Responsive Quaternized PDMAEMA Copolymers with Antimicrobial Action
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
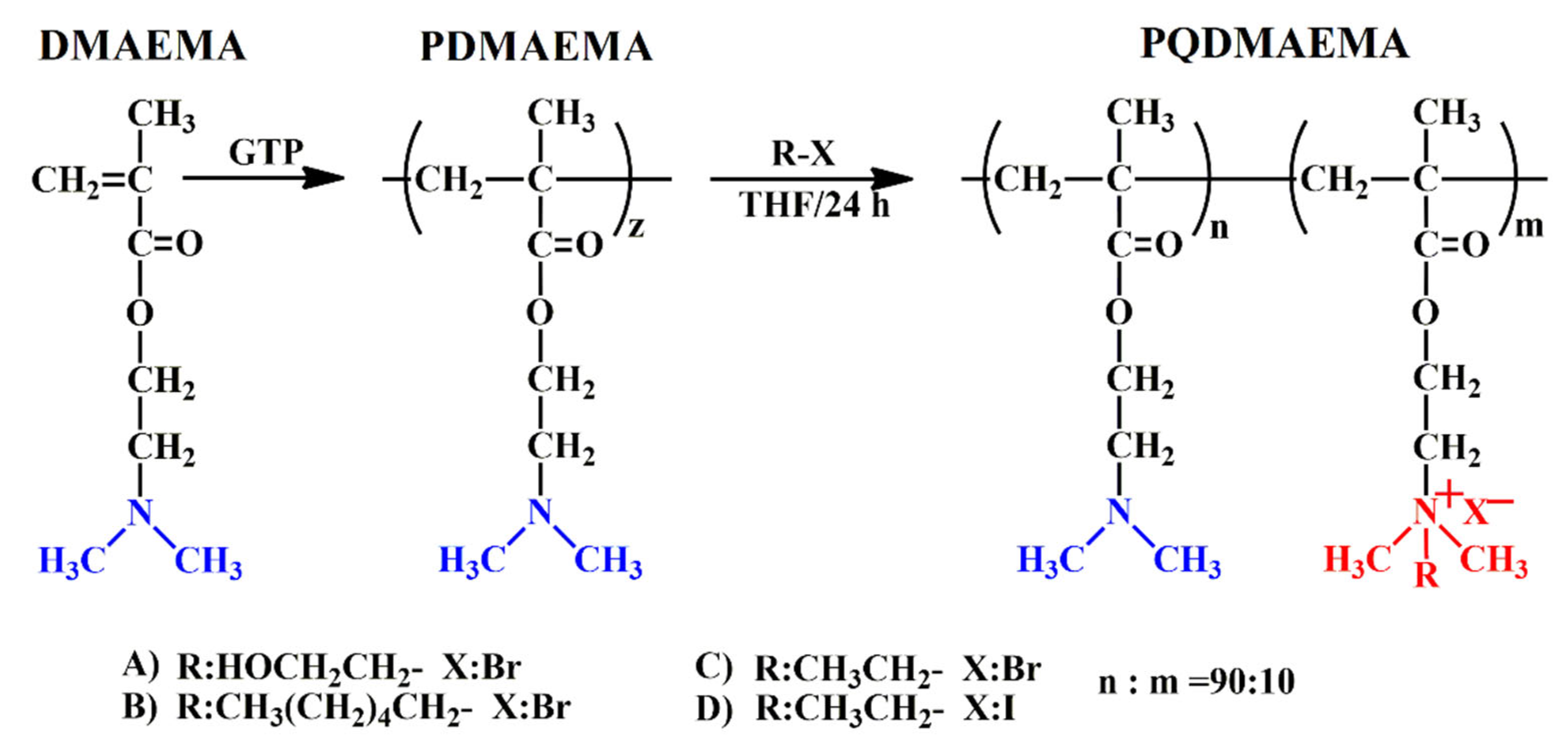
2.1.1. Polymer Synthesis
2.1.2. Quaternization Reaction
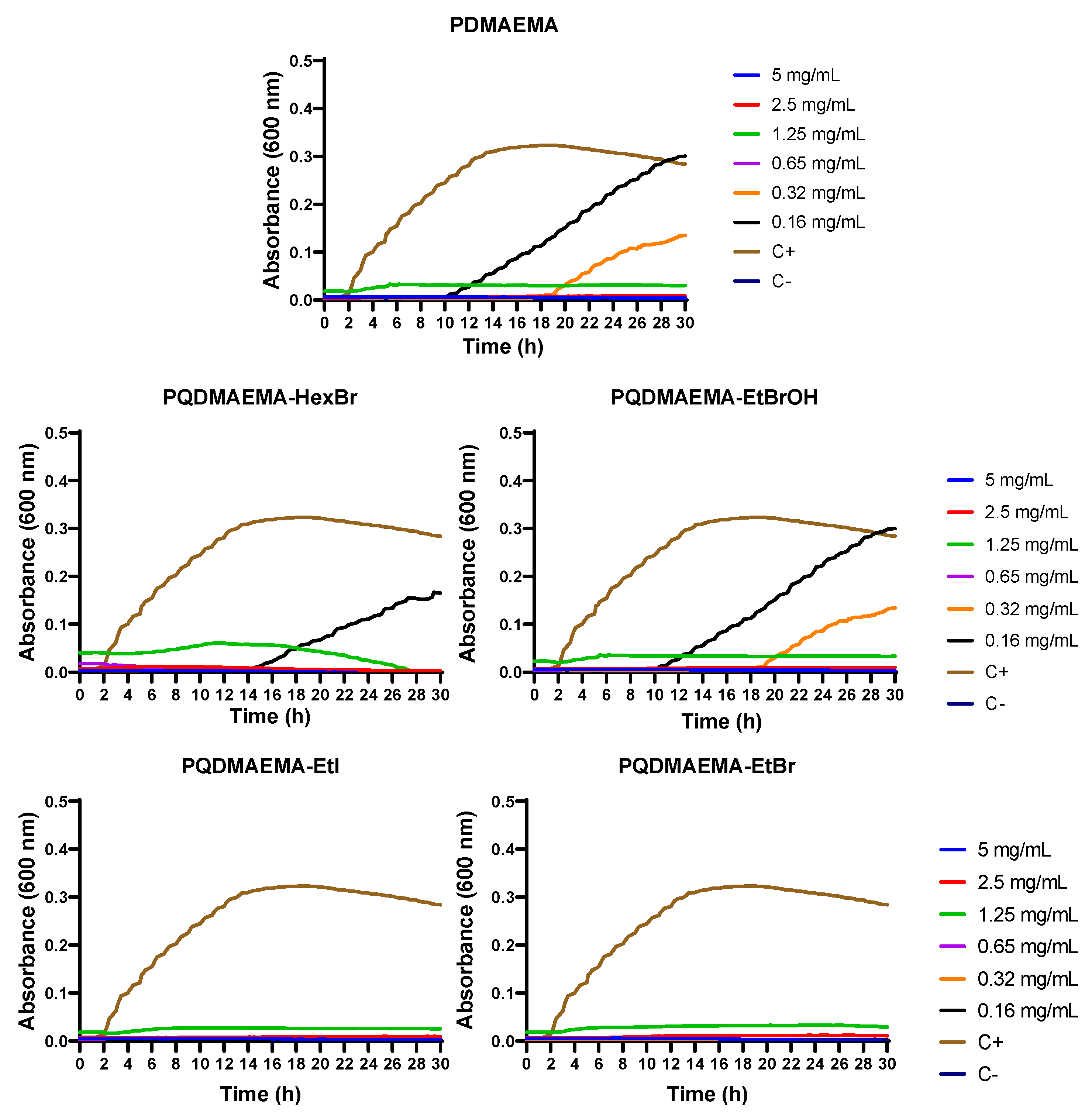
2.2. Antimicrobial Evaluation
2.2.1. Preparation of the Bacterial Suspension
2.2.2. MIC and MBC Tests
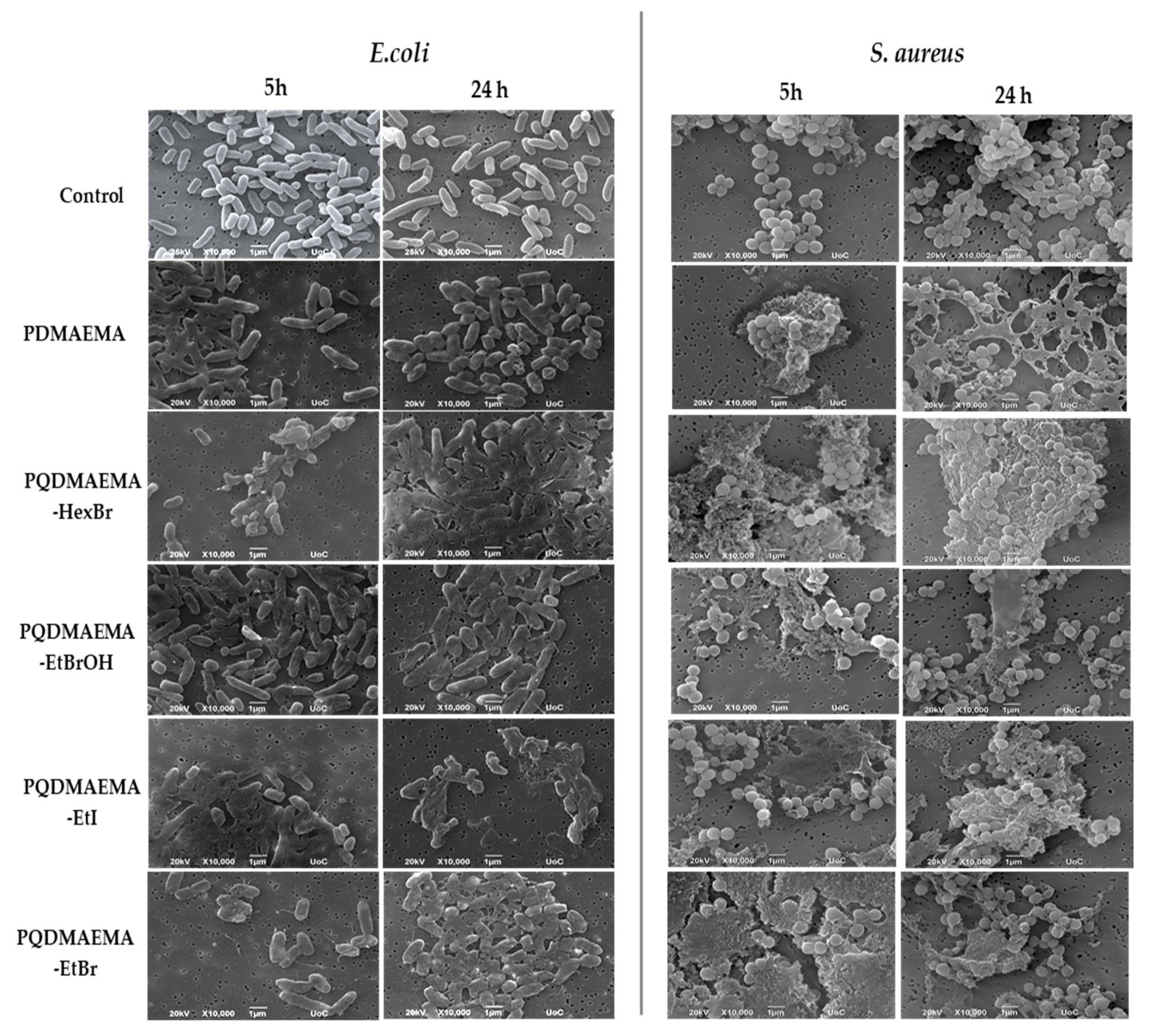
2.2.3. Morphological Observation by SEM
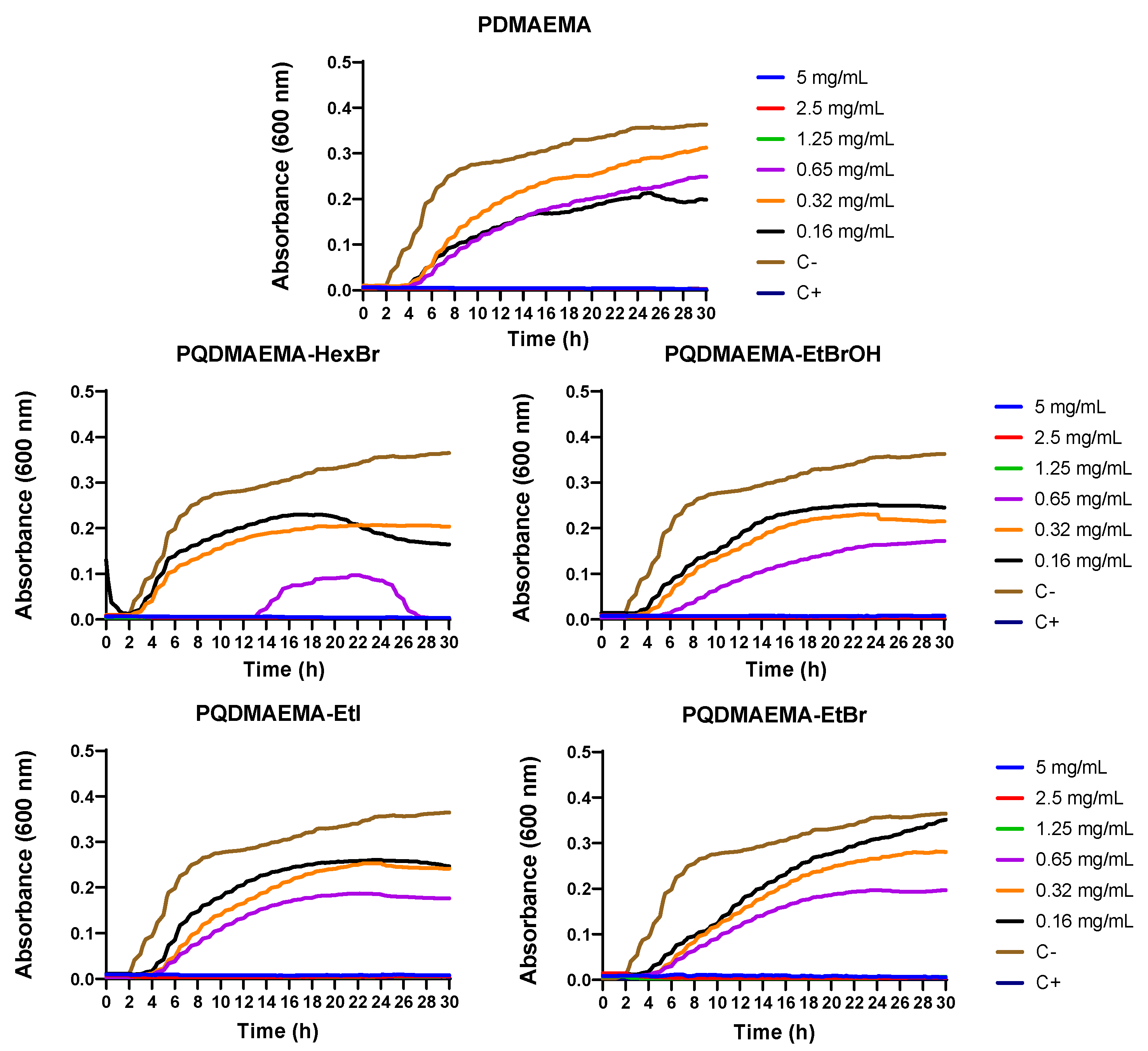
2.2.4. Kinetic Study
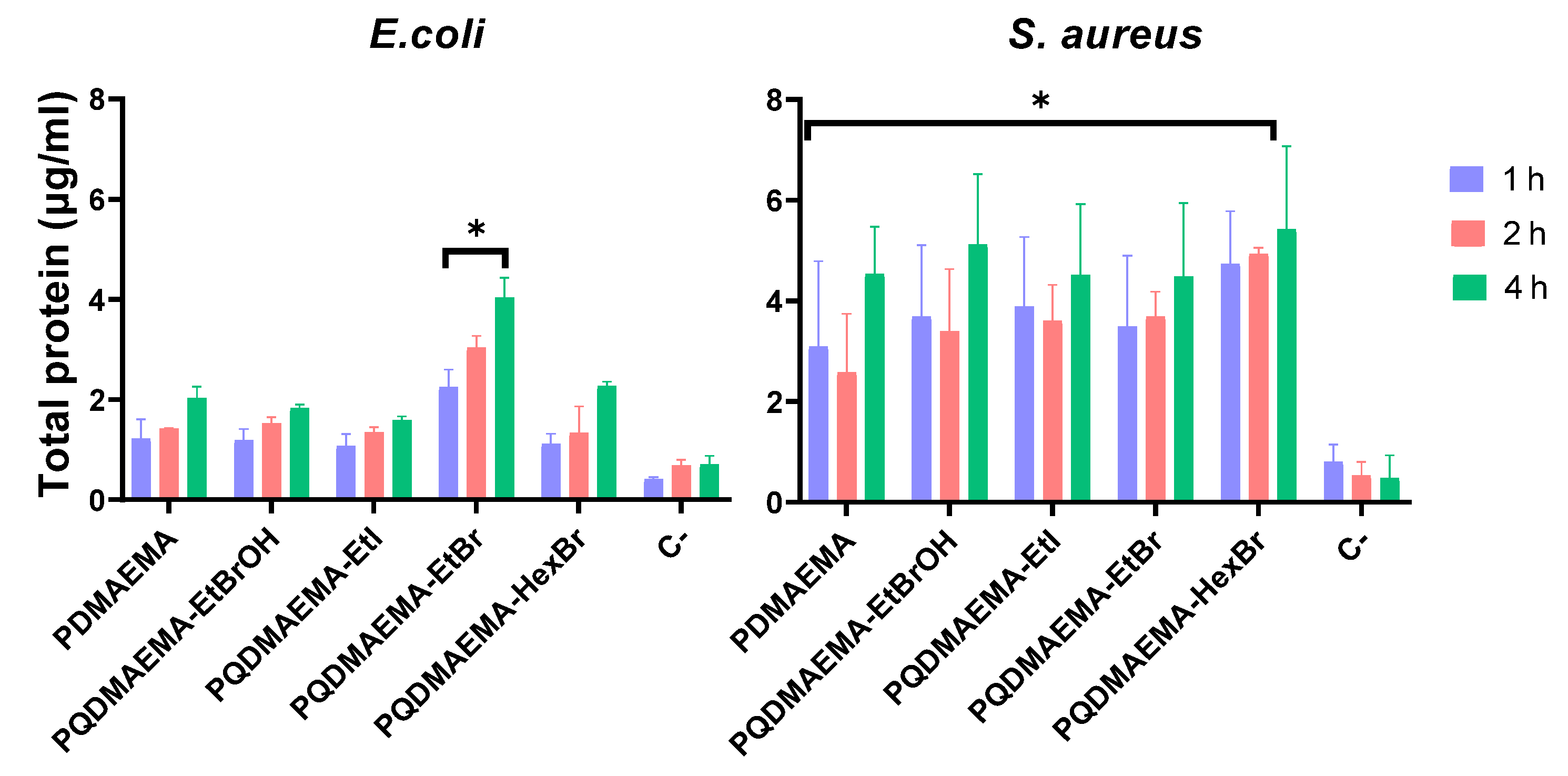
2.2.5. Protein Leakage Test
2.2.6. Statistical Analysis
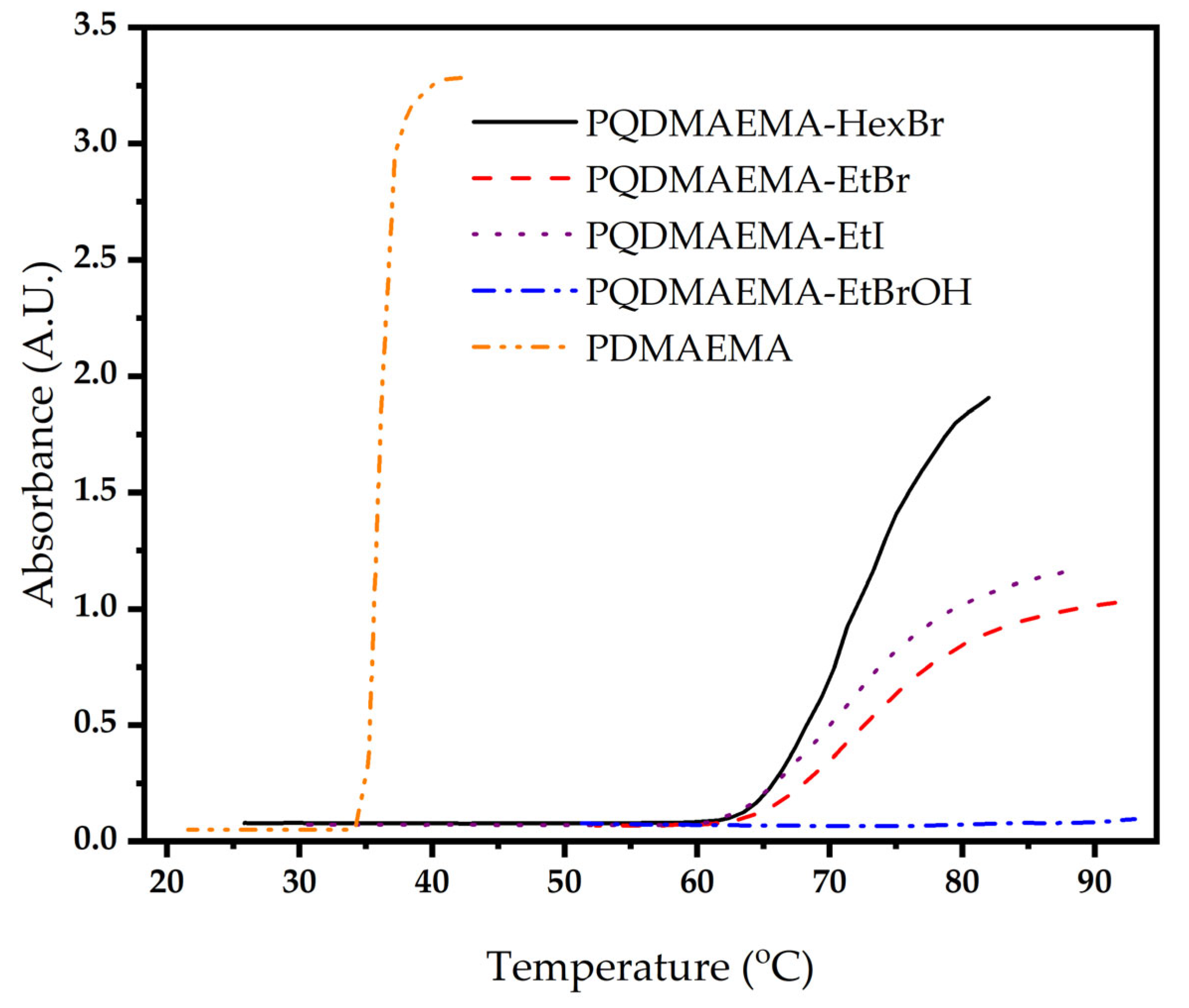
2.3. Characterization Techniques
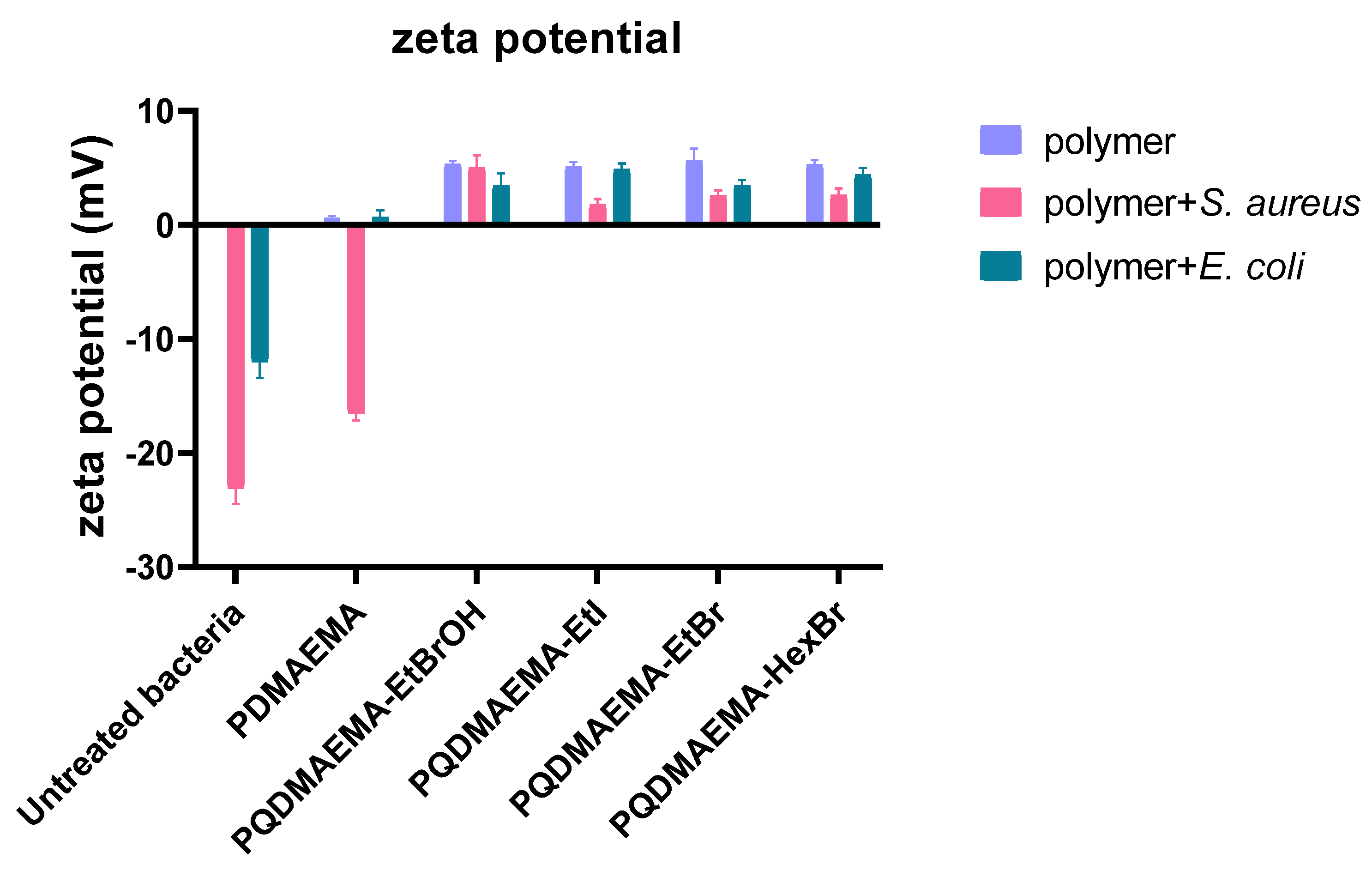
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vouga, M.; Greub, G. Emerging bacterial pathogens: The past and beyond. Clin. Microbiol. Infect. 2016, 22, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Chapter 3—Main Groups of Microorganisms of Relevance for Food Safety and Stability: General Aspects and Overall Description. In Innovative Technologies for Food Preservation; Barba, F.J., Sant’Ana, A.S., Orlien, V., Koubaa, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 53–107. [Google Scholar]
- Rennie, R.P. Current and Future Challenges in the Development of Antimicrobial Agents. In Antibiotic Resistance; Coates, A.R.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 45–65. [Google Scholar]
- Jackson, N.; Czaplewski, L.; Piddock, L.J.V. Discovery and development of new antibacterial drugs: Learning from experience? J. Antimicrob. Chemother. 2018, 73, 1452–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantravadi, P.K.; Kalesh, K.A.; Dobson, R.C.J.; Hudson, A.O.; Parthasarathy, A. The Quest for Novel Antimicrobial Compounds: Emerging Trends in Research, Development, and Technologies. Antibiotics 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Yañez-Macías, R.; Muñoz-Bonilla, A.; De Jesús-Tellez, M.A.; Maldonado-Textle, H.; Guerrero-Sánchez, C.; Schubert, U.S.; Guerrero-Santos, R. Combinations of Antimicrobial Polymers with Nanomaterials and Bioactives to Improve Biocidal Therapies. Polymers 2019, 11, 1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 2019, 9, 17439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, R.J.; Molina, R.; Xu, J.; Dobson, P.J.; Thompson, I.P. Comparison of TiO2 and ZnO nanoparticles for photocatalytic degradation of methylene blue and the correlated inactivation of gram-positive and gram-negative bacteria. J. Nanoparticle Res. 2013, 15, 1432. [Google Scholar] [CrossRef]
- Bogdanović, U.; Lazić, V.; Vodnik, V.; Budimir, M.; Marković, Z.; Dimitrijević, S. Copper nanoparticles with high antimicrobial activity. Mater. Lett. 2014, 128, 75–78. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfei, S.; Schito, A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers 2020, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Manouras, T.; Koufakis, E.; Vasilaki, E.; Peraki, I.; Vamvakaki, M. Antimicrobial Hybrid Coatings Combining Enhanced Biocidal Activity under Visible-Light Irradiation with Stimuli-Renewable Properties. ACS Appl. Mater. Interfaces 2021, 13, 17183–17195. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.M.; Tamer, T.M.; Khalifa, R.E.; Eltaweil, A.S.; Agwa, M.M.; Sabra, S.; Abd-Elmonem, M.S.; Mohy-Eldin, M.S.; Ziora, Z.M. Formulation and Antibacterial Activity Evaluation of Quaternized Aminochitosan Membrane for Wound Dressing Applications. Polymers 2021, 13, 2428. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Piatti, G.; Caviglia, D.; Schito, A.M. Synthesis, Characterization, and Bactericidal Activity of a 4-Ammoniumbuthylstyrene-Based Random Copolymer. Polymers 2021, 13, 1140. [Google Scholar] [CrossRef] [PubMed]
- Vamvakaki, M.; Billingham, N.C.; Armes, S.P. Synthesis of Controlled Structure Water-Soluble Diblock Copolymers via Oxyanionic Polymerization. Macromolecules 1999, 32, 2088–2090. [Google Scholar] [CrossRef]
- Manouras, T.; Koufakis, E.; Anastasiadis, S.H.; Vamvakaki, M. A facile route towards PDMAEMA homopolymer amphiphiles. Soft Matter 2017, 13, 3777–3782. [Google Scholar] [CrossRef] [PubMed]
- Koufakis, E.; Manouras, T.; Anastasiadis, S.H.; Vamvakaki, M. Film Properties and Antimicrobial Efficacy of Quaternized PDMAEMA Brushes: Short vs Long Alkyl Chain Length. Langmuir 2020, 36, 3482–3493. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Dong, C.; Liuyang, R.; Guo, Q.; Zeng, H.; Lin, Y.; Zhang, A. Controllable synthesis and antimicrobial activities of acrylate polymers containing quaternary ammonium salts. React. Funct. Polym. 2017, 121, 110–118. [Google Scholar] [CrossRef]
- Dicker, I.B.; Cohen, G.M.; Farnham, W.B.; Hertler, W.R.; Laganis, E.D.; Sogah, D.Y. Oxyanions catalyze group-transfer polymerization to give living polymers. Macromolecules 1990, 23, 4034–4041. [Google Scholar] [CrossRef]
- Sezonov, G.; Joseleau-Petit, D.; D’Ari, R. Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 2007, 189, 8746–8749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skandalis, N.; Dimopoulou, A.; Georgopoulou, A.; Gallios, N.; Papadopoulos, D.; Tsipas, D.; Theologidis, I.; Michailidis, N.; Chatzinikolaidou, M. The Effect of Silver Nanoparticles Size, Produced Using Plant Extract from Arbutus unedo, on Their Antibacterial Efficacy. Nanomaterials 2017, 7, 178. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wilbon, P.A.; Chen, Y.P.; Zhou, J.H.; Nagarkatti, M.; Wang, C.P.; Chu, F.X.; Decho, A.W.; Tang, C.B. Amphipathic antibacterial agents using cationic methacrylic polymers with natural rosin as pendant group. RSC Adv. 2012, 2, 10275–10282. [Google Scholar] [CrossRef]
- Lin, S.; Wu, J.H.; Jia, H.Q.; Hao, L.M.; Wang, R.Z.; Qi, J.C. Facile preparation and antibacterial properties of cationic polymers derived from 2-(dimethylamino)ethyl methacrylate. RSC Adv. 2013, 3, 20758–20764. [Google Scholar] [CrossRef]
- Panja, S.; Bharti, R.; Dey, G.; Lynd, N.A.; Chattopadhyay, S. Coordination-Assisted Self-Assembled Polypeptide Nanogels to Selectively Combat Bacterial Infection. ACS Appl. Mater. Interfaces 2019, 11, 33599–33611. [Google Scholar] [CrossRef] [PubMed]
| Materials | E. coli | S. aureus | ||
|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| PDMAEMA | 0.5 | 0.8 | 1.0 | 2.4 |
| PQDMAEMA-EtBr | 0.2 | 0.2 | 0.4 | 0.8 |
| PQDMAEMA-EtI | 0.2 | 1.6 | 0.8 | 1.6 |
| PQDMAEMA-HexBr | 0.2 | 0.2 | 0.4 | 0.8 |
| PQDMAEMA-EtBrOH | 0.2 | 0.2 | 0.4 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manouras, T.; Platania, V.; Georgopoulou, A.; Chatzinikolaidou, M.; Vamvakaki, M. Responsive Quaternized PDMAEMA Copolymers with Antimicrobial Action. Polymers 2021, 13, 3051. https://doi.org/10.3390/polym13183051
Manouras T, Platania V, Georgopoulou A, Chatzinikolaidou M, Vamvakaki M. Responsive Quaternized PDMAEMA Copolymers with Antimicrobial Action. Polymers. 2021; 13(18):3051. https://doi.org/10.3390/polym13183051
Chicago/Turabian StyleManouras, Theodore, Varvara Platania, Anthie Georgopoulou, Maria Chatzinikolaidou, and Maria Vamvakaki. 2021. "Responsive Quaternized PDMAEMA Copolymers with Antimicrobial Action" Polymers 13, no. 18: 3051. https://doi.org/10.3390/polym13183051
APA StyleManouras, T., Platania, V., Georgopoulou, A., Chatzinikolaidou, M., & Vamvakaki, M. (2021). Responsive Quaternized PDMAEMA Copolymers with Antimicrobial Action. Polymers, 13(18), 3051. https://doi.org/10.3390/polym13183051








