Extraction of Microcrystalline Cellulose from Washingtonia Fibre and Its Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. MCC Extraction
2.3. Characterization
2.3.1. Chemical Composition and Yield
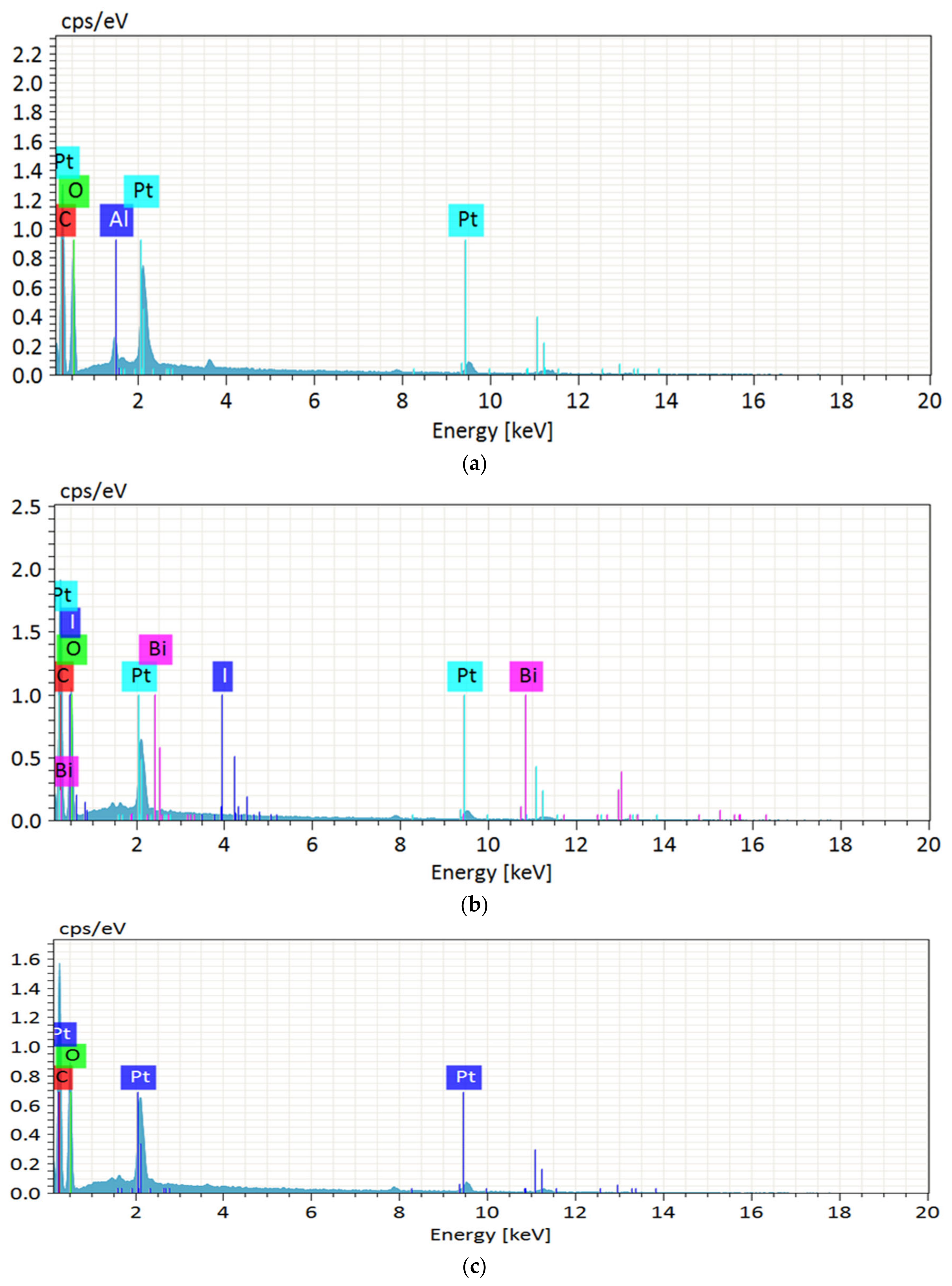
2.3.2. Morphological Feature, Particle Size, and Elemental Composition
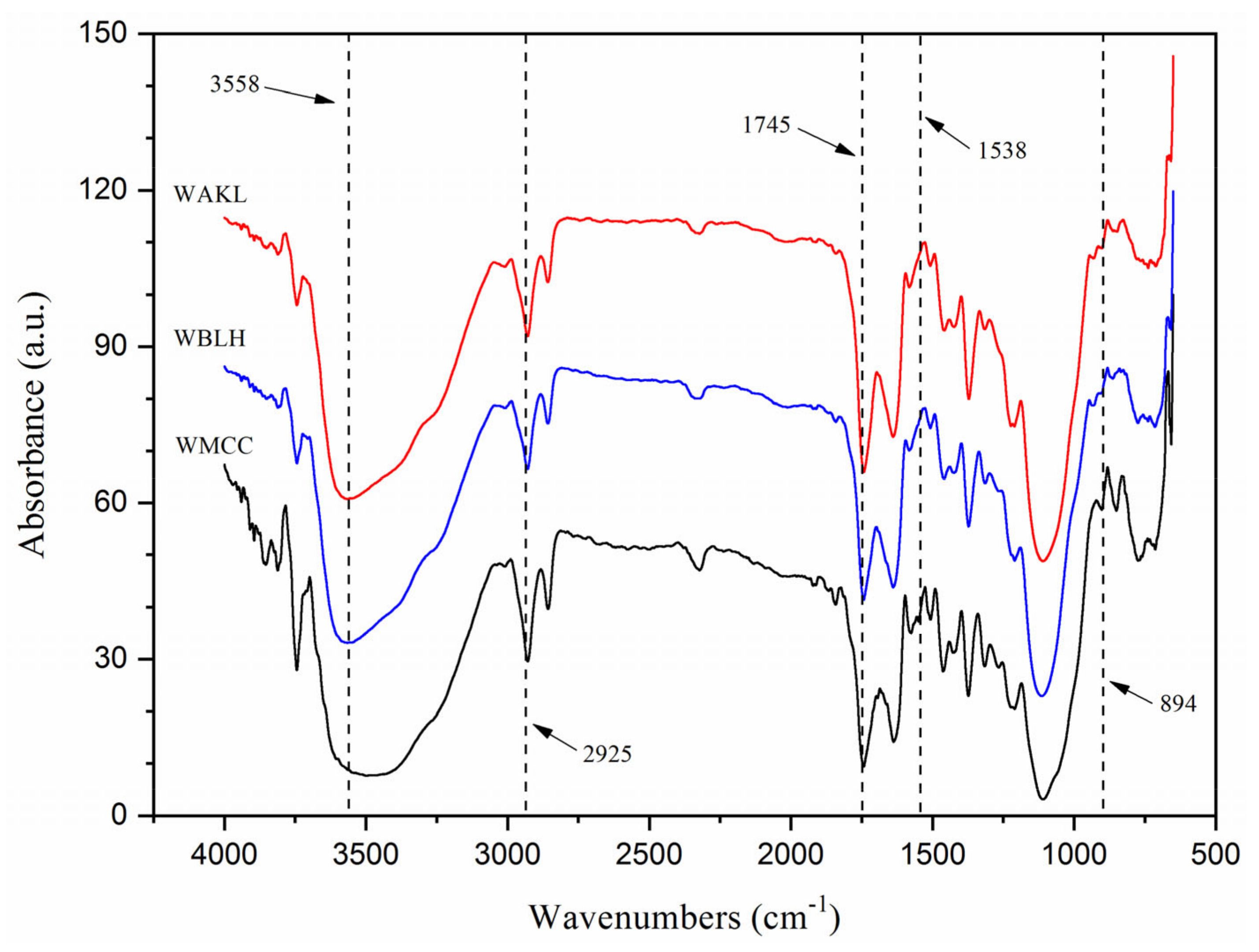
2.3.3. Fourier Transform Infrared-Ray (FTIR) Spectroscopy
2.3.4. X-ray Diffraction (XRD)
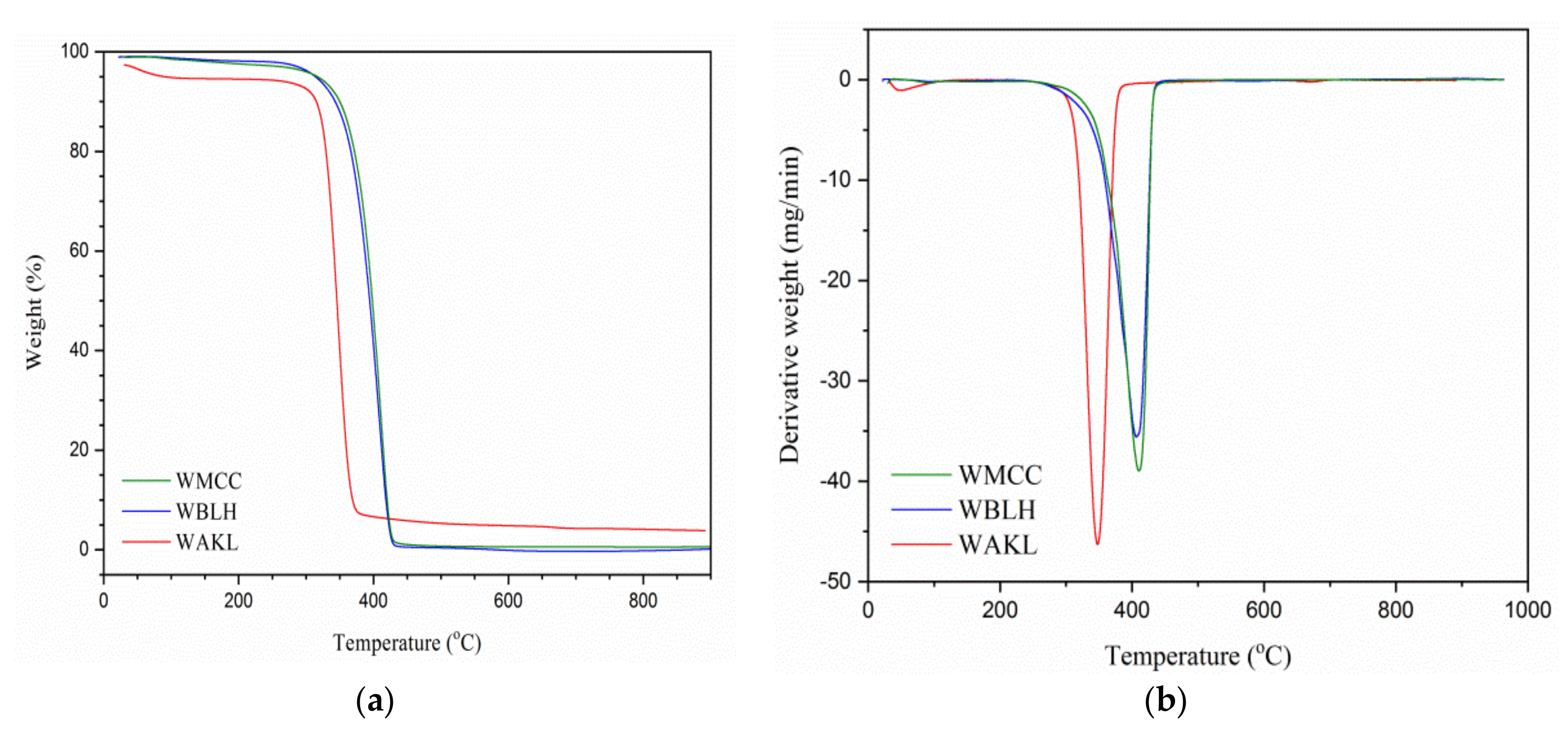
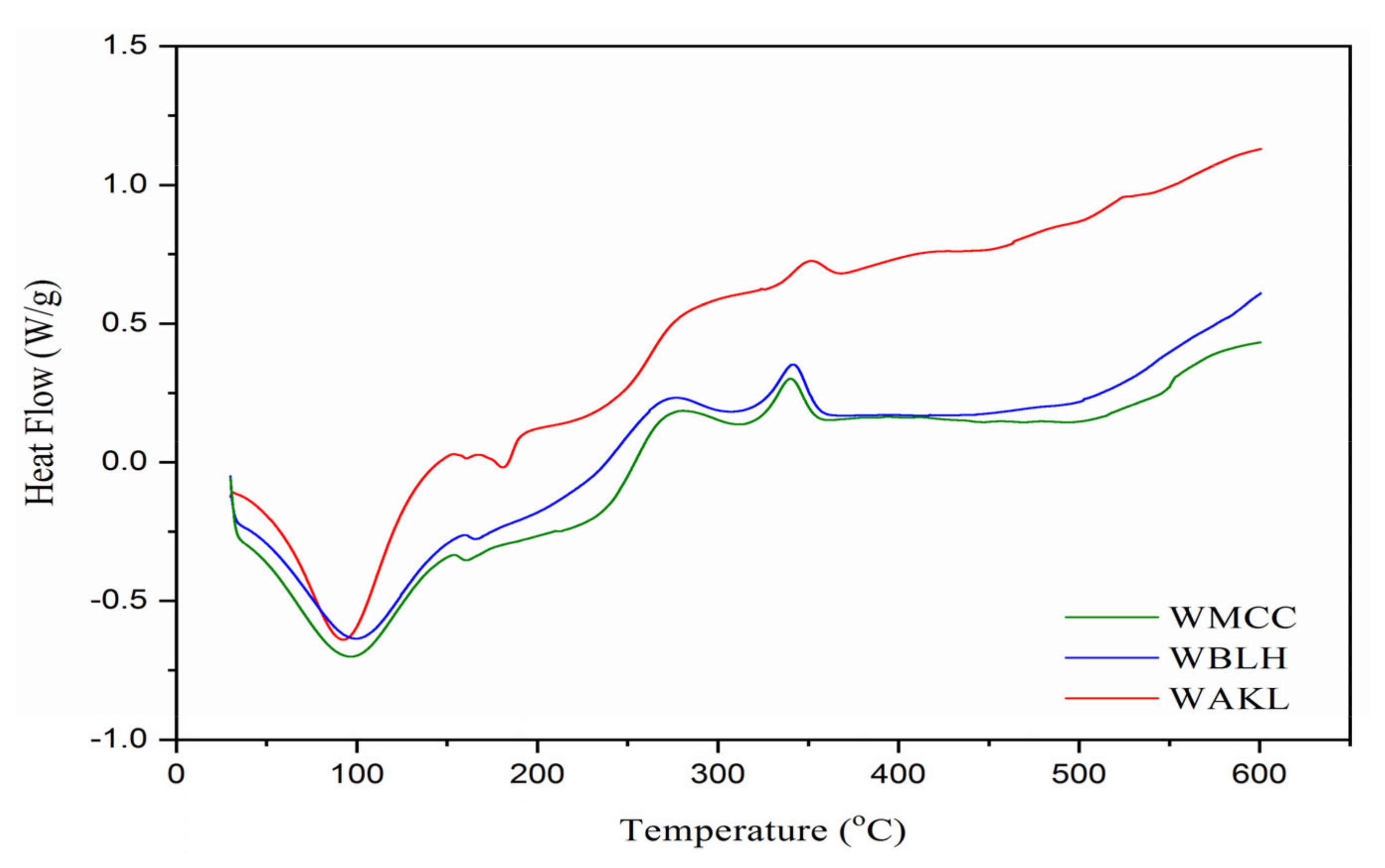
2.3.5. Thermal Analysis
3. Results and Discussion
3.1. Morphological, Chemical Composition and Elemental Composition
3.2. FTIR
3.3. XRD
3.4. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katakojwala, R.; Mohan, S.V. Microcrystalline cellulose production from sugarcane bagasse: Sustainable process development and life cycle assessment. J. Clean. Prod. 2020, 249, 119342. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Derradji, M.; Bessa, W. Ecofriendly isolation and characterization of microcrystalline cellulose from giant reed using various acidic media. Cellulose 2019, 26, 7635–7651. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, Z.; Lin, X.; Ren, Z.; Li, B.; Zhang, Y. Preparation and characterization of microcrystalline cellulose (MCC) from tea waste. Carbohydr. Polym. 2018, 184, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Cangelosi, B.; Clematis, F.; Monroy, F.; Roversi, P.F.; Troiano, R.; Curir, P.; Lanzotti, V. Filiferol, a chalconoid analogue from Washingtonia filifera possibly involved in the defence against the Red Palm Weevil Rhynchophorus ferrugineus Olivier. Phytochemistry 2015, 115, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Nehdi, I.A. Characteristics and composition of Washingtonia filifera (Linden ex André) H. Wendl. seed and seed oil. Food Chem. 2011, 126, 197–202. [Google Scholar] [CrossRef]
- Hou, W.; Ling, C.; Shi, S.; Yan, Z. Preparation and characterization of microcrystalline cellulose from waste cotton fabrics by using phosphotungstic acid. Int. J. Biol. Macromol. 2019, 123, 363–368. [Google Scholar] [CrossRef]
- Kian, L.K.; Saba, N.; Jawaid, M.; Fouad, H. Characterization of microcrystalline cellulose extracted from olive fiber. Int. J. Biol. Macromol. 2020, 156, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Yavorov, N.; Valchev, I.; Radeva, G.; Todorova, D. Kinetic investigation of dilute acid hydrolysis of hardwood pulp for microcrystalline cellulose production. Carbohydr. Res. 2020, 488, 107910. [Google Scholar] [CrossRef]
- Benzannache, N.; Belaadi, A.; Boumaaza, M.; Bourchak, M. Improving the mechanical performance of biocomposite plaster/ Washingtonian filifira fibres using the RSM method. J. Build. Eng. 2021, 33, 101840. [Google Scholar] [CrossRef]
- Gaagaia, D.E.; Bouakba, M.; Layachi, A. Thermo-physico-chemical and statistical mechanical properties of Washingtonian filifera new lignocellulosic fiber. Eng. Solid Mech. 2019, 7, 137–150. [Google Scholar] [CrossRef]
- Jawaid, M.; Kian, L.K.; Fouad, H.; Saba, N.; Alothman, O.Y.; Hashem, M. New cellulosic fibers from washingtonia tree agro-wastes: Structural, morphological, and thermal properties. J. Nat. Fibers 2021, 1–11. [Google Scholar] [CrossRef]
- French, A.D.; Cintron, M.S. Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- Trache, D.; Khimeche, K.; Mezroua, A.; Benziane, M. Physicochemical properties of microcrystalline nitrocellulose from Alfa grass fibres and its thermal stability. J. Therm. Anal. Calorim. 2016, 124, 1485–1496. [Google Scholar] [CrossRef]
- Xiang, L.Y.; Mohammed, M.A.; Baharuddin, A.S. Characterisation of microcrystalline cellulose from oil palm fibres for food applications. Carbohydr. Polym. 2016, 148, 11–20. [Google Scholar] [CrossRef]
- Ferrer, A.; Salas, C.; Rojas, O.J. Physical, thermal, chemical and rheological characterization of cellulosic microfibrils and microparticles produced from soybean hulls. Ind. Crop. Prod. 2016, 84, 337–343. [Google Scholar] [CrossRef]
- Owolabi, A.F.; Haafiz, M.K.M.; Hossain, M.S.; Hussin, M.H.; Fazita, M.R.N. Influence of alkaline hydrogen peroxide pre-hydrolysis on the isolation of microcrystalline cellulose from oil palm fronds. Int. J. Biol. Macromol. 2017, 95, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Kugo, Y.; Erata, T. 13C NMR and XRD studies on the enhancement of cellulose II crystallinity with low concentration NaOH post-treatments. Cellulose 2020, 27, 3553–3563. [Google Scholar] [CrossRef]
- Merci, A.; Urbano, A.; Grossmann, M.V.E.; Tischer, C.A.; Mali, S. Properties of microcrystalline cellulose extracted from soybean hulls by reactive extrusion. Food Res. Int. 2015, 73, 38–43. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, A.; Ibrahim, S.A.; Yang, H.; Huang, W. Isolation and characterization of microcrystalline cellulose from pomelo peel. Int. J. Biol. Macromol. 2018, 111, 717–721. [Google Scholar] [CrossRef]
- Sonia, A.; Dasan, K.P. Chemical, morphology and thermal evaluation of cellulose microfibers obtained from Hibiscus sabdariffa. Carbohydr. Polym. 2013, 92, 668–674. [Google Scholar] [CrossRef]
- Zeni, M.; Favero, D. Preparation of Microcellulose (Mcc) and Nanocellulose (Ncc) from Eucalyptus Kraft Ssp Pulp. Polym. Sci. 2016, 1, 1–5. [Google Scholar] [CrossRef]
- Hussin, M.H.; Pohan, N.A.; Garba, Z.N.; Kassim, M.J.; Rahim, A.A.; Brosse, N.; Haafiz, M.K.M. Physicochemical of microcrystalline cellulose from oil palm fronds as potential methylene blue adsorbents. Int. J. Biol. Macromol. 2016, 92, 11–19. [Google Scholar] [CrossRef]



| Samples | α-Cellulose (%) | Hemicellulose (%) | Lignin (%) | Yield (%) |
|---|---|---|---|---|
| WTSR | 41.6 ± 0.83 | 20.9 ± 0.16 | 21.7 ± 0.17 | - |
| WAKL | 72.8 ± 0.53 | 12.7 ± 0.15 | 7.4 ± 0.06 | 46.2 ± 0.35 |
| WBLH | 75.1 ± 0.57 | 9.1 ± 0.17 | 5.6 ± 0.08 | 39.4 ± 0.38 |
| WMCC | 82.9 ± 0.65 | 5.2 ± 0.21 | 2.1 ± 0.13 | 25.1 ± 0.32 |
| Samples | C (%) a | O (%) b | Al (%) c | Bi (%) d | I (%) e |
|---|---|---|---|---|---|
| WAKL | 61.03 | 37.02 | 0.89 | 0.04 | 0.03 |
| WBLH | 67.05 | 32.11 | 0.00 | 0.15 | 0.22 |
| WMCC | 61.55 | 37.63 | 0.00 | 0.00 | 0.00 |
| Samples | TOD (°C) a | TMD (°C) b | WTL (%) c | WCR (%) d |
|---|---|---|---|---|
| WAKL | 320.8 | 346.1 | 92.5 | 3.9 |
| WBLH | 354.7 | 401.2 | 94.7 | 1.5 |
| WMCC | 355.1 | 413.4 | 94.3 | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azum, N.; Jawaid, M.; Kian, L.K.; Khan, A.; Alotaibi, M.M. Extraction of Microcrystalline Cellulose from Washingtonia Fibre and Its Characterization. Polymers 2021, 13, 3030. https://doi.org/10.3390/polym13183030
Azum N, Jawaid M, Kian LK, Khan A, Alotaibi MM. Extraction of Microcrystalline Cellulose from Washingtonia Fibre and Its Characterization. Polymers. 2021; 13(18):3030. https://doi.org/10.3390/polym13183030
Chicago/Turabian StyleAzum, Naved, Mohammad Jawaid, Lau Kia Kian, Anish Khan, and Maha Moteb Alotaibi. 2021. "Extraction of Microcrystalline Cellulose from Washingtonia Fibre and Its Characterization" Polymers 13, no. 18: 3030. https://doi.org/10.3390/polym13183030
APA StyleAzum, N., Jawaid, M., Kian, L. K., Khan, A., & Alotaibi, M. M. (2021). Extraction of Microcrystalline Cellulose from Washingtonia Fibre and Its Characterization. Polymers, 13(18), 3030. https://doi.org/10.3390/polym13183030









