Abstract
A new type of conjugated polybenzimidazole (CPBI) was synthesized through a simple polycondensation reaction without metal catalysis, and N-alkylation modification was carried out to solve the problems of solubility and fluorescence properties. A series of nano-microsphere polymers CPBIn with large conjugation, good solubility, and strong fluorescence has been successfully used as “turn-off” fluorescent probes for the first time. The results show that, under suitable N-alkylation conditions, the obtained CPBIn can be used as a highly sensitive and selective fluorescent probe for the detection of Cu2+ and Zn2+ at the same time, and their detection limits are both nM levels. In addition, CPBI2 can be designed as an ultra-sensitive IMPLICATION logic gate at the molecular level, cyclically detecting Cu2+. With the test paper containing CPBI2, easy and quick on-site detection can be achieved. This research provides a new idea for the brief synthesis of multifunctional materials.
1. Introduction
Conjugated polymer molecules are important materials for optoelectronic applications because they provide a relatively direct link between optoelectronic properties and compound structures [1]. Compared with non-backbone conjugated polymeric probes, organic backbone conjugated polymers are composed of unsaturated structural units, such as aromatic hydrocarbons, olefins, or acetylene, making the formation of a large delocalized polarizable π-electronic domain easy [2]. For their excellent optical and electrical properties, more and more attention has been paid to them [3]. At the same time, their molecular chains may function as “molecular lines”, along which energy and electric charges can move. This unique performance determines the ability to detect ultra-low concentrations of analytes [4,5,6]. Therefore, the synthesis and application of organic conjugated macromolecular chemical sensors have attracted more and more attention [7,8,9].
Copper ion and zinc ions are essential trace elements in the human body, which play an important role in human health, especially the catalytic auxiliary role in various biological processes such as the function of many cellular enzymes and proteins [10,11,12]. However, when the concentration of absorbed ions is unbalanced, the homeostasis in cells will be affected, leading to a series of diseases [13,14,15]. The multifunctional detection of the same sensor for each detection object is attracting attention due to its high efficiency [16,17,18,19]. As a result, the design and synthesis of new multifunctional fluorescent probes that can simultaneously detect copper and zinc ions are of great significance in biomedical and environmental monitoring applications [20,21].
According to the structural characteristics of the analyte, the design of fluorescent molecules is crucial. Specific recognition units are introduced, such as N-containing heterocyclic compounds such as quinoline [22], imidazole [23,24], and carbazole [25], to provide a definite binding site for metal ion as analyte. Using the availability, strong polarization, and π–π electron transition function of the conjugate system to ensure its energy transfer or electron transfer [26], the multifunctional probe may be constructed. In addition, as an effective method to achieve fluorescence probe regulation [27,28], the introduction of a long alkyl chain at the end of the compound increases the spatial effect of the polymer, significantly affecting the HOMO energy level, keeping the LUMO energy level basically unchanged, increasing the band gap [29], and affecting its optical properties, which is of great research value [30,31,32].
Herein, we used (E)-2-butenedioic acid (E-BA) as a “bridge” by two steps to synthesize a novel conjugated nanosphere structure polybenzimidazole sensor CPBIn (Scheme 1), which can be used for Cu2+ and Zn2+ detection with fluorescence regulation. Additionally, through infrared spectrum (IR), X-ray photoelectron spectroscopy (XPS), scanning electron microscope (SEM), etc., their structures and photophysical properties were studied. The results show that CPBIn with good solubility and optical properties can be successfully obtained by modifying CPBI0 with an alkyl chain and used as a multifunctional fluorescent probe to detect Cu2+ and Zn2+ at the same time, with a low detection limit.

Scheme 1.
Synthesis of serial CPBIs fluorescent materials.
2. Materials and Methods
2.1. Apparatus and Reagents
All reagents and organic solvents were purchased from commercial suppliers and used without further purification. 1H NMR spectra of compounds were recorded on a Bruker 600 MHz instrument (Bruker AVANCE NEO, Bruker, Karlsruhe, Germany). Fluorescence spectra were carried out by a Hitachi F-4600 fluorescence spectrometer (Hitachi, Tokyo, Japan). The Scanning Electron Microscope was obtained by FEI Quanta 250 FEG field emission scanning electron microscope (FEI Quanta 250 FEG, FEI, Hillsboro, OR, USA). The TG data were obtained by TG-209 F3 TG analyzer (NETZSCH-Gerätebau GmbH, Selb, Germany). Quantum yields were calculated by using quinine sulfate (Φfl) 0.55 in 0.5 M H2SO4 solution as a standard. Fluorescence lifetime spectrum was obtained using time-correlated single-photon counting method.
2.2. Synthesis
2.2.1. Synthesis of Intermediate CPBI0
According to the literature [33,34,35], 20 mL polyphosphoric acid (PPA) and 3,3′-diaminobenzidine 1 were added into a 50 mL flask and heated at 160 °C for 2 h. After they were completely dissolved, 3.0 mmol trans-butenedioic acid (E-BA) 2 was added, and the mixture was heated to 170 °C for 48 h. After cooling to room temperature, the pH was adjusted to alkaline with NaOH solution, and then a blue–black solid was obtained by the filtration.
2.2.2. Synthesis of Series of CPBIn
According to reported methods [36,37,38], 1 mmol CPBI0, different molar n-C5H11Br (Table 1), 10 mL MeCN, and moderate NaOH were added into the reaction flask. After refluxing for 24 h, the organic solvent was evaporated in vacuo. The crude product was washed several times with plenty of water to remove NaOH, and then the alkylation product was treated with a mixture of methylene chloride and ethanol. Once the organic phase was collected continuously, the solvent was removed in vacuo. After the desired product was dried in a vacuum drying oven at 40 °C for 24 h, the purified soluble alkylation solid product CPBIn was obtained.

Table 1.
The effects of different feed ratios on the basic structure of serial CPBIn.
3. Results
3.1. Structural Characterization and Basic Properties of CPBIs
The structures of CPBIS were characterized by 1H NMR, IR, XPS, etc. As shown in Figure 1 (see SM for more detailed characterization data of all CPBIs from Figures S1–S7), it can be found that, with the N-alkylation reaction, the signal of N-H (Hd, 13.03 ppm) in the main chain of CPBI0 is becoming weak for the gradual replacement. At the same time, new signals of alkyl chain characteristics, such as Hf (4.06~4.55 ppm), Hg (1.58~1.65 ppm), Hi, Hh (1.23~1.28 ppm), and Hj (0.686~0.90 ppm), can be observed in CPBIn (Figure S7). In addition, the signal of terminal olefin double bond hydrogen (=CH, 6.32–6.67 ppm) in the polymer was used to calculate the number-average molecular weight (Mn) of CPBIn. As shown in Table S1, it can be seen that, with the increase of the feed ratio of n(C5H11Br)/n(CPBI0), the alkylation rate of CPBIn increases continuously and the Mn also increases correspondingly, which is consistent with expectations. Meanwhile, the color of solid product is gradually lightening (Table S1).
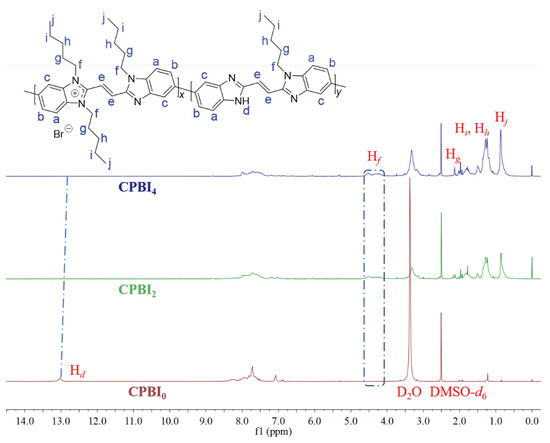
Figure 1.
The changes of 1H NMR spectra of CPBI0 and CPBIn.
In the FT-IR spectra, with the increase of feed ratio [n(C5H11Br)/n(CPBI0)], the benzimidazole N-H stretching vibration at 3300 cm−1 is gradually decreased. On the contrary, some characteristic stretching vibrations of saturated alkyl chains at 2984, 2853, 1479, and 730 cm−1 are strengthened continuously. Of course, the C–N stretching vibration at 1325 cm−1 is also gradually enhanced (Figure S8). These all indicate that the N-alkylation reaction has successfully attached the alkyl chain to the polymer via C–N bond.
As reference method [39,40,41], the XPS energy spectra of CPBI2 shown in Figure S9 were analyzed. It can be seen from Figure S9a that 284.76, 399.76, and 531.76 eV are attributed to C1s, N1s, and O1s on the CPBI2 skeleton, respectively. The sub-peaks of C1s are 284.865 eV, 284.346 eV, 285.982 eV, and 287.795 eV, respectively, belonging to C–C/C=C, C–N/C–O, C=N, and the polymer terminal C=O (Figure S9b). The electron energies of C–N and C=N can also be obtained from the sub-peaks of N1s (Figure S9c). Thus, the XPS data further demonstrate the construction of the polymer skeleton. In a word, CPBI0 has been successfully N-alkylated, which is proved by 1H NMR, IR, and XPS. Additionally, changing the feed ratio [n(C5H11Br)/n(CPBI0)], CPBIn polymers with different alkylation degrees can be obtained, as shown in Table S1. Subsequently, SEM, TG, and XRD of CPBIn were studied and discussed.
The crystallization performance of serial CPBIs was analyzed by XRD (Figure S10). Under low alkylation rate (CPBI1~CPBI4), the polymer is amorphous. However, with the continuous increase of alkylation rate (CPBI5~CPBI7), the polymer morphology tends to become regular, and the crystallinity is increased (Figure S10b). The thermal stabilities of the CPBIs were investigated under an O2 atmosphere (Figure S11). The initial and termination temperatures of thermal decomposition of CPBI5~CPBI7 polymers with high alkylation rate (Table S1) and certain crystallinity are significantly higher than those of amorphous CPBI1~CPBI4 polymers with low alkylation rate (Table S2, and the detailed data analysis can be seen in SM).
Using reported method [42,43,44], SEM was used to observe the morphology of series CPBIs, as shown in Figure S12. The surface of CPBI0 is flat and lamellar. When the feed ratio [n(C5H11Br)/n(CPBI0)] is 1/1, the original lamellar structure is destroyed and gradually transferred to the nano-microsphere structure. When the feed ratio is 2/1, CPBI2 has a nano-microsphere structure with a diameter of 375 nm (Figure 2a). With the continuous increase of alkylation rate, there is still nanosphere structure, e.g., CPBI4 with a diameter of 323 nm (Figure 2b). When n(C5H11Br)/n(CPBI0) is equal to or bigger than 5:1, the nanosphere becomes a larger microsphere (e.g., CPBI5 with a diameter of 7 μm), and begins to be destroyed and accumulated (see Figure S12 in SM). This may be due to the electrostatic repulsion of the polymers being different with the increase of the alkylation rate during the N-alkylation process of CPBI0 and the size of microspheres formed by these polymers under the influence of different electrostatic forces. Therefore, not only can the alkylation rate of CPBI0 be regulated by the feed ratio of reactants, but also, the morphology of the alkylation product can be adjusted, and the nanosphere structure may be formed when the ratio is appropriate (Figure 2).

Figure 2.
SEM imgaes of CPBI2 (a) and CPBI4 (b).
3.2. The Photophysical Properties of Serial CPBIs
As an electron-donating group, the alkyl chain easily produces a p–π conjugate with the benzene ring, resulting in enhanced fluorescence of the probe [31]. In addition, for conjugated polymers, the Aggregation-Caused Quenching (ACQ) effect is weakened by the introduction of long alkyl chains. This improves the solid-state fluorescence of the polymer. In order to illustrate the effect of alkylation rate on the fluorescence intensity of the polymer, the fluorescence test of CPBIn in solid and liquid was carried out, respectively, as shown in Figure 3. It can be found that the fluorescence of solid-state CPBIn is greatly influenced by the alkylation rate, and its fluorescence emission intensity increases with the increase of the alkylation rate (Figure 3a). With a significant blue shift, the solid fluorescence color changes from orange–yellow to yellow (Figure 3c and Table S3).
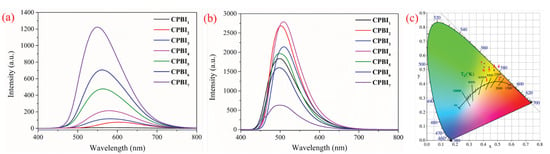
Figure 3.
Solid fluorescence emission spectra (a), liquid fluorescence emission spectra (1 mg/15 mL in DMSO, λex = 375 nm) (b), and solid chromaticity diagram of CPBIn with different feeding ratios (c).
Usually, the use of large conjugated polybenzimidazole in the probe field is limited because of solubility. Here, we modified CPBI0 by N-alkylation to obtain a series of new polymer CPBIn. These polymers (especially CPBI1~CPBI4) are soluble in a variety of organic solvents, such as THF, DMF, DMSO, etc. Among them, DMSO has the best solubility, so DMSO is used as the solvent in the subsequent fluorescence tests. In Figure 3b, it can be found that the fluorescence intensity of CPBIn solution is not only affected by the electron donation of alkyl chain but also greatly related to its structure, solubility, and molecular vibration [32,45,46]. Therefore, there is no obvious regular change in the fluorescence intensity of their solution. Even so, among them, CPBI2 and CPBI4 have relatively strong fluorescence, which may be determined by their nanosphere structure (Figure 2).
The effects of different solvents such as THF, MeCN, EtOH, DMF, DMSO, CH2Cl2, and CHCl3 on the fluorescence properties of the polymer were also investigated. The experimental results are shown in Figure S13. It can be seen that, in different solvents, the difference in fluorescence intensity of CPBI1~CPBI4 is mainly affected by solubility rather than solvent polarity. Additionally, the position of the fluorescence emission peak of CPBIn has little relation with the polarity of the solvent.
The metal ions are soluble in water, and they are generally tested in the experiments as salt solutions. In order to eliminate the influence of the presence of water on the detection of metal ions, the changes in the fluorescence intensity of CPBI1~CPBI4 with different water content were studied (Figure S14). With the increase of water content as DMSO/H2O (v/v), the fluorescence emission intensity of CPBIn is decreased sharply and basically reaches complete quenching (ACQ) at 50% water content (50:50, v/v). Therefore, to avoid the interference of higher water content, DMSO/H2O (90:10, v/v) is used as the mixed solvent in the following experiments to study the sensing performance of the polymer towards metal ions.
3.3. Sensing Performance of CPBIn towards Metal Ions
The solubility and photophysical properties of CPBI0 are improved by N-alkylation reaction. In DMSO/H2O (90:10, v/v) solvents, the effect of alkylation rate on the fluorescence properties of the polymer was analyzed by studying the corresponding CPBIn to different metal ions. The selectivity of CPBI2 (37.4% alkylation) for 18 metal ions was investigated in DMSO/H2O system. Only Cu2+ and Zn2+ are tightly bound by probe CPBI2, resulting in the fluorescence quenching of CPBI2 at λem = 504 nm with the excitation at 375 nm (Figure 4a), and the fluorescence quenching rate is 89.4% and 91.5%, respectively. Compared with the response of Cu2+, after the addition of Zn2+, the fluorescence color of CPBI2 probe solution is changed from bright green to yellow under 365 nm UV light (Figure 4c), and λem is red-shifted from 504 nm to 519 nm. For the other metals evaluated, only Fe3+ has a slightly similar effect, but this can be negligible in comparison with Cu2+ and Zn2+ (Figure 4b). Therefore, CPBI2 can be used as a “turn-off” probe for the simultaneous detection of Cu2+ and Zn2+.

Figure 4.
The fluorescence selectivity study of different metal ions (100 μM) in CPBI2 (1 mg/15 mL in DMSO/H2O, v/v, 9/1) solution, λex = 375 nm (a); the influence of adding different metal ions (100 μM) on CPBI2 fluorescence intensity at 504 nm (b); and the fluorescence color change of the solution when different metal ions are added under a 365 nm UV lamp (c).
We also studied the possibility of other common metal ions interfering with the detection of Cu2+ and Zn2+. As seen in Figure S15, the presence of other metal ions has no effect on the detection of Cu2+ and Zn2+ by the probe CPBI2. The results show that CPBI2 has good anti-interference ability for the detection of Cu2+ and Zn2+. The fluorescence intensity of CPBI2 is gradually decreased at λem = 504 nm with the addition of Cu2+ and Zn2+ (0~100 μM), as shown in Figure 5a,c. According to the calculation method reported in the literature [47,48,49], the fluorescence detection limit of sensor CPBI2 for Cu2+ and Zn2+ can be calculated as 5.98 × 10−9 M and 6.02 × 10−9 M, respectively (Figure 5b,d). Compared with the data reported before, the results indicate that sensor CPBI2 is more sensitive than most known Cu2+ and Zn2+ sensors [50,51,52,53,54] (more comparisons can be seen in Tables S5 and S6).
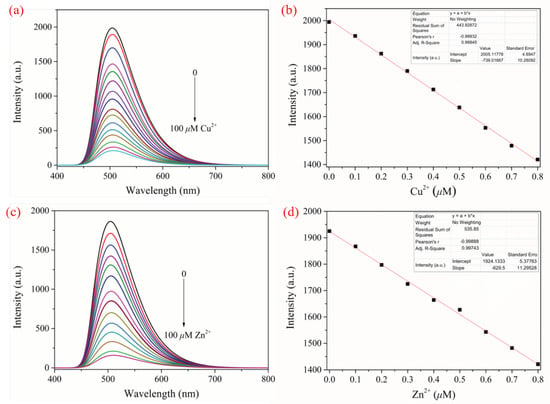
Figure 5.
Fluorescence titration of CPBI2 (1 mg/15 mL in DMSO/H2O, v/v, 9/1) solution with Cu2+ (a,b), Zn2+ (c,d), and their corresponding detection limits (λex = 375 nm).
In addition, as a “turn-off” fluorescence sensor, the fluorescence-quenching process of CPBI2 can be determined by Stern–Volmer (S–V) constant (Ksv), whose calculation formula is: I0/I = 1+Ksv[Q] (Figure 6). The S–V curve has a tendency to bend upward when a higher concentration of Cu2+ or Zn2+ is added to CPBI2 solution. However, the Stern–Volmer equation has a linear-fitting relationship when the concentration of metal ions is lower. This indicates that there are static and dynamic quenching processes of CPBI2 interaction with metal ions. Ksv values for CPBI2 towards Cu2+ and Zn2+ can be calculated to be 8.95 × 104 and 4.52 × 104 M−1, respectively. Fluorescence sensors with dynamic quenching may show good reversibility detection for metal ions [55,56].
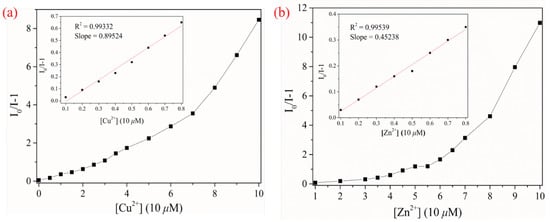
Figure 6.
Stern–Volmer diagram of the interaction of sensors CPBI2, with Cu2+ (a) and Zn2+ (b) (the illustration shows the Stern–Volmer linear diagram at low concentrations).
Similarly, we also conducted the fluorescence tests on other alkylated polymers, such as CPBI1, CPBI3, and CPBI4, as shown in Figures S16–S28. As anticipated, CPBI1, CPBI3, and CPBI4 can selectively recognize Cu2+ and Zn2+ (Figures S16, S20, and S24). For example, it can be found that the Stern–Volmer equation of CPBI4 has a good linear relationship with the concentration of metal ions (Ksv values for CPBI4 towards Cu2+ and Zn2+ can be calculated to be 2.20 × 104 and 1.45 × 104 M−1, respectively), so the fluorescence quenching mode of CPBI4 is static quenching (Figure S28).
Importantly, the sensitivity to metal ions is increasing with the increase of alkylation rate (Table S4). However, the fluorescence-quenching effect is decreasing. This may be due to the increase in electron-donating groups in the polymer as the alkylation rate increases, which makes it easier to combine with electron-deficient metal ions. Nevertheless, the steric hindrance of the polymer is increased likewise, and the combination of metal ions is reduced correspondingly when the alkylation rate is too high, so the fluorescence-quenching effect is reduced. This represents that the different alkylation rates will lead to the different detection performances of polymer fluorescent probes, which further demonstrates the regulation of alkyl chain on the performance of polymer fluorescent probes.
3.4. Sensing Mechanism of CPBIs for Metal Ions
According to the reported method [53,57,58,59], we explored the sensing mechanism of CPBIs before and after the interaction with analytes by IR, SEM, and DFT. Using CPBI2 as an example, in the IR spectra (Figure 7a), the C=N stretching vibration (1654 cm−1), C–N stretching vibration (1327 cm−1), and N-H stretching vibration of imidazole ring in probe CPBI2 are greatly weakened after the addition of Cu2+. This suggests that Cu2+ can interact with C=N and not alkylated C–N in the imidazole ring of the CPBI2 backbone. Of course, after complexing with metal ions, there is an aggregation for CPBI2, which limits the movement of the alkyl chain, resulting in an obvious weakening of the saturated C-H flexural vibration (1461 cm−1) also. Importantly, a new absorption can be observed at 1121 cm−1 when CPBI2 interacts with Cu2+. It is consistent with the results in the literature [16,60,61,62]. In addition, the results of SEM further demonstrate that the original nanoparticle structure of CPBI2 (Figure 7d) has aggregated into a massive flaky structure (Figure 7c) after the interaction of CPBI2 with Cu2+.
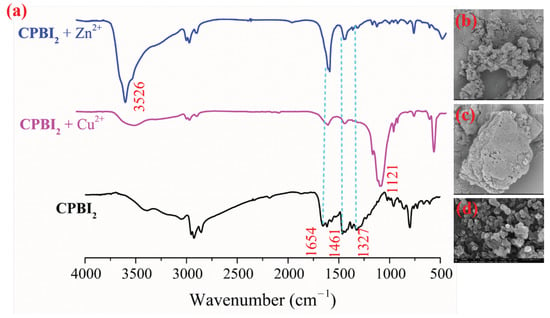
Figure 7.
FT-IR spectra (a), SEM images of CPBI2 before and after the addition of Cu2+ or Zn2+ ((b): CPBI2+Zn2+; (c): CPBI2+Cu2+; (d): CPBI2).
Similarly, the C–N stretching vibration (1327 cm−1) of CPBI2 is also greatly weakened when CPBI2 interacts with Zn2+, while the C=N stretching vibration intensity (1654 cm−1) is basically unchanged (Figure 7a). This shows that Zn2+ is mainly coordinated with C–N in the main chain of benzimidazole. Additionally, the flexural vibration of alkyl chain (1461 cm−1) is also limited when coordinated with Zn2+. Moreover, a new absorption appears at 3540 cm−1, which is also consistent with the results in the literature [63,64]. At the same time, the image of SEM (Figure 7b) also shows the interaction between CPBI2 and Zn2+. Therefore, the interaction between CPBI2 and Cu2+ and Zn2+ can be proved by both IR and SEM. For other series of CPBIn polymers, such as CPBI1, CPBI3, and CPBI4, their IR and SEM morphologies after the interaction with metal ions are similar (Figures S29–S31 in SM).
In addition, we further investigated the quenching process of the probe by changing the fluorescence lifetime [65]. It can be found that the presence of metal ions has no effect on the decay of the fluorescence lifetime of CPBI2 and CPBI4 when the concentration of metal ions is low (Figure S32 in SM). Therefore, it is also verified that the fluorescence-quenching process of CPBI2 and CPBI4 is static quenching in the low concentration range of metal ions.
Using the reported method [66,67,68,69], Gauss DFT-B3LYP/6-31G method was used to optimize the structure of CPBI2, CPBI2–Cu2+ complex, and CPBI0. As shown in Figure 8, the energy of the lowest unoccupied molecular orbital (LUMO) before and after alkylation (CPBI0) is mainly distributed on the carboxylic acid group side at the end of the polymer, and the energy is −2.24 eV. On the contrary, the energy of the highest occupied molecular orbital (HOMO) is mainly distributed on the side containing NH2 at the end of the polymer. After the introduction of the alkyl chain, only HOMO is slightly affected, which makes the HOMO-LUMO band gap (∆E) slightly larger. The structure of the complex combined with metal ions was optimized by the Cu2+ complex. It can be found that the ∆E is greatly reduced when CPBI2 is complexed with Cu2+ (Figure 8). This indicates that a stable metal complex has been formed, which is further proof of coordination between metal ions and polymers [70,71].
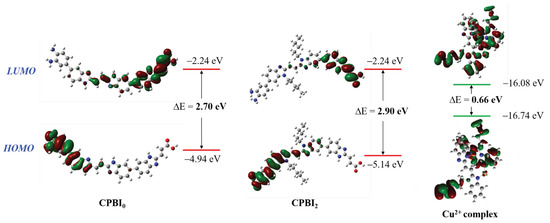
Figure 8.
The optimized geometries of CPBI2, its Cu2+ complex, and CPBI0 (B3LYP/6-31G basis set CPBI0, CPBI2, and B3LYP/6-31G basis set for C, H, N; LanL2DZ for Cu2+ in complex).
3.5. Logic Gate Construction
The reversibility of the sensor is an important indicator of whether the sensor can be reused multiple times. CPBI2 was selected for the logic gate cycle experiment (Figure 9) because of its good fluorescence-quenching effect and obvious detection phenomenon when sensing copper ions. According to the fluorescence titration experiment, when EDTA is added to CPBI2–Cu2+ complex, Cu2+ in the complex will bind to EDTA due to the stronger complexing ability of EDTA so that CPBI2 becomes free and the corresponding fluorescence intensity is also recovered (Figure 9b). In addition, this fluorescent “off/on” switching behavior can be observed at 365 nm UV lamp (Figure 9a). As a chemical sensor, CPBI2 has shown good stability and reversibility; it is expected to be designed as a chemical sensor based on molecular logic gate [72,73].
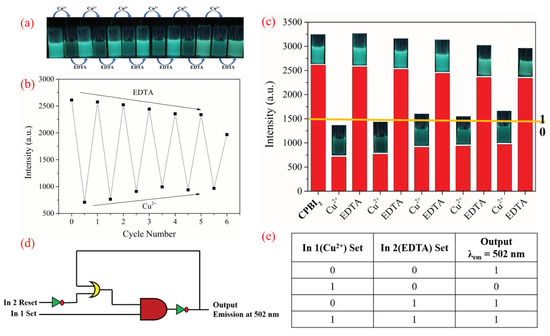
Figure 9.
Fluorescence experiment of the probe CPBI2 in cyclic use: alternately adding Cu2+ and EDTA into the CPBI2 solution (λex = 375 nm), the color change diagram of the solution under the 365 nm UV lamp (a); the fluorescence intensity cycle fluorescence diagram (b); input (In 1 = Cu2+ and In 2 = EDTA) histogram (c); implication logic gate (d); corresponding truth table (e).
Due to the above fluorescence quenching–restoration cycle of probe CPBI2 with Cu2+ and EDTA, a logic gate can be implemented on Boolean logic operations (Figure 9d). This is an IMPLICATION logic gate displaying memory unit with two inputs (In 1 and In 2) and one output (Figure 9e). We set Cu2+ and EDTA to inputs In 1 and In 2, respectively. Their presence and absence are denoted as 1 and 0. The output signal is the emission intensity at 504 nm, and the threshold is set to 1500 a.u. (Figure 9c). When the fluorescence intensity is higher than the threshold, the output signal is on (1). Additionally, when the fluorescence intensity is below the threshold, the output signal is off (0). Based on the above basic logic gate, when both Cu2+ and EDTA are present or absent, the output signal is on (1). When In 1 and In 2 are in the state (1, 0), the output signal is off (0). When In 1 and In 2 are in the state (0, 1), the output signal is on (1) (Figure 9e). Therefore, the IMPLICATION logic gate can be constructed at the molecular level by monitoring the emission intensity value at 504 nm of the output signal through the input signal (Cu2+ and EDTA) [16,74,75,76].
3.6. Visual Detection of Cu2+ in Solid State
According to the literature method [50,77,78], the thin layer chromatography (TLC) plate with CPBI2 solution adsorbed shows green fluorescence under 365 nm UV lamp. The fluorescence in the center of CPBI2 visible to the naked eye is rapidly quenched when Cu2+ solution is added to the TLC plate adsorbed with CPBI2, as shown in Figure 10a. Therefore, the TLC plate adsorbed with CPBI2 can be used to detect Cu2+, and its visual detection of Cu2+ in solution can be achieved.

Figure 10.
Fluorescence image (under 365 nm UV light) of sensor CPBI2 adsorbed on a TLC plate with a spot of Cu2+ solution on sensor CPBI2 (a); fluorescence changes (under 365 nm UV lamp) of test strips for detecting Cu2+ in aqueous solution (b).
In addition, according to the method reported in the literature [47,79], the Whatman test paper is immersed in DMSO solution containing CPBI2, and then vacuum drying makes CPBI2 test paper. The test paper shows green fluorescence under the 356 nm UV lamp, and the fluorescence is quickly quenched when Cu2+ solution is added to the test paper (Figure 10b). Therefore, CPBI2 can be made into not only solution-coated thin layer chromatography plates but also Whatman test paper to realize solid-state visual detection of Cu2+.
4. Conclusions
In summary, a novel large conjugation polybenzimidazole compound CPBI0 was synthesized by a simple green-metal-free catalytic reaction. In order to improve its solubility and fluorescence properties, a series of different alkylated polymers CPBIn were obtained by N-alkylation reaction. An alkyl chain is an electron-donor group; the difference in the alkylation rate means that the number of electron donors in the polymer is different, resulting in a different binding ability with electron-deficient metal ions. Therefore, although CPBIn-series polymers (CPBI1~CPBI4) show a good recognition ability for Cu2+ and Zn2+, they have different sensitivities, indicating the regulation of the alkyl chain on the performance of a polymer fluorescent probe. In addition, CPBI2 with a nano-microsphere can be designed as an ultra-sensitive IMPLICATION logic gate at the molecular level to cyclically detect Cu2+. Furthermore, TLC plates and test papers containing CPBI2 have been developed for the solid visual detection of Cu2+ in a solution. This study provides a new idea and method for simple synthesis and fluorescence regulation of multifunctional fluorescent probes.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/polym13183091/s1, Figure S1. 1H NMR spectrum of CPBI0; Figure S2. 1H NMR spectrum of CPBI1; Figure S3. 1H NMR spectrum of CPBI2; Figure S4. 1H NMR spectrum of CPBI3; Figure S5. 1H NMR spectrum of CPBI4; Figure S6. 1H NMR spectrum of CPBI5; Figure S7. The changes of 1H NMR spectra of CPBIs with different molar feed ratios [n(C5H11Br)/n(CPBI0)]; Figure S8. The FT-IR spectra of CPBIs with different molar feed ratios [n(C5H11Br)/n(CPBI0)]; Figure S9. The full XPS spectra of CPBI2 (a) and its C1s (b), N1s (c) peaks; Figure S10. The XRD analysis of CPBIn with different molar feed ratios [n(C5H11Br)/n(CPBI0)]; Figure S11. TG analysis of CPBIs with different molar feed ratios [n(C5H11Br)/n(CPBI0)]; Figure S12. SEM analysis of CPBIn with different molar feed ratios; Figure S13. Fluorescence emission spectra in different polar solvents (λex = 375 nm) of CPBI1 (a), CPBI2 (b), CPBI3 (c) and CPBI4 (d); Figure S14. Fluorescence emission spectra in DMSO solutions (1 mg/15 mL) with different water content as DMSO/H2O (v/v) of CPBI1 (a), CPBI2 (b), CPBI3 (c) and CPBI4 (d), λex = 375 nm; Figure S15. Fluorescence emission spectra of sensor CPBI2 solution (1 mg/15 mL in DMSO/H2O, v/v, 9/1) with Zn2+ (100 μM) and other metal ions (100 μM) (a) and comparison of fluorescence quenching rate (b), fluorescence emission spectra of Cu2+ (100 μM) and other metal ions (c) and comparison of fluorescence quenching rate (d), λex = 375 nm; Figure S16. The fluorescence selectivity study of different metal ions (100 μM) in CPBI1 (1 mg/15 mL in DMSO/H2O, v/v, 9/1) solution (a), the influence of adding different metal ions (100 μM) on CPBI1 fluorescence intensity at 500 nm (b), λex = 375 nm; Figure S17. Fluorescence emission spectra of sensor CPBI1 solution (1 mg/15 mL in DMSO/H2O, v/v, 9/1) with Cu2+ (100 μM) and other metal ions (100 μM) (the upper), fluorescence emission spectra of Zn2+ (100 μM) and other metal ions (100 μM) (the lower), λex = 375 nm; Figure S18. Fluorescence emission spectra of CPBI1 (1 mg/15 mL in DMSO/H2O, v/v, 9/1) solution with different concentrations of Cu2+ and the linear relationship between CPBI1 and low concentrations of Cu2+, λex = 375 nm; Figure S19. Fluorescence emission spectra of CPBI1 (1 mg/15 mL in DMSO/H2O, v/v, 9/1) solution with different concentrations of Zn2+ and the linear relationship between CPBI1 and low concentrations of Zn2+, λex = 375 nm; Figure S20. The fluorescence selectivity study of different metal ions (100 μM) in CPBI3 (1 mg/15 mL in DMSO/H2O, v/v, 9/1) solution (a), the influence of adding different metal ions (100 μM) on CPBI3 fluorescence intensity at 510 nm (b), λex = 375 nm; Figure S21. Fluorescence emission spectra of sensor CPBI3 solution (1 mg/15 mL in DMSO/H2O, v/v, 9/1) with Cu2+ (100 μM) and other metal ions (100 μM) (the upper), fluorescence emission spectra of Zn2+ (100 μM) and other metal ions (100 μM) (the lower), λex = 375 nm; Figure S22. Fluorescence emission spectra of CPBI3 (1 mg/15 mL in DMSO/H2O, v/v, 9/1) solution with different concentrations of Cu2+ and the linear relationship between CPBI3 and low concentrations of Cu2+, λex = 375 nm; Figure S23. Fluorescence emission spectra of CPBI3 (1 mg/15 mL in DMSO/H2O, v/v, 9/1) solution with different concentrations of Zn2+ and the linear relationship between CPBI3 and low concentrations of Zn2+, λex = 375 nm; Figure S24. The fluorescence selectivity study of different metal ions (100 μM) in CPBI4 (1 mg/15 mL in DMSO/H2O, v/v, 9/1) solution, λex = 375 nm (a), the influence of adding different metal ions (100 μM) on CPBI4 fluorescence intensity at 510 nm (b) and the color change of the solution (c); Figure S25. Fluorescence emission spectra of sensor CPBI4 solution (1 mg/15 mL in DMSO/H2O, v/v, 9/1) with Zn2+ (100 μM) and other metal ions (100 μM) (a) and comparison of fluorescence quenching rate (b), fluorescence emission spectra of Cu2+ (100 μM) and other metal ions (100 μM) (c) and comparison of fluorescence quenching rate (d), λex = 375 nm; Figure S26. Fluorescence emission spectra of CPBI4 (1 mg/15 mL in DMSO/H2O, v/v, 9/1) solution with different concentrations of Cu2+ and the linear relationship between CPBI4 and low concentrations of Cu2+, λex = 375 nm; Figure S27. Fluorescence emission spectra of CPBI4 (1 mg/15 mL in DMSO/H2O, v/v, 9/1) solution with different concentrations of Zn2+ and the linear relationship between CPBI4 and low concentrations of Zn2+, λex = 375 nm; Figure S28. Stern-Volmer diagram of the interaction of sensors CPBI4 with Cu2+ (a) and Zn2+ (b) (the illustration shows the Stern-Volmer linear diagram at low concentrations); Figure S29. FT-IR spectra (a), SEM images of CPBI1 before and after the addition of Cu2+ or Zn2+ (b: CPBI1 + Zn2+; c: CPBI1 + Cu2+; d: CPBI1); Figure S30. FT-IR spectra (a), SEM images of CPBI3 before and after the addition of Cu2+ or Zn2+ (b: CPBI3 + Zn2+; c: CPBI3 + Cu2+; d: CPBI3); Figure S31. FT-IR spectra (a), SEM images of CPBI4 before and after the addition of Cu2+ or Zn2+ (b: CPBI4 + Zn2+; c: CPBI4+ Cu2+; d: CPBI4); +; Figure S32. Time-correlated single photon counting (TCSPC) plot for CPBI2 (left) and CPBI4 (right) interacted with Cu2+ and Zn2+ (λex = 375 nm, λem = 504 nm for CPBI2 and λem = 510 nm for CPBI4, respectively). Table S1. The effects of different feed ratios on yield, color and actual alkylation rate of CPBIn; Table S2. The thermal decomposition temperatures of CPBIs with different feed ratios; Table S3. Photophysical properties of CPBIn with different feed ratios. Table S4. Comparison of LOD when the sensor CPBIn detects Cu2+ and Zn2+; Table S5. The comparison of probe CPBIn with the reported Cu2+ probes in solution; Table S6. The comparison of probe CPBIn with the reported Zn2+ probes in solution.
Author Contributions
Conceptualization, X.-Y.C. and Z.-Y.W.; methodology, X.-Y.C. and C.-M.P.; software, Y.X.; validation, S.-H.L., W.-Q.X. and Z.-Y.W.; formal analysis, Y.X.; investigation, J.-P.H.; data curation, C.-M.P.; writing—original draft preparation, X.-Y.C.; writing—review and editing, Z.-Y.W., X.-Y.C., C.-M.P., Y.X., S.-H.L., W.-Q.X. and J.-P.H.; visualization, S.-H.L.; supervision, Z.-Y.W.; project administration, Z.-Y.W.; funding acquisition, Z.-Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from the National Natural Science Foundation of China (20772035), Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515012342), and Guangdong Provincial Science and Technology Project (No. 2017A010103016) is greatly appreciated.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be made available from the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, K.-N.; Liu, L.-Y.; Mao, D.; Xu, S.D.; Tan, C.-P.; Cao, Q.; Mao, Z.-W.; Liu, B. A Polarity-Sensitive Ratiometric Fluorescence Probe for Monitoring Changes in Lipid Droplets and Nucleus during Ferroptosis. Angew. Chem. Int. Ed. 2021, 60, 15095–15100. [Google Scholar] [CrossRef]
- Wan, W.; Huang, Y.; Xia, Q.; Bai, Y.; Chen, Y.; Jin, W.; Wang, M.; Shen, D.; Lyu, H.; Tang, Y.; et al. Covalent Probes for Aggregated Protein Imaging via Michael Addition. Angew. Chem. Int. Ed. 2021, 60, 11335–11343. [Google Scholar] [CrossRef] [PubMed]
- Maimaitiyiming, X.; Shi, C. Poly(1,4-diethynylphenylene-4,6-pyrimidine)s for fluorescence detection of mercury(II) ion. Mater. Chem. Phys. 2021, 257, 123783. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Lee, T.S. Size-dependent fluorescence of conjugated polymer dots and correlation with the fluorescence in solution and in the solid phase of the polymer. Nanoscale 2020, 12, 2492–2497. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, W.X.; Hou, J.-T.; Won, M.; An, J.S.; Chen, X.; Shu, J.; Kim, J.S. Fluorescence imaging of pathophysiological microenvironments. Chem. Soc. Rev. 2021, 50, 8887–8902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Z.; Yang, J.; Yi, L.; Liao, L.; Xiao, X. Development of a method for the detection of Cu2+ in the envi-ronment and live cells using a synthesized spider web-like fluorescent probe. Biosens. Bioelectron. 2021, 182, 113174. [Google Scholar] [CrossRef] [PubMed]
- Kometani, A.; Inagaki, Y.; Mutoh, K.; Abe, J. Red or near-infrared light operating negative photochromism of a binaph-thyl-bridged imidazole dimer. J. Am. Chem. Soc. 2020, 142, 7995–8005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Fan, Z.; Qiao, Y.; Chen, Y.; Wang, S.; Yue, X.; Shen, T.; Liu, W.; Yang, J.; Gao, H.; et al. AIEgens Conjugation Improves the Photothermal Efficacy and Near-Infrared Imaging of Heptamethine Cyanine IR-780. ACS Appl. Mater. Interfaces 2020, 12, 16114–16124. [Google Scholar] [CrossRef]
- Mehta, P.K.; Neupane, L.N.; Park, S.-H.; Lee, K.-H. Ratiometric fluorescent detection of silver nanoparticles in aqueous samples using peptide-based fluorogenic probes with aggregation-induced emission characteristics. J. Hazard. Mater. 2021, 411, 125041. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, R.; Dehaen, W.; Fang, Y.; Qian, S.; Ren, Y.; Cheng, F.; Guo, Y.; Guo, C.; Li, Y.; et al. Specific recognition, intracellular assay and detoxification of fluorescent curcumin derivative for copper ions. J. Hazard. Mater. 2021, 420, 126490. [Google Scholar] [CrossRef]
- Portelinha, J.; Duay, S.S.; Yu, S.I.; Heilemann, K.; Libardo, M.D.J.; Juliano, S.A.; Klassen, J.L.; Angeles-Boza, A.M. Antimicrobial Peptides and Copper(II) Ions: Novel Therapeutic Opportunities. Chem. Rev. 2021, 121, 2648–2712. [Google Scholar] [CrossRef]
- Arvas, B.; Ucar, B.; Acar, T.; Arvas, M.B.; Sahin, Y.; Aydogan, F.; Yolacan, C. A new coumarin based Schiff base fluorescence probe for zinc ion. Tetrahedron 2021, 88, 132127. [Google Scholar] [CrossRef]
- Dai, C.; Qian, H.-L.; Yan, X.-P. Facile room temperature synthesis of ultra-small sized porous organic cages for fluorescent sensing of copper ion in aqueous solution. J. Hazard. Mater. 2021, 416, 125860. [Google Scholar] [CrossRef]
- Wang, X.; Shen, C.; Zhou, C.; Bu, Y.; Yan, X. Methods, principles and applications of optical detection of metal ions. Chem. Eng. J. 2021, 417, 129125. [Google Scholar] [CrossRef]
- Chakraborty, G.; Katiyar, V.; Pugazhenthi, G. Improvisation of polylactic acid (PLA)/exfoliated graphene (GR) nanocomposite for detection of metal ions (Cu2+). Compos. Sci. Technol. 2021, 213, 108877. [Google Scholar] [CrossRef]
- Zeng, S.; Li, S.-J.; Sun, X.-J.; Liu, T.-T.; Xing, Z.-Y. A dual-functional chemosensor for fluorescent on-off and ratiometric detection of Cu2+ and Hg2+ and its application in cell imaging. Dye. Pigment. 2019, 170, 107642. [Google Scholar] [CrossRef]
- Pang, C.-M.; Chen, S.-H.; Cao, X.-Y.; Zhang, J.-R.; Xiao, Y.; Li, X.-D.; Luo, S.-H.; Wang, Z.-Y. A multifunctional probe based on the conjugate of four fused N-heterocycles: Detecting picric acid, Cu2+ and Al3+ in ethanol solution system. J. Photochem. Photobiol. A Chem. 2020, 403, 112835. [Google Scholar] [CrossRef]
- Pavadai, R.; Amalraj, A.; Subramanian, S.; Perumal, P. High catalytic activity of fluorophore-labeled Y-shaped DNAzyme/3D MOF-MoS2NBs as a versatile biosensing platform for the simultaneous detection of Hg2+, Ni2+, and Ag+ ions. ACS Appl. Mater. Interfaces 2021, 13, 31710–31724. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Wang, W.; Tian, Y. Real-time Tracking and Sensing of Cu+ and Cu2+ with a Single SERS Probe in the Live Brain: Toward Understanding Why Copper Ions Were Increased upon Ischemia. Angew. Chem. Int. Ed. 2021. [Google Scholar] [CrossRef]
- Pang, C.-M.; Luo, S.-H.; Jiang, K.; Wang, B.-W.; Chen, S.-H.; Wang, N.; Wang, Z.-Y. A dual-channel sensor containing multiple nitrogen heterocycles for the selective detection of Cu2+, Hg2+ and Zn2+ in same solvent system by different mechanism. Dye. Pigment. 2019, 170, 107651. [Google Scholar] [CrossRef]
- Desai, M.L.; Basu, H.; Saha, S.; Singhal, R.K.; Kailasa, S.K. One pot synthesis of fluorescent gold nanoclusters from Curcuma longa extract for independent detection of Cd2+, Zn2+ and Cu2+ ions with high sensitivity. J. Mol. Liq. 2020, 304, 112697. [Google Scholar] [CrossRef]
- Xiao, Y.; Ma, J.; Li, D.; Liu, L.; Wang, H. Preparation 4′-Quinolin-2-yl-[2,2′;6′,2″] terpyridine as a ratiometric fluorescent probe for cadmium ions and zinc ions in aqueous. J. Photochem. Photobiol. A 2020, 399, 112613. [Google Scholar] [CrossRef]
- Shellaiah, M.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.-P.; Lin, M.-C.; Ohta, N. Novel rhodamine probe for colorimetric and fluorescent detection of Fe3+ ions in aqueous media with cellular imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 118757. [Google Scholar] [CrossRef]
- Liu, A.; Ji, R.; Shen, S.; Cao, X.; Ge, Y. A ratiometric fluorescent probe for sensing sulfite based on a pyrido[1,2-a]benzimidazole fluorophore. New J. Chem. 2017, 41, 10096–10100. [Google Scholar] [CrossRef]
- Xu, P.; Liu, X.; Liu, L.; Zhu, W.; Li, C.; Fang, M. Carbazole-based colorimetric and fluorescent probe for Cu 2+ and its utility in bio-imaging and real water samples. J. Chin. Chem. Soc. 2020, 68, 106–113. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, W.; Wang, T.; Fu, Y.; Cao, H.; He, Q.; Cheng, J. More Interaction Sites and Enhanced Fluorescence for Highly Sensitive Fluorescence Detection of Methamphetamine Vapor via Sidechain Terminal Functionalization of Conjugated Polymers. ChemistrySelect 2020, 5, 8328–8337. [Google Scholar] [CrossRef]
- Saes, B.W.H.; Lutz, M.; Wienk, M.M.; Meskers, S.C.J.; Janssen, R.A.J. Tuning the Optical Characteristics of Diketopyrrolopyrrole Molecules in the Solid State by Alkyl Side Chains. J. Phys. Chem. C 2020, 124, 25229–25238. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Duan, X.; Cai, Y.; Jia, S.; Chen, C.; Zhao, Z.; Li, Y.; Peng, H.-Q.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Simultaneously boosting the conjugation, brightness and solubility of organic fluorophores by using AIEgens. Chem. Sci. 2020, 11, 8438–8447. [Google Scholar] [CrossRef] [PubMed]
- Chochos, C.L.; Choulis, S.A. How the structural deviations on the backbone of conjugated polymers influence their optoelec-tronic properties and photovoltaic performance. Prog. Polym. Sci. 2011, 36, 1326–1414. [Google Scholar] [CrossRef]
- Yang, Q.; Hu, Z.; Zhu, S.; Ma, R.; Ma, H.; Ma, Z.; Wan, H.; Zhu, T.; Jiang, Z.; Liu, W.; et al. Donor Engineering for NIR-II Molecular Fluorophores with Enhanced Fluorescent Performance. J. Am. Chem. Soc. 2018, 140, 1715–1724. [Google Scholar] [CrossRef]
- Huang, Y.; You, X.; Wang, L.; Zhang, G.; Gui, S.; Jin, Y.; Zhao, R.; Zhang, D. Pyridinium-substituted tetra-phenylethylenes functionalized with alkyl chains as autophagy modulators for cancer therapy. Angew. Chem. Int. Ed. 2020, 59, 10042–10051. [Google Scholar] [CrossRef]
- Jia, P.; Xu, L.; Hu, Y.; Li, W.; Wang, X.; Ling, Q.; Shi, X.; Yin, G.; Li, X.; Sun, H.; et al. Orthogonal self-assembly of a two-step fluorescence-resonance energy transfer system with improved photosensitization effi-ciency and photooxidation activity. J. Am. Chem. Soc. 2021, 143, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Pingitore, A.T.; Benicewicz, B.C. Electrochemical Hydrogen Separation from Reformate Using High-Temperature Polybenzimidazole (PBI) Membranes: The Role of Chemistry. ACS Sustain. Chem. Eng. 2020, 8, 6234–6242. [Google Scholar] [CrossRef]
- Konovalova, A.; Stock, D.; Schröder, S.; Park, H.S.; Jang, J.H.; Kim, H.-J.; Han, J.; Schröder, D.; Henkensmeier, D. Partially methylated polybenzimidazoles as coating for alkaline zinc anodes. J. Membr. Sci. 2020, 610, 118254. [Google Scholar] [CrossRef]
- Aili, D.; Yang, J.S.; Jankova, K.; Henkensmeier, D.; Li, Q. From polybenzimidazoles to polybenzimidazoliums and polyben-zimidazolides. J. Mater. Chem. A 2020, 8, 12854–12886. [Google Scholar] [CrossRef]
- Hussaini, S.Y.; Haque, R.A.; Fatima, T.; Agha, T.M.; Majid, A.M.S.A.; Abdallah, H.H.; Razali, M.R. Nitrile functionalized silver(I) N-heterocyclic carbene complexes: DFT calculations and antitumor studies. Transit. Met. Chem. 2018, 43, 301–312. [Google Scholar] [CrossRef]
- Shannon, M.S.; Hindman, M.S.; Danielsen, S.P.O.; Tedstone, J.M.; Gilmore, R.D.; Bara, J.E. Properties of alkylbenzimidazoles for CO2 and SO2 capture and comparisons to ionic liquids. Sci. China Chem. 2012, 55, 1638–1647. [Google Scholar] [CrossRef]
- Shahini, C.; Achar, G.; Budagumpi, S.; Tacke, M.; Patil, S.A. Synthesis, structural investigation and antibacterial studies of non-symmetrically p-nitrobenzyl substituted benzimidazole N-heterocyclic carbene-silver(I) complexes. Inorg. Chim. Acta 2017, 466, 432–441. [Google Scholar] [CrossRef]
- Du, Y.; Song, Y.; Hao, J.; Cai, K.; Liu, N.; Yang, L.; Wang, L. Ratiometric fluorescence detection of O-2 (center dot-) based on dual-emission Schiff base polymer/rhodamine-B nanocomposites. Talanta 2019, 198, 316–322. [Google Scholar] [CrossRef]
- Dong, S.; Wang, S.; Wang, X.; Zhai, L. Superparamagnetic nanocomposite Fe3O4@SiO2-NH2/CQDs as fluorescent probe for copper (II) detection. Mater. Lett. 2020, 278, 128404. [Google Scholar] [CrossRef]
- Guo, X.; Huang, J.; Wang, M.; Wang, L. A dual-emission water-soluble g-C3N4@AuNCs-based fluorescent probe for label-free and sensitive analysis of trace amounts of ferrous (II) and copper (II) ions. Sens. Actuators B 2020, 309, 127766. [Google Scholar] [CrossRef]
- He, Y.; Li, N.; Li, W.; Zhang, X.; Zhang, X.; Liu, Z.; Liu, Q. 5,10,15,20-tetrakis (4-carboxylphenyl) porphyrin func-tionalized NiCo2S4 yolk-shell nanospheres: Excellent peroxidase-like activity, catalytic mechanism and fast cascade colorimetric biosensor for cholesterol. Sens. Actuators B 2021, 326, 128850. [Google Scholar] [CrossRef]
- Giri, D.; Bankura, A.; Patra, S.K. Poly(benzodithieno-imidazole-alt-carbazole) based π-conjugated copolymers: Highly selective and sensitive turn-off fluorescent probes for Hg2+. Polymer 2018, 158, 338–353. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Hu, X.; Shi, Y.; Li, Z.; Huang, X.; Zhang, W.; Zhang, D.; Zou, X.; Shi, J. A smartphone-integrated ratiometric fluorescence sensor for visual detection of cadmium ions. J. Hazard. Mater. 2021, 408, 124872. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Xu, H.; Zong, S.; Gong, Y.; Hao, Y.; Tang, X.; Li, Z. Detection of Tyrosinase in Real Food Samples and Living Cells by a Novel Near-Infrared Fluorescence Probe. J. Agric. Food Chem. 2021, 69, 1994–2000. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Pan, X.; Meng, X.; Liu, Y.; Wei, D.; Ma, W.; Huo, L.; Sun, X.; Lee, T.; Huang, M.; et al. Alkyl Side-Chain Engineering in Wide-Bandgap Copolymers Leading to Power Conversion Efficiencies over 10%. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Kaewnok, N.; Sirirak, J.; Jungsuttiwong, S.; Wongnongwa, Y.; Kamkaew, A.; Petdum, A.; Panchan, W.; Sahasithiwat, S.; Sooksimuang, T.; Charoenpanich, A.; et al. Detection of hazardous mercury ion using [5] helicene-based fluo-rescence probe with “turn-ON” sensing response for practical applications. J. Hazard. Mater. 2021, 418, 126242. [Google Scholar] [CrossRef]
- Xia, Q.; Feng, S.; Hong, J.; Feng, G. One probe for multiple targets: A NIR fluorescent rhodamine-based probe for ONOO− and lysosomal pH detection in live cells. Sens. Actuators B Chem. 2021, 337, 129732. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, F.; Yao, X.; Liu, W.; Zhu, W.; Qian, X.; Liu, Y. A novel near-infrared fluorescent probe to in vivo evaluate the release efficiency of H2S prodrug. Sens. Actuators B Chem. 2021, 339, 129881. [Google Scholar] [CrossRef]
- Jung, J.M.; Lee, S.Y.; Nam, E.; Lim, M.H.; Kim, C. A highly selective turn-on chemosensor for Zn2+ in aqueous media and living cells. Sens. Actuators B Chem. 2017, 244, 1045–1053. [Google Scholar] [CrossRef]
- Wang, Y.; Lao, S.; Ding, W.; Zhang, Z.; Liu, S. A novel ratiometric fluorescent probe for detection of iron ions and zinc ions based on dual-emission carbon dots. Sens. Actuators B Chem. 2019, 284, 186–192. [Google Scholar] [CrossRef]
- Du, T.; Wang, J.; Zhang, T.; Zhang, L.; Yang, C.; Yue, T.; Sun, J.; Li, T.; Zhou, M.; Wang, J. An Integrating Platform of Ratiometric Fluorescent Adsorbent for Unconventional Real-Time Removing and Monitoring of Copper Ions. ACS Appl. Mater. Interfaces 2020, 12, 13189–13199. [Google Scholar] [CrossRef]
- Sonkar, A.K.; Rai, A.; Tripathi, K.; Yadav, R.; Shukla, M.; Chauhan, B.S.; Srikrishna, S.; Mishra, L. A dual optical probe with larger stokes shift for simultaneous detection of Cu2+ and Zn2+ ions and aggregation induced enhanced emission empowering selective detection of Cu2+ ions. Sens. Actuators B Chem. 2021, 327, 129011. [Google Scholar] [CrossRef]
- Aysha, T.S.; El-Sedik, M.S.; Mohamed, M.B.I.; Gaballah, S.T.; Kamel, M.M. Dual functional colorimetric and turn-off fluores-cence probe based on pyrrolinone ester hydrazone dye derivative for Cu2+ monitoring and pH change. Dyes Pigment. 2019, 170, 107549. [Google Scholar] [CrossRef]
- Erdemir, S.; Malkondu, S. Dual-channel responsive fluorescent sensor for the logic-controlled detection and bioimaging of Zn2+ and Hg2+. J. Mol. Liq. 2021, 326, 115279. [Google Scholar] [CrossRef]
- Xie, H.-F.; Yu, C.-J.; Huang, Y.-L.; Xu, H.; Zhang, Q.-L.; Sun, X.-H.; Feng, X.; Redshaw, C. A turn-off fluorescent probe for the detection of Cu2+ based on a tetraphenylethylene-functionalized salicylaldehyde Schiff-base. Mater. Chem. Front. 2020, 4, 1500–1506. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Tian, F.-F.; Fan, X.-Y.; Jiang, F.-L.; Liu, Y. Fabrication of an acylhydrazone based fluorescence probe for Al3+. Sens. Actuators B Chem. 2017, 240, 916–925. [Google Scholar] [CrossRef]
- Li, R.-Y.; Wei, Z.-L.; Wang, L.; Zhang, Y.; Ru, J.-X. A new salamo-based fluorescence probe to visually detect aluminum(III) ion and bio-imaging in zebrafish. Microchem. J. 2021, 162, 105720. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Yin, J.; Yang, Y.; Luo, H.; Song, J.; Xu, X.; Wang, S. A novel camphor-based “turn-on” fluorescent probe with high specificity and sensitivity for sensing mercury(II) in aqueous medium and its bioimaging application. ACS Sustain. Chem. Eng. 2020, 8, 12348–12359. [Google Scholar] [CrossRef]
- Song, Y.; Tao, J.; Wang, Y.; Cai, Z.; Fang, X.; Wang, S.; Xu, H. A novel dual-responsive fluorescent probe for the detection of copper(II) and nickel(II) based on BODIPY derivatives. Inorg. Chim. Acta 2021, 516, 120099. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Z.; Fan, C.; Liu, G.; Pu, S. A naphthalene–dansylhydrazine based ratiometric fluorescence probe for selectively detecting Cu2+. Tetrahedron Lett. 2020, 61, 151427. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, L.; Kong, F.; Yu, L. Design and preparation of poly(tannic acid) nanoparticles with intrinsic fluorescence: A sensitive detector of picric acid. Chem. Eng. J. 2021, 416, 129090. [Google Scholar] [CrossRef]
- Musib, D.; Devi, L.R.; Raza, M.K.; Chanu, S.B.; Roy, M. A New Thiophene-based Aggregation-induced Emission Chemosensor for Selective Detection of Zn2+ Ions and Its Turn Off. Chem. Lett. 2020, 49, 473–476. [Google Scholar] [CrossRef]
- Liu, T.-T.; Xu, J.; Liu, C.-G.; Zeng, S.; Xing, Z.-Y.; Sun, X.-J.; Li, J.-L. A novel dual-function probe for recognition and differentiation of Zn2+ and Al3+ and its application. J. Mol. Liq. 2020, 300, 112250. [Google Scholar] [CrossRef]
- Wang, Z.; Toffoletti, A.; Hou, Y.; Zhao, J.; Barbon, A.; Dick, B. Insight into the drastically different triplet lifetimes of BODIPY obtained by optical/magnetic spectroscopy and theoretical computations. Chem. Sci. 2021, 12, 2829–2840. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Deng, Y.; Wang, X.; Liu, M. Polymer fluorescent probe for Hg(II) with thiophene, benzothiazole and quinoline groups. Sens. Actuators B Chem. 2017, 245, 441–447. [Google Scholar] [CrossRef]
- Ghosh, K.; Saha, I. Selective sensing of Zn(II) ion by a simple anthracene-based tripodal chemo- sensor. Tetrahedron Lett. 2010, 51, 4995–4999. [Google Scholar] [CrossRef]
- Cui, X.; Si, Z.; Li, Y.; Duan, Q. Synthesis of telechelic PNIPAM ended with 9,10-dihydroacridine group as a recyclable and specific Fe3+ detection fluorescent sensor. Dye. Pigment. 2020, 173, 107873. [Google Scholar] [CrossRef]
- Tang, X.; Liu, W.; Wu, J.; Lee, C.-S.; You, J.; Wang, P. Synthesis, Crystal Structures, and Photophysical Properties of Triphenylamine-Based Multicyano Derivatives. J. Org. Chem. 2010, 75, 7273–7278. [Google Scholar] [CrossRef]
- Ravichandiran, P.; Prabakaran, D.S.; Maroli, N.; Kim, A.R.; Park, B.-H.; Han, M.-K.; Ramesh, T.; Ponpandian, S.; Yoo, D.J. Mitochondria-targeted acridine-based dual-channel fluorescence chemosensor for detection of Sn4+ and Cr2O72- ions in water and its application in discriminative detection of cancer cells. J. Hazard. Mater. 2021, 419, 126409. [Google Scholar] [CrossRef]
- Mu, X.; Shi, L.; Yan, L.; Tang, N. A 2-Hydroxy-1-naphthaldehyde Schiff Base for Turn-on Fluorescence Detection of Zn2+ Based on PET Mechanism. J. Fluoresc. 2021, 31, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, L.Y.; Wu, J.; Yang, X.; Lin, P.; Wang, M. A dual-functional colorimetric and fluorescent peptide-based probe for sequential detection of Cu2+ and S2- in 100% aqueous buffered solutions and living cells. J. Hazard. Mater. 2021, 407, 124388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Zhao, L.; Xu, B. A dual-responsive and highly sensitive fluorescent probe for Cu2+ and pH based on a dansyl derivative. Dye. Pigment. 2020, 180, 108513. [Google Scholar] [CrossRef]
- Wu, Y.C.; Shi, C.Q.; Chen, Z.G.; Zhou, Y.B.; Liu, S.M.; Zhao, J.Q. A novel hydroxyl-containing polyimide as a colorimetric and ratiometric chemosensor for the reversible detection of fluoride ions. Polym. Chem. 2019, 10, 1399–1406. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, S.; Chen, Z.; Zhao, J. Synthesis and properties of cardo-type polyimides containing hydroxyl groups for application in specific detection of fluoride ion. Dye. Pigment. 2020, 173, 107924. [Google Scholar] [CrossRef]
- Magri, D.C.; Brown, G.J.; McClean, G.D.; De Silva, A.P. Communicating Chemical Congregation: A Molecular AND Logic Gate with Three Chemical Inputs as a “Lab-on-a-Molecule” Prototype. J. Am. Chem. Soc. 2006, 128, 4950–4951. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Luo, S.-H.; Cao, L.; Jiang, K.; Wang, L.-Y.; Xie, J.-C.; Wang, Z.-Y. Self-assembled structures of N-alkylated bisbenzi- midazolyl naphthalene in aqueous media for highly sensitive detection of picric acid. Anal. Chim. Acta 2017, 976, 74–83. [Google Scholar] [CrossRef]
- Jiang, K.; Luo, S.-H.; Pang, C.-M.; Wang, B.-W.; Wu, H.-Q.; Wang, Z.-Y. A functionalized fluorochrome based on quinoline- benzimidazole conjugate: From facile design to highly sensitive and selective sensing for picric acid. Dyes Pigment. 2019, 162, 367–376. [Google Scholar] [CrossRef]
- Kumar, A.; Chae, P.S. Aggregation induced emission enhancement behavior of conformationally rigid pyreneamide-based probe for ultra-trace detection of picric acid (PA). Dye. Pigment. 2018, 156, 307–317. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).