Novel Cellulose Triacetate (CTA)/Cellulose Diacetate (CDA) Blend Membranes Enhanced by Amine Functionalized ZIF-8 for CO2 Separation
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Materials
2.2. ZIF-8 Synthesis
2.3. Amine Modification of ZIF-8
2.4. Membrane Fabrication
2.5. Characterizations
2.6. Gas Permeation Measurements
2.6.1. Pure Gas Tests
2.6.2. Mixed Gas Tests
3. Results and Discussion
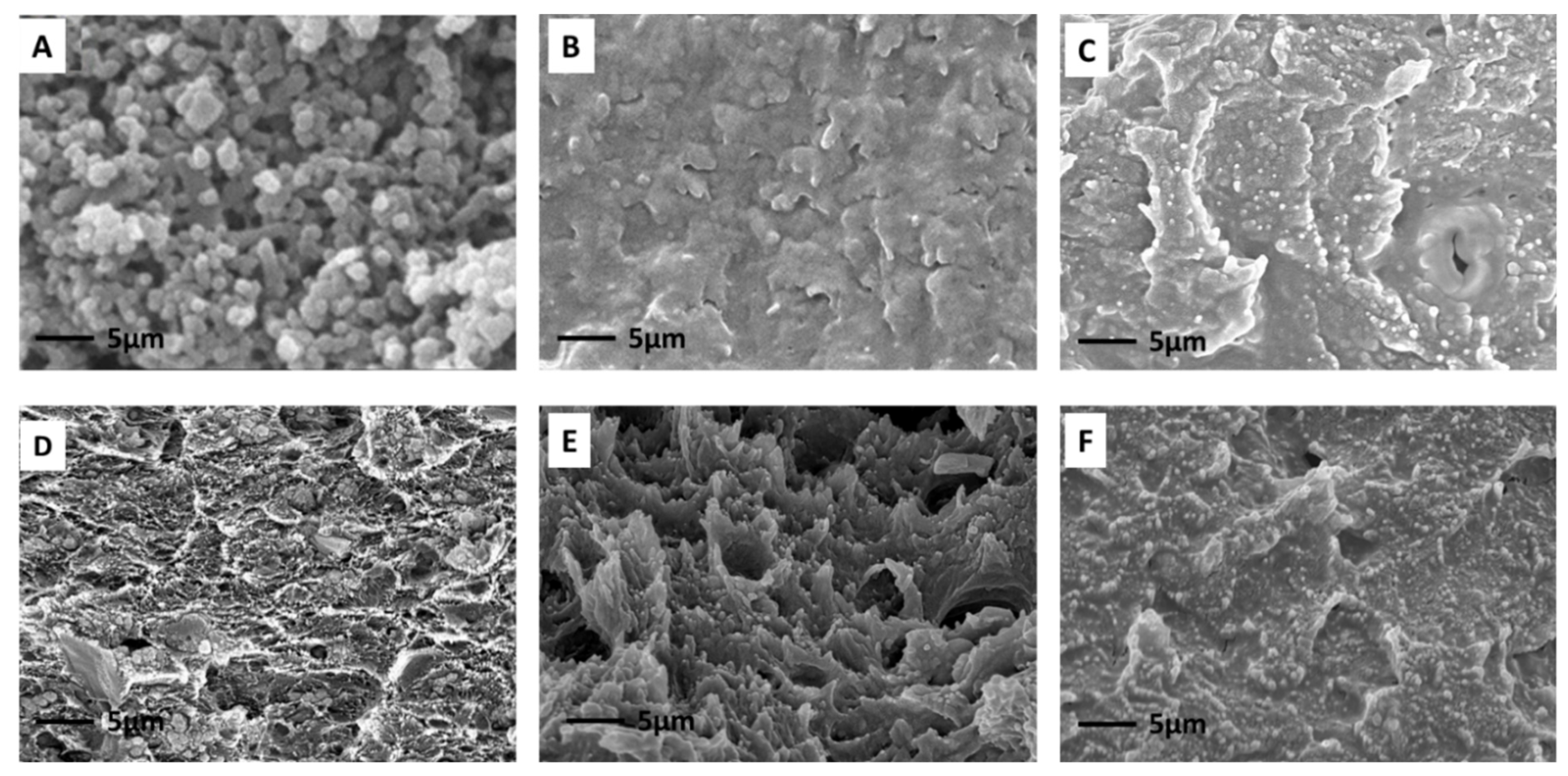
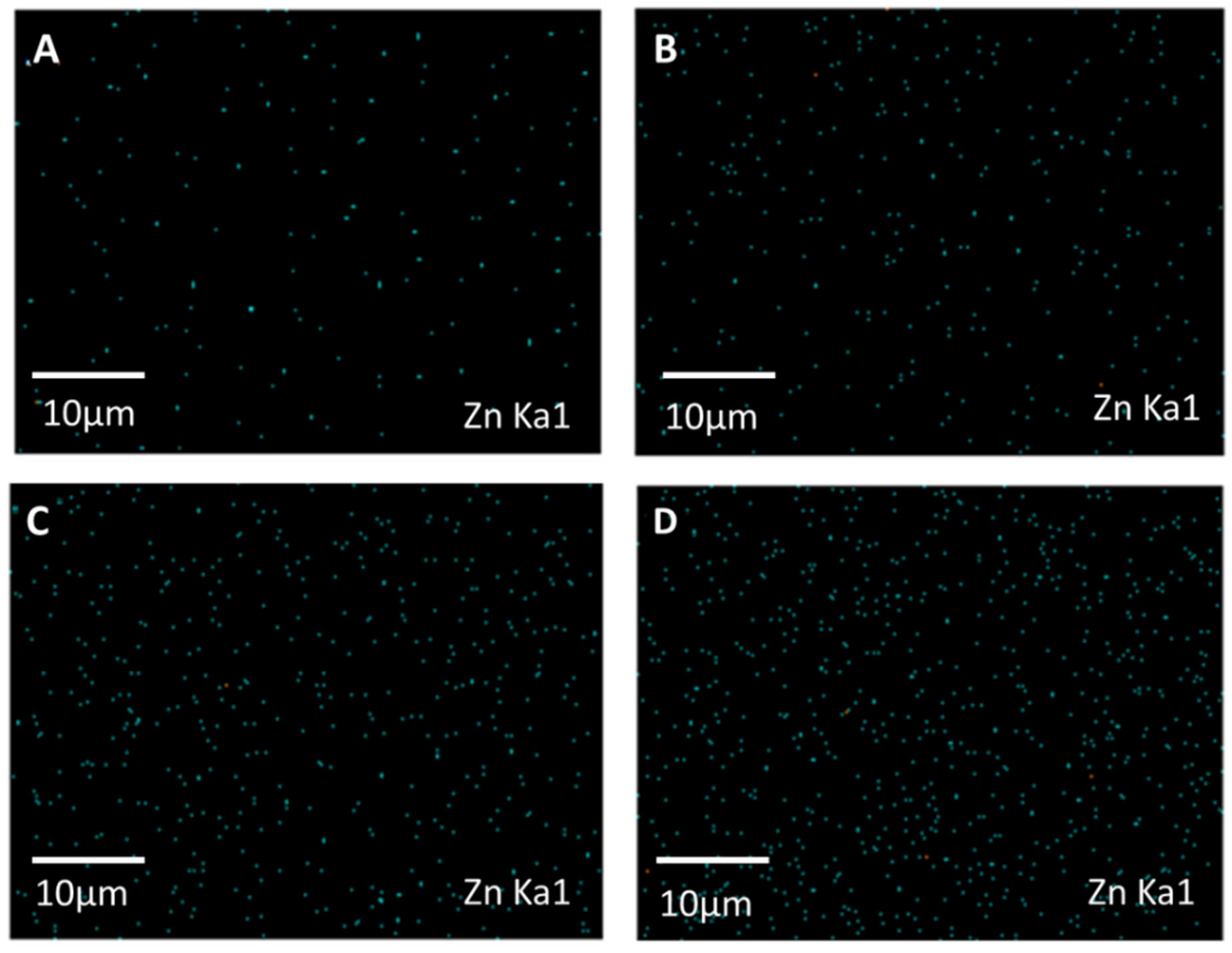
3.1. Characterizations
3.2. Gas Transport Properties
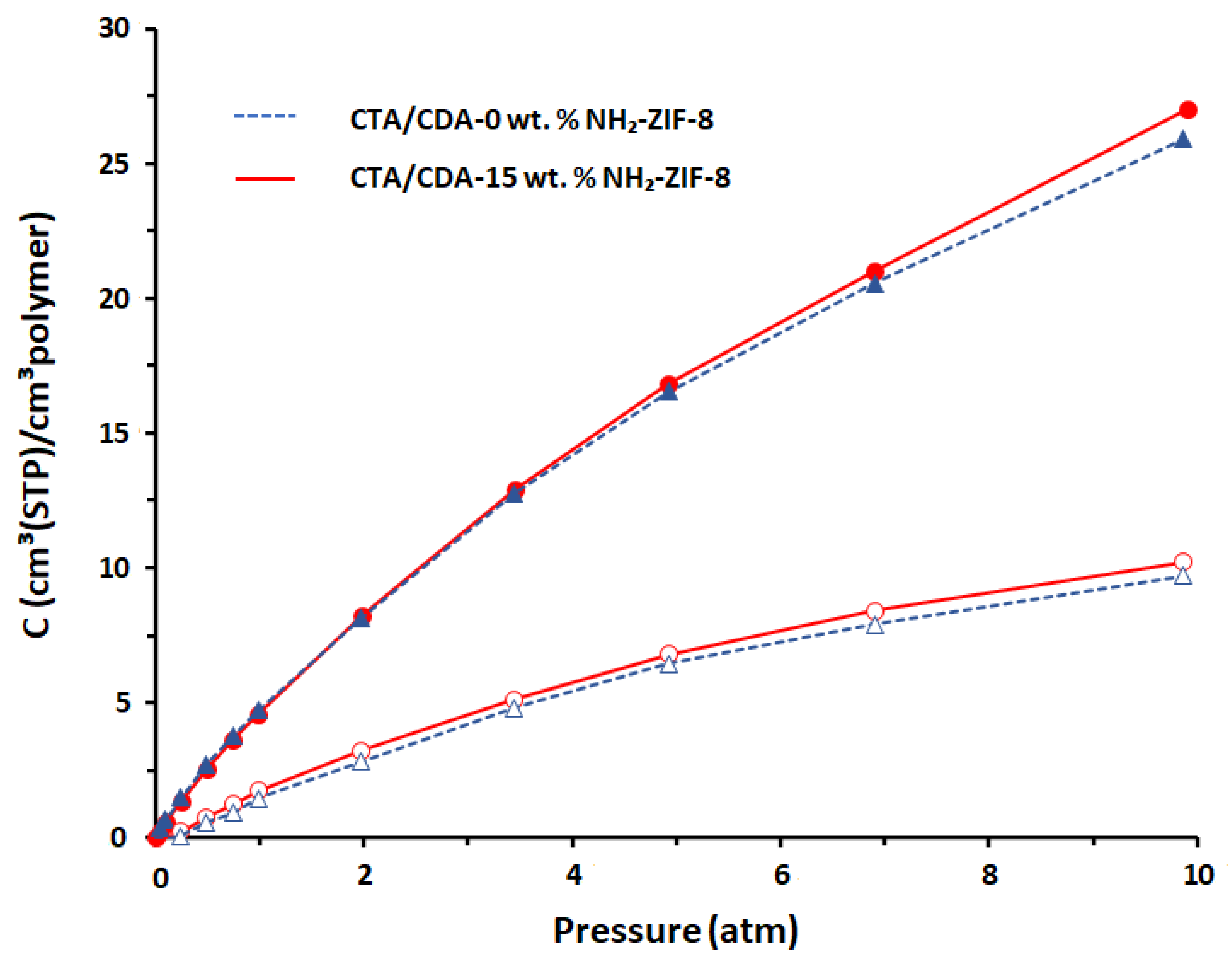
3.2.1. Sorption Behavior of CO2 and CH4
3.2.2. Plasticization Behavior and Mixed Gas Tests
3.3. Benchmark with the Literature
4. Conclusions
- There is a linear relationship between permeability and NH2-ZIF-8 loading. However, the relationship changes to a ˄-shape between CO2/CH4 selectivity and NH2-ZIF-8 loading due to void formation. Thus, there is an optimum loading to fabricate CTA/CDA based MMMs with high gas separation performance.
- The optimized MMM contained 15 wt.% of NH2-ZIF-8 nanoparticles and had a CO2 permeability of 11.33 Barrer which was 50% higher than the pristine CTA/CDA membrane. In addition, the former had a superior CO2/CH4 selectivity of 33.33 to the latter of 27 under pure gas tests.
- The enhanced molecular sieving capability and additional free volume provided by NH2-ZIF-8 nanoparticles improved the CO2 diffusivity and solubility coefficients by 44% and 4%, respectively, under pure gas tests when 15 wt.% NH2-ZIF-8 was incorporated into the CTA/CDA membrane.
- The CO2/CH4 selectivity can be further increased to 41 under mixed gas tests due to the competitive diffusion and sorption between CO2 and CH4 molecules.
- A notable improvement in CO2-induced plasticization pressure from 10.4 to 21.47 bar was observed owing to the good compatibility and chain interactions between CTA/CDA and NH2-ZIF-8 molecules.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, R.W.; Lokhandwala, K. Natural gas processing with membranes: An overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Shah, M.S.; Tsapatsis, M.; Siepmann, J.I. Hydrogen sulfide capture: From absorption in polar liquids to oxide, zeolite, and metal–organic framework adsorbents and membranes. Chem. Rev. 2017, 117, 9755–9803. [Google Scholar] [CrossRef]
- Mazyan, W.; Ahmadi, A.; Ahmed, H.; Hoorfar, M. Market and technology assessment of natural gas processing: A review. J. Nat. Gas Sci. Eng. 2016, 30, 487–514. [Google Scholar] [CrossRef]
- Bhide, B.; Stern, S. Membrane processes for the removal of acid gases from natural gas. II. Effects of operating conditions, economic parameters, and membrane properties. J. Membr. Sci. 1993, 81, 239–252. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley & Sons: Newark, CA, USA, 2012; pp. 325–373. [Google Scholar]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Xiao, Y.; Low, B.T.; Hosseini, S.S.; Chung, T.S.; Paul, D.R. The strategies of molecular architecture and modification of polyimide-based membranes for CO2 removal from natural gas—A review. Prog. Polym. Sci. 2009, 34, 61–580. [Google Scholar] [CrossRef]
- Koros, W.; Fleming, G.; Jordan, S.; Kim, T.; Hoehn, H. Polymeric membrane materials for solution-diffusion based permeation separations. Prog. Polym. Sci. 1988, 13, 339–401. [Google Scholar] [CrossRef]
- Dong, G.; Li, H.; Chen, V. Challenges and opportunities for mixed-matrix membranes for gas separation. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Liang, C.Z.; Chung, T.S.; Lai, J.-Y. A review of polymeric composite membranes for gas separation and energy production. Prog. Polym. Sci. 2019, 97, 101141. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Z. Nanostructured membrane materials for CO2 capture: A critical review. J. Nano Sci. 2019, 19, 3173–3179. [Google Scholar] [CrossRef]
- Shah, S.; Shah, M.; Shah, A.; Shah, M. Evolution in the membrane-based materials and comprehensive review on carbon capture and storage in industries. Emerg. Mater. 2020, 3, 33–44. [Google Scholar] [CrossRef]
- Lin, H.; Yavari, M. Upper bound of polymeric membranes for mixed-gas CO2/CH4 separations. J. Membr. Sci. 2015, 475, 101–109. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Suleman, M.S.; Lau, K.K.; Yeong, Y.F. Plasticization and swelling in polymeric membranes in CO2 removal from natural gas. Chem. Eng. Technol. 2016, 39, 1604–1616. [Google Scholar] [CrossRef]
- Minelli, M.; Oradei, S.; Fiorini, M.; Sarti, G.C. CO2 plasticization effect on glassy polymeric membranes. Polymer 2019, 163, 29–35. [Google Scholar] [CrossRef]
- Ismail, A.F.; Lorna, W. Penetrant-induced plasticization phenomenon in glassy polymers for gas separation membrane. Sep. Purif. Technol. 2002, 27, 173–194. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.M. High performance polymer membranes for CO2 separation. Curr. Opin. Chem. Eng. 2013, 2, 238–244. [Google Scholar] [CrossRef]
- Yong, W.F.; Zhang, H. Recent advances in polymer blend membranes for gas separation and pervaporation. Prog. Mater. Sci. 2020, 116, 100713. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Guan, W.; Dai, Y.; Dong, C.; Yang, X.; Xi, Y. Zeolite imidazolate framework (ZIF)-based mixed matrix membranes for CO2 separation: A review. J. Appl. Polym. Sci. 2020, 137, 48968. [Google Scholar] [CrossRef]
- Wang, B.; Sheng, M.; Xu, J.; Zhao, S.; Wang, J.; Wang, Z. Recent advances of gas transport channels constructed with different dimensional nanomaterials in mixed-matrix membranes for CO2 separation. Small Methods 2020, 4, 1900749. [Google Scholar] [CrossRef]
- Askari, M.; Chung, T.S. Natural gas purification and olefin/paraffin separation using thermal cross-linkable co-polyimide/ZIF-8 mixed matrix membranes. J. Membr. Sci. 2013, 444, 173–183. [Google Scholar] [CrossRef]
- Lai, Z. Development of ZIF-8 membranes: Opportunities and challenges for commercial applications. Curr. Opin. Chem. Eng. 2018, 20, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Wang, Y.; Kuang, T. ZIF-8-based membranes for carbon dioxide capture and separation. ACS Sustain. Chem. Eng. 2017, 5, 11204–11214. [Google Scholar] [CrossRef]
- Hopfenberg, H.; Paul, D.R. Transport phenomena in polymer blends. In Polymer Blends, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1978; Volume 1, pp. 445–489. [Google Scholar] [CrossRef]
- Mahajan, R.; Burns, R.; Schaeffer, M.; Koros, W.J. Challenges in forming successful mixed matrix membranes with rigid polymeric materials. J. Appl. Polym. Sci. 2002, 86, 881–890. [Google Scholar] [CrossRef]
- Chung, T.S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Li, J.; Nagai, K.; Nakagawa, T.; Wang, S. Preparation of polyethyleneglycol (PEG) and cellulose acetate (CA) blend membranes and their gas permeabilities. J. Appl. Polym. Sci. 1995, 58, 1455–1463. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Nagai, K.; Nakagawa, T.; Mau, A.W. Effect of polyethyleneglycol (PEG) on gas permeabilities and permselectivities in its cellulose acetate (CA) blend membranes. J. Membr. Sci. 1998, 138, 143–152. [Google Scholar] [CrossRef]
- Sajjan, P.; Nayak, V.; Padaki, M.; Zadorozhnyy, V.Y.; Klyamkin, S.N.; Konik, P. Fabrication of cellulose acetate film through blending technique with palladium acetate for hydrogen gas separation. Energy Fuel 2020, 34, 11699–11707. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Ahmadi, R.; Sinaei, M.; Kargari, A. Pebax-modified cellulose acetate membrane for CO2/N2 separation. J. Membr. Sci. 2019, 5, 25–32. [Google Scholar]
- Akbarzadeh, E.; Shockravi, A.; Vatanpour, V. High performance compatible thiazole-based polymeric blend cellulose acetate membrane as selective CO2 absorbent and molecular sieve. Carbohydr. Polym. 2021, 252, 117215. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Hsiao, M.-Y.; Nagai, K.; Lin, H. Suppressed crystallization and enhanced gas permeability in thin films of cellulose acetate blends. Polymer 2020, 205, 122790. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.A.H.M.; Racha, S.M.; Matsuura, T.; Misdan, N.; Sani, N.A.A.; Ismail, A.F.; Mustafa, A. Facile modification of ZIF-8 mixed matrix membrane for CO2/CH4 separation: Synthesis and preparation. RSC Adv. 2015, 5, 43110–43120. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Y.; Xia, Q.; Li, Z.; Xi, H. Experimental and molecular simulation studies of CO2 adsorption on zeolitic imidazolate frameworks: ZIF-8 and amine-modified ZIF-8. Adsorption 2013, 19, 25–37. [Google Scholar] [CrossRef]
- Chen, H.Z.; Xiao, Y.C.; Chung, T.S. Synthesis and characterization of poly (ethylene oxide) containing copolyimides for hydrogen purification. Polymer 2010, 51, 4077–4086. [Google Scholar] [CrossRef]
- Yong, W.F.; Chung, T.S. Mechanically strong and flexible hydrolyzed polymers of intrinsic microporosity (PIM1) membranes. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 344–354. [Google Scholar] [CrossRef]
- Wang, R.; Liu, S.; Lin, T.; Chung, T.S. Characterization of hollow fiber membranes in a permeator using binary gas mixtures. Chem. Eng. Sci. 2002, 57, 967–976. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Nataraj, S.; Roussenova, M.V.; Tan, J.C.; Hughes, D.J.; Li, W.; Bourgoin, P.; Alam, M.A.; Cheetham, A.K.; Al-Muhtaseb, S.A. Zeolitic imidazolate framework (ZIF-8) based polymer nanocomposite membranes for gas separation. Energy Environ. Sci. 2012, 5, 8359–8369. [Google Scholar] [CrossRef]
- Chatterjee, P.K.; Conrad, C.M. Thermogravimetric analysis of cellulose. J. Polym. Sci. Part A1 Polym. Chem. 1968, 6, 3217–3233. [Google Scholar] [CrossRef]
- Kamal, H.; Abd-Elrahim, F.; Lotfy, S. Characterization and some properties of cellulose acetate-co-polyethylene oxide blends prepared by the use of gamma irradiation. J. Radiat. Res. Appl. Sci. 2014, 7, 146–153. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Thanikaivelan, P.; Srinivasn, K.; Mohan, D.; Rajendran, M. Synthesis, characterization and thermal studies on cellulose acetate membranes with additive. Eur. Polym. J. 2004, 40, 2153–2159. [Google Scholar] [CrossRef]
- Abedini, R.; Mousavi, S.M.; Aminzadeh, R. A novel cellulose acetate (CA) membrane using TiO2 nanoparticles: Preparation, characterization and permeation study. Desalination 2011, 277, 40–45. [Google Scholar] [CrossRef]
- Jamil, A.; Zulfiqar, M.; Arshad, U.; Mahmood, S.; Iqbal, T.; Rafiq, S.; Iqbal, M.Z. Development and performance evaluation of cellulose acetate-bentonite mixed matrix membranes for CO2 separation. Adv. Polym. Technol. 2020, 2020. [Google Scholar] [CrossRef]
- Kamide, K.; Saito, M. Thermal analysis of cellulose acetate solids with total degrees of substitution of 0.49, 1.75, 2.46, and 2.92. Polym. J. 1985, 17, 919–928. [Google Scholar] [CrossRef] [Green Version]
- Lam, B.; Wei, M.; Zhu, L.; Luo, S.; Guo, R.; Morisato, A.; Alexandridis, P.; Lin, H. Cellulose triacetate doped with ionic liquids for membrane gas separation. Polymer 2016, 89, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nordin, N.A.H.M.; Ismail, A.F.; Misdan, N.; Nazri, N.A.M. Modified ZIF-8 mixed matrix membrane for CO2/CH4 separation. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2017. [Google Scholar]
- Isanejad, M.; Arzani, M.; Mahdavi, H.R.; Mohammadi, T. Novel amine modification of ZIF-8 for improving simultaneous removal of cationic dyes from aqueous solutions using supported liquid membrane. J. Mol. Liq. 2017, 225, 800–809. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy, 5th ed.; Nelson Education: Stanford, CA, USA, 2014; pp. 14–86. [Google Scholar]
- Yuan, J.; Li, Q.; Shen, J.; Huang, K.; Liu, G.; Zhao, J.; Duan, J.; Jin, W. Hydrophobic functionalized ZIF8 nanoparticles incorporated PDMS membranes for high selective separation of propane/nitrogen. Asia-Pac. J. Chem. Eng. 2017, 12, 110–120. [Google Scholar] [CrossRef]
- Wang, S.; Cui, J.; Zhang, S.; Xie, X.; Xia, W. Enhancement thermal stability and CO2 adsorption property of ZIF-8 by pre-modification with polyaniline. Mater. Res. Express 2020, 7, 025304. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Lau, K.K.; Shariff, A.M. Mixed matrix membranes comprising of ZIF-8 nanofillers for enhanced gas transport properties. Procedia Eng. 2016, 148, 1259–1265. [Google Scholar] [CrossRef] [Green Version]
- Yeong, Y.F.; Wang, H.; Pramoda, K.P.; Chung, T.S. Thermal induced structural rearrangement of cardo-copolybenzoxazole membranes for enhanced gas transport properties. J. Membr. Sci. 2012, 397, 51–65. [Google Scholar] [CrossRef]
- Heering, C.; Boldog, I.; Vasylyeva, V.; Sanchiz, J.; Janiak, C. Bifunctional pyrazolate–carboxylate ligands for isoreticular cobalt and zinc MOF-5 analogs with magnetic analysis of the {Co4(μ4-O)} node. CrystEngComm 2013, 15, 9757–9768. [Google Scholar] [CrossRef]
- Sada, E.; Kumazawa, H.; Xu, P.; Wang, S.T. Permeation of pure carbon dioxide and methane and binary mixtures through cellulose acetate membranes. J. Polym. Sci. 1990, 28, 113–125. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, G.; Jiang, J. Adsorption and diffusion of CO2 and CH4 in zeolitic imidazolate framework-8: Effect of structural flexibility. J. Phys. Chem. C 2014, 118, 8788–8794. [Google Scholar] [CrossRef]
- Japip, S.; Wang, H.; Xiao, Y.; Chung, T.S. Highly permeable zeolitic imidazolate framework (ZIF)-71 nano-particles enhanced polyimide membranes for gas separation. J. Membr. Sci. 2014, 467, 162–174. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, Y.; Johnson, J.R.; Karvan, O.; Koros, W.J. High performance ZIF-8/6FDA-DAM mixed matrix membrane for propylene/propane separations. J. Membr. Sci. 2012, 389, 34–42. [Google Scholar] [CrossRef]
- Nguyen, H.; Wang, M.; Hsiao, M.-Y.; Nagai, K.; Ding, Y.; Lin, H. Suppression of crystallization in thin films of cellulose diacetate and its effect on CO2/CH4 separation properties. J. Membr. Sci. 2019, 586, 7–14. [Google Scholar] [CrossRef]
- Kim, W.G.; Lee, J.S.; Bucknall, D.G.; Koros, W.J.; Nair, S. Nanoporous layered silicate AMH-3/cellulose acetate nanocomposite membranes for gas separations. J. Membr. Sci. 2013, 441, 129–136. [Google Scholar] [CrossRef]
- Moghadassi, A.; Rajabi, Z.; Hosseini, S.; Mohammadi, M. Fabrication and modification of cellulose acetate based mixed matrix membrane: Gas separation and physical properties. J. Ind. Eng. Chem. 2014, 20, 1050–1060. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Tanczyk, M.; Warmuzinski, K.; Jaschik, M.; Sandru, M.; Dahl, P.I.; Genua, A.; Lois, S.; Sheridan, E. The performance of affordable and stable cellulose-based poly-ionic membranes in CO2/N2 and CO2/CH4 gas separation. J. Membr. Sci. 2018, 564, 552–561. [Google Scholar] [CrossRef]
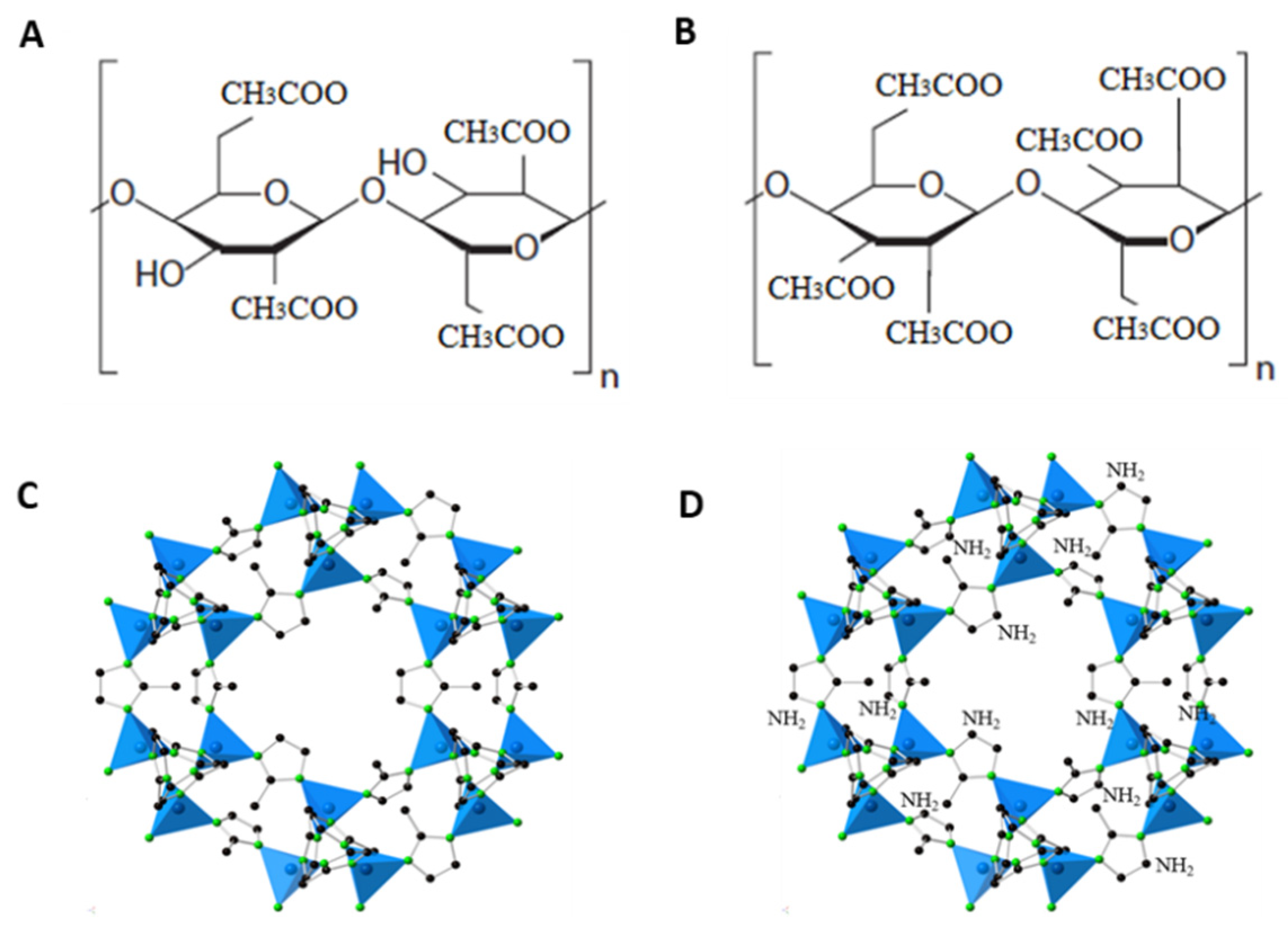
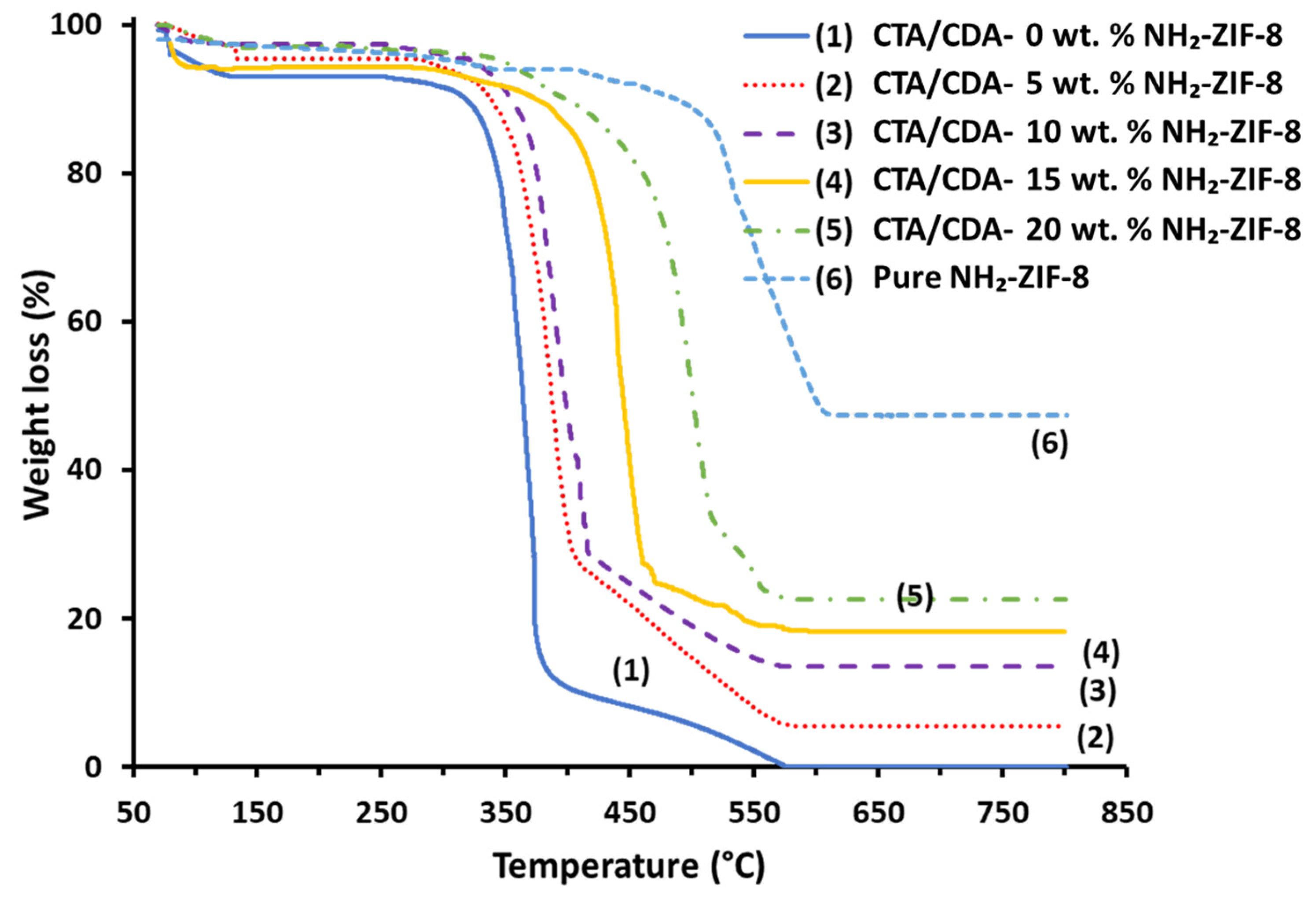
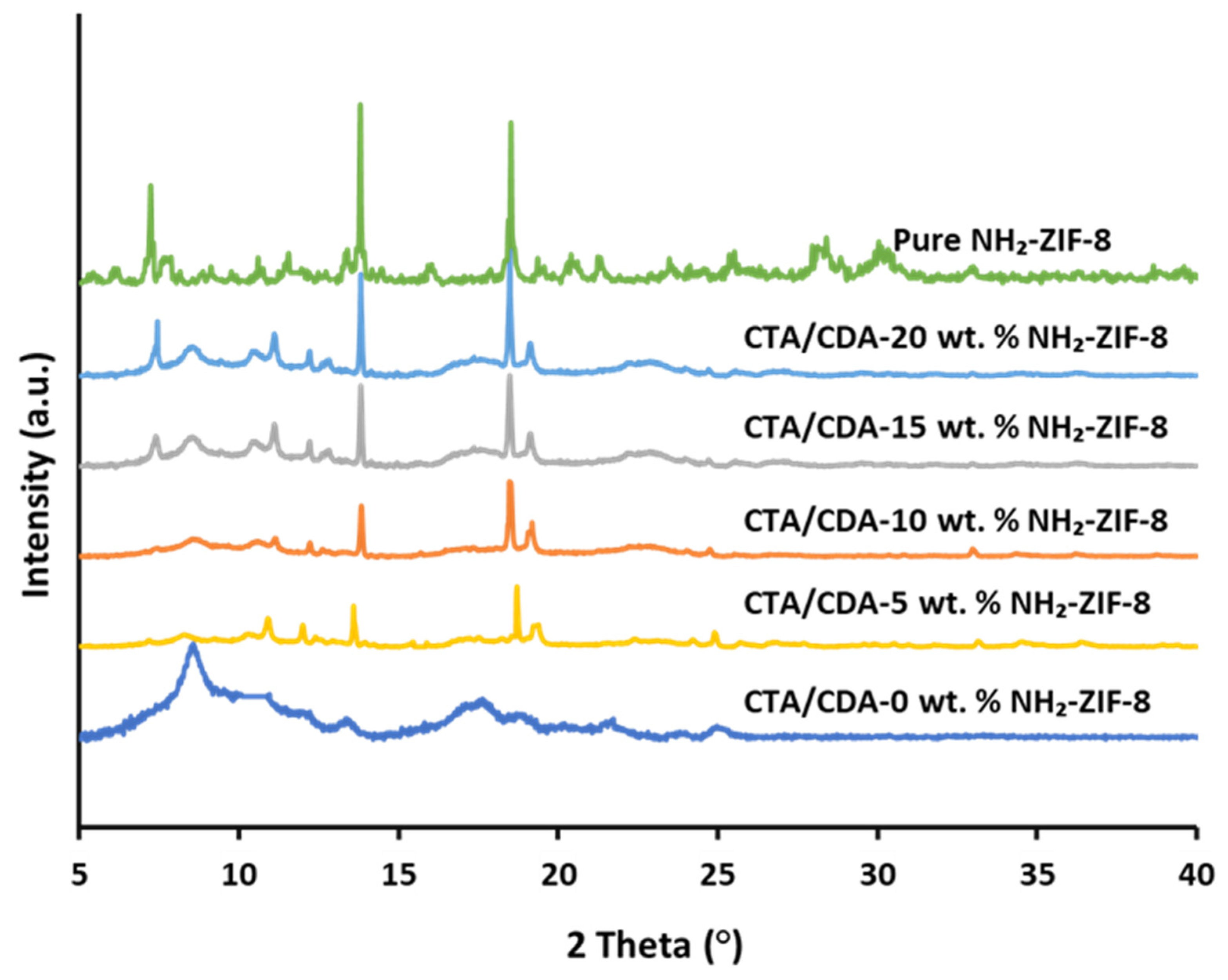
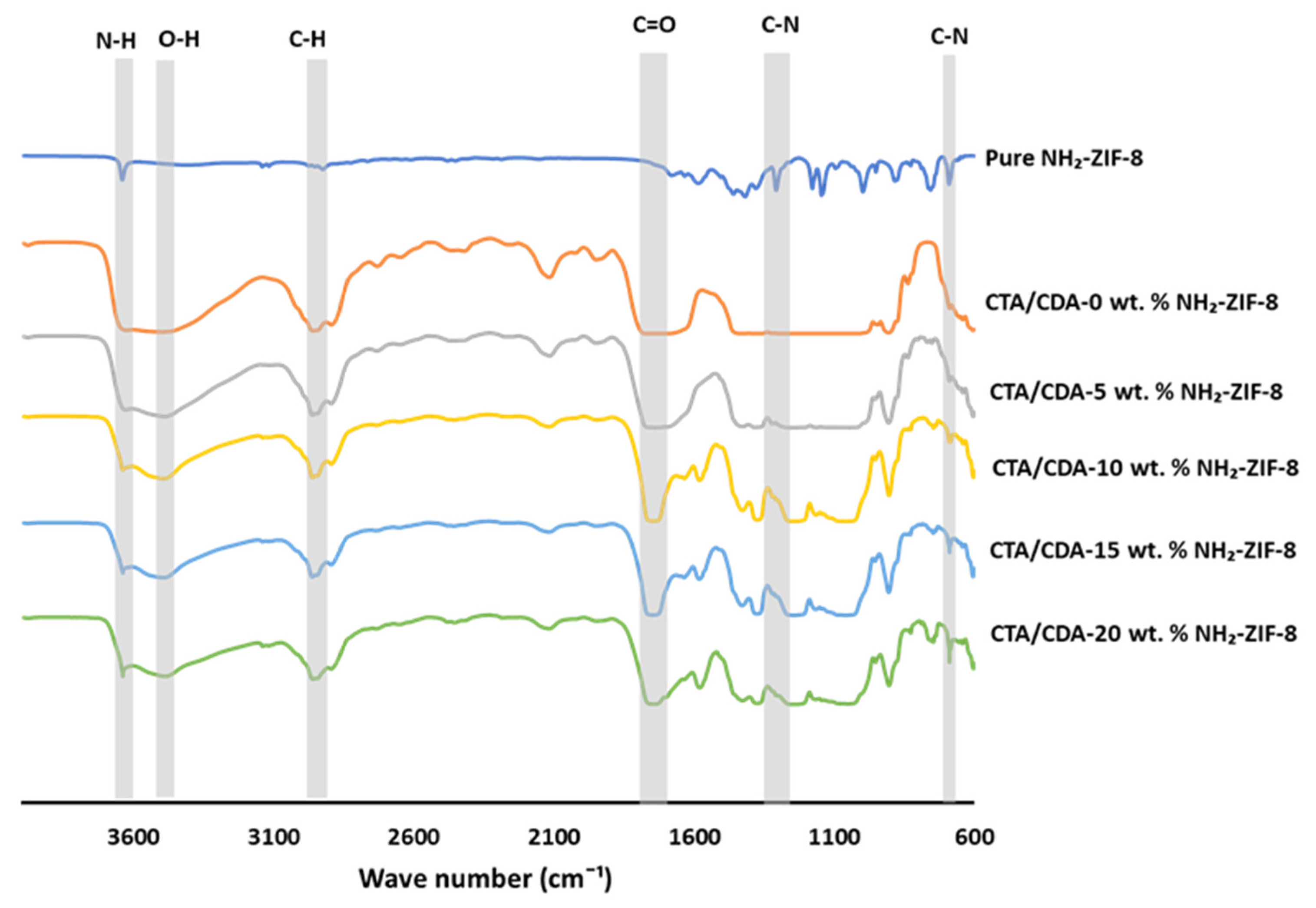
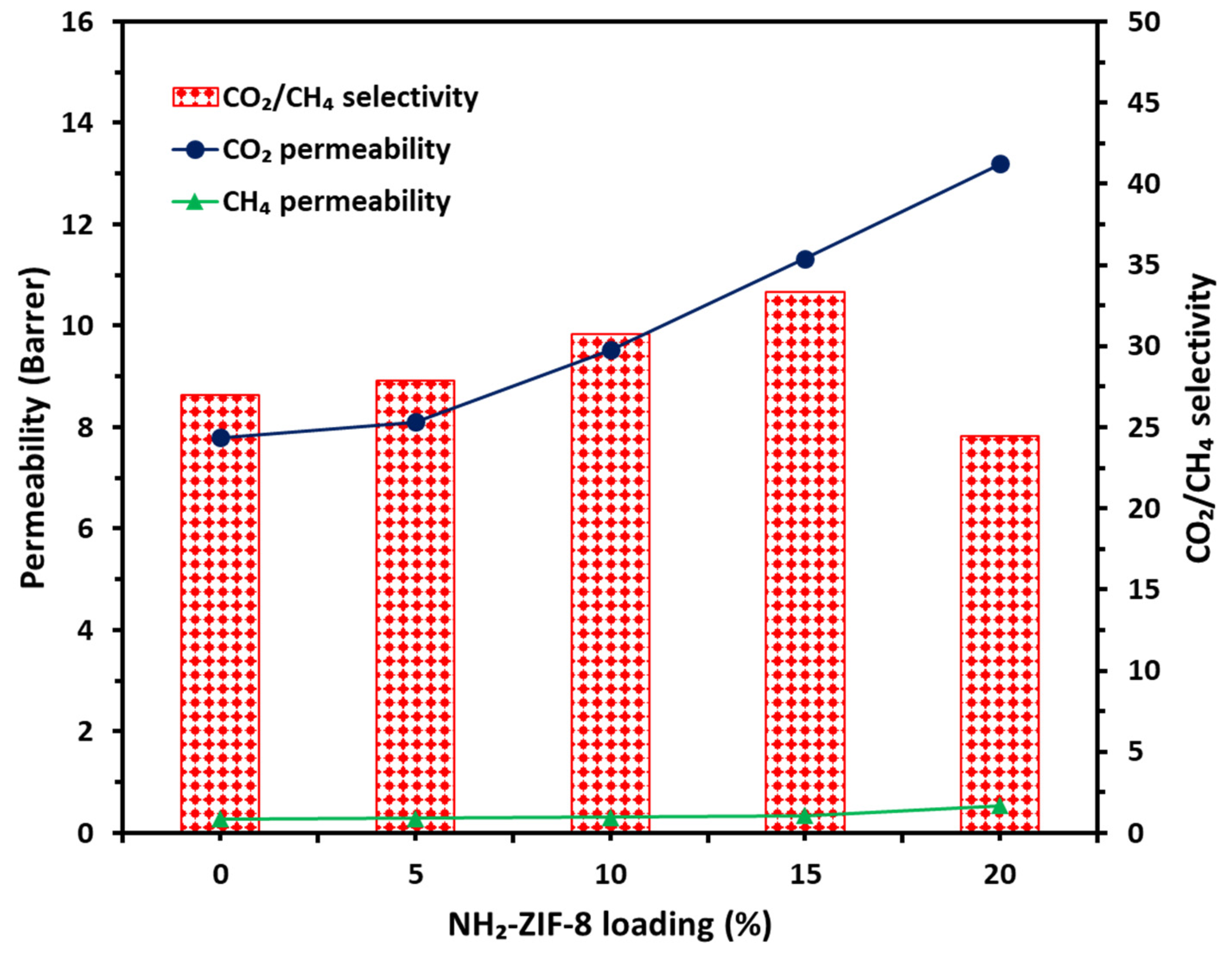

| Sample Name | Actual NH2-ZIF-8 Loading (%) | Td (°C) | Pure Gas Permeabilities (Barrer) | CO2/CH4 Selectivity | |
|---|---|---|---|---|---|
| CO2 | CH4 | ||||
| CTA/CDA- 0 wt.% NH2-ZIF-8 | 0 | 310 | 7.56 ± 0.17 | 0.28 ± 0.02 | 27.00 |
| CTA/CDA- 5 wt.% NH2-ZIF-8 | 5.13 | 320 | 8.10 ± 0.07 | 0.29 ± 0.03 | 27.93 |
| CTA/CDA- 10 wt.% NH2-ZIF-8 | 10.58 | 338 | 9.52 ± 0.05 | 0.31 ± 0.01 | 30.71 |
| CTA/CDA- 15 wt.% NH2-ZIF-8 | 15.80 | 363 | 11.33 ± 0.09 | 0.34 ± 0.02 | 33.32 |
| CTA/CDA- 20 wt.% NH2-ZIF-8 | 22.37 | 390 | 13.20 ± 0.21 | 0.54 ± 0.01 | 24.44 |
| Sample Name | Pure CO2 | Pure CH4 | CO2/CH4 Selectivity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P a | S b | D c | P a | S b | D c | ||||
| CTA/CDA-0 wt.% NH2-ZIF-8 | 7.56 | 3.47 | 2.18 | 0.28 | 1.29 | 0.22 | 27.00 | 2.69 | 10.04 |
| CTA/CDA-15 wt.% NH2-ZIF-8 | 11.33 | 3.61 | 3.14 | 0.34 | 1.35 | 0.25 | 33.33 | 2.67 | 12.46 |
| Sample Name | Pure Gas | Mixed Gas | ||||
|---|---|---|---|---|---|---|
| CO2 (Barrer) | CH4 (Barrer) | Selectivity CO2/CH4 | CO2 (Barrer) | CH4 (Barrer) | Selectivity CO2/CH4 | |
| CTA/CDA-0 wt.% NH2-ZIF-8 | 7.56 | 0.28 | 27 | 6.70 | 0.22 | 30.22 |
| CTA/CDA-15 wt.% NH2-ZIF-8 | 11.33 | 0.34 | 33.32 | 9.50 | 0.23 | 41.09 |
| Membrane Material | Pres. (Bar) | Temp. (°C ) | CO2 (Barrer) | CH4 (Barrer) | CO2/CH4 | Ref. |
|---|---|---|---|---|---|---|
| CDA | 11 | 35 | 3.9 | 0.11 | 35 | [62] |
| CTA | 4 | 35 | 6 | 0.3 | 20 | [49] |
| CDA-CTA a | 11 | 35 | 7.1 | 0.27 | 26 | [34] |
| CA/nanoporous layered silicate AMH-3 | 4.6 | - | 10.36 | 0.35 | 30.03 | [63] |
| CA/MWCNTs | 2 | Room temperature | 14.21 | 0.66 | 21.20 | [64] |
| P[CA][TF2N] b | 1 | 25 | 8.9 | 0.4 | 22.25 | [65] |
| CTA/[emim][BF4] c | 4 | 35 | 12 | 0.6 | 20 | [49] |
| CTA/CDA-0 wt.% NH2-ZIF-8 | 5 | 35 | 7.56 | 0.28 | 27 | This work |
| CTA/CDA-15 wt.% NH2-ZIF-8 | 5 | 35 | 11.33 | 0.34 | 33.33 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, A.; Japip, S.; Liang, C.Z.; Farrukh, S.; Hussain, A.; Chung, T.-S. Novel Cellulose Triacetate (CTA)/Cellulose Diacetate (CDA) Blend Membranes Enhanced by Amine Functionalized ZIF-8 for CO2 Separation. Polymers 2021, 13, 2946. https://doi.org/10.3390/polym13172946
Raza A, Japip S, Liang CZ, Farrukh S, Hussain A, Chung T-S. Novel Cellulose Triacetate (CTA)/Cellulose Diacetate (CDA) Blend Membranes Enhanced by Amine Functionalized ZIF-8 for CO2 Separation. Polymers. 2021; 13(17):2946. https://doi.org/10.3390/polym13172946
Chicago/Turabian StyleRaza, Ayesha, Susilo Japip, Can Zeng Liang, Sarah Farrukh, Arshad Hussain, and Tai-Shung Chung. 2021. "Novel Cellulose Triacetate (CTA)/Cellulose Diacetate (CDA) Blend Membranes Enhanced by Amine Functionalized ZIF-8 for CO2 Separation" Polymers 13, no. 17: 2946. https://doi.org/10.3390/polym13172946
APA StyleRaza, A., Japip, S., Liang, C. Z., Farrukh, S., Hussain, A., & Chung, T.-S. (2021). Novel Cellulose Triacetate (CTA)/Cellulose Diacetate (CDA) Blend Membranes Enhanced by Amine Functionalized ZIF-8 for CO2 Separation. Polymers, 13(17), 2946. https://doi.org/10.3390/polym13172946








