Polymer Vesicles for Antimicrobial Applications
Abstract
1. Introduction
2. Strategies to Integrate Polymer Vesicles with Antimicrobial Agents
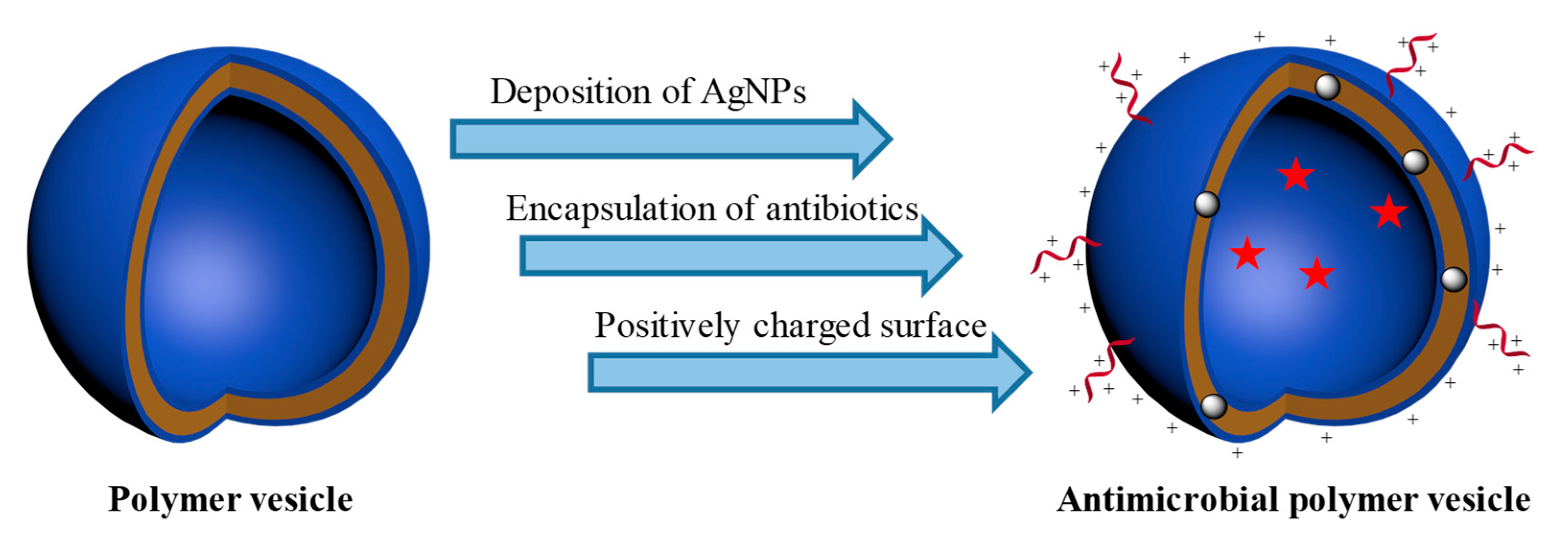
2.1. Deposition of Silver Nanoparticles (AgNPs) onto the Membrane of Polymer Vesicles
2.2. Encapsulation of Antibiotics
2.3. Introduction of Positively Charged Coronas
2.4. Using Antibacterial Polypeptides as Building Blocks
3. Antimicrobial Applications of Polymer Vesicles
3.1. Broad-Spectrum Antibacterial
3.2. Selective Antimicrobial and Anti-MDR Bacteria
3.3. Antimicrobial Drug Carrier
3.4. Anti-Biofilm, Wound Healing, and Tissue Engineering
4. Conclusions and Future Perspectives
- (1)
- How to improve the loading content of antimicrobial active components? The antimicrobial agents such as antibiotics are encapsulated in the cavity or membrane of polymer vesicles during self-assembly. This method limits the loading content of antibiotics. To improve the concentration of antibiotics is very important to ensure the entire elimination of bacteria, preventing the generation of drug resistance. Introduction of non-covalent interactions such as π−π interaction, hydrogen bonding, etc. between antibiotics and polymer vesicles might be an effective method to significantly improve the loading content of antibiotics.
- (2)
- How to unleash the advantages of polymer vesicles in combating MDR bacteria? MDR bacteria such as MRSA have threatened the life safety of human beings. Taking advantage of the multifunctional regions of polymer vesicles, different antimicrobial agents including antibiotics, silver nanoparticles, and AMPs could be loaded simultaneously. The synergy effect between those antimicrobial agents might exhibit unexpected antimicrobial activity to MDR bacteria.
- (3)
- How to increase the selectivity toward bacteria and mammalian cells? Antimicrobial agents to kill bacteria by physical interactions with the cell membrane of bacteria such as AMPs and positively charged polymeric nanostructure often show high cytotoxicity to mammalian cells. Shielding of the positive charges before targeting bacteria is the key to increase selectivity. The on-demand release of antimicrobial agents or exposure of the positively charged surfaces of polymer vesicles in response to the bacterial stimulus, such as bacterial toxins or external environmental changes such as pH, concentration of glucose, and so forth is believed to be a feasible strategy. Besides, the decoration of signal molecules on the surface of vesicles to target the cell membrane of bacteria is another alternative.
- (4)
- How to achieve antibacterial and anticancer simultaneously? Considering that the tumor site is commonly accompanied with the bacterial infections due to the decrease of resistance of patients, the simultaneous realization of antibacterial and anticancer is of special significance. One option is to use polymer vesicles with intrinsic antimicrobial activity as “armed” drug carriers, while the other is to take advantage of the high cytotoxicity of antimicrobial agents such as AMPs to kill cancer cells. The synergy between antimicrobial agents and anticancer drugs may bring new insight into the field of cancer treatment.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
- Warren, N.J.; Armes, S.P. Polymerization-Induced Self-Assembly of Block Copolymer Nano-objects via RAFT Aqueous Dispersion Polymerization. J. Am. Chem. Soc. 2014, 136, 10174–10185. [Google Scholar] [CrossRef]
- Cui, H.; Chen, Z.; Zhong, S.; Wooley, K.L.; Pochan, D.J. Block Copolymer Assembly via Kinetic Control. Science 2007, 317, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guerin, G.; Wang, H.; Wang, Y.; Manners, I.; Winnik, M.A. Cylindrical Block Copolymer Micelles and Co-Micelles of Controlled Length and Architecture. Science 2007, 317, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Du, J. Plasmonic vesicles with tailored collective properties. Nanoscale 2018, 10, 17354–17361. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, D.; Du, J. Nanobowls with controlled openings and interior holes driven by the synergy of hydrogen bonding and π‒π interaction. Chem. Sci. 2019, 10, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Du, J. Intramolecular Cyclization-Induced Crystallization-Driven Self-Assembly of an Amorphous Poly(amic acid). Macromolecules 2020, 53, 11033–11039. [Google Scholar] [CrossRef]
- Foster, J.C.; Varlas, S.; Couturaud, B.; Coe, Z.; O’Reilly, R.K. Getting into Shape: Reflections on a New Generation of Cylindrical Nanostructures’ Self-Assembly Using Polymer Building Blocks. J. Am. Chem. Soc. 2019, 141, 2742–2753. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Zhu, Y.; Han, L.; Wang, M.; He, F. Rectangular Platelet Micelles with Controlled Aspect Ratio by Hierarchical Self-Assembly of Poly(3-hexylthiophene)-b-poly(ethylene glycol). Macromolecules 2020, 53, 6555–6565. [Google Scholar] [CrossRef]
- Varlas, S.; Lawrenson, S.B.; Arkinstall, L.A.; O’Reilly, R.K.; Foster, J.C. Self-assembled nanostructures from amphiphilic block copolymers prepared via ring-opening metathesis polymerization (ROMP). Prog. Polym. Sci. 2020, 107, 101278. [Google Scholar] [CrossRef]
- Wong, C.K.; Qiang, X.; Mueller, A.H.E.; Groeschel, A.H. Self-Assembly of block copolymers into internally ordered microparticles. Prog. Polym. Sci. 2020, 102, 101211. [Google Scholar] [CrossRef]
- Du, J.; Sun, H. Polymer/TiO2 hybrid vesicles for excellent UV screening and effective encapsulation of antioxidant agents. ACS Appl. Mater. Interfaces 2014, 6, 13535–13541. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, Y.; Yang, B.; Wang, Y.; Wu, Y.; Du, J. Template-free fabrication of nitrogen-doped hollow carbon spheres for high-performance supercapacitors based on a scalable homopolymer vesicle. J. Mater. Chem. A 2016, 4, 12088–12097. [Google Scholar] [CrossRef]
- Xiao, Y.; Sun, H.; Du, J. Sugar-Breathing Glycopolymersomes for Regulating Glucose Level. J. Am. Chem. Soc. 2017, 139, 7640–7647. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jiang, J.; Xiao, Y.; Du, J. Efficient Removal of Polycyclic Aromatic Hydrocarbons, Dyes, and Heavy Metal Ions by a Homopolymer Vesicle. ACS Appl. Mater. Interfaces 2018, 10, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Eisenberg, A. Polymer Vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Yang, B.; Chen, S.; Du, J.Z. Polymer vesicles: Mechanism, preparation, application, and responsive behavior. Prog. Polym. Sci. 2017, 64, 1–22. [Google Scholar] [CrossRef]
- Palivan, C.G.; Goers, R.; Najer, A.; Zhang, X.; Car, A.; Meier, W. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem. Soc. Rev. 2016, 45, 377–411. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.P.; Cao, W.; Zhang, X. Selenium-Containing Polymers: Promising Biomaterials for Controlled Release and Enzyme Mimics. Acc. Chem. Res. 2013, 46, 1647–1658. [Google Scholar] [CrossRef]
- Hu, X.; Zhai, S.; Liu, G.; Xing, D.; Liang, H.; Liu, S. Concurrent Drug Unplugging and Permeabilization of Polyprodrug-Gated Crosslinked Vesicles for Cancer Combination Chemotherapy. Adv. Mater. 2018, 30, 1706307. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Li, R.; Khan, S.; Clanton, R.; Zhang, F.; Lin, Y.N.; Song, Y.; Wang, H.; Fan, J.; Hernandez, S.; et al. Chemical Design of Both a Glutathione-Sensitive Dimeric Drug Guest and a Glucose-Derived Nanocarrier Host to Achieve Enhanced Osteosarcoma Lung Metastatic Anticancer Selectivity. J. Am. Chem. Soc. 2018, 140, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Gaitzsch, J.; Hirschi, S.; Freimann, S.; Fotiadis, D.; Meier, W. Directed Insertion of Light-Activated Proteorhodopsin into Asymmetric Polymersomes from an ABC Block Copolymer. Nano Lett. 2019, 19, 2503–2508. [Google Scholar] [CrossRef]
- Bellomo, E.G.; Wyrsta, M.D.; Pakstis, L.; Pochan, D.J.; Deming, T.J. Stimuli-responsive polypeptide vesicles by conformation-specific assembly. Nat. Mater. 2004, 3, 244–248. [Google Scholar] [CrossRef]
- Ratcliffe, L.P.D.; Derry, M.J.; Ianiro, A.; Tuinier, R.; Armes, S.P. A Single Thermoresponsive Diblock Copolymer Can Form Spheres, Worms or Vesicles in Aqueous Solution. Angew. Chem. Int. Ed. 2019, 58, 18964–18970. [Google Scholar] [CrossRef] [PubMed]
- Jaggers, R.W.; Chen, R.; Bon, S.A.F. Control of vesicle membrane permeability with catalytic particles. Mater. Horiz. 2016, 3, 41–46. [Google Scholar] [CrossRef]
- Nishimura, T.; Hirose, S.; Sasaki, Y.; Akiyoshi, K. Substrate-Sorting Nanoreactors Based on Permeable Peptide Polymer Vesicles and Hybrid Liposomes with Synthetic Macromolecular Channels. J. Am. Chem. Soc. 2020, 142, 154–161. [Google Scholar] [CrossRef]
- Sun, H.; Wang, F.; Du, J. Preparation, application and perspective in polymer vesicles with an inhomogeneous membrane. Sci. Sin. Chim. 2019, 49, 877–890. [Google Scholar] [CrossRef][Green Version]
- Liu, D.; Sun, H.; Xiao, Y.; Chen, S.; Cornel, E.J.; Zhu, Y.; Du, J. Design principles, synthesis and biomedical applications of polymer vesicles with inhomogeneous membranes. J. Control. Release 2020, 326, 365–386. [Google Scholar] [CrossRef]
- Song, Z.; Han, Z.; Lv, S.; Chen, C.; Chen, L.; Yin, L.; Cheng, J. Synthetic polypeptides: From polymer design to supramolecular assembly and biomedical application. Chem. Soc. Rev. 2017, 46, 6570–6599. [Google Scholar] [CrossRef]
- Nishimura, T.; Shishi, S.; Sasaki, Y.; Akiyoshi, K. Thermoresponsive Polysaccharide Graft Polymer Vesicles with Tunable Size and Structural Memory. J. Am. Chem. Soc. 2020, 142, 11784–11790. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhou, J.; Duan, H. Self-Assembled Plasmonic Vesicles of SERS-Encoded Amphiphilic Gold Nanoparticles for Cancer Cell Targeting and Traceable Intracellular Drug Delivery. J. Am. Chem. Soc. 2012, 134, 13458–13469. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Li, J.; Mohammed, F.; Wang, Y.; Tou, K.; Liu, X.; Wen, P.; Kinoh, H.; Anraku, Y.; Chen, H.; et al. Therapeutic Polymersome Nanoreactors with Tumor-Specific Activable Cascade Reactions for Cooperative Cancer Therapy. ACS Nano 2019, 13, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Yu, K.; Lo, J.C.; Yan, M.; Yang, X.; Brooks, D.E.; Hancock, R.E.; Lange, D.; Kizhakkedathu, J.N. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 2017, 116, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Fan, L.; Liu, Q.; Wei, J.; Ren, T.; Du, J. Preparation of water-dispersible silver-decorated polymer vesicles and micelles with excellent antibacterial efficacy. Polym. Chem. 2012, 3, 2217–2227. [Google Scholar] [CrossRef]
- Sun, H.; Fan, L.; Zou, K.; Zhu, H.; Du, J. Decoration of homopolymer vesicles by antibacterial ultrafine silver nanoparticles. RSC Adv. 2014, 4, 41331–41335. [Google Scholar] [CrossRef]
- Liu, R.; Chen, X.; Falk, S.P.; Masters, K.S.; Weisblum, B.; Gellman, S.H. Nylon-3 Polymers Active against Drug-Resistant Candida albicans Biofilms. J. Am. Chem. Soc. 2015, 137, 2183–2186. [Google Scholar] [CrossRef]
- Lam, S.J.; O’Brien-Simpson, N.M.; Pantarat, N.; Sulistio, A.; Wong, E.H.; Chen, Y.Y.; Lenzo, J.C.; Holden, J.A.; Blencowe, A.; Reynolds, E.C.; et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016, 1, 16162. [Google Scholar] [CrossRef]
- Melo, M.N.; Ferre, R.; Castanho, M.A.R.B. Antimicrobial peptides: Linking partition, activity and high membrane-bound concentrations. Nat. Rev. Microbiol. 2009, 7, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W. Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect. Dis. 2005, 5, 209–218. [Google Scholar] [CrossRef]
- Lam, S.J.; Wong, E.H.H.; Boyer, C.; Qiao, G.G. Antimicrobial polymeric nanoparticles. Prog. Polym. Sci. 2018, 76, 40–64. [Google Scholar] [CrossRef]
- Mikhalevich, V.; Craciun, I.; Kyropoulou, M.; Palivan, C.G.; Meier, W. Amphiphilic Peptide Self-Assembly: Expansion to Hybrid Materials. Biomacromolecules 2017, 18, 3471–3480. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Shimada, N.; Maruyama, A. A Thermoresponsive Cationic Comb-Type Copolymer Enhances Membrane Disruption Activity of an Amphiphilic Peptide. Biomacromolecules 2018, 19, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, G.J.; Pool, J.G.; Som, A.; Dabkowski, J.M.; Coughlin, E.B.; Muthukurnar, M.; Tew, G.N. Interactions between Antimicrobial Polynorbornenes and Phospholipid Vesicles Monitored by Light Scattering and Microcalorimetry. Langmuir 2008, 24, 12489–12495. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; Araujo, P.M. Antimicrobial Polymer-Based Assemblies: A Review. Int. J. Mol. Sci. 2021, 22, 5424. [Google Scholar] [CrossRef]
- Zou, K.; Liu, Q.; Chen, J.; Du, J. Silver-decorated biodegradable polymer vesicles with excellent antibacterial efficacy. Polym. Chem. 2014, 5, 405–411. [Google Scholar] [CrossRef]
- Wayakanon, K.; Thornhill, M.H.; Douglas, C.W.I.; Lewis, A.L.; Warren, N.J.; Pinnock, A.; Armes, S.P.; Battaglia, G.; Murdoch, C. Polymersome-mediated intracellular delivery of antibiotics to treat Porphyromonas gingivalis-infected oral epithelial cells. FASEB J. 2013, 27, 4455–4465. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.D.; Su, F.Y.; Chiu, D.Y.; Srinivasan, S.; Wilson, J.T.; Ratner, D.M.; Stayton, P.S.; Convertine, A.J. Dynamic intracellular delivery of antibiotics via pH-responsive polymersomes. Polym. Chem. 2015, 6, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, Y.; Zhou, C.; Yuan, W.; Du, J. Antibacterial vesicles by direct dissolution of a block copolymer in water. Polym. Chem. 2013, 4, 255–259. [Google Scholar]
- Wang, M.; Zhou, C.; Chen, J.; Xiao, Y.; Du, J. Multifunctional Biocompatible and Biodegradable Folic Acid Conjugated Poly(ε-caprolactone)-Polypeptide Copolymer Vesicles with Excellent Antibacterial Activities. Bioconjugate Chem. 2015, 26, 725–734. [Google Scholar] [CrossRef]
- Gao, J.Y.; Wang, M.Z.; Wang, F.Y.K.; Du, J.Z. Synthesis and Mechanism Insight of a Peptide-Grafted Hyperbranched Polymer Nanosheet with Weak Positive Charges but Excellent Intrinsically Antibacterial Efficacy. Biomacromolecules 2016, 17, 2080–2086. [Google Scholar]
- Liu, Z.G.; Deshazer, H.; Rice, A.J.; Chen, K.; Zhou, C.H.; Kallenbach, N.R. Multivalent antimicrobial peptides from a reactive polymer scaffold. J. Med. Chem. 2006, 49, 3436–3439. [Google Scholar] [CrossRef]
- Deming, T.J. Polypeptide materials: New synthetic methods and applications. Adv. Mater. 1997, 9, 299–311. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Mohan, Y.M.; Bajpai, M.; Tankhiwale, R.; Thomas, V. Synthesis of polymer stabilized silver and gold nanostructures. J. Nanosci. Nanotechnol. 2007, 7, 2994–3010. [Google Scholar]
- Abdo, H.S.; Khalil, K.A.; Al-Deyab, S.S.; Altaleb, H.; Sherif, E.-S.M. Antibacterial effect of carbon nanofibers containing Ag nanoparticles. Fibers Polym. 2013, 14, 1985–1992. [Google Scholar]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Juby, K.A.; Dwivedi, C.; Kumar, M.; Kota, S.; Misra, H.S.; Bajaj, P.N. Silver nanoparticle-loaded PVA/gum acacia hydrogel: Synthesis, characterization and antibacterial study. Carbohydr. Polym. 2012, 89, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Klasen, H.J. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 2000, 26, 117–130. [Google Scholar] [CrossRef]
- Kong, H.; Jang, J. Antibacterial properties of novel poly(methyl methacrylate) nanofiber containing silver nanoparticles. Langmuir 2008, 24, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Hassan, S.E.-D.; Abdo, A.M.; El-Gamal, M.S. Antimicrobial, Antioxidant and Larvicidal Activities of Spherical Silver Nanoparticles Synthesized by Endophytic Streptomyces spp. Biol. Trace Elem. Res. 2020, 195, 707–724. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Duran, N.; Diez, M.C.; Martinez, M.; Parada, J.; Seabra, A.B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Mostafavi, E.; Afifi, A.M.; Izadiyan, Z.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Wound dressings functionalized with silver nanoparticles: Promises and pitfalls. Nanoscale 2020, 12, 2268–2291. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Chimisso, V.; Maffeis, V.; Huerlimann, D.; Palivan, C.G.; Meier, W. Self-Assembled Polymeric Membranes and Nanoassemblies on Surfaces: Preparation, Characterization, and Current Applications. Macromol. Biosci. 2020, 20, 1900257. [Google Scholar] [CrossRef]
- Zhen, J.-B.; Kang, P.-W.; Zhao, M.-H.; Yang, K.-W. Silver Nanoparticle Conjugated Star PCL-b-AMPs Copolymer as Nanocomposite Exhibits Efficient Antibacterial Properties. Bioconjugate Chem. 2020, 31, 51–63. [Google Scholar] [CrossRef]
- Bassous, N.J.; Webster, T.J. The Binary Effect on Methicillin-Resistant Staphylococcus aureus of Polymeric Nanovesicles Appended by Proline-Rich Amino Acid Sequences and Inorganic Nanoparticles. Small 2019, 15, 1804247. [Google Scholar] [CrossRef]
- Dann, A.B.; Hontela, A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2011, 31, 285–311. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E., Jr.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Nederberg, F.; Zhang, Y.; Tan, J.P.K.; Xu, K.; Wang, H.; Yang, C.; Gao, S.; Guo, X.D.; Fukushima, K.; Li, L.; et al. Biodegradable nanostructures with selective lysis of microbial membranes. Nat. Chem. 2011, 3, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, G.; Wang, X.; Hu, J.; Liu, S. Enzyme-Responsive Polymeric Vesicles for Bacterial-Strain-Selective Delivery of Antimicrobial Agents. Angew. Chem. Int. Ed. 2016, 55, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.P.D.S.; Arcisio-Mirandaa, M.; Costa, S.T.B.; Konno, K.; Ruggiero, J.R.; Procopio, J.; Neto, J.R. Study of the mechanism of action of anoplin, a helical antimicrobial decapeptide with ion channel-like activity, and the role of the amidated C-terminus. J. Pept. Sci. 2008, 14, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Blin, T.; Purohit, V.; Leprince, J.; Jouenne, T.; Glinel, K. Bactericidal Microparticles Decorated by an Antimicrobial Peptide for the Easy Disinfection of Sensitive Aqueous Solutions. Biomacromolecules 2011, 12, 1259–1264. [Google Scholar] [CrossRef]
- Shih, Y.-H.; Chang, K.-W.; Hsia, S.-M.; Yu, C.-C.; Fuh, L.-J.; Chi, T.-Y.; Shieh, T.-M. In vitro antimicrobial and anticancer potential of hinokitiol against oral pathogens and oral cancer cell lines. Microbiol. Res. 2013, 168, 254–262. [Google Scholar] [CrossRef]
- Siriwardena, T.N.; Stach, M.; He, R.; Gan, B.-H.; Javor, S.; Heitz, M.; Ma, L.; Cai, X.; Chen, P.; Wei, D.; et al. Lipidated Peptide Dendrimers Killing Multidrug-Resistant Bacteria. J. Am. Chem. Soc. 2018, 140, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Quarta, N.; Zhang, H.; Lu, Z.; Hof, M.; Sachl, R.; Liu, R.; Hoernke, M. Hidden complexity in membrane permeabilization behavior of antimicrobial polycations. Phys. Chem. Chem. Phys. 2021, 23, 1475–1488. [Google Scholar] [CrossRef]
- Lienkamp, K.; Kumar, K.-N.; Som, A.; Nuesslein, K.; Tew, G.N. “Doubly Selective” Antimicrobial Polymers: How Do They Differentiate between Bacteria? Chem.-Eur. J. 2009, 15, 11710–11714. [Google Scholar] [CrossRef] [PubMed]
- Siano, A.; Humpola, M.V.; Rey, M.C.; Simonetta, A.; Tonarelli, G.G. Interaction of Acylated and Substituted Antimicrobial Peptide Analogs with Phospholipid-Polydiacetylene Vesicles. Correlation with their Biological Properties. Chem. Biol. Drug Des. 2011, 78, 85–93. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Zueva, I.V.; Petrov, K.A.; Lukashenko, S.S.; Nizameev, I.R.; Kulik, N.V.; Voloshina, A.D.; Almasy, L.; Kadirov, M.K.; Masson, P.; et al. Mixed cationic liposomes for brain delivery of drugs by the intranasal route: The acetylcholinesterase reactivator 2-PAM as encapsulated drug model. Colloids Surf. B 2018, 171, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Barman, R.; Mondal, T.; Sarkar, J.; Sikder, A.; Ghosh, S. Self-Assembled Polyurethane Capsules with Selective Antimicrobial Activity against Gram-Negative E. coli. ACS Biomater. Sci. Eng. 2020, 6, 654–663. [Google Scholar] [CrossRef]
- Blackman, L.D.; Oo, Z.Y.; Qu, Y.; Gunatillake, P.A.; Cass, P.; Locock, K.E.S. Antimicrobial Honey-Inspired Glucose-Responsive Nanoreactors by Polymerization-Induced Self-Assembly. ACS Appl. Mater. Interfaces 2020, 12, 11353–11362. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Kanaoka, S.; Sato, T.; Aoshima, S.; Kuroda, K. Block versus Random Amphiphilic Copolymers as Antibacterial Agents. Biomacromolecules 2011, 12, 3581–3591. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.Z.; Wei, J.R.; Lu, H.; Fan, L.; Du, J.Z. Water-dispersible and biodegradable polymer micelles with good antibacterial efficacy. Chem. Commun. 2012, 48, 6857–6859. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, J.; Ran, R.; Liu, Y.; Zhang, Z.; Gao, H.; He, Q. Development of an anti-microbial peptide-mediated liposomal delivery system: A novel approach towards pH-responsive anti-microbial peptides. Drug Deliv. 2016, 23, 1163–1170. [Google Scholar] [CrossRef]
- Shi, Z.L.; Neoh, K.G.; Kang, E.T.; Wang, W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials 2006, 27, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Jui, M.S.; Bam, M.; Cha, Y.; Luat, E.; Alabresm, A.; Nagarkatti, M.; Decho, A.; Tang, C. Facial Amphiphilicity-Induced Polymer Nanostructures for Antimicrobial Applications. ACS Appl. Mater. Interfaces 2020, 12, 21221–21230. [Google Scholar] [CrossRef]
- Zhu, H.; Geng, Q.; Chen, W.; Zhu, Y.; Chen, J.; Du, J. Antibacterial high-genus polymer vesicle as an “armed” drug carrier. J. Mater. Chem. B 2013, 1, 5496–5504. [Google Scholar] [CrossRef]
- Wyrsta, M.D.; Cogen, A.L.; Deming, T.J. A parallel synthetic approach for the analysis of membrane interactive copolypeptides. J. Am. Chem. Soc. 2001, 123, 12919–12920. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Su, X.; Tan, Z.; Zhou, C. Synthesis of triblock amphiphilic copolypeptides with excellent antibacterial activity. Eur. Polym. J. 2018, 106, 175–181. [Google Scholar] [CrossRef]
- Sun, V.Z.; Li, Z.; Deming, T.J.; Kamei, D.T. Intracellular Fates of Cell-Penetrating Block Copolypeptide Vesicles. Biomacromolecules 2011, 12, 10–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, H.; Hong, Y.; Xi, Y.; Zou, Y.; Gao, J.; Du, J. Synthesis, Self-Assembly, and Biomedical Applications of Antimicrobial Peptide-Polymer Conjugates. Biomacromolecules 2018, 19, 1701–1720. [Google Scholar] [CrossRef]
- Zhou, C.C.; Wang, M.Z.; Zou, K.D.; Chen, J.; Zhu, Y.Q.; Du, J.Z. Antibacterial Polypeptide-Grafted Chitosan-Based Nanocapsules As an “Armed” Carrier of Anticancer and Antiepileptic Drugs. ACS Macro Lett. 2013, 2, 1021–1025. [Google Scholar] [CrossRef]
- Xi, Y.J.; Song, T.; Tang, S.; Wang, N.; Du, J.Z. Preparation and Antibacterial Mechanism Insight of Polypeptide-Based Micelles with Excellent Antibacterial Activities. Biomacromolecules 2016, 17, 3922–3930. [Google Scholar] [CrossRef]
- Hong, Y.; Xi, Y.; Zhang, J.; Wang, D.; Zhang, H.; Yan, N.; He, S.; Du, J. Polymersome-hydrogel composites with combined quick and long-term antibacterial activities. J. Mater. Chem. B 2018, 6, 6311–6321. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Chen, L.-S.; Sun, M.; Wang, C.-Y.; Fan, Z.; Du, J.-Z. Biodegradable Polypeptide-based Vesicles with Intrinsic Blue Fluorescence for Antibacterial Visualization. Chin. J. Polym. Sci. 2021. [Google Scholar] [CrossRef]
- Yang, G.; Chen, S.; Zhang, J. Bioinspired and Biomimetic Nanotherapies for the Treatment of Infectious Diseases. Front. Pharmacol. 2019, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Wang, Y.; Gao, J.; Xiao, Y.; Du, J. Dual Corona Vesicles with Intrinsic Antibacterial and Enhanced Antibiotic Delivery Capabilities for Effective Treatment of Biofilm-Induced Periodontitis. ACS Nano 2019, 13, 13645–13657. [Google Scholar] [CrossRef]
- Stulz, A.; Vogt, A.; Saar, J.S.; Akil, L.; Lienkamp, K.; Hoernke, M. Quantified Membrane Permeabilization Indicates the Lipid Selectivity of Membrane-Active Antimicrobials. Langmuir 2019, 35, 16366–16376. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Barman, R.; Ghosh, S. Tunable nanostructures by directional assembly of donor-acceptor supramolecular copolymers and antibacterial activity. J. Mater. Chem. B 2020, 8, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.; Najer, A.; Kloeckner, A.; Zhang, S.; Holme, M.N.; Nele, V.; Che, J.; Massi, L.; Penders, J.; Saunders, C.; et al. Controlled Dendrimersome Nanoreactor System for Localized Hypochlorite-Induced Killing of Bacteria. ACS Nano 2020, 14, 17333–17353. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, J.; Jeong, S.; Im, B.N.; Kim, M.-K.; Yang, S.-G.; Na, K. Lipase-Sensitive Transfersomes Based on Photosensitizer/Polymerizable Lipid Conjugate for Selective Antimicrobial Photodynamic Therapy of Acne. Adv. Healthcare Mater. 2016, 5, 3139–3147. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chu, G.; Qi, M.; Liu, Y.; Huang, P.; Pan, H.; Wang, Y.; Chen, Y.; Zhou, Y. Porphyrin Alternating Copolymer Vesicles for Photothermal Drug-Resistant Bacterial Ablation and Wound Disinfection. ACS Appl. Bio. Mater. 2020, 3, 9117–9125. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, Q.; Li, S.; Yan, Z.; Li, J.; Li, Y.; Mou, L.; Zhang, B.; Yang, W.; Miao, X.; et al. Novel antimicrobial peptide CPF-C1 analogs with superior stabilities and activities against multidrug-resistant bacteria. Chem. Biol. Drug Des. 2017, 90, 690–702. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; He, J.; Zhou, C. Polycaprolactone-Based Mimetic Antimicrobial Peptide Copolymers Vesicles as an Effective Drug-Carrier for Cancer Therapy. Polymers 2019, 11, 1783. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Chua, R.R.; Bow, H.; Tambyah, P.A.; Hadinoto, K.; Leong, S.S. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015, 15, 127–138. [Google Scholar] [CrossRef]
- Geilich, B.M.; Gelfat, I.; Sridhar, S.; van de Ven, A.L.; Webster, T.J. Superparamagnetic iron oxide-encapsulating polymersome nanocarriers for biofilm eradication. Biomaterials 2017, 119, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cornel, E.J.; Du, J. Advances and Prospects of Polymeric Particles for the Treatment of Bacterial Biofilms. ACS Appl. Polym. Mater. 2021, 3, 2218–2232. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yuan, Y.; Zhou, P.; Wang, F.; Hong, Y.; Wang, N.; Xu, S.; Du, J. Highly Effective Antibacterial Vesicles Based on Peptide-Mimetic Alternating Copolymers for Bone. Biomacromolecules 2017, 18, 4154–4162. [Google Scholar] [CrossRef] [PubMed]
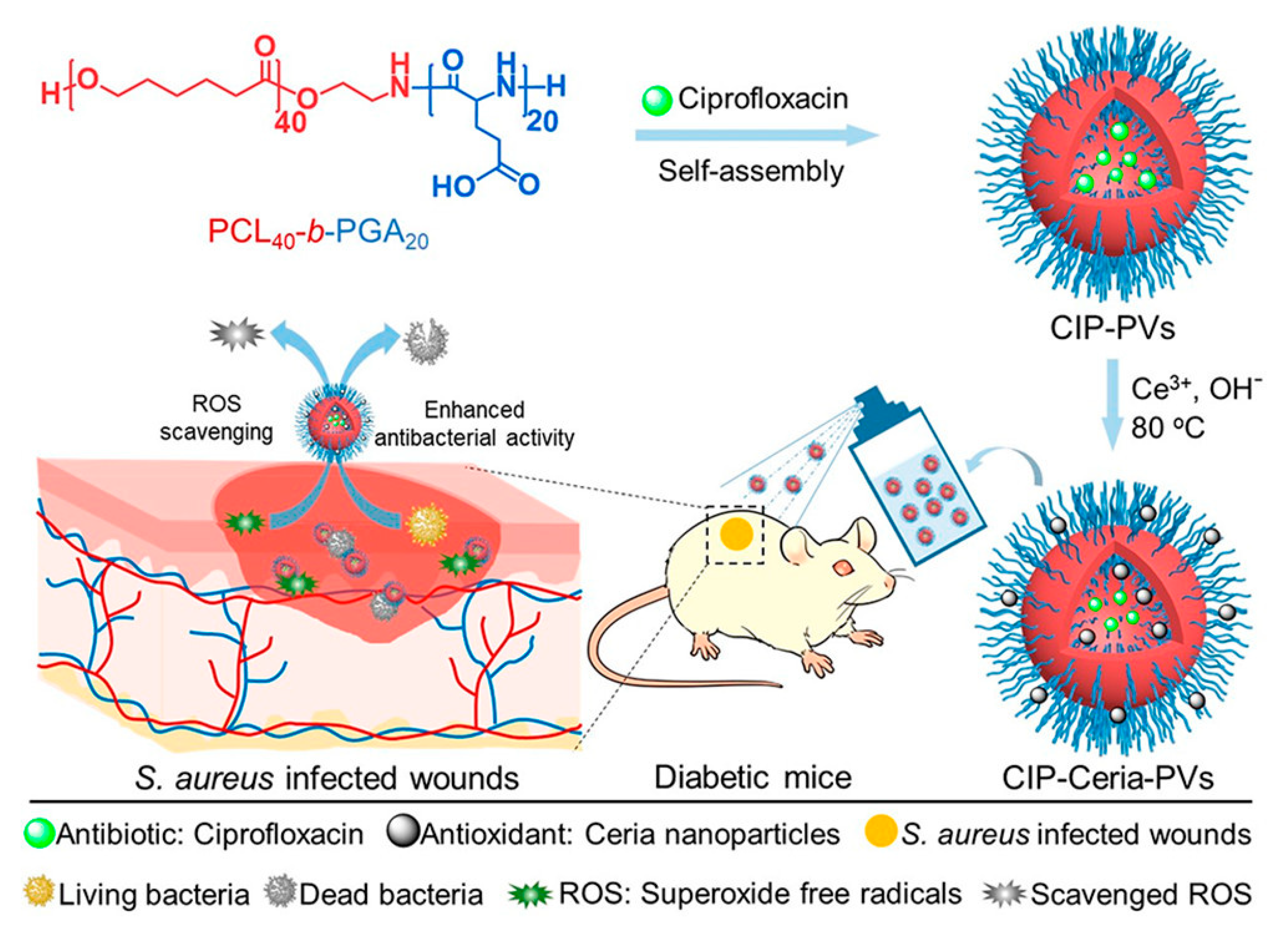
- Wang, T.; Li, Y.; Cornel, E.J.; Li, C.; Du, J. Combined Antioxidant-Antibiotic Treatment for Effectively Healing Infected Diabetic Wounds Based on Polymer Vesicles. ACS Nano 2021, 15, 9027–9038. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Wang, Y.; Song, J. Polymer Vesicles for Antimicrobial Applications. Polymers 2021, 13, 2903. https://doi.org/10.3390/polym13172903
Sun H, Wang Y, Song J. Polymer Vesicles for Antimicrobial Applications. Polymers. 2021; 13(17):2903. https://doi.org/10.3390/polym13172903
Chicago/Turabian StyleSun, Hui, Yin Wang, and Jiahui Song. 2021. "Polymer Vesicles for Antimicrobial Applications" Polymers 13, no. 17: 2903. https://doi.org/10.3390/polym13172903
APA StyleSun, H., Wang, Y., & Song, J. (2021). Polymer Vesicles for Antimicrobial Applications. Polymers, 13(17), 2903. https://doi.org/10.3390/polym13172903