Physical, Chemical, and Electrochemical Properties of Redox-Responsive Polybenzopyrrole as Electrode Material for Faradaic Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis Procedure of Polybenzopyrrole (Pbp)
2.3. Analysis and Characterization
2.4. Electrochemical Characterization
3. Results and Discussion
3.1. Optimisation of the Synthesis Conditions
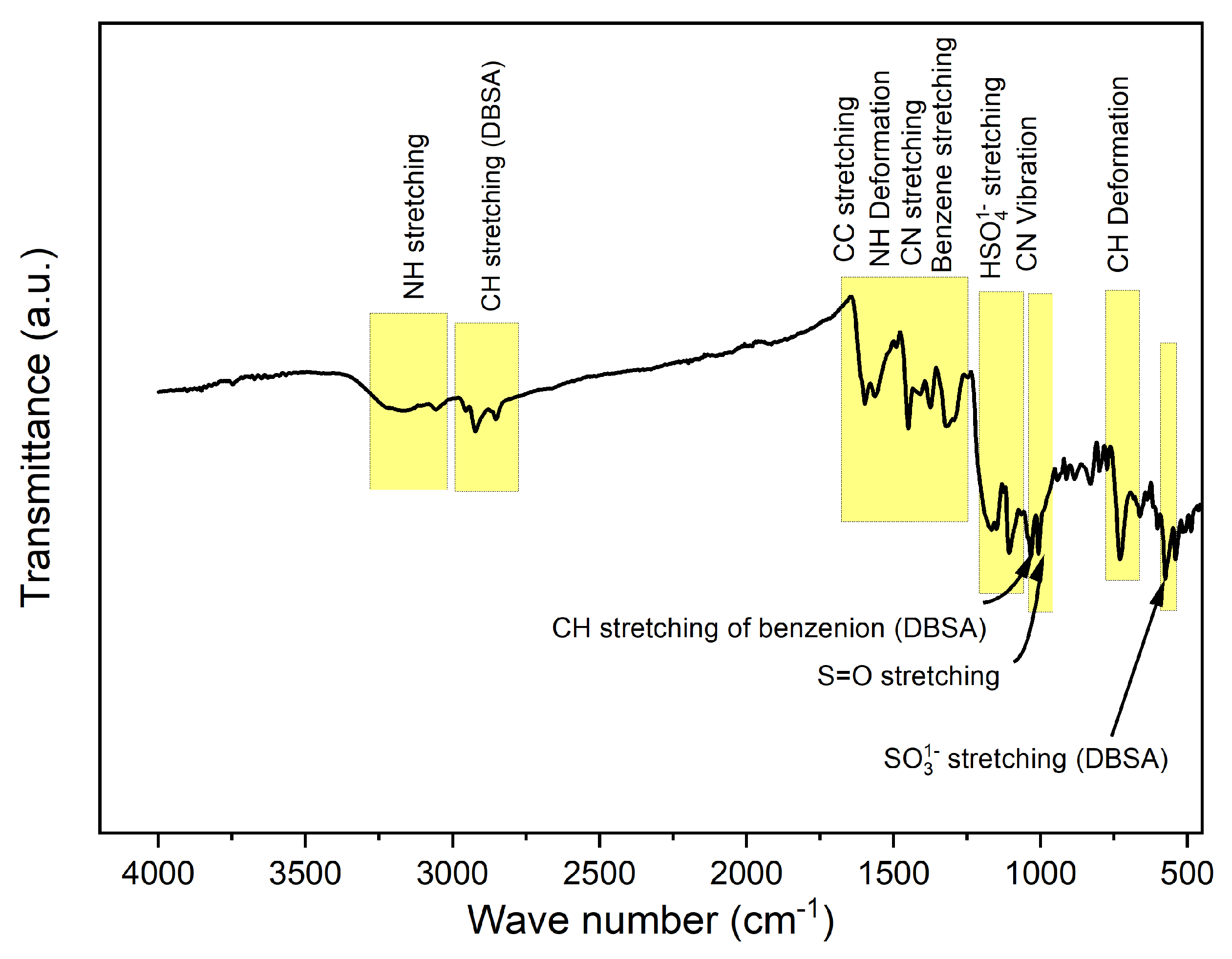
3.2. FTIR Spectroscopy
3.3. Morphological Analysis
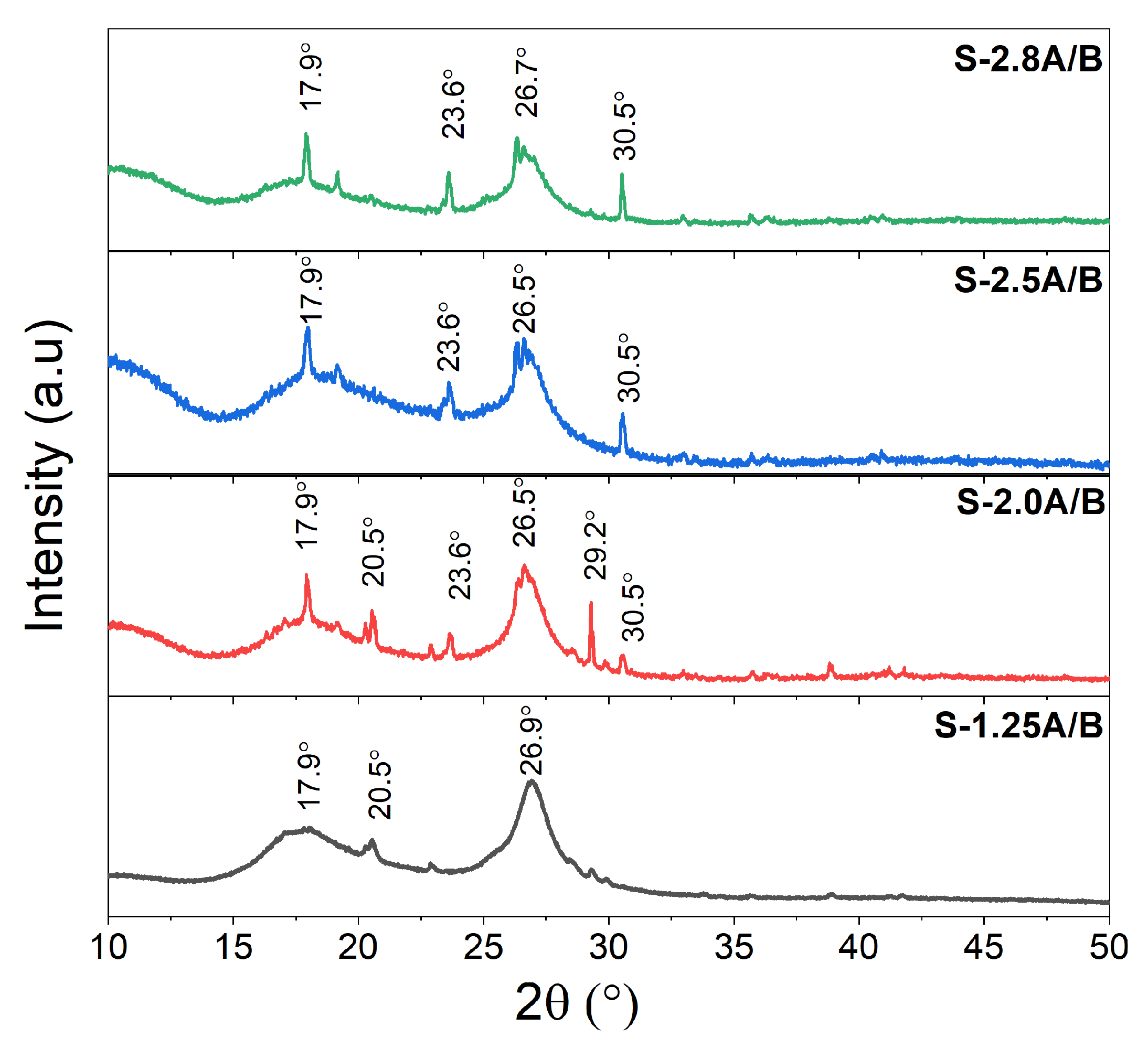
3.4. XRD Analysis
3.5. Photochemical Properties of the Polybenzopyrrols
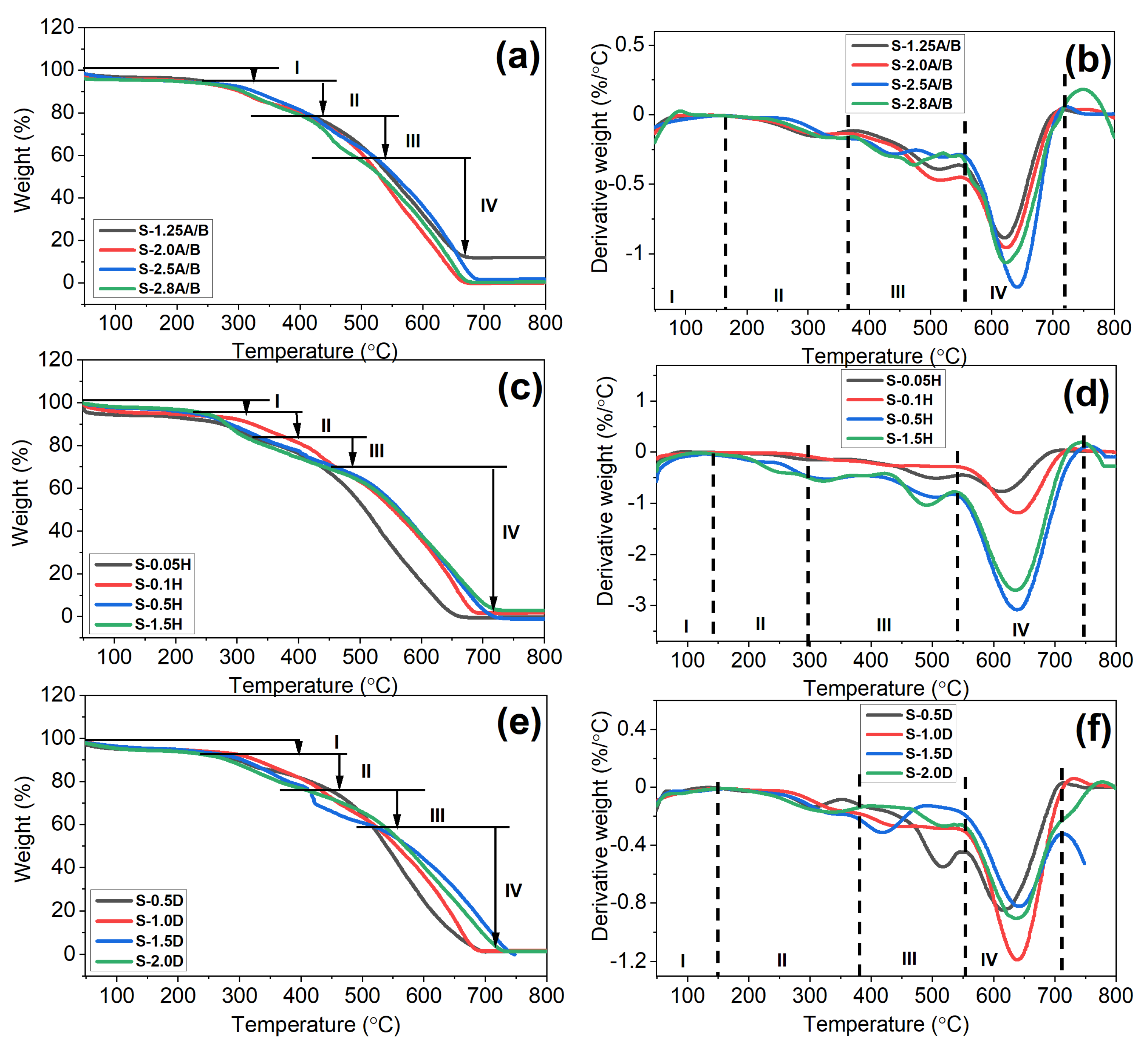
3.6. Thermogravimetric Analysis
3.7. Three-Electrode Cell Electrochemical Performance
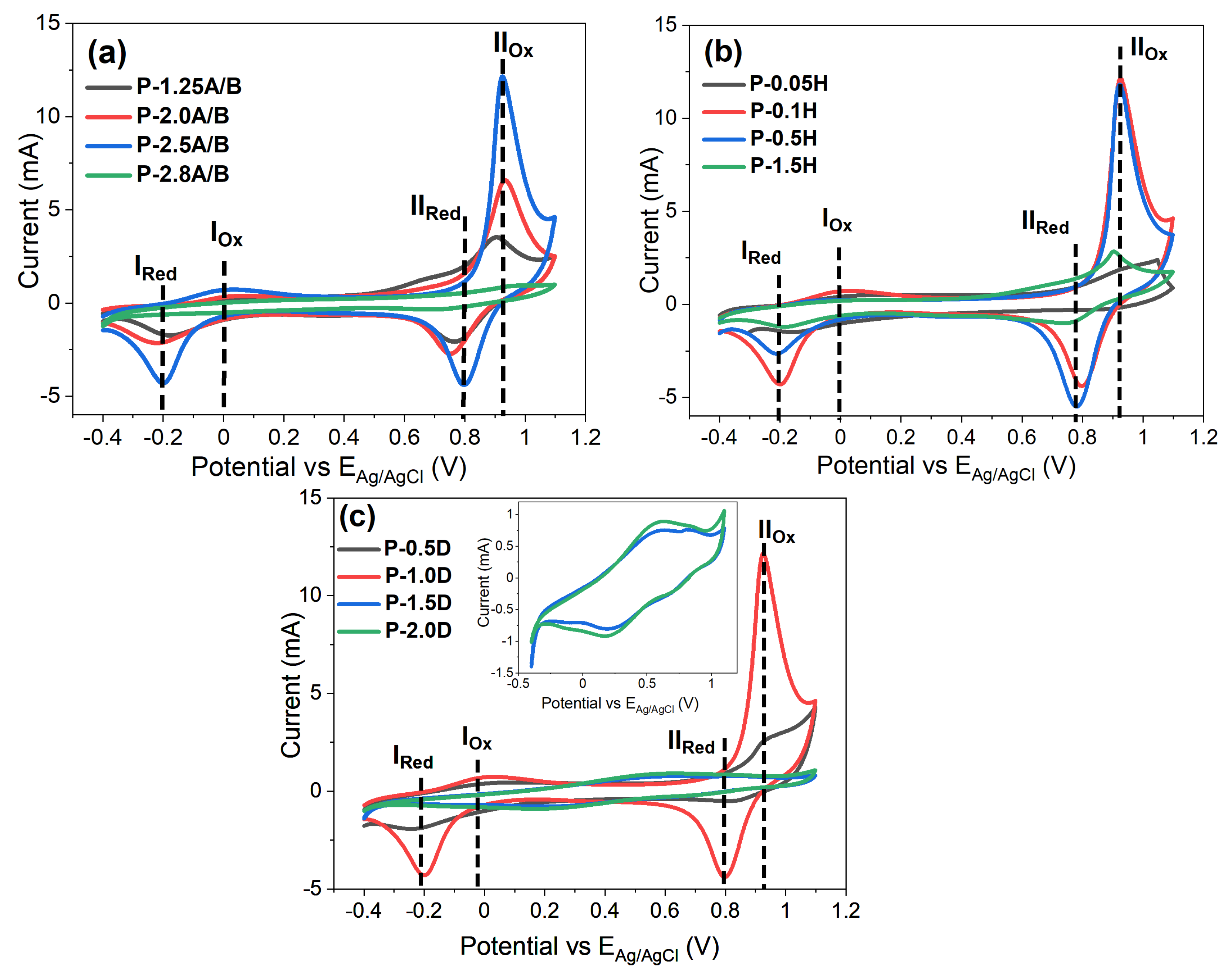
3.7.1. Cyclic Voltammetry
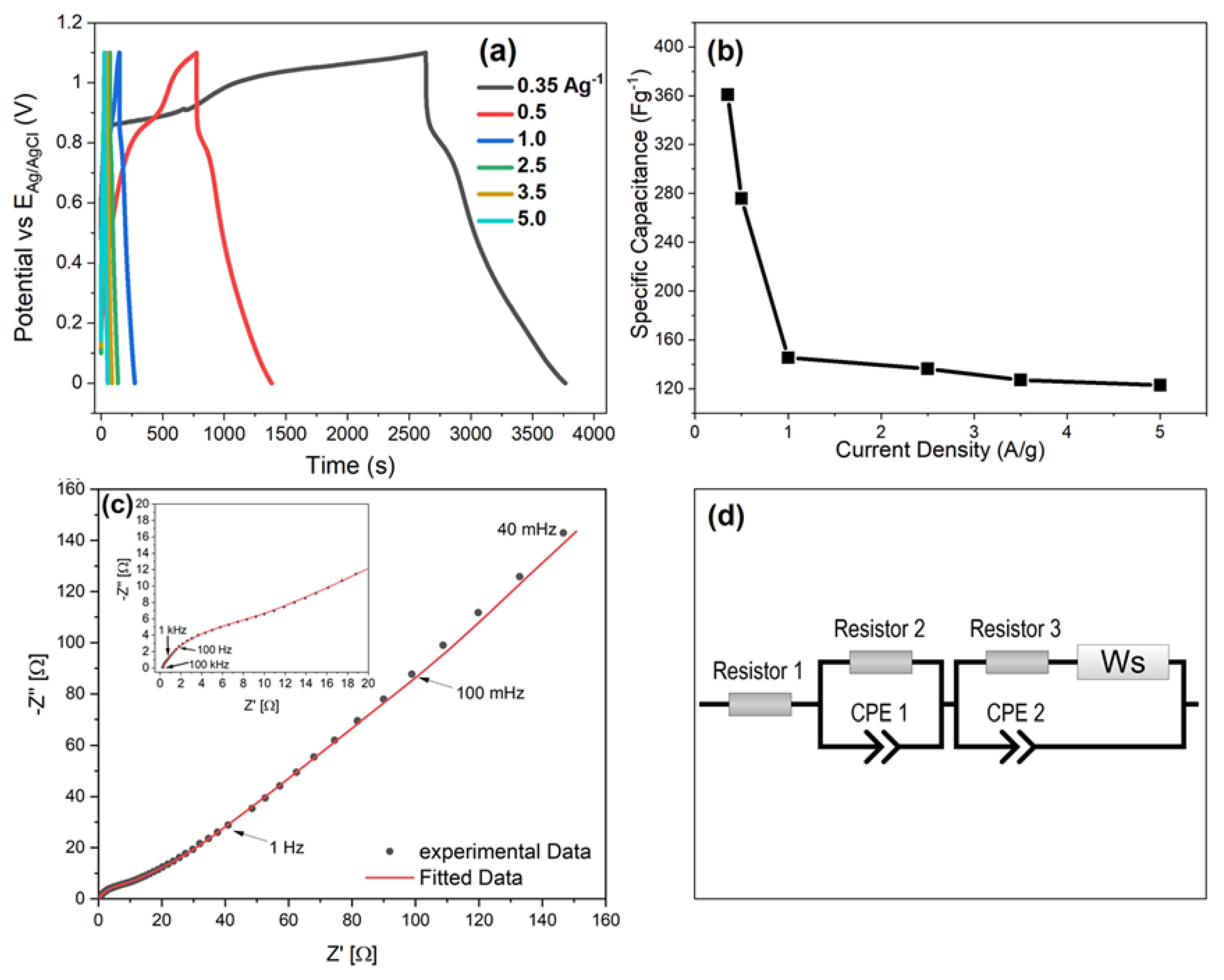
3.7.2. Galvanostatic Charge-Discharge
3.7.3. Electrochemical Impedance Spectroscopy
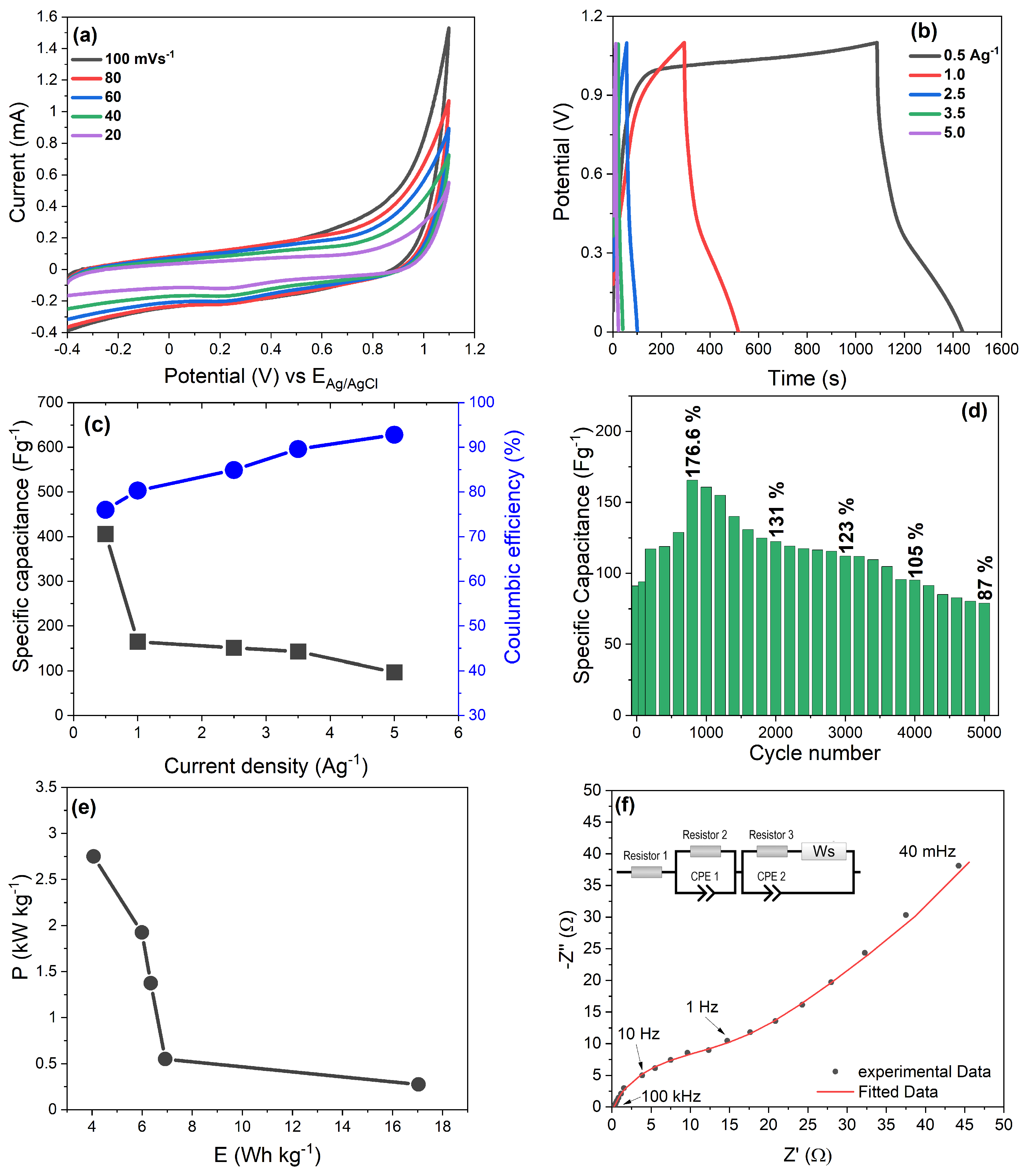
3.8. Two-Electrode Cell Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Kim, J.H.; Ariga, K. Redox-Active Polymers for Energy Storage Nanoarchitectonics. Joule 2017, 1, 739–768. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Chen, Z.; Gong, Y.; Zhang, R.; Lui, H.; Tang, P.; Chen, X.; Passerini, S.; Lui, J. The role of cation vacancies in electrode materials for enhanced electrochemical energy storage: Synthesis, advanced characterization, and fundamentals. Adv. Energy Mater. 2020, 10, 1903780–1903804. [Google Scholar] [CrossRef]
- Santino, L.M.; Lu, Y.; Acharya, S.; Bloom, L.; Cotton, D.; Wayne, A.; D’Arcy, J.M. Enhancing Cycling Stability of Aqueous Polyaniline Electrochemical Capacitors. J. Appl. Mater. Interfaces 2016, 8, 29452–29460. [Google Scholar] [CrossRef]
- Zhao, C.; Jia, X.; Shu, K.; Yu, C.; Wallace, G.G.; Wang, C. Conducting polymer composites for unconventional solid-state supercapacitors. J. Mater. Chem. A 2020, 8, 4677–4699. [Google Scholar] [CrossRef]
- Chakraborty, I.K.; Chakrabarty, N.; Senapati, A.; Chakraborty, A.K. CuO@NiO/Polyaniline/MWCNT Nanocomposite as High Performance Electrode for Supercapacitor. J. Phys. Chem. C 2018, 122, 27180–27190. [Google Scholar] [CrossRef]
- Rashti, A.; Wang, B.; Hassani, E.; Feyzbar-Khalkhali-Nejad, F.; Zhang, X.; Oh, T.-S. Electrophoretic deposition of nickel cobaltite/polyaniline/rGO composite electrode for high performance all-solid-state asymmetric supercapacitors. Energy Fuels 2020, 5, 6448–6461. [Google Scholar] [CrossRef]
- Mudila, H.; Prasher, P.; Kumar, M.; Kumar, A.; Zaidi, M.G.H.; Kumar, A. Critical analysis of polyindole and its composites in supercapacitor application. Mat. Renew. Sustain. Energy 2019, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Torvi, A.I.; Munavalli, B.B.; Naik, S.R.; Kariduraganavar, M.Y. Scalable fabrication of a flexible interdigital micro-supercapacitor device by in-situ polymerization of pyrrole into hybrid PVA-TEOS membrane. Electrochim. Acta 2018, 282, 469–479. [Google Scholar] [CrossRef]
- Shoeb, M.; Mobin, M.; Rauf, M.A.; Owais, M.; Naqvi, A.H. In vitro and in vivo antimicrobial evaluation of graphene−polyindole (gr@pin) nanocomposite against methicillin-resistant staphylococcus aureus pathogen. ACS Omega 2018, 3, 9431–9440. [Google Scholar] [CrossRef] [Green Version]
- Tebyetekerwaa, M.; Yanga, S.; Pengb, S.; Xua, Z.; Shaoa, W.; Pana, D.; Ramakrishnab, S.; Zhu, M. Unveiling Polyindole: Freestanding As-electrospun Polyindole Nanofibers and Polyindole/Carbon Nanotubes Composites as Enhanced Electrodes for Flexible All-solid-state Supercapacitors. Electrochim. Acta 2017, 247, 400–409. [Google Scholar] [CrossRef]
- Goel, S.; Mazumdar, N.A.; Gupta, A. Growth of one-dimensional polyindole nanostructures. J. Nanosci. Nanotechnol. 2011, 11, 10164–10172. [Google Scholar] [CrossRef] [PubMed]
- Mudila, H.; Rana, S.; Zaidi, M.G.H.; Alam, S. Polyindole/Graphene Oxide Nanocomposites: The Novel Material for Electrochemical Energy Storage. Fuller. Nanotub. Carbon Nanostruct. 2014, 23, 20–26. [Google Scholar] [CrossRef]
- Cai, Z.-J.; Zhang, Q.; Song, X.-Y. Improved Electrochemical Performance of Polyindole/Carbon Nanotubes Composite as Electrode Material for Supercapacitors. Electron. Mater. Lett. 2016, 12, 830–840. [Google Scholar] [CrossRef]
- Jayakrishnan, P.; Ramesan, M.T. Synthesis, characterization, electrical conductivity and material properties of magnetite/polyindole/poly(vinyl alcohol) blend nanocomposite. J. Inorg. Organomet. Polym. 2017, 27, 323–333. [Google Scholar] [CrossRef]
- Phasuksom, K.; Sirivat, A. Synthesis of nano-sized polyindole via emulsion polymerization and doping. Synth. Met. 2016, 219, 142–153. [Google Scholar] [CrossRef]
- Phasuksom, K.; Prissanaroon-Ouajai, W.; Sirivat, A. Electrical conductivity response of methanol sensor based on conductive polyindole. Sens. Actuators B 2018, 262, 1013–1023. [Google Scholar] [CrossRef]
- Shah, A.H.A.; Kamran, M.; Bilal, S.; Ullah, R. Cost Effective Chemical Oxidative Synthesis of Soluble and Electroactive Polyaniline Salt and Its Application as Anticorrosive Agent for Steel. Materials 2019, 12, 1527. [Google Scholar] [CrossRef] [Green Version]
- Rajesh, M.; Manikandan, R.; Kim, B.C.; Becuwe, M.; Yu, K.H.; Raj, C.J. Electrochemical polymerization of chloride doped PEDOT hierarchical porous nanostructure on graphite as a potential electrode for high performance supercapacitor. Electrochim. Acta 2020, 354, 136669. [Google Scholar] [CrossRef]
- Waikar, M.R.; Rasal, A.S.; Shinde, N.S.; Dhas, S.D.; Moholkar, A.V.; Shirsat, M.D.; Chakarvarti, S.K.; Sonkawade, R.G. Electrochemical performance of Polyaniline based symmetrical energy storage device. Mat. Sci. Semicond. Proc. 2020, 120, 105291. [Google Scholar] [CrossRef]
- Mucuk, Z.; Karakışla, M.; Saçak, M. Synthesis of poly (o-toluidine) in DMF/water mixture using benzoyl peroxide. Int. J. Polym. Anal. Charact. 2009, 14, 403–417. [Google Scholar] [CrossRef]
- Bilal, S.; Gul, S.; Holze, R.; Shah, A.H.A. An impressive emulsion polymerization route for the synthesis of highly soluble and conducting polyaniline salts. Syn. Met. 2015, 206, 131–144. [Google Scholar] [CrossRef]
- Han, D.; Chu, Y.; Yang, L.; Liu, Y.; Lv, Z. Reversed micelle polymerization: A new route for the synthesis of DBSA-polyaniline nanoparticles. Coll. Surf. A Physicochem. Eng. Asp. 2005, 259, 175–187. [Google Scholar] [CrossRef]
- Shakeel, I.N.; Ahamed, M.I.; Kanchi, S.; Kashmery, H.A. Green synthesis of ZnO nanoparticles decorated on polyindole functionalized-MCNTs and used as anode material for enzymatic biofuel cell applications. Sci. Rep. 2020, 10, 5052–5062. [Google Scholar]
- Verma, C.J.; Keshari, A.S.; Dubey, P.; Prakash, R. Polyindole modifed g-C3N4 nanohybrids via in-situ chemical polymerization for its improved electrochemical performance. Vacuum 2020, 177, 109363. [Google Scholar] [CrossRef]
- Oh, M.; Kim, S. Effect of dodecyl benzene sulfonic acid on the preparation of polyaniline/activated carbon composites by in situ emulsion polymerization. Electrochim. Acta 2012, 59, 196–201. [Google Scholar]
- Qui, B.; Wang, J.; Li, Z.; Wang, X.; Li, X. Influence of acidity and oxidant concentration in the nanostructures and electrochemical performance of polyaniline during fast microwave-assisted chemical polymerization. Polymers 2020, 12, 310–324. [Google Scholar]
- Song, H.; Li, T.; Han, Y.; Wang, R.; Zhang, C.; Wang, Q. Optimizing the polymerization conditions of conductive polypyrrole. J. Photopolym. Sci. Technol. 2016, 29, 803–808. [Google Scholar] [CrossRef] [Green Version]
- Han, M.G.; Cho, S.K.; Oh, S.G.; In, S.S. Preparation and characterization of polyaniline nanoparticles synthesized from DBSA micellar solution. Synth. Met. 2002, 126, 53–60. [Google Scholar] [CrossRef]
- Gahlout, P.; Choudhary, V. Tailoring of polypyrrole backbone by optimizing synthesis parameters for efficient EMI shielding properties in X-band (8.2-12.4 GHz). Synth. Met. 2016, 222, 170–179. [Google Scholar] [CrossRef]
- Djara, R.; Holade, Y.; Merzouki, A.; Masquelez, N.; Cot, D.; Rebiere, B.; Petit, E.; Huguet, P.; Canaff, C.; Morisset, S.; et al. Insights from the physico-chemical and electrochemical screening of the potentiality of the chemically synthesized polyaniline. J. Electrochem. Soc. 2020, 167, 066503. [Google Scholar] [CrossRef]
- Ram, M.S.; Palaniappan, S. A process for the preparation of polyaniline salt doped with acid and surfactant groups using benzoyl peroxide. J. Mater. Sci. 2004, 39, 3069–3077. [Google Scholar] [CrossRef]
- Majumder, M.; Choudhary, R.B.; Koiry, S.P.; Thakur, A.K.; Kumar, U. Gravimetric and volumetric capacitive performance of polyindole/carbon black/MoS2 hybrid electrode material for supercapacitor applications. Electrochim. Acta 2017, 248, 98–111. [Google Scholar] [CrossRef]
- Chhattise, P.; Handore, K.; Horne, A.; Mohite, K.; Chaskar, A.; Dallavalled, S.; Chabukswar, V. Synthesis and characterization of Polyindole and its catalytic performance study as a heterogeneous catalyst. J. Chem. Sci. 2016, 128, 467–475. [Google Scholar] [CrossRef]
- Olvera-Gracia, M.; Aguilar-Hernandez, J.R. Conductivity and crystallinity of polyethylene oxide/polyaniline microfibers obtained by electrospinning. J. Appl. Res. Technol. 2014, 12, 598–601. [Google Scholar] [CrossRef] [Green Version]
- Bhagat, D.J.; Dhokane, G.R. Novel synthesis and DC electrical studies of polyindole/poly(vinyl acetate) composite films, Chem. Phys. Lett. 2015, 619, 27–31. [Google Scholar]
- Tebyetekerwa, M.; Wang, X.; Marriam, I.; Dan, P.; Yang, S.; Zhu, M. Green approach to fabricate polyindole composite nanofibers for energy and sensor applications. Mat. Lett. 2017, 209, 400–403. [Google Scholar] [CrossRef]
- Hassanien, R.; Al-Hinai, M.; Al-Said, S.A.F.; Little, R.; Siller, L.; Wright, N.G.; Houltom, A.; Horrocks, B.R. Preparation and characterization of conductive and photoluminescent DNA-templetaed polyindole nanowires. ACS Nano 2010, 4, 2149–2159. [Google Scholar] [CrossRef]
- Bhagat, D.J.; Dhokane, G.R. Novel photoluminescence and optical investigation of poly(vinylacetate)/polyindole composites synthesized via chromium chloride as oxidant. Appl. Surf. Sci. 2015, 351, 1140–1145. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, X.; Qian, W.; Li, Y.; Li, X.; Yu, D.-G. Bulk synthesis, optimization, and characterization of highly dispersible polypyrrole nanoparticles toward protein separation using nanocomposite membranes. J. Coll. Interface Sci. 2012, 386, 148–157. [Google Scholar] [CrossRef]
- Han, Y.-G.; Kusunose, T.; Sekino, T. One-step reverse micelle polymerization of organic dispersible polyaniline nanoparticles. Synth. Met. 2009, 159, 123–131. [Google Scholar] [CrossRef]
- Unal, H.I.; Sahan, B.; Erol, O. Investigation of electrokinetic and electrorheological properties of polyindole prepared in the presence of a surfactant. Mat. Chem. Phys. 2012, 134, 382–391. [Google Scholar] [CrossRef]
- Bilal, S.; Begum, B.; Gul, S.; Shah, A.H.A. PANI/DBSA/H2SO4; A promising and highly efficient electrode material for aqueous supercapacitors. Synth. Met. 2018, 235, 1–15. [Google Scholar] [CrossRef]
- Xu, G.; Wang, E.; Qu, X.; Yin, Y.; Chu, L.; He, B.; Wu, H.; Fang, J.; Bao, Y.; Liang, L. Electrochemical properties of polyaniline in p-toluene sulfonic acid solution. Eur. Polym. J. 2009, 45, 2701–2707. [Google Scholar] [CrossRef]
- Syduly, B.; Palaniappan, S.S.; Sirnivas, P. Nano fibre polyaniline containing long chain and a small molecule dopants and carbon composites for supercapacitors. Electrochim. Acta 2013, 95, 251–259. [Google Scholar]
- Fathi, M.; Shaghafi, M.; Mahboubi, F.; Mohajerzadeh, S. Synthesis and electrochemical investigation of polyaniline/unzipped carbon nanotube composites as electrode material in supercapacitors. Synth. Met. 2014, 198, 345–356. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q.; Liang, A.; Zhou, W.; Chang, Y.; Li, D.; Wu, J.; Ye, G.; Xu, J.; Ren, Y. Two-step preparation of carbon nanotudes/RuO2/polyindole ternary nanocomposites and their application as high-performance supercapacitors. Front. Mater. Sci. 2020, 14, 109–119. [Google Scholar] [CrossRef]
- Ogihara, N.; Itou, Y.; Sasaki, T.; Takeuchi, Y. Impedance Spectroscopy Characterization of Porous Electrodes under Different Electrode Thickness Using a Symmetric Cell for High-Performance Lithium-Ion Batteries. J. Phys. Chem. C 2015, 119, 4612–4619. [Google Scholar] [CrossRef]
- Choudhary, R.B.; Ansari, S.; Purty, B. Robust electrochemical performance of polypyrrole (PPy) and polyindole (PIn) based hybrid electrode materials for supercapacitor application: A review. J. Energy Storage 2020, 29, 101302. [Google Scholar] [CrossRef]
- Majumder, M.; Choudhary, R.B.; Thakur, A.K.; Rout, C.S.; Gupta, G. Rare earth metal oxide (RE2O3; RE = Nd, Gd, and Yb) incorporated polyindole composites: Gravimetric and volumetric capacitive performance for supercapacitor applications. New J. Chem. 2018, 42, 5295–5308. [Google Scholar] [CrossRef]
- Wu, J.; Xu, L.; Zhou, W.; Jiang, F.; Liu, P.; Zhang, H.; Jiang, Q.; Xu, J. Fishnet-like, nitrogen-doped carbon films directly anchored on carbon cloths as binder-free electrodes for high-performance supercapacitor. Glob. Chall. 2020, 4, 1900086–1900095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Zhu, D.; Ma, X.; Mo, D.; Jiang, F.; Xu, J.; Zhou, W. PEDOT:PSS-assisted polyindole hollow nanospheres modified carbon cloth as high performance electrochemical capacitor electrodes. Electrochim. Acta 2016, 212, 662–670. [Google Scholar] [CrossRef]
- Choudhary, R.B.; Majumder, M.; Thakur, A.K. Two-dimensional exfoliated MoS2 flakes integrated with polyindole for supercapacitor application. Chem. Select 2019, 4, 6906–6912. [Google Scholar]

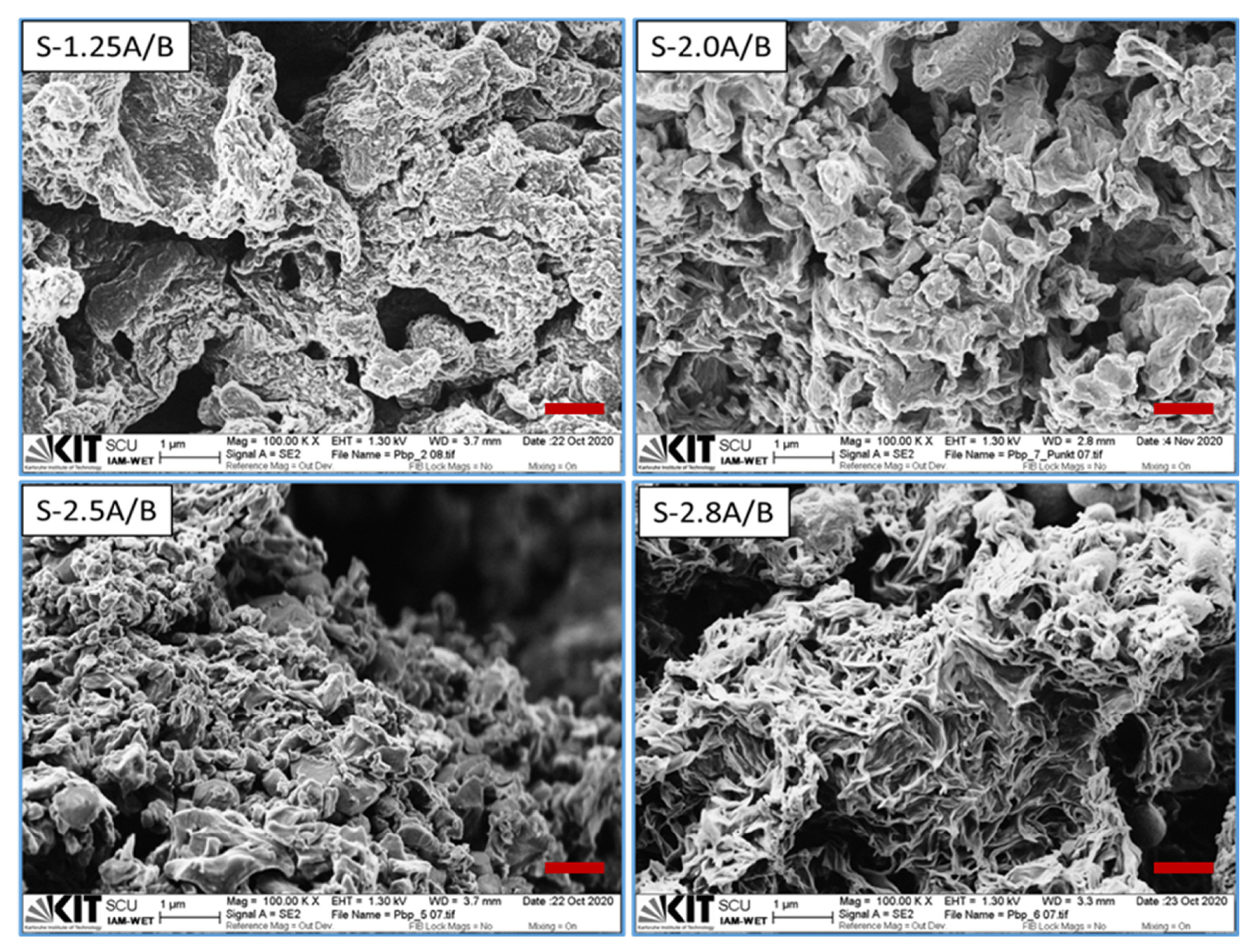
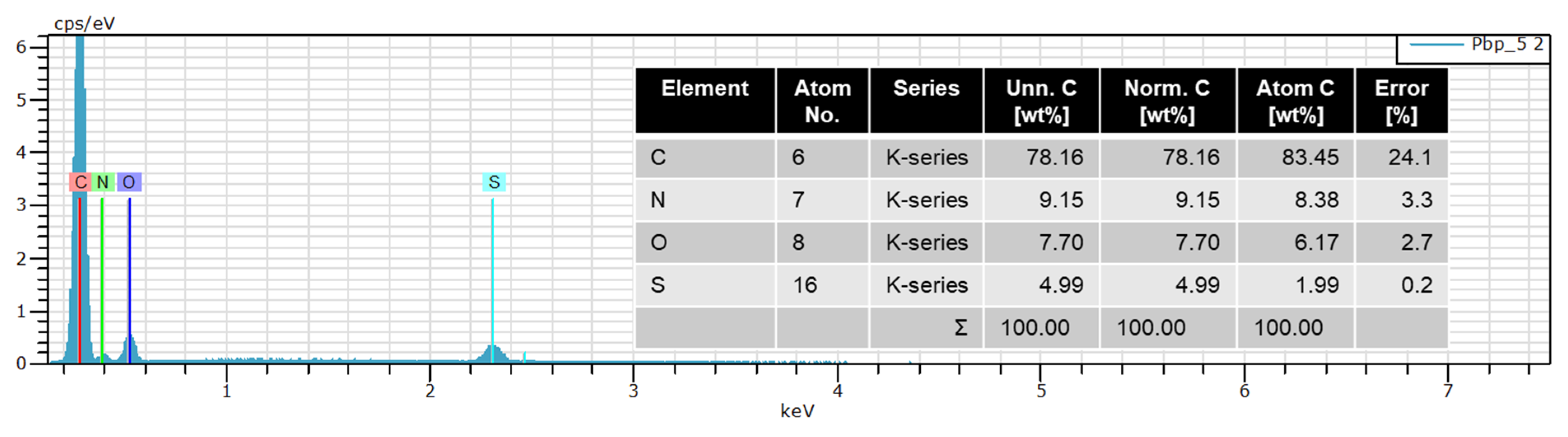
| No. | APS/BP (Mole Ratio) | Concentration of H2SO4 (mol L−1) | Amount of DBSA (mL) | Yield (%) | Sample Code (a) |
|---|---|---|---|---|---|
| Effect of APS/BP mole ratio | |||||
| 1 | 0.25 | 0.1 | 1.0 | 2.5% | S-0.25A/B |
| 2 | 1.0 | 0.1 | 1.0 | 9.3% | S-1.0A/B |
| 3 | 1.25 | 0.1 | 1.0 | 24.7% | S-1.25A/B |
| 4 | 2.0 | 0.1 | 1.0 | 38.2% | S-2.0A/B |
| 5 (b) | 2.5 | 0.1 | 1.0 | 55.9% | S-2.5A/B |
| 6 | 2.8 | 0.1 | 1.0 | 15.7% | S-2.8A/B |
| 7 | 3.0 | 0.1 | 1.0 | 3.0% | S-3.0A/B |
| Effect of H2SO4 concentration | |||||
| 8 | 2.5 | 0.05 | 1.0 | 13.4% | S-0.05H |
| 9 (b) | 2.5 | 0.1 | 1.0 | 55.9% | S-0.1H |
| 10 | 2.5 | 0.5 | 1.0 | 35.0% | S-0.5H |
| 11 | 2.5 | 1.5 | 1.0 | 24.0% | S-1.5H |
| 12 | 2.5 | 2.0 | 1.0 | 18.6% | S-2.0H |
| Effect of DBSA amount | |||||
| 13 | 2.5 | 0.1 | 0.5 | 37.2% | S-0.5D |
| 14 (b) | 2.5 | 0.1 | 1.0 | 55.9% | S-1.0D |
| 15 | 2.5 | 0.1 | 1.5 | 28.6% | S-1.5D |
| 16 | 2.5 | 0.1 | 2.0 | 24.0% | S-2.0D |
| Electrode Material | Specific Capacitance/F g−1 | Energy Density/Wh kg−1 | Power Density/W kg−1 | Cycle Stability | Reference |
|---|---|---|---|---|---|
| Pure Pbp | 23.8 (1 A g−1) | 2.11 (1 A g−1) | 135 (1 A g−1) | 82% (after 5000 cycles at 1 A g−1) | [32] |
| Pure Pbp | 117 (1 A g−1) | 2.60 (1 A g−1) | - | 73% (after 5000 cycles at 1 A g−1) | [49] |
| Pbp Nanospheres | 36.0 (0.5 A g−1) | - | - | - | [51] |
| Pure Ppb | 110 (1 A g−1) | 3.81 (1 A g−1) | 248 (1 A g−1) | 78% (after 5000 cycles at 5 A g−1) | [52] |
| Pbp@HD | 166 ± 2.0 (1 A g−1) | 17.0 (1 A g−1) 4.10 (5 A g−1) | 550 (1 A g−1) & 2750 (5 A g−1) | 87% (after 5000 cycles at 5 A g−1) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begum, B.; Bilal, S.; Shah, A.u.H.A.; Röse, P. Physical, Chemical, and Electrochemical Properties of Redox-Responsive Polybenzopyrrole as Electrode Material for Faradaic Energy Storage. Polymers 2021, 13, 2883. https://doi.org/10.3390/polym13172883
Begum B, Bilal S, Shah AuHA, Röse P. Physical, Chemical, and Electrochemical Properties of Redox-Responsive Polybenzopyrrole as Electrode Material for Faradaic Energy Storage. Polymers. 2021; 13(17):2883. https://doi.org/10.3390/polym13172883
Chicago/Turabian StyleBegum, Bushra, Salma Bilal, Anwar ul Haq Ali Shah, and Philipp Röse. 2021. "Physical, Chemical, and Electrochemical Properties of Redox-Responsive Polybenzopyrrole as Electrode Material for Faradaic Energy Storage" Polymers 13, no. 17: 2883. https://doi.org/10.3390/polym13172883
APA StyleBegum, B., Bilal, S., Shah, A. u. H. A., & Röse, P. (2021). Physical, Chemical, and Electrochemical Properties of Redox-Responsive Polybenzopyrrole as Electrode Material for Faradaic Energy Storage. Polymers, 13(17), 2883. https://doi.org/10.3390/polym13172883








