Biopolymer Nanocomposite Materials Based on Poly(L-lactic Acid) and Inorganic Fullerene-like WS2 Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Processing
2.2. Measurements
3. Results
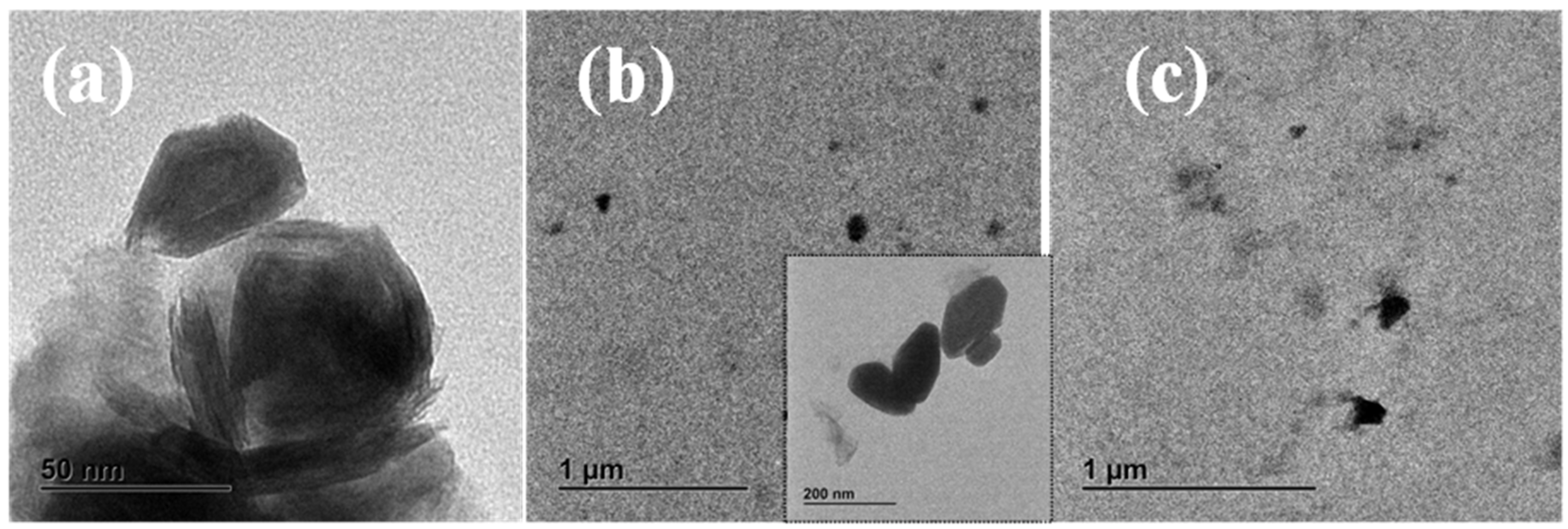
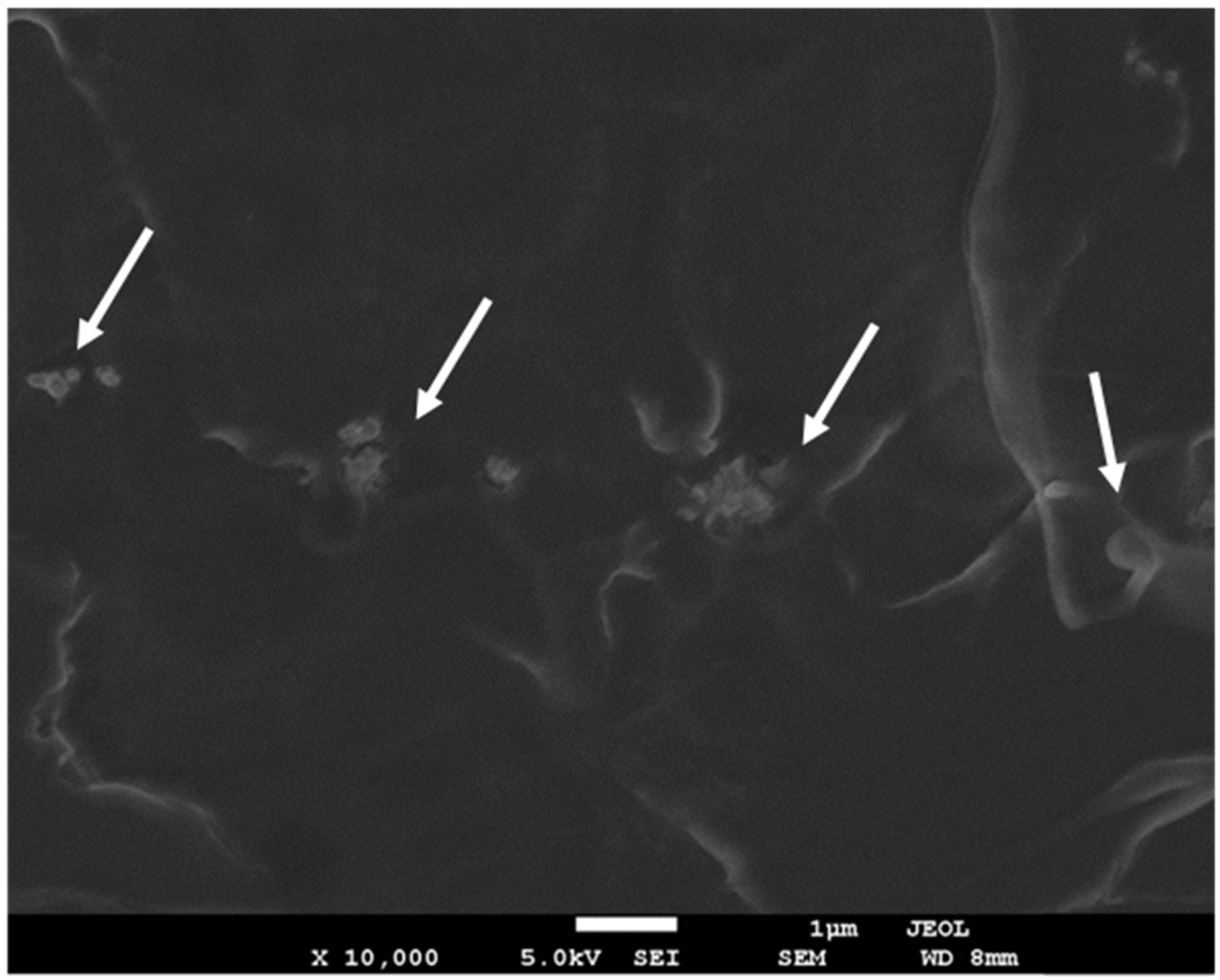
3.1. Morphology
3.2. Non-Isothermal Crystallization and Melting Behaviour
3.3. Lui Analysis
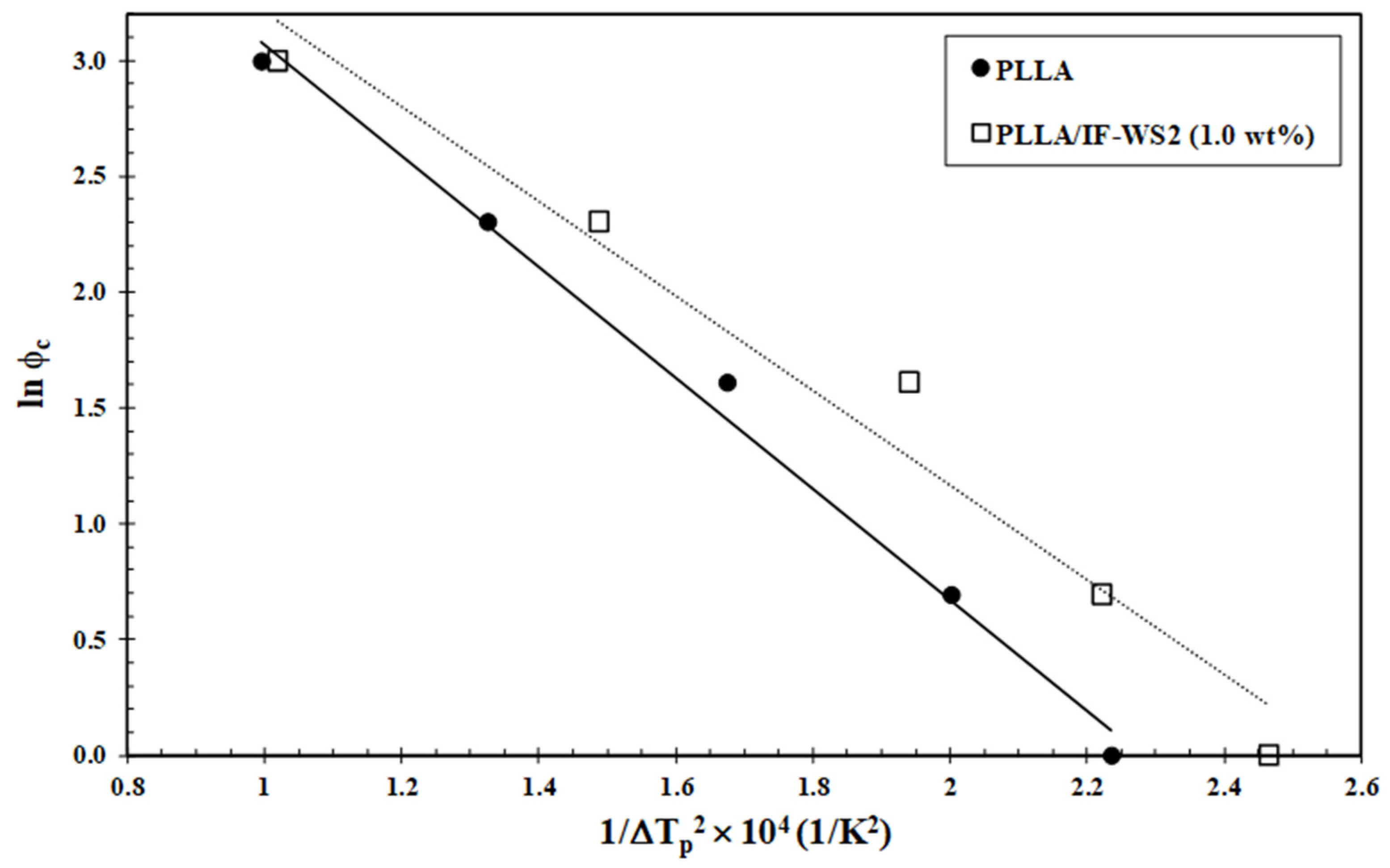
3.4. Nucleation Activity
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [Green Version]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janrkor, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Saeidou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Maitra, U.; Waghmare, U.V. Extraordinary attributes of 2-dimensional MoS2 nanosheets. Chem. Phys. Lett. 2014, 609, 172–183. [Google Scholar] [CrossRef]
- Tenne, R.; Margulis, L.; Genut, M.; Hodes, G. Polyhedral and cylindrical structures of WS2. Nature 1992, 360, 444–445. [Google Scholar] [CrossRef]
- Margulis, L.; Salitra, G.; Tenne, R.; Talianker, M. Nested fullerene-like structures. Nature 1993, 365, 113–114. [Google Scholar] [CrossRef]
- Tenne, R.; Redlich, M. Recent progress in the research of inorganic fullerene-like nanoparticles and inorganic nanotubes. Chem. Soc. Rev. 2010, 39, 1423–1434. [Google Scholar] [CrossRef]
- Naffakh, M.; Díez-Pascual, A.M.; Marco, C.; Ellis, G.; Gómez-Fatou, M.A. Opportunities and challenges in the use of inorganic fullerene-like nanoparticles to produce advanced polymer nanocomposites. Prog. Polym. Sci. 2013, 38, 1163–1231. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Otorgust, G.; Idelevich, A.; Regev, O.; Lapsker, I.; Lewitus, D.Y.; Zak, A. Reinforcement of poly(methyl methacrylate) by WS2 nanotubes towards antiballistic applications. Compos. Sci. Technol. 2021, 207, 108736. [Google Scholar] [CrossRef]
- Zhang, X.; Teng, S.Y.; Loy, A.C.M.; How, B.S.; Leong, W.D.; Tao, X. Transition metal dichalcogenides for the application of pollution reduction: A Review. Nanomaterials 2020, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhang, Y.; Wang, H.; Wang, L.; Kong, T.; Zhang, H.; Meng, S. Recent advances on TMDCs for medical diagnosis. Biomaterials 2021, 269, 120471. [Google Scholar] [CrossRef] [PubMed]
- Levin, T.; Sade, H.; Binyamini, R.B.S.; Pour, M.; Nachman, I.; Lellouche, J.P. Tungsten disulfide-based nanocomposites for photothermal therapy. Beilstein J. Nanotechnol. 2019, 10, 811–822. [Google Scholar] [CrossRef]
- Naffakh, M.; Remskar, M.; Marco, M.; Gómez-Fatou, M.A.; Jiménez, I. Towards a new generation of polymer nanocomposites based on inorganic nanotubes. J. Mater. Chem. 2011, 21, 3574–3578. [Google Scholar] [CrossRef]
- Naffakh, M.; Diez-Pascual, A.M.; Gómez-Fatou, M.A. New hybrid nanocomposites containing carbon nanotubes, inorganic fullerene-like WS2 nanoparticles and poly(ether ether ketone) (PEEK). J. Mater. Chem. 2011, 21, 7425–7433. [Google Scholar] [CrossRef]
- Reddy, C.S.; Zak, A.; Zussman, E. WS2 Nanotubes Embedded in PMMA Nanofibers as Energy Absorptive Material. J. Mater. Chem. 2011, 21, 16086–16093. [Google Scholar] [CrossRef]
- Zhou, K.; Gao, R.; Gui, Z.; Hu, Y. The effective reinforcements of functionalized MoS2 nanosheets in polymer hybrid composites by sol-gel technique. Compos. Part A 2017, 94, 1–9. [Google Scholar] [CrossRef]
- Lalwani, G.; Henslee, A.M.; Farshid, B.; Parmar, P.; Lin, L.; Qin, Y.X.; Kasper, F.K.; Mikos, A.G.; Sitharaman, B. Tungsten disulfide nanotubes reinforced biodegradable polymers for bone tissue engineering. Acta Biomater. 2013, 9, 8365–8373. [Google Scholar] [CrossRef] [Green Version]
- Naffakh, M.; Díez-Pascual, A.M. Nanocomposite biomaterials based on poly(etherether-ketone) (PEEK) and WS2 inorganic nanotubes. J. Mater. Chem. B 2014, 2, 4509–4520. [Google Scholar] [CrossRef] [Green Version]
- Naffakh, M.; Marco, C.; Ellis, G.; Cohen, S.R.; Laikhtman, A.; Rapoport, L.; Zak, A. Novel poly(3-hydroxybutyrate) nanocomposites containing WS2 inorganic nanotubes with improved thermal, mechanical and tribological properties. Mater. Chem. Phys. 2014, 147, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Naffakh, M.; Shuttleworth, P.S.; Ellis, G. Bio-based polymer nanocomposites based on nylon 11 and WS2 inorganic nanotubes. RSC Adv. 2015, 5, 17879–17887. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Liang, X.; Xu, Y.; Zhou, Y.; Nie, W. Enhanced thermal and mechanical properties of PLA/MoS2 nanocomposites synthesized via the in-situ ring-opening polymerization. Appl. Surf. Sci. 2018, 440, 1143–1149. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Ellis, G. Development of novel melt-processable biopolymer nanocomposites based on poly(L-lactic acid) and WS2 inorganic nanotubes. CrystEngComm 2014, 16, 5062–5072. [Google Scholar] [CrossRef] [Green Version]
- Naffakh, M.; Fernández, M.; Shuttleworth, P.S.; García, A.M.; Moreno, D.A. Nanocomposite materials with poly(l-lactic acid) and transition-metal dichalcogenide nanosheets 2D-TMDCs WS2. Polymers 2020, 12, 2699. [Google Scholar] [CrossRef]
- Naffakh, M.; Martín, Z.; Fanegas, N.; Marco, C.; Gómez, M.A.; Jiménez, I. Influence of Inorganic fullerene-like WS2 nanoparticles on the thermal behavior of isotactic polypropylene. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 2309–2321. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Pan, P.; Liang, Z.; Cao, A.; Inoue, Y. Layered metal phosphonate reinforced poly(l-lactide) composites with a highly enhanced crystallization rate. ACS Appl. Mater. Interfaces 2009, 1, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Enyashin, A.N.; Glazyrina, P.Y. On the crystallization of polymer composites with inorganic fullerene-like particles. Phys. Chem. Chem. Phys. 2012, 14, 7104–7111. [Google Scholar] [CrossRef] [PubMed]
- Naffakh, M.; Marco, C.; Ellis, G. Non-isothermal cold-crystallization behavior and kinetics of poly(l-lactic acid)/WS2 inorganic nanotube nanocomposites. Polymers 2015, 7, 2175–2189. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II. Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 128, 150–158. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Gómez, M.A.; Jiménez, I. Unique nucleation activity of inorganic fullerene-like WS2 nanoparticles in polyphenylene sulfide nanocomposites: Isokinetic and isoconversional study of dynamic crystallization kinetics. J. Phys. Chem. B 2009, 113, 7107–7115. [Google Scholar] [CrossRef]
- Dobreva, A.; Gutzow, I. Activity of substrates in the catalyzed nucleation of glass-forming melts. I. Theory. J. Non Cryst. Solids 1993, 162, 1–12. [Google Scholar] [CrossRef]
- Dobreva, A.; Gutzow, I. Activity of substrates in the catalyzed nucleation of glass-forming melts. II. Experimental evidence. J. Non Cryst. Solids 1993, 162, 13–25. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C. Isothermal crystallization kinetics and melting behavior of poly(L-lactic acid)/WS2 inorganic nanotube nanocomposites. J. Mater. Sci. 2015, 50, 6066–6074. [Google Scholar] [CrossRef]
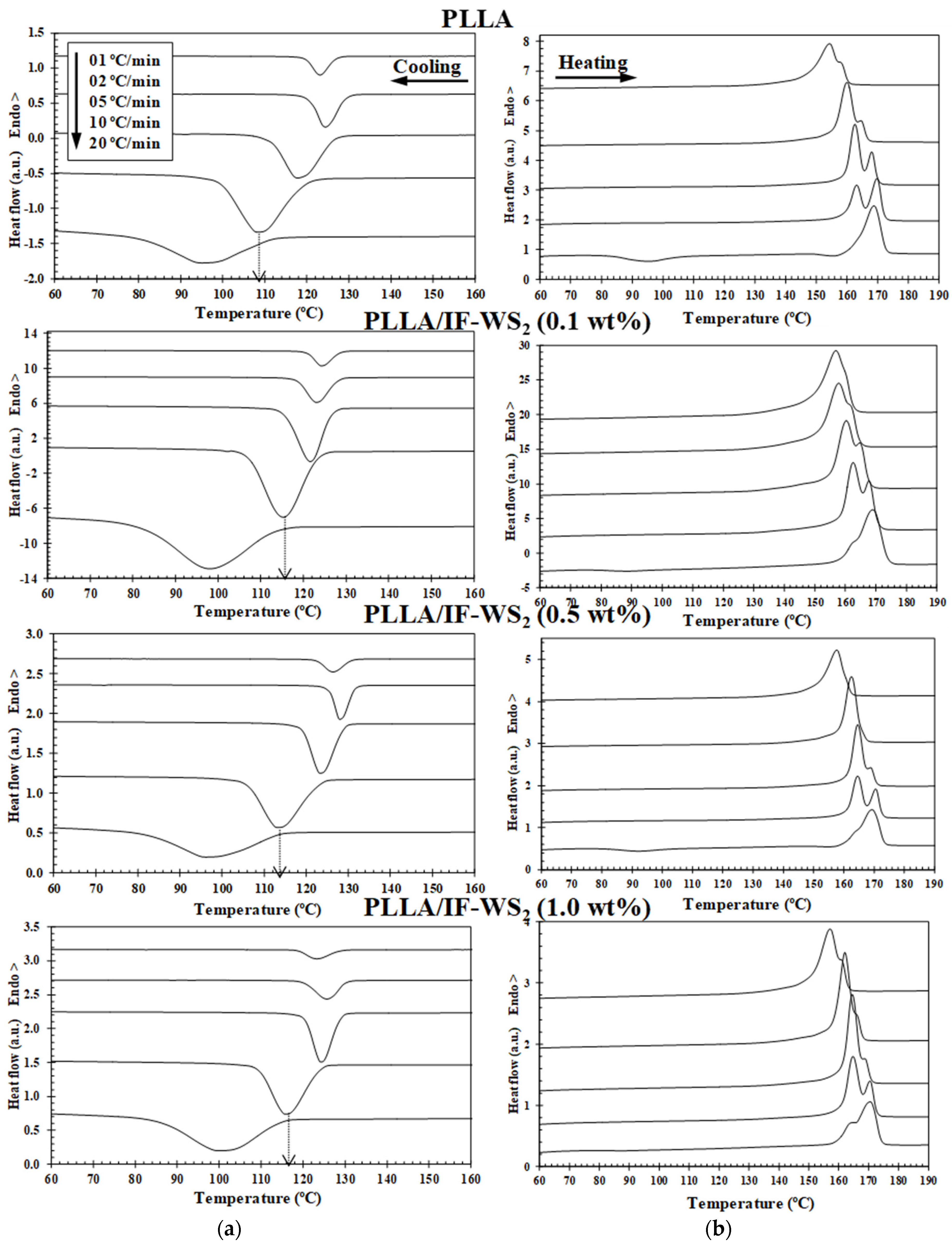

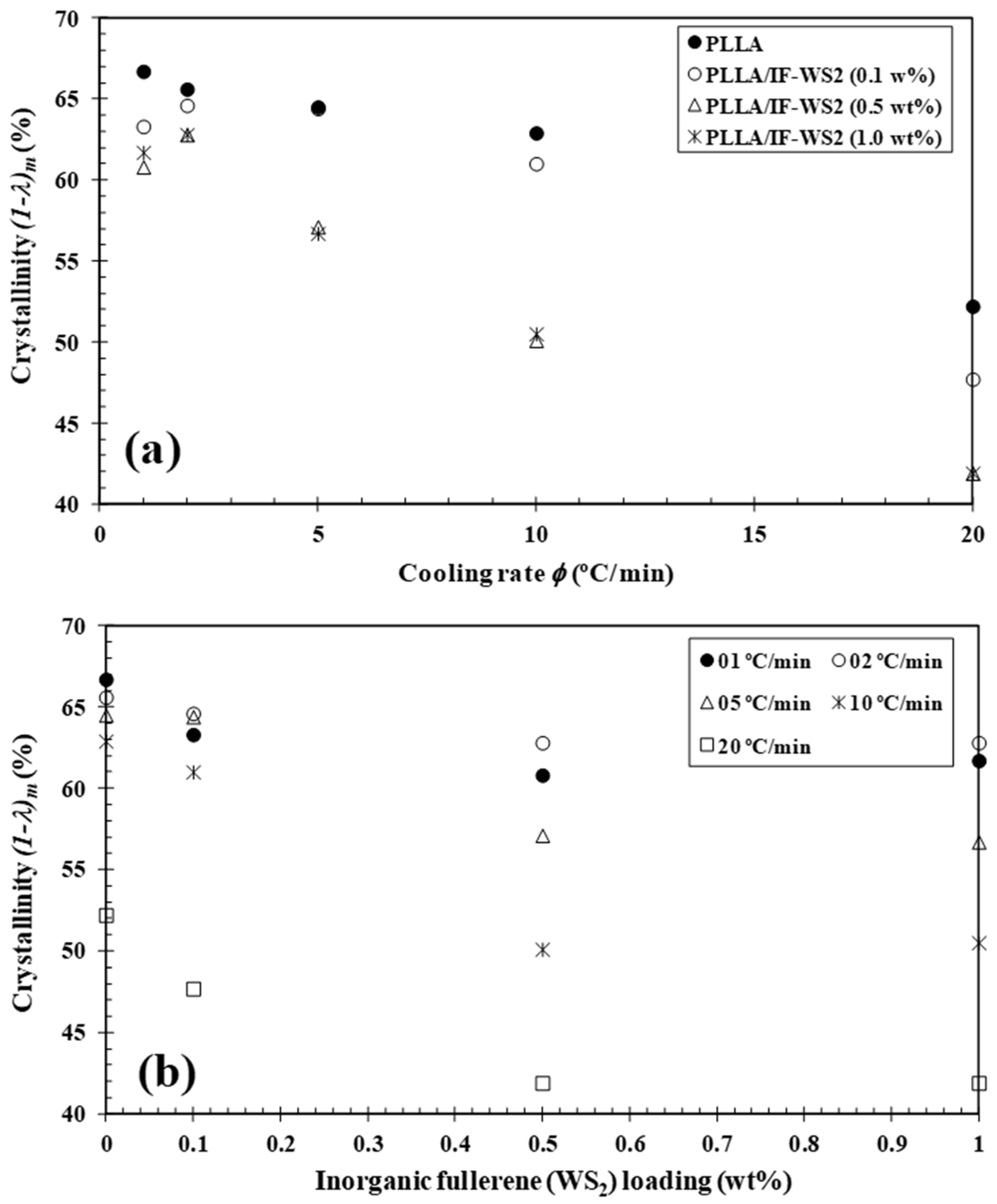
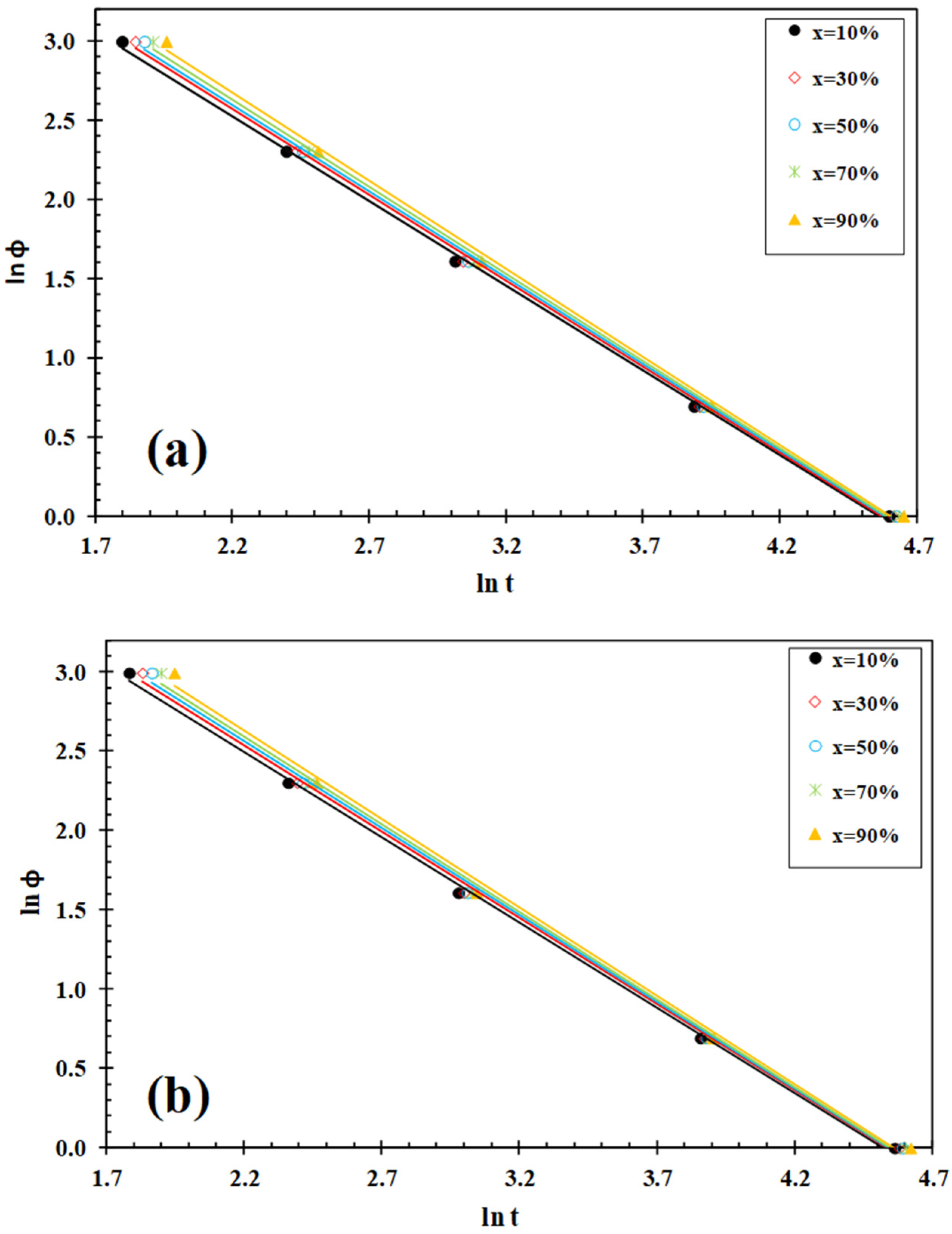
| IF-WS2 (wt%) | φc (°C/min) | Tc (°C) | (1−λ)c (%) | Tcc (°C) | (1−λ)cc (%) | Tm1 (°C) | Tm2 (°C) | (1−λ)m (%) |
|---|---|---|---|---|---|---|---|---|
| 0.0 | 1 2 5 10 20 | 123.1 124.3 117.7 108.1 94.7 | 58.1 57.5 53.8 49.7 28.0 | - - - - 95.0 | - - - - 19.7 | 154.2 159.0 162.5 163.0 - | 157.2 164.8 168.1 169.7 168.7 | 66.7 65.6 64.5 62.9 52.2 |
| 0.1 | 1 2 5 10 20 | 124.2 122.9 121.5 115.1 97.9 | 56.1 57.0 55.8 49.8 33.6 | - - - - 88.8 | - - - - 1.8 | - 157.8 160.3 162.5 163.2 | 156.8 162.0 164.5 167.5 168.9 | 63.3 64.6 64.4 61.0 47.7 |
| 0.5 | 1 2 5 10 20 | 126.3 127.9 123.2 113.1 96.0 | 55.8 57.0 51.8 43.4 22.5 | - - - - 92.1 | - - - - 5.7 | - - 164.5 164.5 - | 157.6 162.5 169.1 170.3 169.2 | 60.8 62.8 57.1 50.1 41.9 |
| 1.0 | 1 2 5 10 20 | 126.3 127.9 123.2 113.0 95.9 | 55.7 57.0 51.8 43.6 23.6 | - - - - 92.0 | - - - - 5.9 | 157.6 161.6 164.9 164.5 165.0 | 160.5 165.9 168.6 170.3 169.2 | 61.7 62.8 56.7 50.5 41.9 |
| IF-WS2 (wt%) | x (%) | α | f(T) |
|---|---|---|---|
| 0.0 | 10 30 50 70 90 | 1.07 1.08 1.09 1.10 1.11 | 4.88 4.96 5.00 5.05 5.12 |
| 0.1 | 10 30 50 70 90 | 1.06 1.07 1.08 1.09 1.10 | 4.83 4.90 4.94 4.98 5.04 |
| 0.5 | 10 30 50 70 90 | 1.07 1.09 1.10 1.10 1.11 | 4.86 4.92 4.97 5.01 5.08 |
| 1.0 | 10 30 50 70 90 | 1.06 1.07 1.07 1.08 1.08 | 4.81 4.86 4.90 4.93 4.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naffakh, M. Biopolymer Nanocomposite Materials Based on Poly(L-lactic Acid) and Inorganic Fullerene-like WS2 Nanoparticles. Polymers 2021, 13, 2947. https://doi.org/10.3390/polym13172947
Naffakh M. Biopolymer Nanocomposite Materials Based on Poly(L-lactic Acid) and Inorganic Fullerene-like WS2 Nanoparticles. Polymers. 2021; 13(17):2947. https://doi.org/10.3390/polym13172947
Chicago/Turabian StyleNaffakh, Mohammed. 2021. "Biopolymer Nanocomposite Materials Based on Poly(L-lactic Acid) and Inorganic Fullerene-like WS2 Nanoparticles" Polymers 13, no. 17: 2947. https://doi.org/10.3390/polym13172947
APA StyleNaffakh, M. (2021). Biopolymer Nanocomposite Materials Based on Poly(L-lactic Acid) and Inorganic Fullerene-like WS2 Nanoparticles. Polymers, 13(17), 2947. https://doi.org/10.3390/polym13172947






