Bioinspired Design of Sericin/Chitosan/Ag@MOF/GO Hydrogels for Efficiently Combating Resistant Bacteria, Rapid Hemostasis, and Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ag@MOF
2.3. Preparation of GO Sheets
2.4. Synthesis of Ag@MOF–GO
2.5. Preparation of CS/SS/Ag@MOF and CS/SS/GO Hydrogels
2.6. Preparation of CS/SS/Ag@MO–GO Nanocomposite Hydrogels
2.7. Characterization
2.8. Thermogravimetric Analysis
2.9. Water Solubility (WS) of the Hydrogels
2.10. Water Vapor Permeability
2.11. Water Retention Studies
2.12. In Vitro Antibacterial Study and Ag Ion Release Measurement
2.13. Antibacterial Effects of the Extract Liquid of Samples
2.14. Morphological Characterization of Bacteria
2.15. In Vitro Cytotoxicity
2.16. Hemostatic Properties of Hydrogels
2.16.1. Blood Clotting Kinetics and Clotting Blood Time (CBT)
2.16.2. Blood Plasma Clotting Analysis
2.17. Adhesion of Erythrocyte and Platelets
2.18. Hemolysis Assay
2.19. Cell Migration Assay
2.20. In Vivo Animal Experiment
2.21. Statistical Analysis
3. Results and Discussion
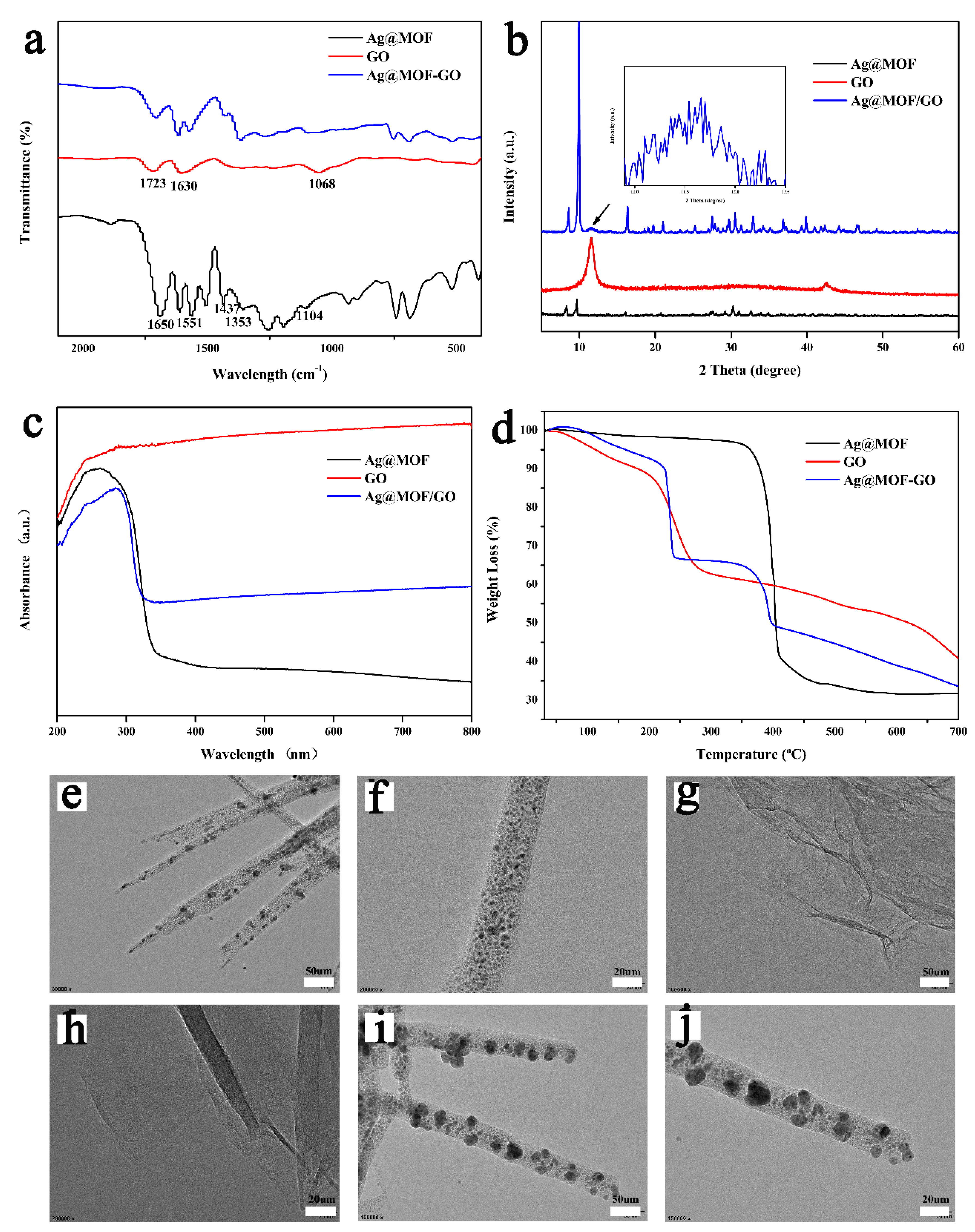
3.1. Characterization of Ag@MOF–GO
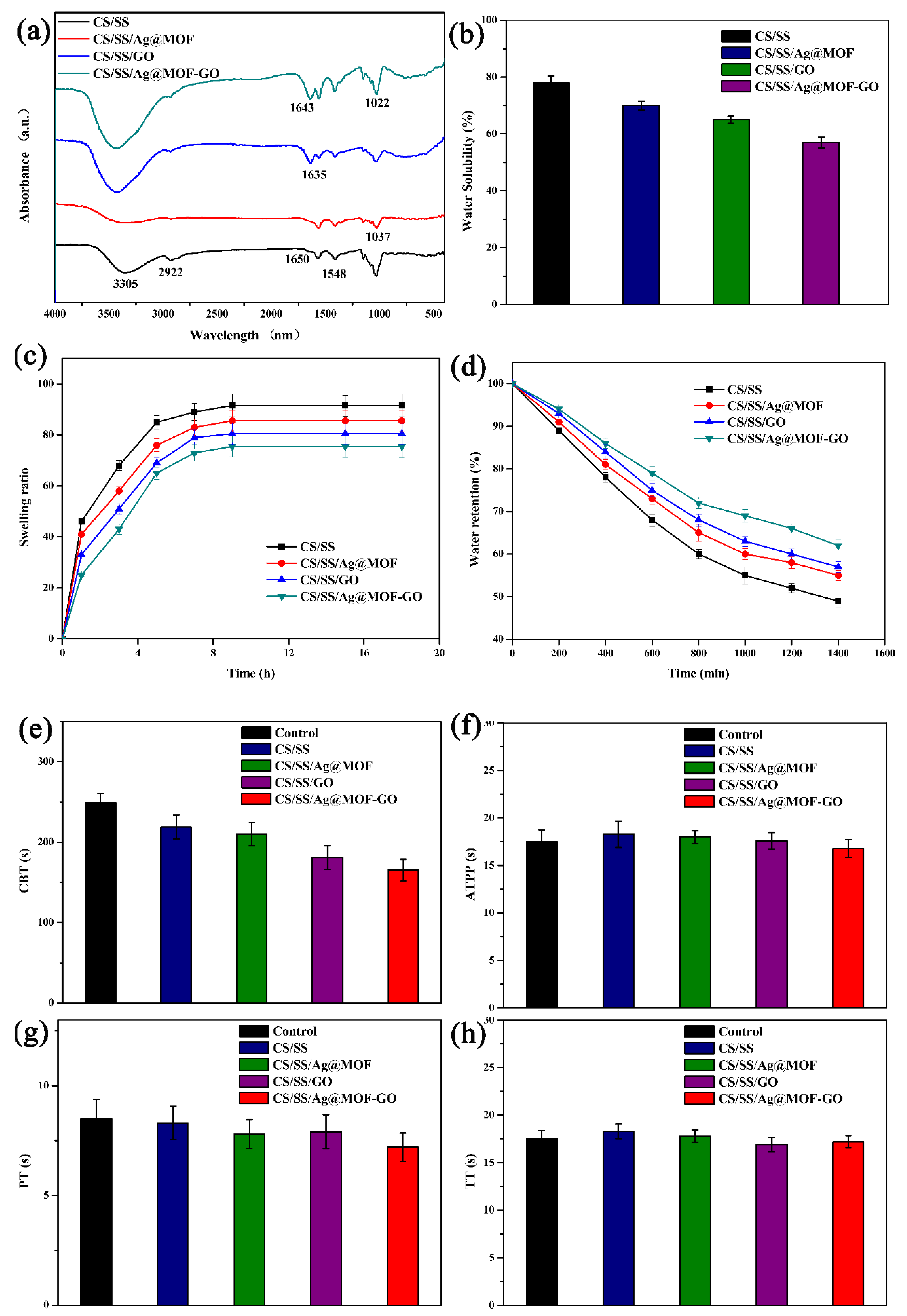
3.2. FTIR of Composite Dresssings
3.3. Water Solubility, Swelling Degree, and Water Retention
3.4. Hemostatic Properties of Dressings
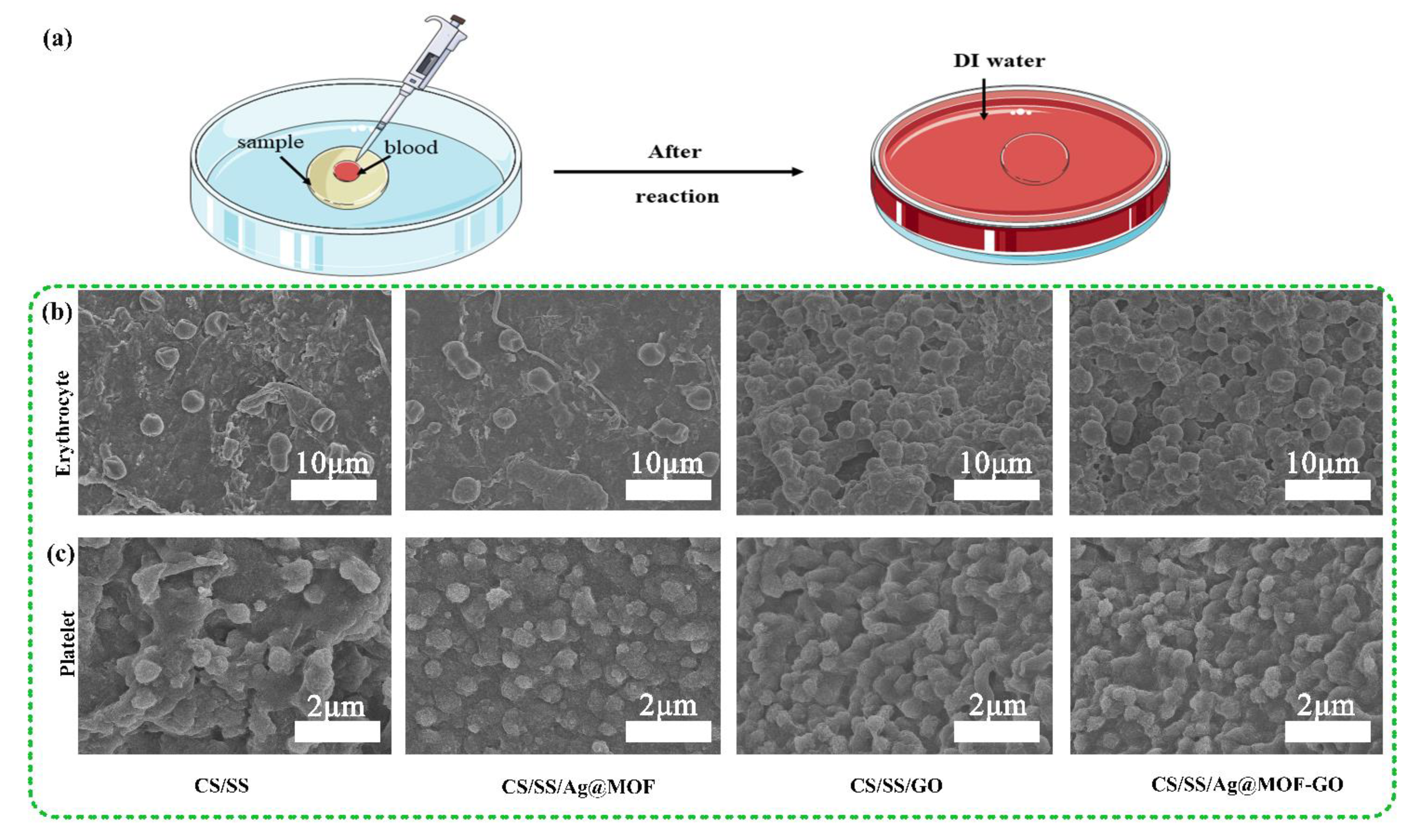
3.5. Adhesion of Erythrocyte and Platelets
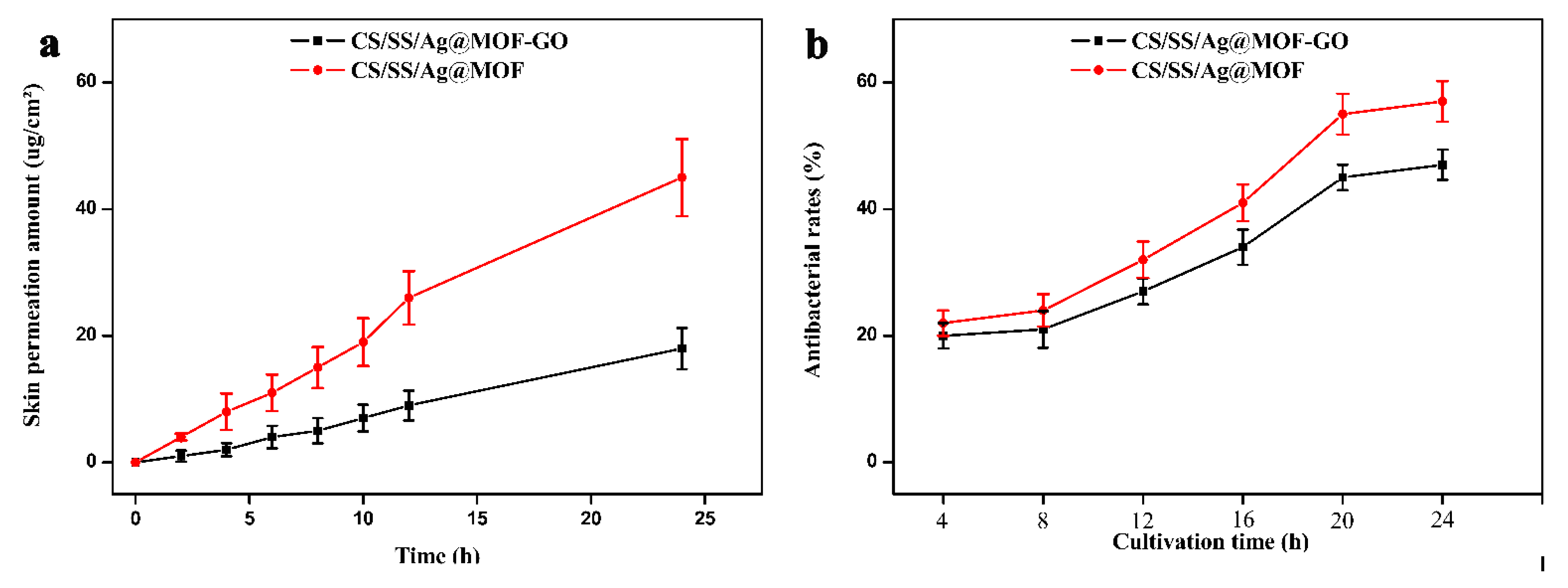
3.6. Antibacterial Activities and Ag Ion Release of the Dressings
3.7. Biocompatibility of Dressings
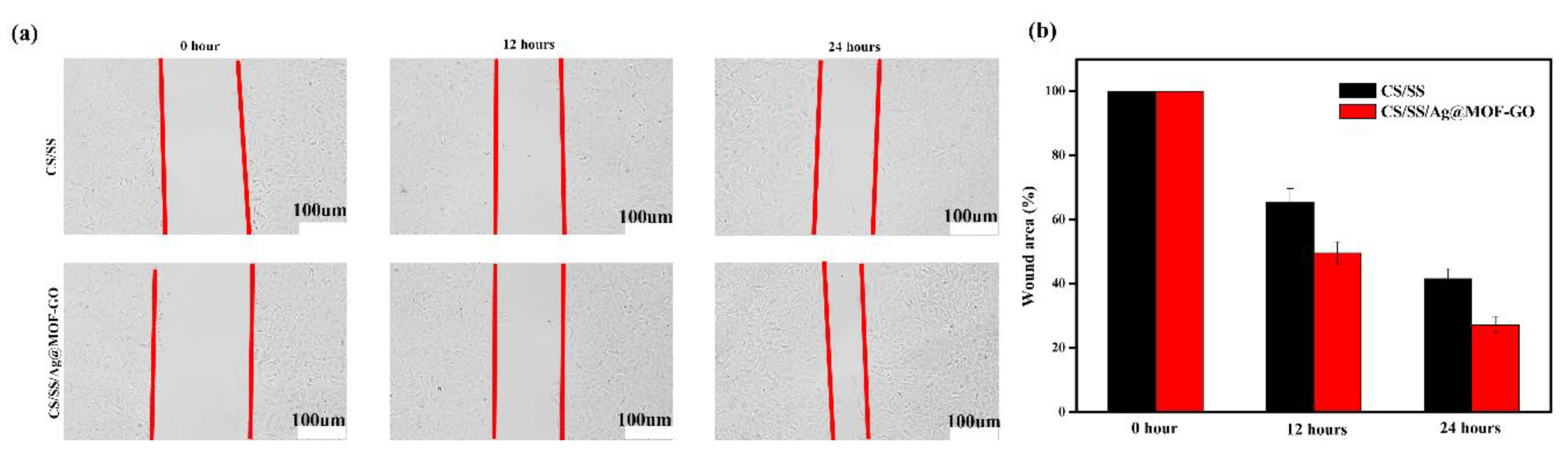
3.8. In Vitro Cell Migration Assay of Dressings
3.9. In Vivo Wound Healing
3.10. Histological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; MansooriMoghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef]
- Wang, L.; Yang, K.; Li, X.; Zhang, X.; Zhang, D.; Wang, L.N.; Lee, C.S. A double-crosslinked self-healing antibacterial hydrogel with enhanced mechanical performance for wound treatment. Acta Biomater. 2021. [Google Scholar] [CrossRef]
- Qian, Y.; Xu, C.; Xiong, W.; Jiang, N.; Zheng, Y.; He, X.; Ding, F.; Lu, X.; Shen, J. Dual cross-linked organic-inorganic hybrid hydrogels accelerate diabetic skin wound healing. Chem. Eng. J. 2021, 417, 129335. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Thi, P.L.; Lee, Y.; Tran, D.L.; Thi, T.T.H.; Kang, J.I.; Park, K.M.; Park, K.D. In situ forming and reactive oxygen species-scavenging gelatin hydrogels for enhancing wound healing efficacy. Acta Biomater. 2020, 103, 142–152. [Google Scholar] [CrossRef]
- Zandi, N.; Dolatyar, B.; Lotfi, R.; Shallageh, Y.; Shokrgozar, M.A.; Tamjid, E.; Annabi, N.; Simchi, A. Biomimetic nanoengineered scaffold for enhanced full-thickness cutaneous wound healing. Acta Biomater. 2021, 124, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.T.; Zhang, Y.Q. Processing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedicines. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 940–952. [Google Scholar] [CrossRef]
- Gilotra, S.; Chouhan, D.; Bhardwaj, N.; Nandi, S.K.; Mandal, B.B. Potential of silk sericin based nanofibrous mats for wound dressing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 420–432. [Google Scholar] [CrossRef]
- Tao, G.; Cai, R.; Wang, Y.; Liu, L.; Zuo, H.; Zhao, P.; Umar, A.; Mao, C.; Xia, Q.; He, H. Bioinspired design of AgNPs embedded silk sericin-based sponges for efficiently combating bacteria and promoting wound healing. Mater. Des. 2019, 180, 107940. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020, 149, 513–521. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, B.B. Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef]
- He, H.; Cai, R.; Wang, Y.; Tao, G.; Guo, P.; Zuo, H.; Chen, L.; Liu, X.; Zhao, P.; Xia, Q. Preparation and characterization of silk sericin/PVA blend film with silver nanoparticles for potential antimicrobial application. Int. J. Biol. Macromol. 2017, 104, 457–464. [Google Scholar] [CrossRef]
- Doyan, A.; Susilawati, S.; Prayogi, S.; Bilad, M.R.; Arif, M.F.; Ismail, N.M. Polymer Film Blend of Polyvinyl Alcohol, Trichloroethylene and Cresol Red for Gamma Radiation Dosimetry. Polymers 2021, 13, 1866. [Google Scholar] [CrossRef]
- Akhlaq, M.; Azad, A.K.; Ullah, I.; Nawaz, A.; Safdar, M.; Bhattacharya, T.; Uddin, A.B.M.H.; Abbas, S.A.; Mathews, A.; Kundu, S.K.; et al. Methotrexate-Loaded Gelatin and Polyvinyl Alcohol (Gel/PVA) Hydrogel as a pH-Sensitive Matrix. Polymers 2021, 13, 2300. [Google Scholar] [CrossRef]
- Khan, N.A.; Niazi, M.B.K.; Sher, F.; Jahan, Z.; Noor, T.; Azhar, O.; Rashid, T.; Iqbal, N. Metal Organic Frameworks Derived Sustainable Polyvinyl Alcohol/Starch Nanocomposite Films as Robust Materials for Packaging Applications. Polymers 2021, 13, 2307. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhao, S.X.; Yin, X.L.; Wang, H.Y.; Wei, Z.G.; Zhang, Y.Q. Silk sericin has significantly hypoglycaemic effect in type 2 diabetic mice via anti-oxidation and anti-inflammation. Int. J. Biol. Macromol. 2020, 150, 1061–1071. [Google Scholar] [CrossRef]
- Lee, S.J.; Nah, H.; Heo, D.N.; Kim, K.-H.; Seok, J.M.; Heo, M.; Moon, H.-J.; Lee, D.; Lee, J.S.; An, S.Y.; et al. Induction of osteogenic differentiation in a rat calvarial bone defect model using an In situ forming graphene oxide incorporated glycol chitosan/oxidized hyaluronic acid injectable hydrogel. Carbon 2020, 168, 264–277. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Napiwocki, B.N.; Peng, X.-F.; Turng, L.-S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Shen, T.; Dai, K.; Yu, Y.; Wang, J.; Liu, C. Sulfated chitosan rescues dysfunctional macrophages and accelerates wound healing in diabetic mice. Acta Biomater. 2020, 117, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-García, A.; Caicedo, C.; Jiménez-Regalado, E.J.; Morales, G.; Aguirre-Loredo, R.Y. Effects of Poloxamer Content and Storage Time of Biodegradable Starch-Chitosan Films on Its Thermal, Structural, Mechanical, and Morphological Properties. Polymers 2021, 13, 2341. [Google Scholar] [CrossRef]
- Sánchez-Cardona, Y.; Echeverri-Cuartas, C.; López, M.; Moreno-Castellanos, N. Chitosan/Gelatin/PVA Scaffolds for Beta Pancreatic Cell Culture. Polymers 2021, 13, 2372. [Google Scholar] [CrossRef]
- Thamer, B.M.; Esmail, G.A.; Al-Dhabi, N.A.; Moydeen, A.; Arasu, M.V.; Al-Enizi, A.M.; El-Newehy, M.H. Fabrication of biohybrid electrospun nanofibers for the eradication of wound infection and drug-resistant pathogens. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125691. [Google Scholar] [CrossRef]
- Cobos, M.; De-La-Pinta, I.; Quindós, G.; Fernández, M.J.; Fernández, M.D. One-step eco-friendly synthesized silver-graphene oxide/poly(vinyl alcohol) antibacterial nanocomposites. Carbon 2019, 150, 101–116. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, F.; Hou, Z.; Chen, Y.; Luo, X. Injectable self-healing CuS nanoparticle complex hydrogels with antibacterial, anti-cancer, and wound healing properties. Chem. Eng. J. 2021, 409, 128224. [Google Scholar] [CrossRef]
- Khan, M.I.; Paul, P.; Behera, S.K.; Jena, B.; Tripathy, S.K.; Stålsby Lundborg, C.; Mishra, A. To decipher the antibacterial mechanism and promotion of wound healing activity by hydrogels embedded with biogenic Ag@ZnO core-shell nanocomposites. Chem. Eng. J. 2020, 409, 128025. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Bhardwaj, S.K.; Mehta, J.; Kim, K.H.; Deep, A. MOF-Bacteriophage Biosensor for Highly Sensitive and Specific Detection of Staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9, 33589–33598. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Hao, S.; Fan, R.; Qin, M.; Chen, W.; Wang, P.; Yang, Y. Hydrophobicity-Adjustable MOF Constructs Superhydrophobic MOF-rGO Aerogel for Efficient Oil-Water Separation. ACS Appl. Mater. Interfaces 2020, 12, 56435–56444. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xia, Q.; Xing, H.; Chen, D.; Wang, H. Construction of Pillared-Layer MOF as Efficient Visible-Light Photocatalysts for Aqueous Cr(VI) Reduction and Dye Degradation. ACS Sustain. Chem. Eng. 2017, 5, 4449–4456. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Forgan, R.S. Application of zirconium MOFs in drug delivery and biomedicine. Coord. Chem. Rev. 2019, 380, 230–259. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ying, T.; Li, J.; Xu, Y.; Wang, R.; Ke, Q.; Shen, S.G.F.; Xu, H.; Lin, K. Hierarchical micro/nanofibrous scaffolds incorporated with curcumin and zinc ion eutectic metal organic frameworks for enhanced diabetic wound healing via anti-oxidant and anti-inflammatory activities. Chem. Eng. J. 2020, 402, 126273. [Google Scholar] [CrossRef]
- Guan, Q.L.; Han, C.; Bai, F.Y.; Liu, J.; Xing, Y.H.; Shi, Z.; Sun, L.X. Bismuth-MOF based on tetraphenylethylene derivative as a luminescent sensor with turn-off/on for application of Fe3+ detection in serum and bioimaging, as well as emissive spectra analysis by TRES. Sens. Actuators B Chem. 2020, 325, 128767. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Li, D.; Zhao, M.; Wu, H.; Shen, B.; Liu, P.; Ding, S. Electrochemical biosensor for ultrasensitive exosomal miRNA analysis by cascade primer exchange reaction and MOF@Pt@MOF nanozyme. Biosens. Bioelectron. 2020, 168, 112554. [Google Scholar] [CrossRef]
- Han, D.; Li, Y.; Liu, X.; Yeung, K.W.K.; Zheng, Y.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Wang, X.; et al. Photothermy-strengthened photocatalytic activity of polydopamine-modified metal-organic frameworks for rapid therapy of bacteria-infected wounds. J. Mater. Sci. Technol. 2021, 62, 83–95. [Google Scholar] [CrossRef]
- Lu, X.; Ye, J.; Zhang, D.; Xie, R.; Bogale, R.F.; Sun, Y.; Zhao, L.; Zhao, Q.; Ning, G. Silver carboxylate metal-organic frameworks with highly antibacterial activity and biocompatibility. J. Inorg. Biochem. 2014, 138, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Gao, J.; He, Q.; Wu, J.; Liang, D.; Yang, H.; Chen, R. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr. Polym. 2017, 156, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Travlou, N.A.; Algarra, M.; Alcoholado, C.; Cifuentes-Rueda, M.; Labella, A.M.; Lázaro-Martínez, J.M.; Rodríguez-Castellón, E.; Bandosz, T.J. Carbon Quantum Dot Surface-Chemistry-Dependent Ag Release Governs the High Antibacterial Activity of Ag-Metal–Organic Framework Composites. ACS Appl. Bio Mater. 2018, 1, 693–707. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, G.; Wang, D.; Zheng, Y.; Li, Y.; Meng, W.; Zhang, X.; Du, F.; Lee, S. Ag@MOF-loaded chitosan nanoparticle and polyvinyl alcohol/sodium alginate/chitosan bilayer dressing for wound healing applications. Int. J. Biol. Macromol. 2021, 175, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Berchel, M.; Gall, T.L.; Denis, C.; Hir, S.L.; Quentel, F.; Elléouet, C.; Montier, T.; Rueff, J.-M.; Salaün, J.-Y.; Haelters, J.-P.; et al. A silver-based metal–organic framework material as a ‘reservoir’ of bactericidal metal ions. New J. Chem. 2011, 35, 1000–1003. [Google Scholar] [CrossRef]
- Venkataprasanna, K.S.; Prakash, J.; Vignesh, S.; Bharath, G.; Venkatesan, M.; Banat, F.; Sahabudeen, S.; Ramachandran, S.; Devanand Venkatasubbu, G. Fabrication of Chitosan/PVA/GO/CuO patch for potential wound healing application. Int. J. Biol. Macromol. 2020, 143, 744–762. [Google Scholar] [CrossRef]
- Zhao, R.; Kong, W.; Sun, M.; Yang, Y.; Liu, W.; Lv, M.; Song, S.; Wang, L.; Song, H.; Hao, R. Highly Stable Graphene-Based Nanocomposite (GO-PEI-Ag) with Broad-Spectrum, Long-Term Antimicrobial Activity and Antibiofilm Effects. ACS Appl. Mater. Interfaces 2018, 10, 17617–17629. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, R.; Pandey, U.P.; Sahoo, B. Role of oxygen functionalities of GO in corrosion protection of metallic Fe. Carbon 2021, 173, 350–363. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Moore, R.B.; Mahajan, R.L. An Al-assisted GO/rGO Janus film: Fabrication and hygroscopic properties. Carbon 2021, 171, 585–596. [Google Scholar] [CrossRef]
- Khalil, W.F.; El-Sayyad, G.S.; El Rouby, W.M.A.; Sadek, M.A.; Farghali, A.A.; El-Batal, A.I. Graphene oxide-based nanocomposites (GO-chitosan and GO-EDTA) for outstanding antimicrobial potential against some Candida species and pathogenic bacteria. Int. J. Biol. Macromol. 2020, 164, 1370–1383. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Karmakar, R.S.; Lu, Y.J.; Chan, S.H.; Wu, M.C.; Lin, K.J.; Chen, C.K.; Wei, K.C.; Hsu, Y.H. Miniaturized Flexible Piezoresistive Pressure Sensors: Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) Copolymers Blended with Graphene Oxide for Biomedical Applications. ACS Appl. Mater. Interfaces 2019, 11, 34305–34315. [Google Scholar] [CrossRef]
- Kwon, Y.; Liu, M.; Castilho, C.; Saleeba, Z.; Hurt, R.; Külaots, I. Controlling pore structure and conductivity in graphene nanosheet films through partial thermal exfoliation. Carbon 2021, 174, 227–239. [Google Scholar] [CrossRef]
- Shuai, Y.; Yang, S.; Li, C.; Zhu, L.; Mao, C.; Yang, M. In situ protein-templated porous protein-hydroxylapatite nanocomposite microspheres for pH-dependent sustained anticancer drug release. J. Mater. Chem. B 2017, 5, 3945–3954. [Google Scholar] [CrossRef]
- Usman, A.; Hussain, Z.; Riaz, A.; Khan, A.N. Enhanced mechanical, thermal and antimicrobial properties of poly(vinyl alcohol)/graphene oxide/starch/silver nanocomposites films. Carbohydr. Polym. 2016, 153, 592–599. [Google Scholar] [CrossRef]
- Rui, L.; Xie, M.; Hu, B.; Zhou, L.; Yin, D.; Zeng, X. A comparative study on chitosan/gelatin composite films with conjugated or incorporated gallic acid. Carbohydr. Polym. 2017, 173, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Shan, X.; Zhao, X.; Zha, H.; Chen, X.; Wang, J.; Cai, C.; Wang, X.; Li, G.; Hao, J.; et al. Spongy bilayer dressing composed of chitosan-Ag nanoparticles and chitosan-Bletilla striata polysaccharide for wound healing applications. Carbohydr. Polym. 2017, 157, 1538–1547. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Wu, H.; Tong, C.; Pang, J.; Wu, C. Multifunctional bionanocomposite films based on konjac glucomannan/chitosan with nano-ZnO and mulberry anthocyanin extract for active food packaging. Food Hydrocoll. 2020, 107, 105942. [Google Scholar] [CrossRef]
- Omidi, S.; Kakanejadifard, A. Modification of chitosan and chitosan nanoparticle by long chain pyridinium compounds: Synthesis, characterization, antibacterial, and antioxidant activities. Carbohydr. Polym. 2019, 208, 477–485. [Google Scholar] [CrossRef]
- Sasidharan, A.; Panchakarla, L.S.; Sadanandan, A.R.; Ashokan, A.; Chandran, P.; Girish, C.M.; Menon, D.; Nair, S.V.; Rao, C.N.; Koyakutty, M. Hemocompatibility and macrophage response of pristine and functionalized graphene. Small 2012, 8, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Chen, L.; Hong, F.F. A Biodegradable Antibacterial Nanocomposite Based on Oxidized Bacterial Nanocellulose for Rapid Hemostasis and Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 3382–3392. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.Z.; Patino, J.; Cuadrado-Collados, C.; Tamayo, A.; Gutierrez, M.C.; Ferrer, M.L.; Silvestre-Albero, J.; Del Monte, F. Carbon-GO Composites with Preferential Water versus Ethanol Uptake. ACS Appl. Mater. Interfaces 2019, 11, 24493–24503. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Zhang, F.; Duan, J.; Jiang, J. Characterization of bacterial cellulose composite films incorporated with bulk chitosan and chitosan nanoparticles: A comparative study. Carbohydr. Polym. 2020, 237, 116167. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, J.; Huang, J.; Hui, M. Production and characterization of bacterial cellulose membranes with hyaluronic acid and silk sericin. Colloids Surf. B Biointerfaces 2020, 195, 111273. [Google Scholar] [CrossRef]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan based-asymmetric membranes for wound healing: A review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yao, X.; Liu, L.; Guan, J.; Liu, M.; Li, Z.; Yang, J.; Huang, S.; Wu, J.; Tian, F.; et al. Blood coagulation evaluation of N-alkylated chitosan. Carbohydr. Polym. 2017, 173, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, R.; Sundarrajan, S.; Vanangamudi, A.; Singh, G.; Matsuura, T.; Ramakrishna, S. Green Processing Mediated Novel Polyelectrolyte Nanofibers and Their Antimicrobial Evaluation. Macromol. Mater. Eng. 2014, 299, 283–289. [Google Scholar] [CrossRef]
- Chandrasekaran, A.R.; Venugopal, J.; Sundarrajan, S.; Ramakrishna, S. Fabrication of a nanofibrous scaffold with improved bioactivity for culture of human dermal fibroblasts for skin regeneration. Biomed. Mater. 2011, 6, 045010. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wang, D.; Ji, N.; Lee, S.; Wang, G.; Zheng, Y.; Zhang, X.; Yang, L.; Qin, Z.; Yang, Y. Bioinspired Design of Sericin/Chitosan/Ag@MOF/GO Hydrogels for Efficiently Combating Resistant Bacteria, Rapid Hemostasis, and Wound Healing. Polymers 2021, 13, 2812. https://doi.org/10.3390/polym13162812
Zhang M, Wang D, Ji N, Lee S, Wang G, Zheng Y, Zhang X, Yang L, Qin Z, Yang Y. Bioinspired Design of Sericin/Chitosan/Ag@MOF/GO Hydrogels for Efficiently Combating Resistant Bacteria, Rapid Hemostasis, and Wound Healing. Polymers. 2021; 13(16):2812. https://doi.org/10.3390/polym13162812
Chicago/Turabian StyleZhang, Meng, Dong Wang, Nana Ji, Shaoxiang Lee, Guohui Wang, Yuqi Zheng, Xin Zhang, Lin Yang, Zhiwei Qin, and Yang Yang. 2021. "Bioinspired Design of Sericin/Chitosan/Ag@MOF/GO Hydrogels for Efficiently Combating Resistant Bacteria, Rapid Hemostasis, and Wound Healing" Polymers 13, no. 16: 2812. https://doi.org/10.3390/polym13162812
APA StyleZhang, M., Wang, D., Ji, N., Lee, S., Wang, G., Zheng, Y., Zhang, X., Yang, L., Qin, Z., & Yang, Y. (2021). Bioinspired Design of Sericin/Chitosan/Ag@MOF/GO Hydrogels for Efficiently Combating Resistant Bacteria, Rapid Hemostasis, and Wound Healing. Polymers, 13(16), 2812. https://doi.org/10.3390/polym13162812




