Novel Approaches to In-Situ ATR-FTIR Spectroscopy and Spectroscopic Imaging for Real-Time Simultaneous Monitoring Curing Reaction and Diffusion of the Curing Agent at Rubber Nanocomposite Surface
Abstract
1. Introduction
2. Materials and Methods
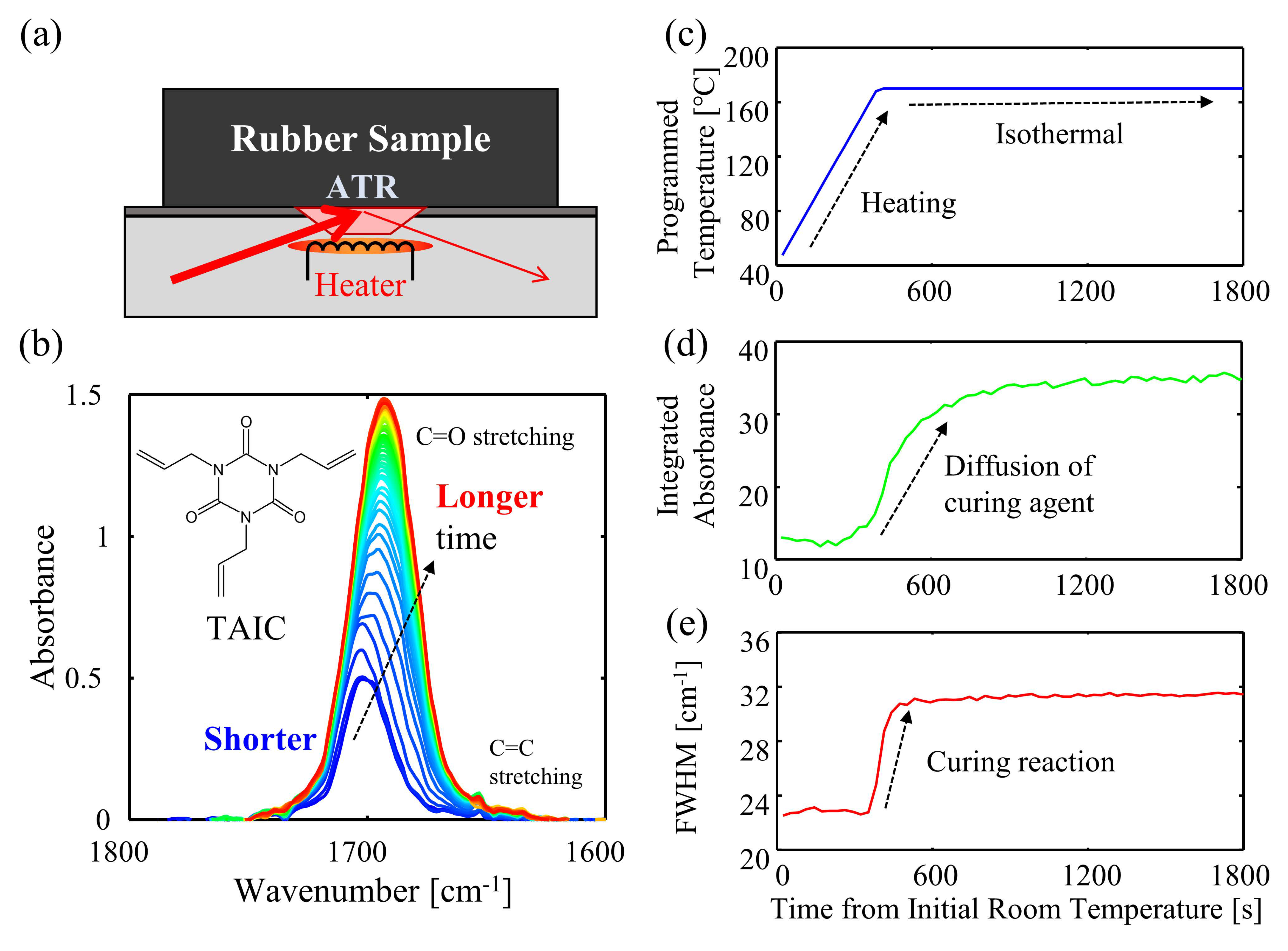
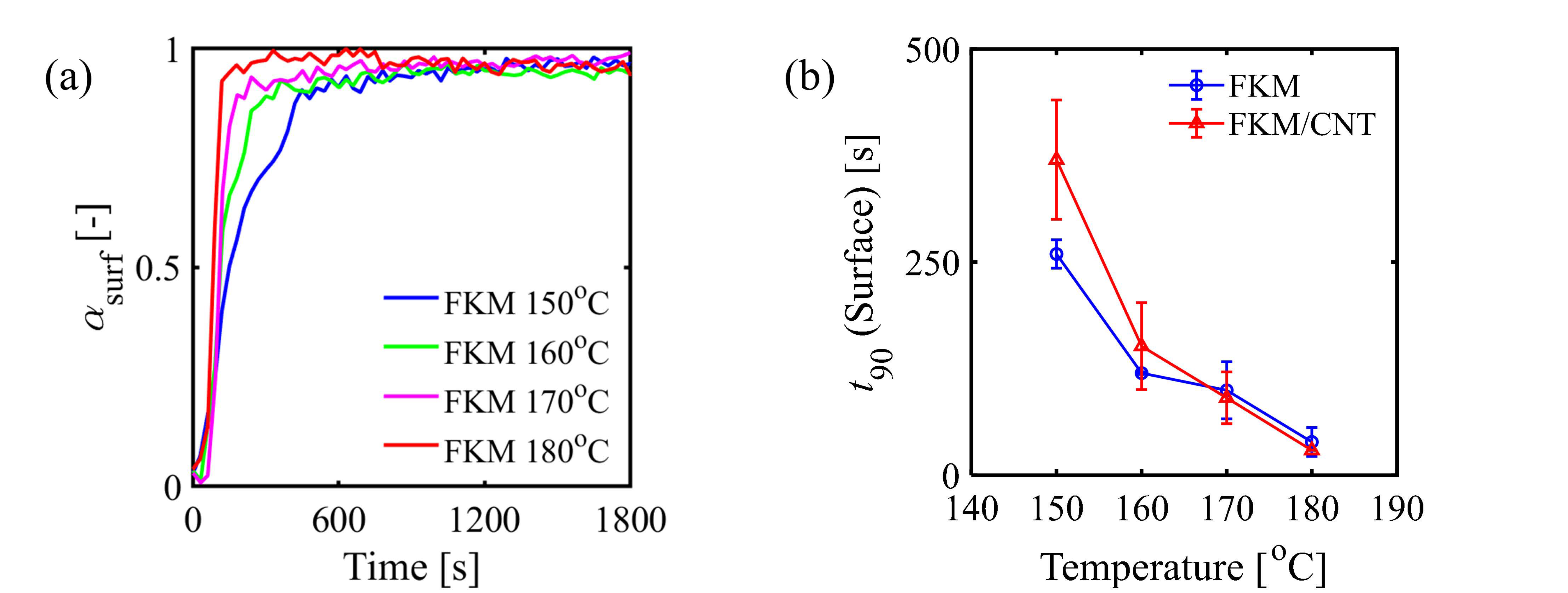
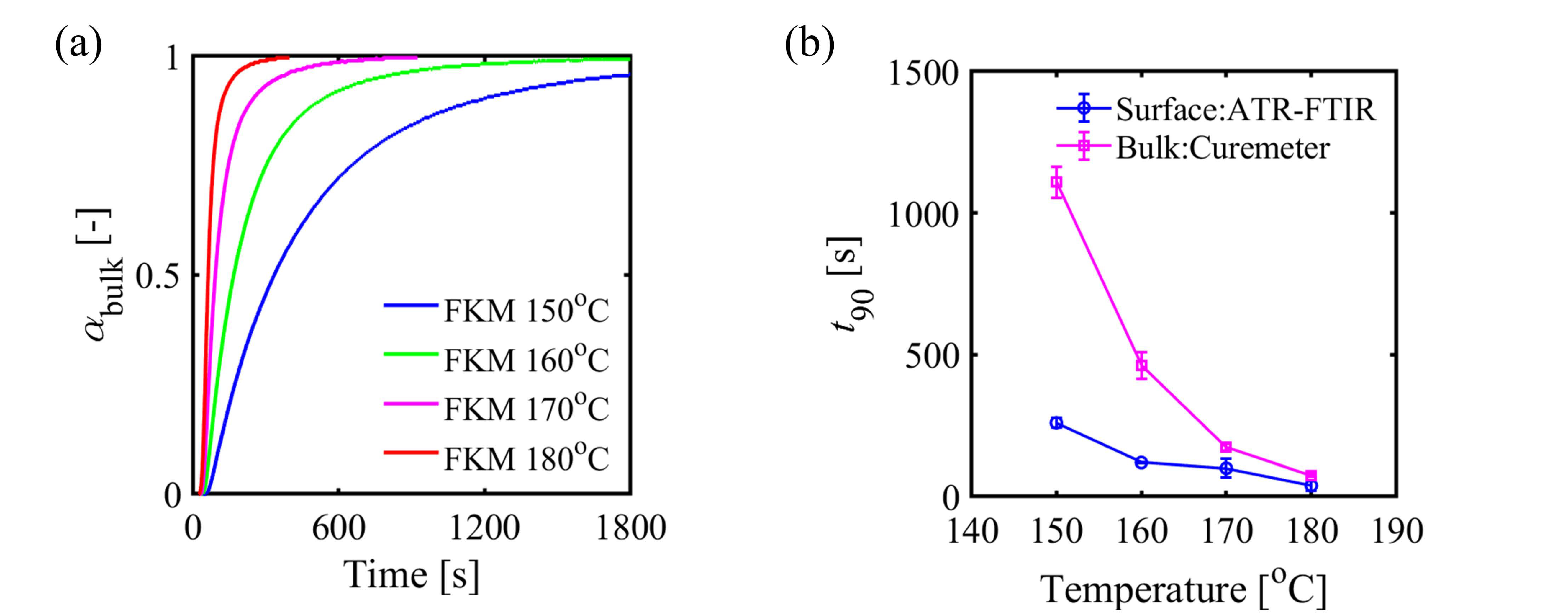
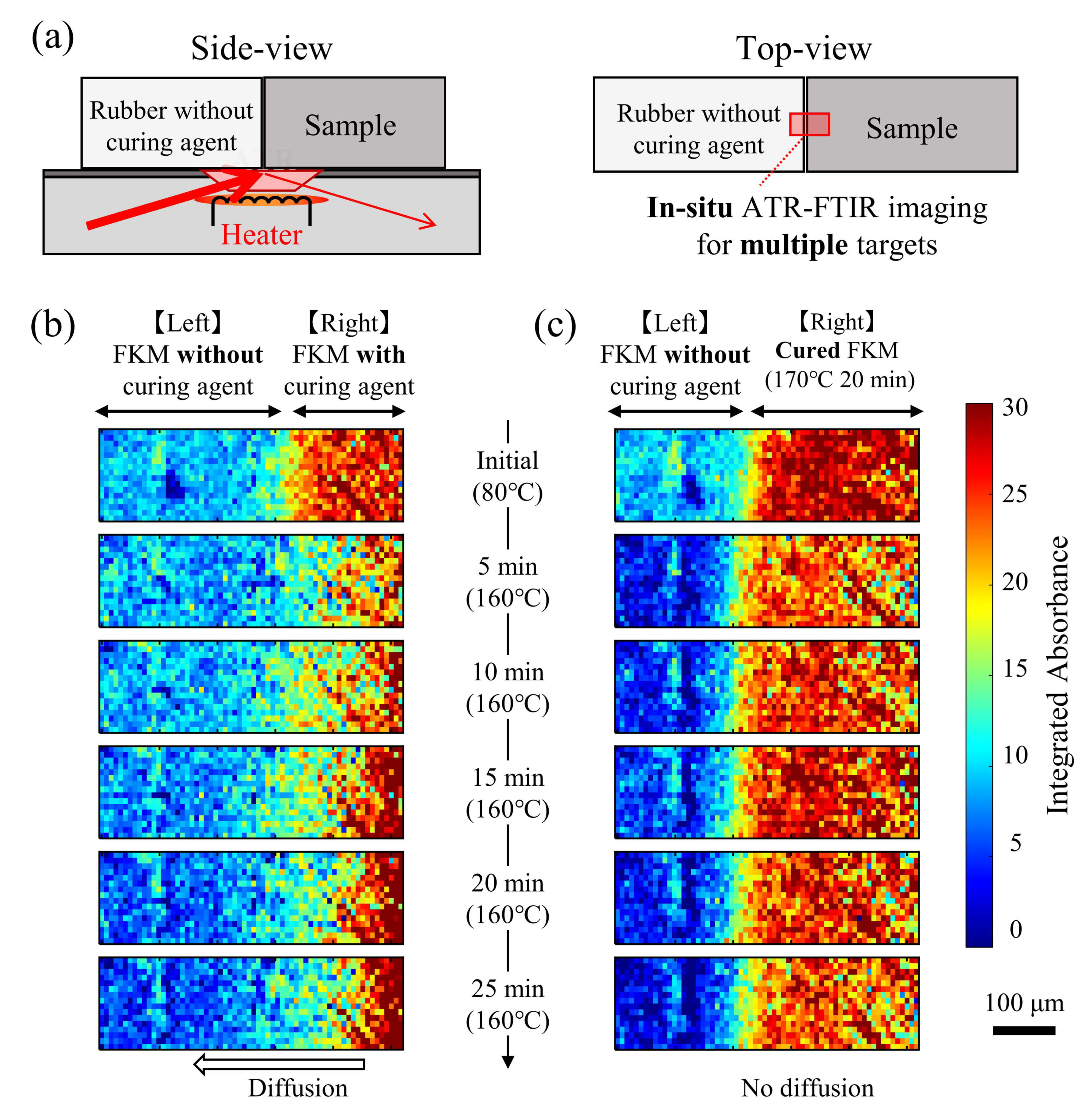
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shajari, S.; Mahmoodi, M.; Rajabian, M.; Karan, K.; Sundararaj, U.; Sudak, L.J. Highly Sensitive and Stretchable Carbon Nanotube/Fluoroelastomer Nanocomposite with a Double-Percolated Network for Wearable Electronics. Adv. Electron. Mater. 2020, 6, 1901067. [Google Scholar] [CrossRef]
- Montes, S.; White, J.L.; Nakajima, N. Rheological behavior of rubber carbon black compounds in various shear flow histories. J. Non-Newton. Fluid Mech. 1988, 28, 183–212. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Razzaghi-Kashani, M. Catalytic and networking effects of carbon black on the kinetics and conversion of sulfur vulcanization in styrene butadiene rubber. Soft Matter 2018, 14, 9194–9208. [Google Scholar] [CrossRef]
- Sarkawi, S.S.; Dierkes, W.K.; Noordermeer, J.W. Elucidation of filler-to-filler and filler-to-rubber interactions in silica-reinforced natural rubber by TEM Network Visualization. Eur. Polym. J. 2014, 54, 118–127. [Google Scholar] [CrossRef]
- Wu, W.; Cong, S. Silica and diatomite fillers modified fluorine rubber composites treated by silane-coupling agents. J. Vinyl Addit. Technol. 2020, 26, 55–61. [Google Scholar] [CrossRef]
- Premphet, K.; Horanont, P. Phase structure of ternary polypropylene/elastomer/filler composites: Effect of elastomer polarity. Polymer 2000, 41, 9283–9290. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Heinrich, G. Tube-like natural halloysite/fluoroelastomer nanocomposites with simultaneous enhanced mechanical, dynamic mechanical and thermal properties. Eur. Polym. J. 2011, 47, 1746–1755. [Google Scholar] [CrossRef]
- Kader, M.A.; Nah, C. Influence of clay on the vulcanization kinetics of fluoroelastomer nanocomposites. Polymer 2004, 45, 2237–2247. [Google Scholar] [CrossRef]
- Gao, W.; Guo, J. A novel processing method namely fast evaporation mixing to prepare fluoroelastomer/montmorillonite composites. Compos. Sci. Technol. 2017, 139, 26–35. [Google Scholar] [CrossRef]
- Ata, S.; Tomonoh, S.; Yamda, T.; Hata, K. Improvement in thermal durability of fluorinated rubber by the addition of single-walled carbon nanotubes as a thermally stable radical scavenger. Polymer 2017, 119, 112–117. [Google Scholar] [CrossRef]
- Endo, M.; Noguchi, T.; Ito, M.; Takeuchi, K.; Hayashi, T.; Kim, Y.A.; Wanibuchi, T.; Jinnai, H.; Terrones, M.; Dresselhaus, M.S. Extreme-Performance Rubber Nanocomposites for and Probing Excavating Deep Oil Resources Using Multi-Walled Carbon Nanotubes. Adv. Funct. Mater. 2008, 18, 3403–3409. [Google Scholar] [CrossRef]
- Deng, F.; Ito, M.; Noguchi, T.; Wang, L.; Ueki, H.; Niihara, K.; Kim, Y.A.; Endo, M.; Zheng, Q. Elucidation of the Reinforcing Mechanism in Carbon Nanotube/Rubber Nanocomposites. ACS Nano 2011, 5, 3858–3866. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, X.; Cong, C.; Zhou, Q. Strategy of Tailoring the Interface Between Multiwalled Carbon Nanotube and Fluoroelastomer. Polym. Compos. 2015, 36, 257–263. [Google Scholar] [CrossRef]
- Shanmugharaj, A.M.; Bae, J.H.; Lee, K.Y.; Noh, W.H.; Lee, S.H.; Ryu, S.H. Physical and chemical characteristics of multiwalled carbon nanotubes functionalized with aminosilane and its influence on the properties of natural rubber composites. Compos. Sci. Technol. 2007, 67, 1813–1822. [Google Scholar] [CrossRef]
- Ahmadi, M.; Shojaei, A. Cure kinetic and network structure of NR/SBR composites reinforced by multiwalled carbon nanotube and carbon blacks. Thermochim. Acta. 2013, 566, 238–248. [Google Scholar] [CrossRef]
- Sui, G.; Zhong, W.H.; Yang, X.P.; Yu, Y.H. Curing kinetics and mechanical behavior of natural rubber reinforced with pretreated carbon nanotubes. Mater. Sci. Eng. A 2008, 485, 524–531. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Wang, L.; Li, J.; Feng, T.; Fan, J.; Chen, L.; Gu, J. Superior wave-absorbing performances of silicone rubber composites via introducing covalently bonded SnO2@MWCNT absorbent with encapsulation structure. Compos. Commun. 2020, 22, 100486. [Google Scholar] [CrossRef]
- Khajehpour, M.; Sadeghi, S.; Yazdi, A.Z.; Sundararaj, U. Tuning the curing behavior of fluoroelastomer (FKM) by incorporation of nitrogen doped graphene nanoribbons (CNx-GNRs). Polymer 2014, 55, 6293–6302. [Google Scholar] [CrossRef]
- Mensah, B.; Gupta, K.C.; Kang, G.; Lee, H.; Nah, C. A comparative study on vulcanization behavior of acrylonitrile-butadiene rubber reinforced with graphene oxide and reduced graphene oxide as fillers. Polym. Test. 2019, 76, 127–137. [Google Scholar] [CrossRef]
- Barghamadi, M.; Ghoreishy, M.H.R.; Karrabi, M.; Mohammadian-Gezaz, S. Investigation on the kinetics of cure reaction of acrylonitrile-butadiene rubber (NBR)/polyvinyl chloride (PVC)/graphene nanocomposite using various models. J. Appl. Polym. Sci. 2020, 137, 48632. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Z.; Zhang, Y.; Chen, L.; Cao, D.; Gu, J. Polymer-based EMI shielding composites with 3D conductive networks: A mini-review. SusMat 2021, 1–19. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Erfanian, M.-R.; Anbarsooz, M.; Moghiman, M. A Three Dimensional Simulation of a Rubber Curing Process Considering Variable Order of Reaction. Appl. Math. Model. 2016, 40, 8592–8604. [Google Scholar] [CrossRef]
- Berger, T.; Kaliske, M. A thermo-mechanical material model for rubber curing and tire manufacturing simulation. Comput. Mech. 2020, 66, 513–535. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Y.; Zhao, J.; Shen, J.; Feng, M. Oil bleed from elastomeric thermal silicone conductive pads. Front. Chem. Sci. Eng. 2016, 10, 509–516. [Google Scholar] [CrossRef]
- Wakabayashi, M.; Kohno, T.; Kimura, T.; Tamura, S.; Endoh, M.; Ohnishi, S.; Nishioka, T.; Tanaka, Y.; Kanai, T. New Bleeding Model of Additives in a Polypropylene Film under Atmospheric Pressure. J. Appl. Polym. Sci. 2007, 104, 3751–3757. [Google Scholar] [CrossRef][Green Version]
- Fernàndez-Francos, X.; Kazarian, S.G.; Ramis, X.; Serra, A. Simultaneous Monitoring of Curing Shrinkage and Degree of Cure of Thermosets by Attenuated Total Reflection Fourier Transform Infrared (ATR FT-IR) Spectroscopy. Appl. Spectrosc. 2013, 67, 1427–1436. [Google Scholar] [CrossRef]
- Mitomo, H.; Kaneda, A.; Quynh, T.M.; Nagasawa, N.; Yoshii, F. Improvement of heat stability Of poly(L-lactic acid) by radiation-induced crosslinking. Polymer 2005, 46, 4695–4703. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Manaila, E.; Zuga, N. The use of polyfunctional monomers in the radical cure of chlorinated polyethylene. Polym. J. 2011, 43, 792–800. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Zhang, H.; Shang, Y.; Wang, X.; Han, B.; Li, Z. Theoretical study on the reaction of triallyl isocyanurate in the UV radiation cross-linking of polyethylene. RSC Adv. 2017, 7, 37095–37104. [Google Scholar] [CrossRef]
- Thite, A.G.; Krishnanand, K.; Panda, P.K. Electron beam-induced crosslinking of silk fibers using triallyl isocyanurate for enhanced properties. J. Appl. Polym. Sci. 2019, 136, 47888. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Cong, C.; Cui, H.; Xu, L.; Zhang, Y.; Meng, X.; Zhou, Q. Thermal and thermo-oxidative degradation of tetrafluoroethylene-propylene elastomer above 300 °C. Polym. Degrad. Stab. 2020, 177, 109180. [Google Scholar] [CrossRef]
- Muroga, S.; Takahashi, Y.; Hikima, Y.; Ata, S.; Ohshima, M.; Okazaki, T.; Hata, K. New evaluation method for the curing degree of rubber and its nanocomposites using ATR-FTIR spectroscopy. Polym. Test. 2021, 93, 106993. [Google Scholar] [CrossRef]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef]
- Lu, H.; Kazarian, S.G.; Sato, H. Simultaneous Visualization of Phase Separation and Crystallization in PHB/PLLA Blends with In Situ ATR-FTIR Spectroscopic Imaging. Macromolecules 2020, 53, 9074–9085. [Google Scholar] [CrossRef]
- Lu, H.; Sato, H.; Kazarian, S.G. Visualization of Inter- and Intramolecular Interactions in PHB/PLLA Blend During Isothermal Melt-crystallization Using ATR FT-IR Spectroscopic Imaging. Appl. Spectrosc. 2021, 75, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Shinzawa, H.; Kazarian, S.G. Intermolecular Interactions in the Polymer Blends Under High-Pressure CO2 Studied Using Two-Dimensional Correlation Analysis and Two-Dimensional Disrelation Mapping. Appl. Spectrosc. 2021, 75, 250–258. [Google Scholar] [CrossRef]
- Yang, S.; Wu, Z.; Yang, W.; Yang, M. Thermal and mechanical properties of chemical crosslinked polylactide (PLA). Polym. Test. 2008, 27, 957–963. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, L.; Zhang, H.; Lin, W.; Wang, Y. Investigation on multifunctional monomer modified polypropylene and its foamability. J. Appl. Polym. Sci. 2013, 130, 1675–1681. [Google Scholar] [CrossRef]
- Yamane, S.; Shinzawa, H.; Ata, S.; Suzuki, Y.; Nishizawa, A.; Mizukado, J. A thermal oxidative degradation study of triallyl isocyanurate crosslinking moiety in fluorinated rubber by two-dimensional infrared correlation spectroscopy. Vib. Spectrosc. 2018, 98, 30–34. [Google Scholar] [CrossRef]
- George, S.C.; Thomas, S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Ata, S.; Mizuno, T.; Nishizawa, A.; Subramaniam, C.; Futaba, D.N.; Hata, K. Influence of matching solubility parameter of polymer matrix and CNT on electrical conductivity of CNT/rubber composite. Sci. Rep. 2014, 4, 7232. [Google Scholar] [CrossRef]
- Ito, M.; Noguchi, T.; Ueki, H.; Inukai, S.; Iinou, S.; Takeuchi, K.; Endo, M. Adhesion and Reinforcement of CNT-fluoroelastomers Composite for Oilfield Applications. J. Adh. Soc. Jpn. 2011, 47, 146–153. [Google Scholar]
- Tagelsir, Y.; Li, S.-X.; Lv, X.; Wang, S.; Wang, S.; Osman, Z. Effect of oxidized and fluorinated MWCNTs on mechanical, thermal and tribological properties of fluoroelastomer/carbon black/MWCNT hybrid nanocomposite. Mater. Res. Express 2018, 5, 065318. [Google Scholar] [CrossRef]
- Ata, S.; Subramaniam, C.; Nishizawa, A.; Yamada, T.; Hata, K. Highly Thermally Conductive Yet Flexible Composite of Carbon Fiber, Carbon Nanotube, and Rubber Obtained by Decreasing the Thermal Resistivity at the Interface between Carbon Fiber and Carbon Nanotube. Adv. Eng. Mater. 2017, 19, 1600596. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muroga, S.; Takahashi, Y.; Hikima, Y.; Ata, S.; Kazarian, S.G.; Ohshima, M.; Okazaki, T.; Hata, K. Novel Approaches to In-Situ ATR-FTIR Spectroscopy and Spectroscopic Imaging for Real-Time Simultaneous Monitoring Curing Reaction and Diffusion of the Curing Agent at Rubber Nanocomposite Surface. Polymers 2021, 13, 2879. https://doi.org/10.3390/polym13172879
Muroga S, Takahashi Y, Hikima Y, Ata S, Kazarian SG, Ohshima M, Okazaki T, Hata K. Novel Approaches to In-Situ ATR-FTIR Spectroscopy and Spectroscopic Imaging for Real-Time Simultaneous Monitoring Curing Reaction and Diffusion of the Curing Agent at Rubber Nanocomposite Surface. Polymers. 2021; 13(17):2879. https://doi.org/10.3390/polym13172879
Chicago/Turabian StyleMuroga, Shun, Yu Takahashi, Yuta Hikima, Seisuke Ata, Sergei G. Kazarian, Masahiro Ohshima, Toshiya Okazaki, and Kenji Hata. 2021. "Novel Approaches to In-Situ ATR-FTIR Spectroscopy and Spectroscopic Imaging for Real-Time Simultaneous Monitoring Curing Reaction and Diffusion of the Curing Agent at Rubber Nanocomposite Surface" Polymers 13, no. 17: 2879. https://doi.org/10.3390/polym13172879
APA StyleMuroga, S., Takahashi, Y., Hikima, Y., Ata, S., Kazarian, S. G., Ohshima, M., Okazaki, T., & Hata, K. (2021). Novel Approaches to In-Situ ATR-FTIR Spectroscopy and Spectroscopic Imaging for Real-Time Simultaneous Monitoring Curing Reaction and Diffusion of the Curing Agent at Rubber Nanocomposite Surface. Polymers, 13(17), 2879. https://doi.org/10.3390/polym13172879





