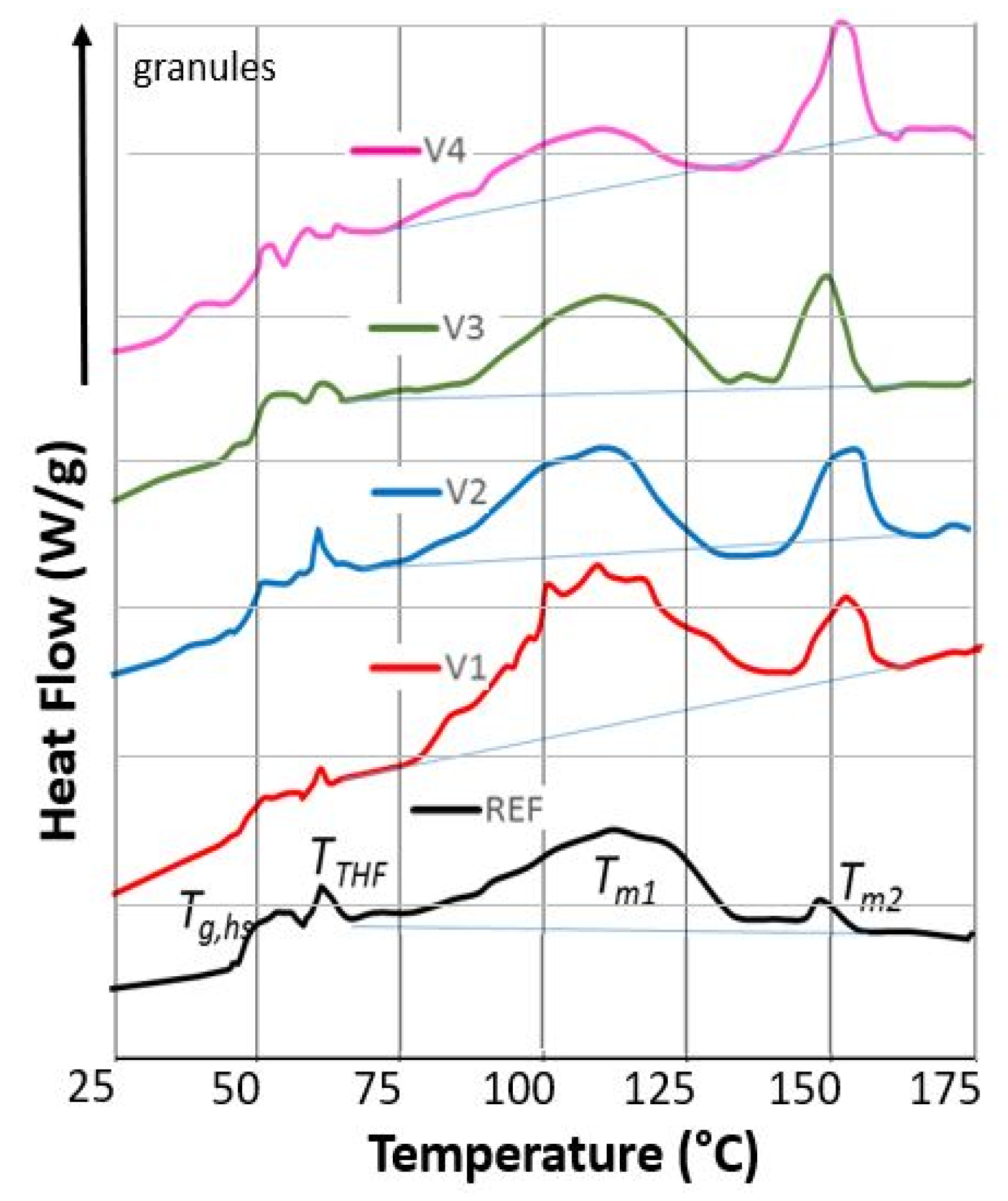
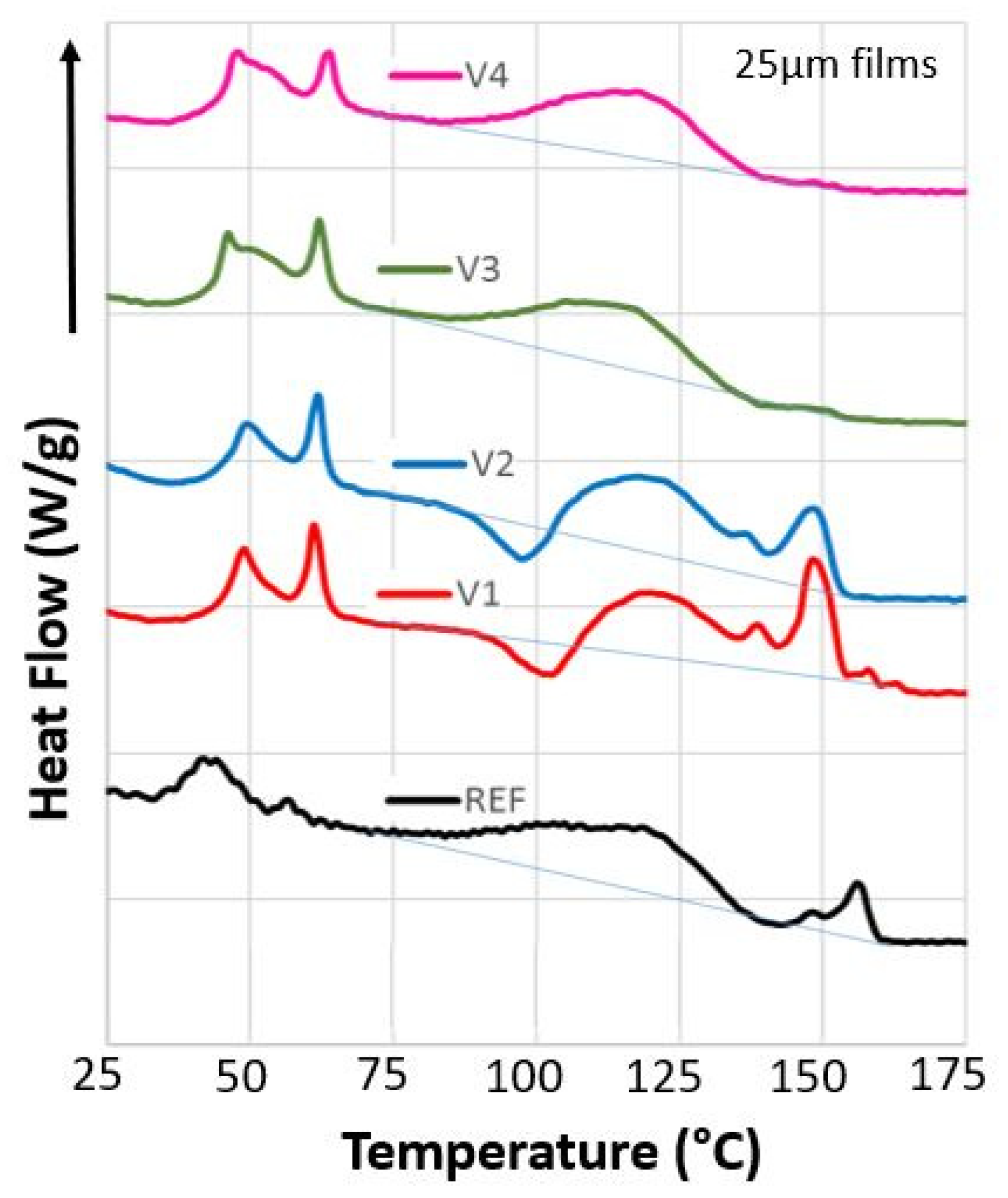
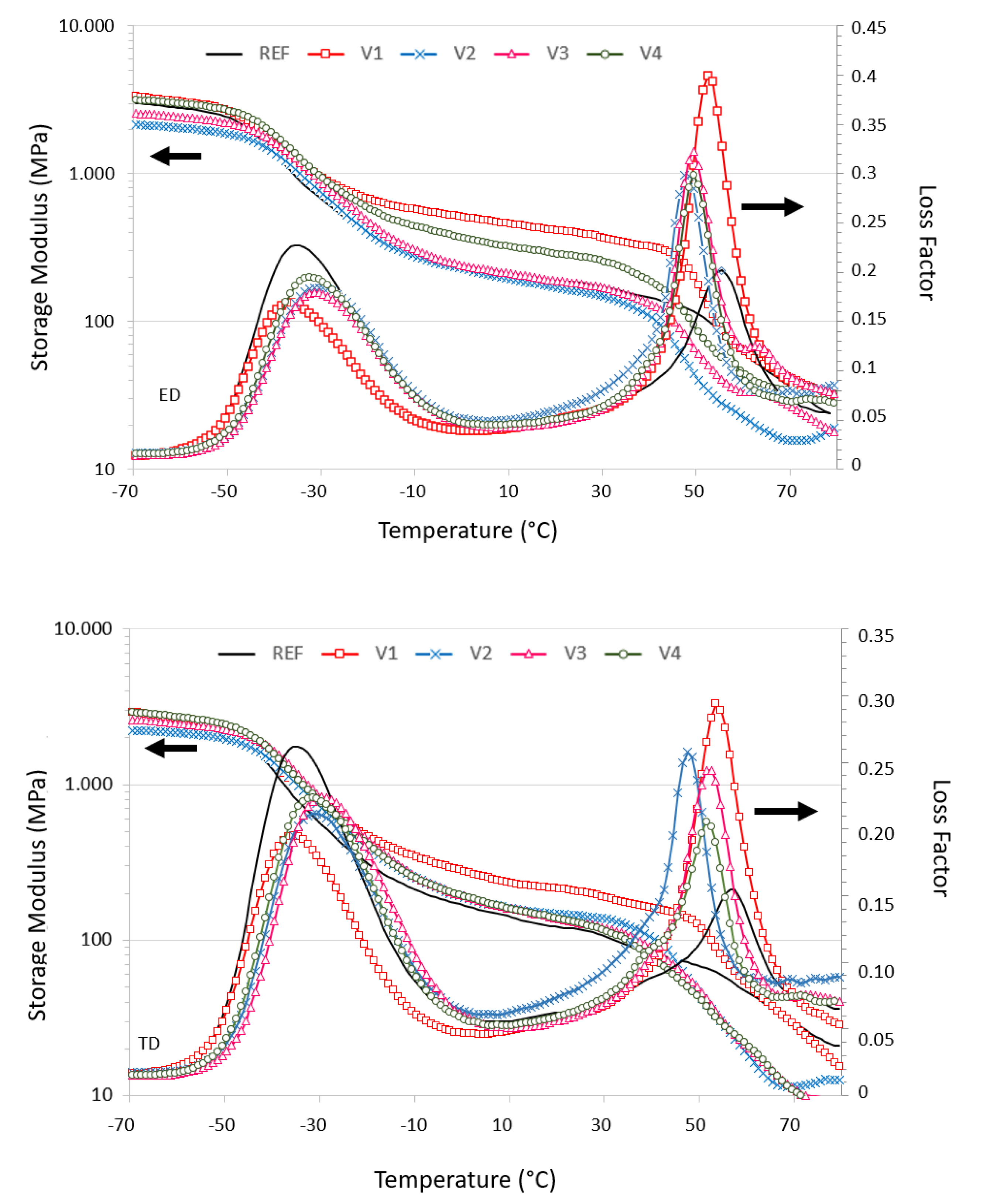
| Code | EED | ETD | σmax,ED | σmax,TD | εbreak,ED | εbreak,TD | Ftear,ED | Ftear,TD | Fseal | MVR |
|---|---|---|---|---|---|---|---|---|---|---|
| MPa | MPa | MPa | MPa | % | % | N mm−1 | N mm−1 | N | cm3 10 min−1 | |
| REF | 457 ± 36 | 181 ± 22 | 28 ± 3 | 29 ± 3 | 400 ± 48 | 450 ± 27 | 107 ± 3 | 54 ± 17 | 7.0 ± 0.3 | 4.0 ± 0.1 |
| V1 | 399 ± 42 | 177 ± 22 | 31 ± 3 | 33 ± 4 | 210 ± 10 | 450 ± 30 | 123 ± 38 | 41 ± 13 | 0.4 ± 0.2 | 5.0 ± 0.1 |
| V2 | 501 ± 31 | 162 ± 45 | 24 ± 2 | 24 ± 2 | 250 ± 31 | 450 ± 30 | 110 ± 34 | 35 ± 11 | 8.5 ± 0.3 | 5.8 ± 0.2 |
| V3 | 407 ± 66 | 147 ± 5 | 22 ± 3 | 25 ± 2 | 250 ± 31 | 460 ± 16 | 118 ± 38 | 40 ± 13 | 8 ± 0.6 | 0.6 ± 0.1 |
| V4 | 627 ± 56 | 380 ± 66 | 33 ± 3 | 35 ± 3 | 240 ± 14 | 440 ± 27 | 112 ± 35 | 40 ± 12 | 10 ± 2 | 1.0 ± 0.1 |
| Change of Properties with Respect to Corresponding Aging States | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Code | EED (MPa) | ETD (MPa) | σmax,ED (MPa) | σmax,TD (MPa) | εbreak,ED (%) | εbreak,TD (%) | Ftear,ED (N mm−1) | Ftear,TD (N mm−1) | Fseal (N) | MVR (cm3 10 min−1) |
| Aging 1 month at 50 °C | ||||||||||
| REF | 437 ± 37 | 238 ± 10 | 20.4 ± 2.6 | 21.8 ± 2.2 | 310 ± 39 | 390 ± 20 | 104 ± 33 | 57 ± 18 | 5.9 ± 0.4 | 4.7 ± 0.1 |
| V1 | 379 ± 21 | 183 ± 10 | 20 ± 1 | 22 ± 2 | 270 ± 17 | 450 ± 23 | 139 ± 43 | 35 ± 11 | 0.4 ± 0.2 | 5.5 ± 0.2 |
| V2 | 422 ± 20 | 182 ± 18 | 22 ± 2 | 23 ± 2 | 260 ± 17 | 480 ± 15 | 142 ± 44 | 31 ± 10 | 6.5 ± 0.3 | 6.3 ± 0.2 |
| V3 | 453 ± 16 | 206 ± 11 | 31 ± 1 | 33 ± 2 | 200 ± 16 | 450 ± 32 | 132 ± 41 | 30 ± 10 | 0.6 ± 0.1 | 0.4 ± 0.1 |
| V4 | 454 ± 38 | 265 ± 33 | 30 ± 1 | 31 ± 1 | 220 ± 11 | 420 ± 19 | 111 ± 35 | 29 ± 9 | 0.9 ± 0.1 | 1.4 ± 0.1 |
| Aging 2 months at 50 °C | ||||||||||
| REF | 363 ± 48 | 197 ± 12 | 21.2 ± 1.5 | 26.1 ± 1.8 | 410 ± 26 | 500 ± 20 | 80 ± 25 | 51 ± 16 | 4.8 ± 0.6 | 5.5 ± 0.2 |
| V1 | 400 ± 11 | 187 ± 23 | 20 ± 2 | 21 ± 1 | 250 ± 24 | 430 ± 14 | 138 ± 43 | 31 ± 13 | 0.6 ± 0.3 | 5.9 ± 0.2 |
| V2 | 436 ± 26 | 207 ± 20 | 32 ± 2 | 36 ± 2 | 200 ± 20 | 480 ± 39 | 130 ± 30 | 32 ± 11 | 5.5 ± 0.9 | 7.9 ± 0.3 |
| V3 | 436 ± 37 | 207 ± 14 | 32 ± 3 | 36 ± 3 | 200 ± 32 | 480 ± 29 | 112 ± 35 | 30 ± 10 | 0.5 ± 0.1 | 0.35 ± 0.1 |
| V4 | 460 ± 27 | 236 ± 25 | 30 ± 2 | 29 ± 3 | 210 ± 10 | 430 ± 33 | 109 ± 34 | 43 ± 14 | - | 1.2 ± 0.1 |
| Properties | Unit | REF | V1 | V2 | V3 | V4 | |
|---|---|---|---|---|---|---|---|
| Tg,hs_1 | °C | Onset | 50.0 | 43.7 | 46.7 | 47.8 | 46.2 |
| Midpoint | 54.1 | 49.0 | 49.8 | 52.8 | 49.7 | ||
| Tm1_1 | °C | Onset | 81.2 | 84.8 | 77.8 | 94.9 | 81.1 |
| Peak | 113.4 | 105.8 | 108.7 | 107.6 | 105.1 | ||
| Tm2_1 | °C | Onset | 143.2 | 144.2 | 143.4 | 142.7 | 143.2 |
| Peak | 149.9 | 152.0 | 152.7 | 149.9 | 152.7 | ||
| Tcr | °C | Onset | 75.4 | 83.4 | 83.0 | 87.3 | 87.2 |
| Peak | 65.3 | 72.6 | 71.9 | 76.2 | 75.2 | ||
| Tg,hs_2 | °C | Onset | 55.9 | 51.1 | 54.9 | 58.4 | 59.2 |
| Midpoint | 57.5 | 55.9 | 57.2 | 60.8 | 60.8 | ||
| Tm1_2 | °C | Onset | 88.4 | 89.1 | 88.9 | 83.6 | 83.8 |
| Peak | 115.6 | 116.6 | 115.2 | 116.3 | 116.6 | ||
| Tm2_2 | °C | Onset | 143.8 | - | 144.0 | - | - |
| Peak | 146.6 | - | 149.2 | - | - | ||
| ΔHm1_1 | J g−1 | 8.5 | 9.4 | 6.9 | 5.1 | 5.2 | |
| ΔHm2_1 | J g−1 | 0.3 | 1.1 | 1.9 | 1.1 | 2.8 | |
| ΔHcr | 11.0 | 11.6 | 10.9 | 9.1 | 10.3 | ||
| ΔHm1_2 | J g−1 | 7.3 | 7.3 | 5.8 | 5.8 | 5.8 | |
| ΔHm2_2 | J g−1 | 0 | 0 | 0.8 | 0 | 0 |
| Code | Tg,hs (°C) | TTHF (J g−1) | ΔHTHF (J g−1) | ΔHm1 (J g−1) | ΔHm2 (J g−1) | ΔHcr (J g−1) | ||
|---|---|---|---|---|---|---|---|---|
| Onset | Midpoint | Endset | PBAT | PLA | ||||
| 25 µm film | ||||||||
| REF | 43.0 | 47.4 | 52.2 | 60.5 | 0.2 | 5.4 | 1.1 | 15.2 |
| STD | 0.7 | 0.0 | 0.5 | 0.4 | 0.1 | 0.4 | 0.1 | 0.9 |
| V1 | 44.9 | 47.6 | 49.3 | 61.3 | 0.3 | 5.4 | 1.2 | 15.1 |
| STD | 0.6 | 0.0 | 1.8 | 0.8 | 0.1 | 0.5 | 0.1 | 0.1 |
| V2 | 44.4 | 48.5 | 51.8 | 63.9 | 0.5 | 5.1 | 2.9 | 14.7 |
| STD | 1.7 | 0.4 | 0.6 | 0.1 | 0.0 | 0.2 | 0.1 | 0.1 |
| V3 | 41.7 | 45.3 | 48.4 | 63.8 | 0.4 | 6.4 | 1.2 | 11.7 |
| STD | 0.4 | 0.3 | 0.1 | 0.7 | 0.0 | 0.1 | 0.0 | 0.3 |
| V4 | 43.3 | 46.4 | 50.4 | 63.1 | 0.3 | 3.7 | 3.5 | 12.7 |
| STD | 0.9 | 0.7 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.6 |
| 100 µm film | ||||||||
| REF | 34.2 | 38.3 | 42.2 | 56.4 | 0.2 | 9.5 | 1.4 | 15.2 |
| STD | 1.1 | 0.4 | 0.1 | 0.3 | 0.1 | 1.8 | 0.1 | 0.3 |
| V1 | 41.4 | 46.2 | 48.9 | 61.4 | 1.0 | 5.8 | 2.6 | 13.5 |
| STD | 0.4 | 0.1 | 0.3 | 0.1 | 0.0 | 0.2 | 0.1 | 0.6 |
| V2 | 43.9 | 46.0 | 48.4 | 61.7 | 0.9 | 4.2 | 1.7 | 10.4 |
| STD | 1.3 | 0.8 | 0.4 | 0.1 | 0.0 | 0.4 | 0.1 | 0.1 |
| V3 | 43.5 | 44.8 | 45.7 | 62.2 | 0.7 | 7.2 | 0.0 | 15.6 |
| STD | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.2 | 0.0 | 0.1 |
| V4 | 44.0 | 45.5 | 47.0 | 63.0 | 0.7 | 8.0 | 0.0 | 14.7 |
| STD | 0.6 | 0.8 | 0.5 | 0.8 | 0.0 | 0.4 | 0.0 | 0.4 |
| Sample | E’ (MPa) | E” (MPa) | Tg,ss (E”) (°C) | Tg,hs (E”) (°C) | Tg,ss (tanδ °C) | Tg,hs (tanδ °C) | |
|---|---|---|---|---|---|---|---|
| REF | ED | 177 ± 33 | 10.3 ± 1.2 | −41.7 ± 0.2 | 52.4 ± 0.8 | −35.5 ± 0.1 | 54.8 ± 0.7 |
| TD | 124 ± 13 | 8.0 ± 1 | −41.9 ± 0.1 | 55.0 ± 0.4 | −35.2 ± 0.1 | 56.7 ± 0.3 | |
| V1 | ED | 387 ± 38 | 21.0 ± 1.4 | −41.7 ± 0.6 | 50.6 ± 1.2 | −36.8 ± 0.9 | 53.3 ± 1.2 |
| TD | 322 ± 17 | 20.0 ± 1.6 | −41.9 ± 0.1 | 51.0 ± 0.1 | −36.1 ± 0.3 | 53.6 ± 0.1 | |
| V2 | ED | 210 ± 33 | 13.0 ± 1.4 | −38.8 ± 0.7 | 50.0 ± 4.2 | −32.7 ± 0.5 | 51.9 ± 4.5 |
| TD | 151 ± 12 | 11.0 ± 0.8 | −38.9 ± 0.9 | 50.0 ± 1.3 | −32.2 ± 0.9 | 52.1 ± 1.1 | |
| V3 | ED | 184 ± 15 | 9.3 ± 1.2 | −36.8 ± 0.7 | 50.5 ± 5.8 | −31.0 ± 0.5 | 52.5 ± 6.2 |
| TD | 126 ± 5 | 7.3 ± 0.5 | −37.2 ± 0.5 | 53.6 ± 3.8 | −30.6 ± 0.5 | 56.1 ± 4.0 | |
| V4 | ED | 270 ± 24 | 15.0 ± 1.9 | −38.7 ± 1.3 | 50.0 ± 4.3 | −32.9 ± 1.9 | 52.0 ± 4.4 |
| TD | 133 ± 6 | 8.7 ± 0.9 | −38.0 ± 0.4 | 49.6 ± 5.7 | −31.8 ± 0.8 | 51.8 ± 6.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, J.V.C.; Dorp, E.R.-v.; Hausnerova, B.; Möginger, B. The Effects of Chain-Extending Cross-Linkers on the Mechanical and Thermal Properties of Poly(butylene adipate terephthalate)/Poly(lactic acid) Blown Films. Polymers 2021, 13, 3092. https://doi.org/10.3390/polym13183092
Azevedo JVC, Dorp ER-v, Hausnerova B, Möginger B. The Effects of Chain-Extending Cross-Linkers on the Mechanical and Thermal Properties of Poly(butylene adipate terephthalate)/Poly(lactic acid) Blown Films. Polymers. 2021; 13(18):3092. https://doi.org/10.3390/polym13183092
Chicago/Turabian StyleAzevedo, Juliana V. C., Esther Ramakers-van Dorp, Berenika Hausnerova, and Bernhard Möginger. 2021. "The Effects of Chain-Extending Cross-Linkers on the Mechanical and Thermal Properties of Poly(butylene adipate terephthalate)/Poly(lactic acid) Blown Films" Polymers 13, no. 18: 3092. https://doi.org/10.3390/polym13183092
APA StyleAzevedo, J. V. C., Dorp, E. R.-v., Hausnerova, B., & Möginger, B. (2021). The Effects of Chain-Extending Cross-Linkers on the Mechanical and Thermal Properties of Poly(butylene adipate terephthalate)/Poly(lactic acid) Blown Films. Polymers, 13(18), 3092. https://doi.org/10.3390/polym13183092






