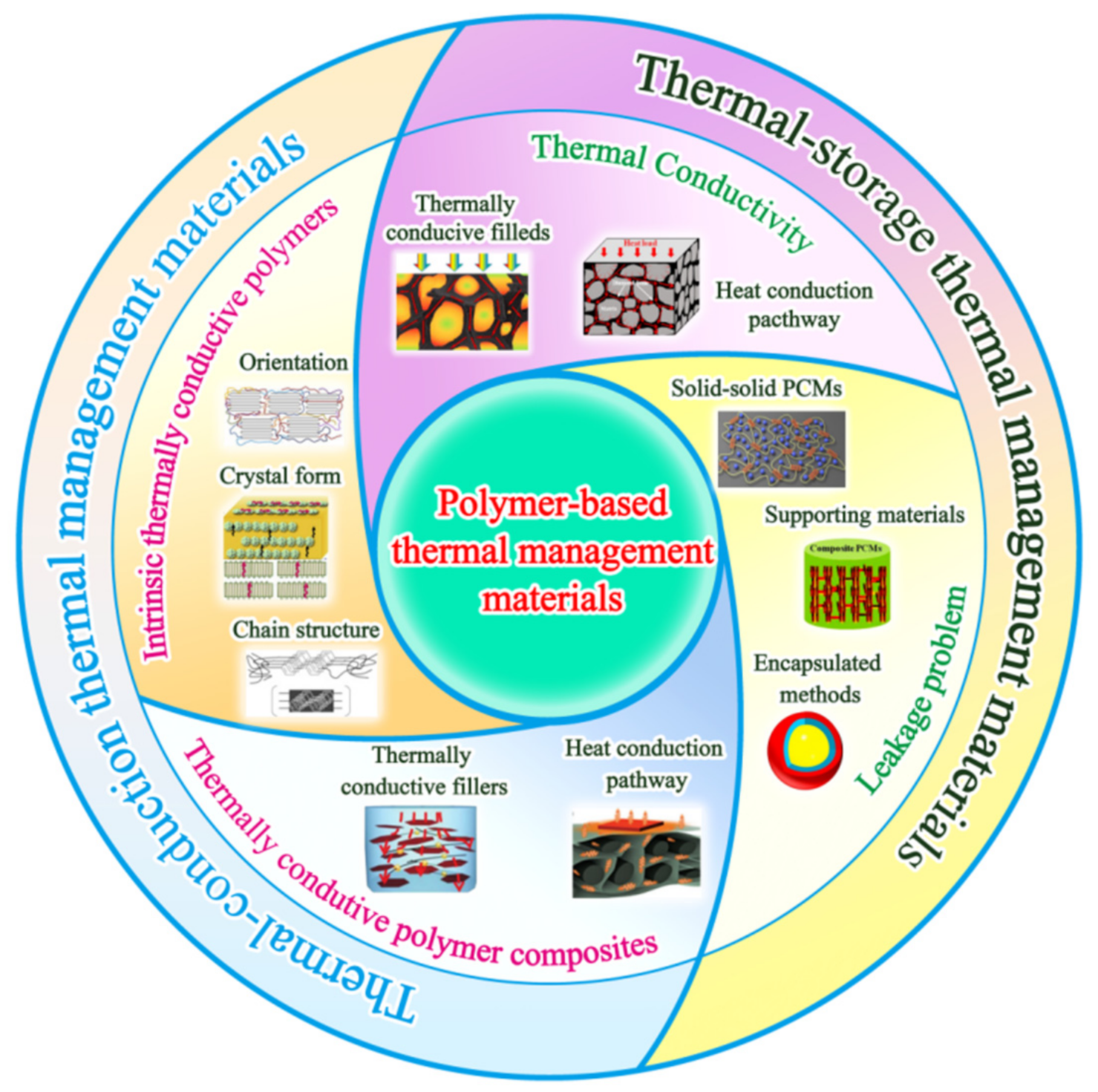
Recent Advances in Design and Preparation of Polymer-Based Thermal Management Material
Abstract
:1. Introduction
2. Intrinsic Thermally Conductive Polymers
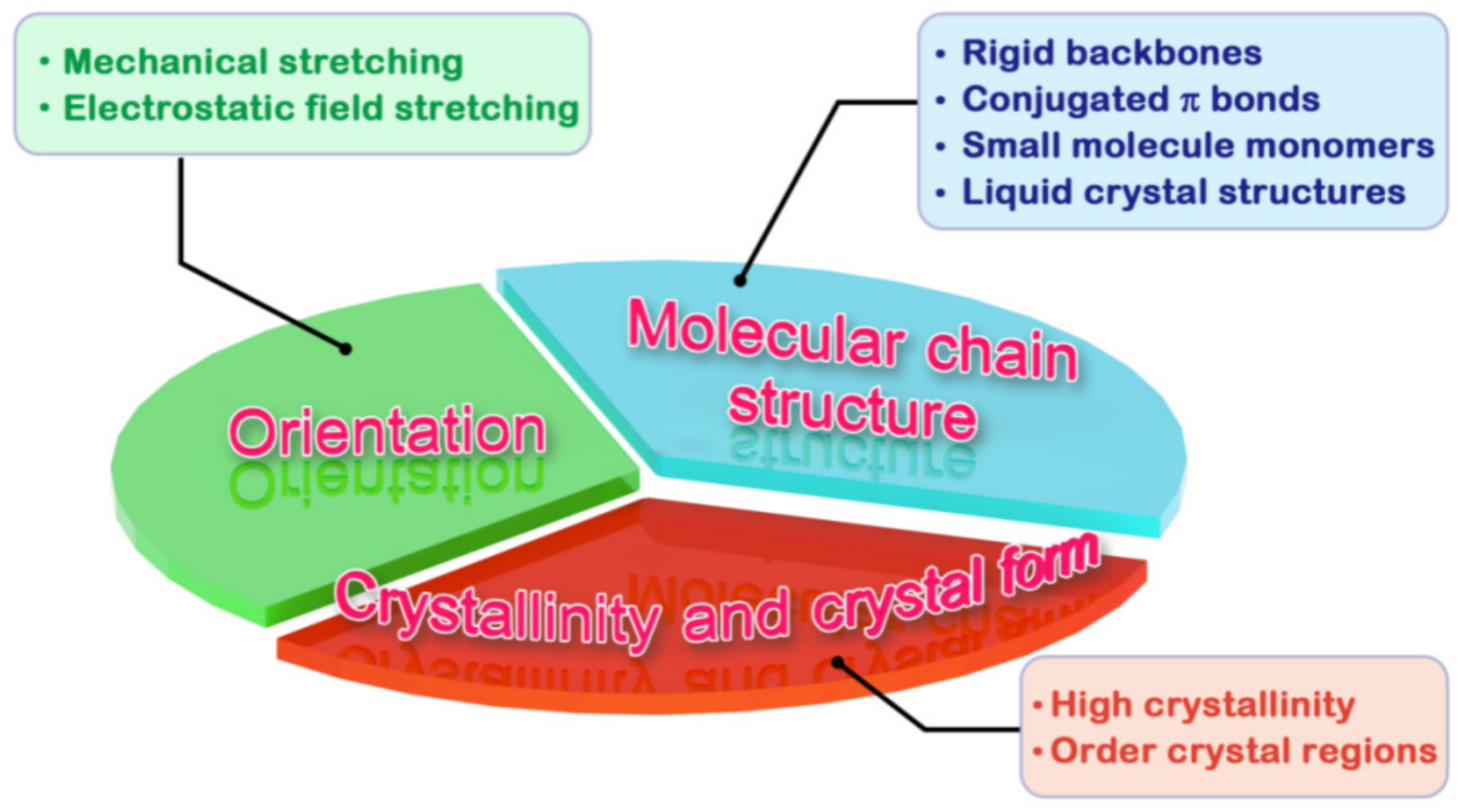
2.1. Molecular Chain Structure
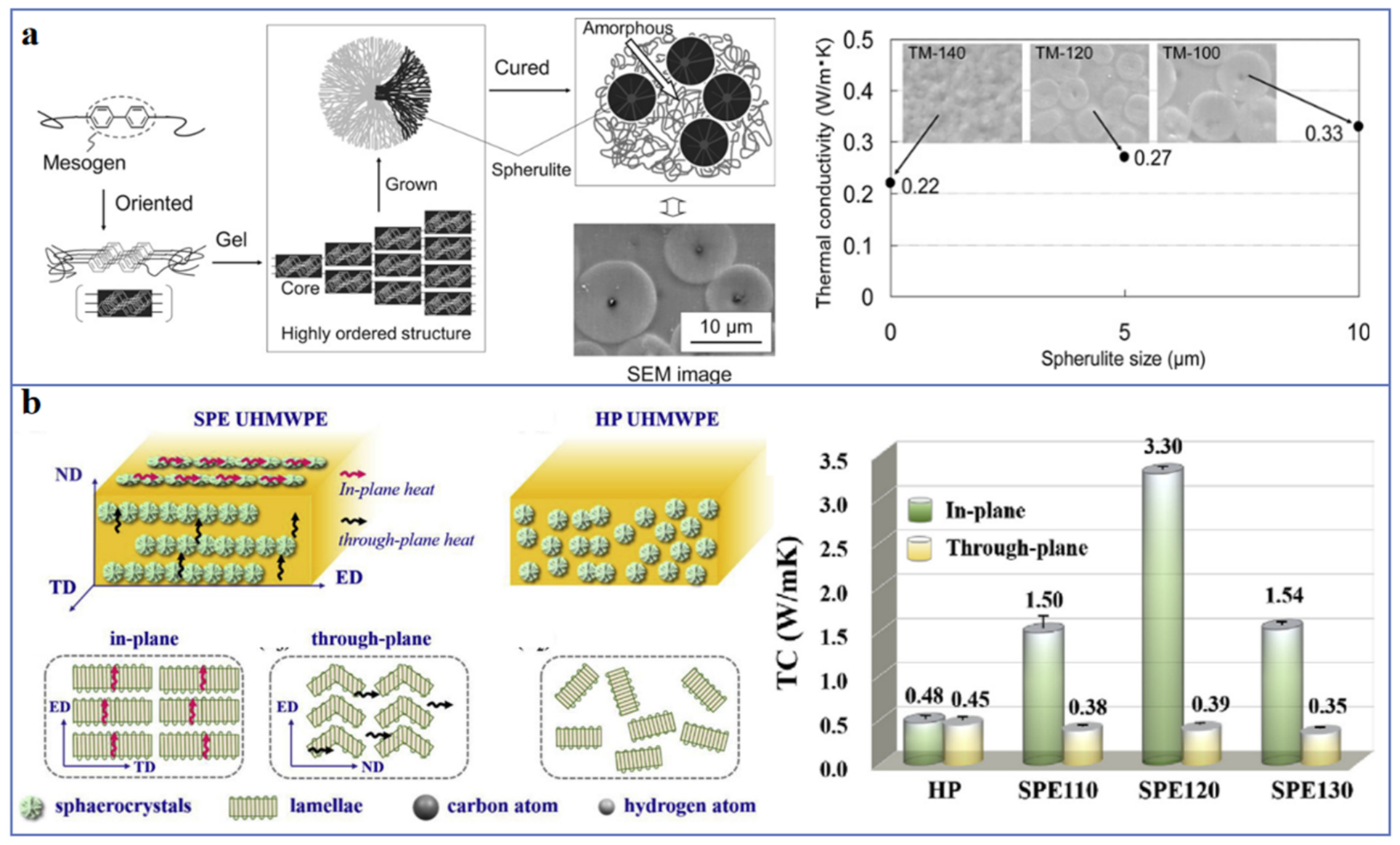
2.2. Crystallinity and Crystal Morphology
2.3. Orientation of Molecular Chains
3. Thermally Conductive Polymer Composites
3.1. Thermally Conductive Fillers
3.1.1. Carbon Material
3.1.2. Metals
3.1.3. Ceramics
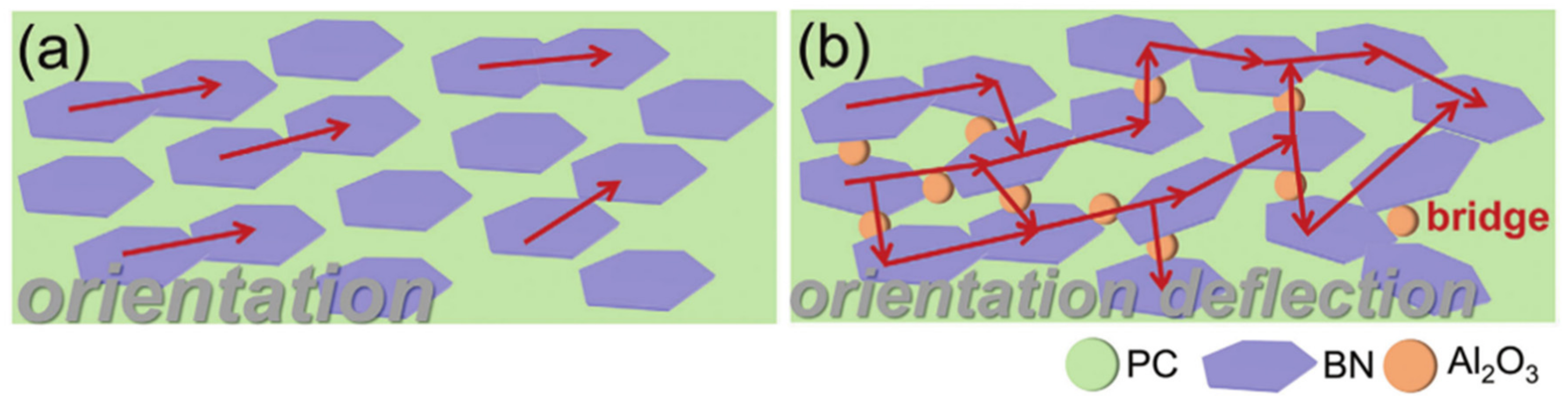
3.2. Strategies to Enhance the Thermal Conductivity
4. Thermally Conductive PCM (Thermal-Storage Thermal Management Material)
4.1. Shape-Stabilized Composite PCM
4.1.1. Encapsulated Composite PCM
4.1.2. Supporting Material
4.1.3. Solid–Solid Composite PCM
4.2. Strategies to Enhance the Thermal Conductivity of PCM
5. Thermal Management Applications
6. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, B.; Li, G.; Xu, L.; Liao, J.; Zhang, X. Nanoporous Boron Nitride Aerogel Film and Its Smart Composite with Phase Change Materials. ACS Nano 2020, 14, 16590–16599. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, F.; Cheng, W.; Chen, X.; Zhao, Q. Study of using enhanced heat-transfer flexible phase change material film in thermal management of compact electronic device. Energy Convers. Manag. 2020, 210, 112680. [Google Scholar] [CrossRef]
- He, X.; Wang, Y. Recent Advances in the Rational Design of Thermal Conductive Polymer Composites. Ind. Eng. Chem. Res. 2021, 60, 1137–1154. [Google Scholar] [CrossRef]
- Huang, C.; Qian, X.; Yang, R. Thermal conductivity of polymers and polymer nanocomposites. Mater. Sci. Eng. R Rep. 2018, 132, 1–22. [Google Scholar] [CrossRef] [Green Version]
- An, D.; Cheng, S.; Zhang, Z.; Jiang, C.; Fang, H.; Li, J.; Liu, Y.; Wong, C.-P. A polymer-based thermal management material with enhanced thermal conductivity by introducing three-dimensional networks and covalent bond connections. Carbon 2019, 155, 258–267. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Sun, B.; Jiang, P. Highly Thermally Conductive Yet Electrically Insulating Polymer/Boron Nitride Nanosheets Nanocomposite Films for Improved Thermal Management Capability. ACS Nano 2019, 13, 337–345. [Google Scholar] [CrossRef]
- Feng, C.P.; Bai, L.; Bao, R.-Y.; Wang, S.-W.; Liu, Z.; Yang, M.-B.; Chen, J.; Yang, W. Superior thermal interface materials for thermal management. Compos. Commun. 2019, 12, 80–85. [Google Scholar] [CrossRef]
- Kou, Y.; Sun, K.; Luo, J.; Zhou, F.; Huang, H.; Wu, Z.-S.; Shi, Q. An intrinsically flexible phase change film for wearable thermal managements. Energy Storage Mater. 2021, 34, 508–514. [Google Scholar] [CrossRef]
- Huang, J.; Wu, B.; Lyu, S.; Li, T.; Han, H.; Li, D.; Wang, J.K.; Zhang, J.; Lu, X.; Sun, D. Improving the thermal energy storage capability of diatom-based biomass/polyethylene glycol composites phase change materials by artificial culture methods. Sol. Energy Mater. Sol. Cells 2021, 219, 110797. [Google Scholar] [CrossRef]
- Yang, X.; Liang, C.; Ma, T.; Guo, Y.; Kong, J.; Gu, J.; Chen, M.; Zhu, J. A review on thermally conductive polymeric composites: Classification, measurement, model and equations, mechanism and fabrication methods. Adv. Compos. Hybrid Mater. 2018, 1, 207–230. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.-J.; Son, Y.-R.; In, I.; An, K.-H.; Kim, B.-J.; Park, S.-J. Recent advanced thermal interfacial materials: A review of conducting mechanisms and parameters of carbon materials. Carbon 2019, 142, 445–460. [Google Scholar] [CrossRef]
- Ma, H.; Gao, B.; Wang, M.; Yuan, Z.; Shen, J.; Zhao, J.; Feng, Y. Strategies for enhancing thermal conductivity of polymer-based thermal interface materials: A review. J. Mater. Sci. 2020, 56, 1064–1086. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef] [Green Version]
- Henry, A.; Chen, G. High thermal conductivity of single polyethylene chains using molecular dynamics simulations. Phys. Rev. Lett. 2008, 101, 235502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Hao, Q. A mini review on thermally conductive polymers and polymer-based composites. Compos. Commun. 2021, 24, 100617. [Google Scholar] [CrossRef]
- Langer, L.; Billaud, D.; Issi, J.-P. Thermal conductivity of stretched and annealed poly (p-phenylene sulfide) films. Solid State Commun. 2003, 126, 353–357. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, K.; Chen, W.; Nan, B.; Qu, Z.; Lu, M. High Intrinsic Thermal Conductivity of Polythiophene by Reducing Steric Hindrance and Enhancing p-π Conjugation. Macromol. Chem. Phys. 2021, 222, 2000418. [Google Scholar] [CrossRef]
- Harada, M.; Hamaura, N.; Ochi, M.; Agari, Y. Thermal conductivity of liquid crystalline epoxy/BN filler composites having ordered network structure. Compos. Part B 2013, 55, 306–313. [Google Scholar] [CrossRef]
- Song, S.-h.; Katagi, H.; Takezawa, Y. Study on high thermal conductivity of mesogenic epoxy resin with spherulite structure. Polymer 2012, 53, 4489–4492. [Google Scholar] [CrossRef]
- Ruan, K.; Guo, Y.; Gu, J. Liquid Crystalline Polyimide Films with High Intrinsic Thermal Conductivities and Robust Toughness. Macromolecules 2021, 54, 4934–4944. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Wang, Z.-G.; Yu, W.-C.; Ren, Y.; Lei, J.; Xu, J.-Z.; Li, Z.-M. Achieving high thermal conductivity and mechanical reinforcement in ultrahigh molecular weight polyethylene bulk material. Polymer 2019, 180, 121760. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Z.; Cheng, Z.; Xu, S.; Wang, X. Thermal Conductivity of Ultrahigh Molecular Weight Polyethylene Crystal: Defect Effect Uncovered by 0 K Limit Phonon Diffusion. ACS Appl. Mater. Interfaces 2015, 7, 27279–27288. [Google Scholar] [CrossRef]
- Yu, J.; Sundqvist, B.; Tonpheng, B.; Andersson, O. Thermal conductivity of highly crystallized polyethylene. Polymer 2014, 55, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Zhao, X.; Bao, R.-Y.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Effect of temperature, crystallinity and molecular chain orientation on the thermal conductivity of polymers: A case study of PLLA. J. Mater. Sci. 2018, 53, 10543–10553. [Google Scholar] [CrossRef]
- Zhong, Z.; Wingert, M.C.; Strzalka, J.; Wang, H.H.; Sun, T.; Wang, J.; Chen, R.; Jiang, Z. Structure-induced enhancement of thermal conductivities in electrospun polymer nanofibers. Nanoscale 2014, 6, 8283–8291. [Google Scholar] [CrossRef]
- Zhang, R.-C.; Huang, Z.; Sun, D.; Ji, D.; Zhong, M.; Zang, D.; Xu, J.-Z.; Wan, Y.; Lu, A. New insights into thermal conductivity of uniaxially stretched high density polyethylene films. Polymer 2018, 154, 42–47. [Google Scholar] [CrossRef]
- Choy, C.L.; Luk, W.H.; Chen, F.C. Thermal conductivity of highly oriented polyethylene. Polymer 1978, 19, 155–162. [Google Scholar] [CrossRef]
- Choy, C.L.; Wong, Y.W.; Yang, G.W.; Kanamoto, T. Elastic modulus and thermal conductivity of ultradrawn polyethylene. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 3359–3367. [Google Scholar] [CrossRef]
- Shen, S.; Henry, A.; Tong, J.; Zheng, R.; Chen, G. Polyethylene nanofibres with very high thermal conductivities. Nat. Nanotechnol. 2010, 5, 251–255. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Hou, X.; Zhan, Z.; Wang, H.; Fu, H.; Lin, C.-T.; Fu, L.; Jiang, N.; Yu, J. Highly thermal conductive and electrical insulating polymer composites with boron nitride. Compos. Part B 2020, 184, 107746. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, Y.; Zhou, S.; Zou, H.; Liang, M. A Concurrent Enhancement of Both In-Plane and Through-Plane Thermal Conductivity of Injection Molded Polycarbonate/Boron Nitride/Alumina Composites by Constructing a Dense Filler Packing Structure. Macromol. Mater. Eng. 2021, 2100267. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.; Liu, Y.; Wang, R.; Gao, C. Multifunctional non-woven fabrics of interfused graphene fibres. Nat. Commun. 2016, 7, 13684. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wu, W.; Liu, C.; Wang, Y.; Chen, Q.; Cui, S. Preparation and mechanism research of bio-inspired dopamine decorated expanded graphite/silicone rubber composite with high thermal conductivity and excellent insulation. Nanotechnology 2021, 32, 325702. [Google Scholar] [CrossRef]
- Guerra, V.; Wan, C.; McNally, T. Thermal conductivity of 2D nano-structured boron nitride (BN) and its composites with polymers. Prog. Mater. Sci. 2019, 100, 170–186. [Google Scholar] [CrossRef]
- Luo, S.; Yu, J.; Yu, S.; Sun, R.; Cao, L.; Liao, W.-H.; Wong, C.-P. Significantly Enhanced Electrostatic Energy Storage Performance of Flexible Polymer Composites by Introducing Highly Insulating-Ferroelectric Microhybrids as Fillers. Adv. Energy Mater. 2019, 9, 1803204. [Google Scholar] [CrossRef]
- Guo, Y.; Ruan, K.; Shi, X.; Yang, X.; Gu, J. Factors affecting thermal conductivities of the polymers and polymer composites: A review. Compos. Sci. Technol. 2020, 193, 108134. [Google Scholar] [CrossRef]
- Lin, W.; Moon, K.-S.; Wong, C.P. A Combined Process of In Situ Functionalization and Microwave Treatment to Achieve Ultrasmall Thermal Expansion of Aligned Carbon Nanotube-Polymer Nanocomposites: Toward Applications as Thermal Interface Materials. Adv. Mater. 2009, 21, 2421–2424. [Google Scholar] [CrossRef]
- Ren, P.-G.; Si, X.-H.; Sun, Z.-F.; Ren, F.; Pei, L.; Hou, S.-Y. Synergistic effect of BN and MWCNT hybrid fillers on thermal conductivity and thermal stability of ultra-high-molecular-weight polyethylene composites with a segregated structure. J. Polym. Res. 2016, 23, 21. [Google Scholar] [CrossRef]
- Ma, J.; Shang, T.; Ren, L.; Yao, Y.; Zhang, T.; Xie, J.; Zhang, B.; Zeng, X.; Sun, R.; Xu, J.-B.; et al. Through-plane assembly of carbon fibers into 3D skeleton achieving enhanced thermal conductivity of a thermal interface material. Chem. Eng. J. 2020, 380, 122550. [Google Scholar] [CrossRef]
- Uetani, K.; Ata, S.; Tomonoh, S.; Yamada, T.; Yumura, M.; Hata, K. Elastomeric thermal interface materials with high through-plane thermal conductivity from carbon fiber fillers vertically aligned by electrostatic flocking. Adv. Mater. 2014, 26, 5857–5862. [Google Scholar] [CrossRef]
- Liu, H.; Gu, S.; Cao, H.; Li, X.; Li, Y. A dense packing structure constructed by flake and spherical graphite: Simultaneously enhanced in-plane and through-plane thermal conductivity of polypropylene/graphite composites. Compos. Commun. 2020, 19, 25–29. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Xie, B.; Zhao, X.; Chen, J.; Chen, Z.; Long, Y. Stearic acid/expanded graphite as a composite phase change thermal energy storage material for tankless solar water heater. Sustain. Cities Soc. 2019, 44, 458–464. [Google Scholar] [CrossRef]
- Luo, D.; Xiang, L.; Sun, X.; Xie, L.; Zhou, D.; Qin, S. Phase-change smart lines based on paraffin-expanded graphite/polypropylene hollow fiber membrane composite phase change materials for heat storage. Energy 2020, 197, 117252. [Google Scholar] [CrossRef]
- Li, Y.; Wei, W.; Wang, Y.; Kadhim, N.; Mei, Y.; Zhou, Z. Construction of highly aligned graphene-based aerogels and their epoxy composites towards high thermal conductivity. J. Mater. Chem. C 2019, 7, 11783–11789. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Jiang, G.; Cano, Z.P.; Yang, J.; Wang, J.; Liu, J.; Chen, X.; Chen, Z. Boosting the Heat Dissipation Performance of Graphene/Polyimide Flexible Carbon Film via Enhanced Through-Plane Conductivity of 3D Hybridized Structure. Small 2020, 16, e1903315. [Google Scholar] [CrossRef]
- Feng, C.P.; Chen, L.B.; Tian, G.L.; Bai, L.; Bao, R.Y.; Liu, Z.Y.; Ke, K.; Yang, M.B.; Yang, W. Robust polymer-based paper-like thermal interface materials with a through-plane thermal conductivity over 9 Wm−1K−1. Chem. Eng. J. 2020, 392, 123784. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, G.; Tang, M.; Zhou, L.; Li, J.; Fan, X.; Shi, X.; Qin, J. Synergistic effect of carbon nanotube and graphene nanoplates on the mechanical, electrical and electromagnetic interference shielding properties of polymer composites and polymer composite foams. Chem. Eng. J. 2018, 353, 381–393. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Pan, S.; Zhang, J.; Liu, Y.; Liu, J.; Lu, H. Highly Oriented Graphite Aerogel Fabricated by Confined Liquid-Phase Expansion for Anisotropically Thermally Conductive Epoxy Composites. ACS Appl. Mater. Interfaces 2020, 12, 27476–27484. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Dai, F. Epoxy Nanocomposites with Reduced Graphene Oxide-Constructed Three-Dimensional Networks of Single Wall Carbon Nanotube for Enhanced Thermal Management Capability with Low Filler Loading. ACS Appl. Mater. Interfaces 2020, 12, 3051–3058. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yao, Y.; Gong, Z.; Wang, F.; Sun, R.; Xu, J.; Wong, C.P. Ice-Templated Assembly Strategy to Construct 3D Boron Nitride Nanosheet Networks in Polymer Composites for Thermal Conductivity Improvement. Small 2015, 11, 6205–6213. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, Y.; Feng, W. Three-dimensional interconnected networks for thermally conductive polymer composites: Design, preparation, properties, and mechanisms. Mater. Sci. Eng. R Rep. 2020, 142, 100580. [Google Scholar] [CrossRef]
- Li, X.-H.; Liu, P.; Li, X.; An, F.; Min, P.; Liao, K.-N.; Yu, Z.-Z. Vertically aligned, ultralight and highly compressive all-graphitized graphene aerogels for highly thermally conductive polymer composites. Carbon 2018, 140, 624–633. [Google Scholar] [CrossRef]
- Yang, S.; Xue, B.; Li, Y.; Li, X.; Xie, L.; Qin, S.; Xu, K.; Zheng, Q. Controllable Ag-rGO heterostructure for highly thermal conductivity in layer-by-layer nanocellulose hybrid films. Chem. Eng. J. 2020, 383, 123072. [Google Scholar] [CrossRef]
- Ma, Z.; Kang, S.; Ma, J.; Shao, L.; Zhang, Y.; Liu, C.; Wei, A.; Xiang, X.; Wei, L.; Gu, J. Ultraflexible and Mechanically Strong Double-Layered Aramid Nanofiber-Ti3C2Tx MXene/Silver Nanowire Nanocomposite Papers for High-Performance Electromagnetic Interference Shielding. ACS Nano 2020, 14, 8368–8382. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Lee, J.W.; Han, T.H.; Park, C.; Kwon, Y.; Hong, S.M.; Koo, C.M. Copper shell networks in polymer composites for efficient thermal conduction. ACS Appl. Mater. Interfaces 2013, 5, 11618–11622. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Xu, P.; Wang, Y.; Shang, Y.; Ma, J.; Su, F.; Feng, Y.; He, C.; Wang, Y.; Liu, C. Flexible polyvinylidene fluoride film with alternating oriented graphene/Ni nanochains for electromagnetic interference shielding and thermal management. Chem. Eng. J. 2020, 395, 125209. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, P.; Xie, L. Ferroelectric polymer/silver nanocomposites with high dielectric constant and high thermal conductivity. Appl. Phys. Lett. 2009, 95, 242901. [Google Scholar] [CrossRef] [Green Version]
- Bhanushali, S.; Ghosh, P.C.; Simon, G.P.; Cheng, W. Copper Nanowire-Filled Soft Elastomer Composites for Applications as Thermal Interface Materials. Adv. Mater. Interfaces 2017, 4, 1700387. [Google Scholar] [CrossRef]
- Jin, Z.; Liang, F.; Lu, W.; Dai, J.; Meng, S.; Lin, Z. Effect of Particle Sizes of Nickel Powder on Thermal Conductivity of Epoxy Resin-Based Composites under Magnetic Alignment. Polymers 2019, 11, 1990. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Y.; Ding, F.; Bai, L.; Li, X.; Hou, G.; Fan, J.; Yuan, F. Design of network Al2O3 spheres for significantly enhanced thermal conductivity of polymer composites. Compos. Part A 2020, 128, 105673. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Wang, L.; Yu, K.; Lin, Z.; He, L.; Bai, Y. Fabrication and characterization of aluminum nitride polymer matrix composites with high thermal conductivity and low dielectric constant for electronic packaging. Mater. Sci. Eng. B 2012, 177, 892–896. [Google Scholar] [CrossRef]
- Dai, W.; Lv, L.; Lu, J.; Hou, H.; Yan, Q.; Alam, F.E.; Li, Y.; Zeng, X.; Yu, J.; Wei, Q.; et al. A Paper-Like Inorganic Thermal Interface Material Composed of Hierarchically Structured Graphene/Silicon Carbide Nanorods. ACS Nano 2019, 13, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Liu, X. Novel Functionalized BN Nanosheets/Epoxy Composites with Advanced Thermal Conductivity and Mechanical Properties. ACS Appl. Mater. Interfaces 2020, 12, 6503–6515. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Wu, K.; Wu, L.; Liu, W.; Du, R.; Chen, F.; Fu, Q. Phase change material with anisotropically high thermal conductivity and excellent shape stability due to its robust cellulose/BNNSs skeleton. J. Mater. Chem. A 2019, 7, 19364–19373. [Google Scholar] [CrossRef]
- Feng, C.-P.; Bai, L.; Bao, R.-Y.; Liu, Z.-Y.; Yang, M.-B.; Chen, J.; Yang, W. Electrically insulating POE/BN elastomeric composites with high through-plane thermal conductivity fabricated by two-roll milling and hot compression. Adv. Compos. Hybrid Mater. 2017, 1, 160–167. [Google Scholar] [CrossRef]
- Han, J.; Du, G.; Gao, W.; Bai, H. An Anisotropically High Thermal Conductive Boron Nitride/Epoxy Composite Based on Nacre-Mimetic 3D Network. Adv. Funct. Mater. 2019, 29, 1900412. [Google Scholar] [CrossRef]
- Cheng, S.; Duan, X.; Liu, X.; Zhang, Z.; An, D.; Zhao, G.; Liu, Y. Achieving significant thermal conductivity improvement via constructing vertically arranged and covalently bonded silicon carbide nanowires/natural rubber composites. J. Mater. Chem. C 2021, 9, 7127–7141. [Google Scholar] [CrossRef]
- Ganguli, S.; Roy, A.K.; Anderson, D.P. Improved thermal conductivity for chemically functionalized exfoliated graphite/epoxy composites. Carbon 2008, 46, 806–817. [Google Scholar] [CrossRef]
- Zhou, W. Effect of coupling agents on the thermal conductivity of aluminum particle/epoxy resin composites. J. Mater. Sci. 2011, 46, 3883–3889. [Google Scholar] [CrossRef]
- Shen, H.; Guo, J.; Wang, H.; Zhao, N.; Xu, J. Bioinspired modification of h-BN for high thermal conductive composite films with aligned structure. ACS Appl. Mater. Interfaces 2015, 7, 5701–5708. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hou, X.; Liao, M.; Dai, W.; Wang, Z.; Yan, C.; Li, H.; Lin, C.-T.; Jiang, N.; Yu, J. Constructing a “pea-pod-like” alumina-graphene binary architecture for enhancing thermal conductivity of epoxy composite. Chem. Eng. J. 2020, 381, 122690. [Google Scholar] [CrossRef]
- Hu, J.; Huang, Y.; Yao, Y.; Pan, G.; Sun, J.; Zeng, X.; Sun, R.; Xu, J.B.; Song, B.; Wong, C.P. Polymer Composite with Improved Thermal Conductivity by Constructing a Hierarchically Ordered Three-Dimensional Interconnected Network of BN. ACS Appl. Mater. Interfaces 2017, 9, 13544–13553. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, J.; Zeng, X.; Sun, R.; Xu, J.B.; Wong, C.P. Construction of 3D Skeleton for Polymer Composites Achieving a High Thermal Conductivity. Small 2018, 14, e1704044. [Google Scholar] [CrossRef]
- Xu, S.; Wang, S.; Chen, Z.; Sun, Y.; Gao, Z.; Zhang, H.; Zhang, J. Electric-Field-Assisted Growth of Vertical Graphene Arrays and the Application in Thermal Interface Materials. Adv. Funct. Mater. 2020, 30, 2003302. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, J.; Wang, H.; Qian, L.; Lan, H. Continuous DLP-based ceramic 3D printing using a composite oxygen-rich film. J. Manuf. Process. 2021, 64, 341–348. [Google Scholar] [CrossRef]
- Zhang, G.; Lan, H.; Qian, L.; Zhao, J.; Wang, F. A Microscale 3D Printing Based on the Electric-Field-Driven Jet. 3d Print. Addit. Manuf. 2020, 7, 37–44. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Z.; Hu, Y.; Li, H.; Yang, J.; Lan, H. Facile fabrication of defogging microlens arrays using electric field-driven jet printing. Opt. Laser Technol. 2020, 123, 105943. [Google Scholar] [CrossRef]
- Li, G.; Hong, G.; Dong, D.; Song, W.; Zhang, X. Multiresponsive Graphene-Aerogel-Directed Phase-Change Smart Fibers. Adv. Mater. 2018, 30, e1801754. [Google Scholar] [CrossRef]
- Song, Q.; Zhu, W.; Deng, Y.; Hai, F.; Wang, Y.; Guo, Z. Enhanced through-plane thermal conductivity and high electrical insulation of flexible composite films with aligned boron nitride for thermal interface material. Compos. Part A 2019, 127, 105654. [Google Scholar] [CrossRef]
- Hyder, M.N.; Kavian, R.; Sultana, Z.; Saetia, K.; Chen, P.-Y.; Lee, S.W.; Shao-Horn, Y.; Hammond, P.T. Vacuum-Assisted Layer-by-Layer Nanocomposites for Self-Standing 3D Mesoporous Electrodes. Chem. Mater. 2014, 26, 5310–5318. [Google Scholar] [CrossRef]
- Wu, Y.; Xue, Y.; Qin, S.; Liu, D.; Wang, X.; Hu, X.; Li, J.; Wang, X.; Bando, Y.; Golberg, D.; et al. BN Nanosheet/Polymer Films with Highly Anisotropic Thermal Conductivity for Thermal Management Applications. ACS Appl. Mater. Interfaces 2017, 9, 43163–43170. [Google Scholar] [CrossRef]
- Zeng, X.; Sun, J.; Yao, Y.; Sun, R.; Xu, J.B.; Wong, C.P. A Combination of Boron Nitride Nanotubes and Cellulose Nanofibers for the Preparation of a Nanocomposite with High Thermal Conductivity. ACS Nano 2017, 11, 5167–5178. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tang, L.-S.; Bai, L.; Bao, R.-Y.; Liu, Z.; Xie, B.-H.; Yang, M.-B.; Yang, W. Photodriven Shape-Stabilized Phase Change Materials with Optimized Thermal Conductivity by Tailoring the Microstructure of Hierarchically Ordered Hybrid Porous Scaffolds. ACS Sustain. Chem. Eng. 2018, 6, 6761–6770. [Google Scholar] [CrossRef]
- Qin, M.; Feng, Y.; Ji, T.; Feng, W. Enhancement of cross-plane thermal conductivity and mechanical strength via vertical aligned carbon nanotube@graphite architecture. Carbon 2016, 104, 157–168. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, H.; Niu, H.; Ren, Y.; Fang, H.; Fang, X.; Lv, R.; Maqbool, M.; Bai, S. Highly Thermally Conductive 3D Printed Graphene Filled Polymer Composites for Scalable Thermal Management Applications. ACS Nano 2021, 15, 6917–6928. [Google Scholar] [CrossRef]
- Lai, K.C.; Lee, L.Y.; Hiew, B.Y.Z.; Thangalazhy-Gopakumar, S.; Gan, S. Environmental application of three-dimensional graphene materials as adsorbents for dyes and heavy metals: Review on ice-templating method and adsorption mechanisms. J. Environ. Sci. 2019, 79, 174–199. [Google Scholar] [CrossRef]
- Xue, F.; Jin, X.Z.; Wang, W.Y.; Qi, X.D.; Yang, J.H.; Wang, Y. Melamine foam and cellulose nanofiber co-mediated assembly of graphene nanoplatelets to construct three-dimensional networks towards advanced phase change materials. Nanoscale 2020, 12, 4005–4017. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, M.; Qi, X.; Li, H.; Zhang, Y.F.; Li, Z.; Peng, Z.; Yang, J.; Qian, L.; Xu, Q.; et al. Templateless, Plating-Free Fabrication of Flexible Transparent Electrodes with Embedded Silver Mesh by Electric-Field-Driven Microscale 3D Printing and Hybrid Hot Embossing. Adv. Mater. 2021, 33, e2007772. [Google Scholar] [CrossRef]
- Guo, P.; Lin, X.; Liu, J.; Xu, J.; Li, J.; Zhang, Y.; Lu, X.; Qu, N.; Lan, H.; Huang, W. Passive behavior of nickel-based superalloys prepared by high-deposition-rate laser solid forming additive manufacturing. Corros. Sci. 2020, 177, 109036. [Google Scholar] [CrossRef]
- Smith, P.M.; Su, L.; Gong, W.; Nakamura, N.; Reeja-Jayan, B.; Shen, S. Thermal conductivity of poly(3,4-ethylenedioxythiophene) films engineered by oxidative chemical vapor deposition (oCVD). RSC Adv. 2018, 8, 19348–19352. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Lv, Z.; Wu, Y.; Guo, Y.; Tian, L.; Qiu, H.; Li, W.; Zhang, Q. Dielectric thermally conductive boron nitride/polyimide composites with outstanding thermal stabilities via in -situ polymerization-electrospinning-hot press method. Compos. Part A 2017, 94, 209–216. [Google Scholar] [CrossRef]
- Yang, J.; Tang, L.-S.; Bai, L.; Bao, R.-Y.; Liu, Z.-Y.; Xie, B.-H.; Yang, M.-B.; Yang, W. High-performance composite phase change materials for energy conversion based on macroscopically three-dimensional structural materials. Mater. Horiz. 2019, 6, 250–273. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, P.; Tang, Z.; Xu, X.; Gao, H.; Wang, G. Carbon-Based Composite Phase Change Materials for Thermal Energy Storage, Transfer, and Conversion. Adv. Sci. 2021, 8, 2001274. [Google Scholar] [CrossRef]
- Cao, R.; Sun, D.; Wang, L.; Yan, Z.; Liu, W.; Wang, X.; Zhang, X. Enhancing solar–thermal–electric energy conversion based on m-PEGMA/GO synergistic phase change aerogels. J. Mater. Chem. A 2020, 8, 13207–13217. [Google Scholar] [CrossRef]
- Lu, X.; Huang, J.; Kang, B.; Yuan, T.; Qu, J. Bio-based poly (lactic acid)/high-density polyethylene blends as shape-stabilized phase change material for thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2019, 192, 170–178. [Google Scholar] [CrossRef]
- Chen, X.; Gao, H.; Tang, Z.; Dong, W.; Li, A.; Wang, G. Optimization strategies of composite phase change materials for thermal energy storage, transfer, conversion and utilization. Energy Environ. Sci. 2020, 13, 4498–4535. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, Y.; Cong, R.; Ran, F.; Fang, G. Improved thermal properties of stearic acid/high density polyethylene/carbon fiber composite heat storage materials. Sol. Energy Mater. Sol. Cells 2021, 219, 110782. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Ok, Y.S.; Kua, H.W.; Kim, S. Thermal properties of composite organic phase change materials (PCMs): A critical review on their engineering chemistry. Appl. Therm. Eng. 2020, 181, 115960. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, H.; Chen, X.; Zhang, Y.; Li, A.; Wang, G. Advanced multifunctional composite phase change materials based on photo-responsive materials. Nano Energy 2021, 80, 105454. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, L.; Shen, C.; Mao, Z.; Xu, H.; Feng, X.; Wang, B.; Sui, X. Boron nitride microsheets bridged with reduced graphene oxide as scaffolds for multifunctional shape stabilized phase change materials. Sol. Energy Mater. Sol. Cells 2020, 209, 110441. [Google Scholar] [CrossRef]
- Wu, H.; Chen, R.; Shao, Y.; Qi, X.; Yang, J.; Wang, Y. Novel Flexible Phase Change Materials with Mussel-Inspired Modification of Melamine Foam for Simultaneous Light-Actuated Shape Memory and Light-to-Thermal Energy Storage Capability. ACS Sustain. Chem. Eng. 2019, 7, 13532–13542. [Google Scholar] [CrossRef]
- Du, X.; Qiu, J.; Deng, S.; Du, Z.; Cheng, X.; Wang, H. Flame-retardant and form-stable phase change composites based on black phosphorus nanosheets/cellulose nanofiber aerogels with extremely high energy storage density and superior solar-thermal conversion efficiency. J. Mater. Chem. A 2020, 8, 14126–14134. [Google Scholar] [CrossRef]
- Wu, S.; Li, T.; Wu, M.; Xu, J.; Hu, Y.; Chao, J.; Yan, T.; Wang, R. Highly thermally conductive and flexible phase change composites enabled by polymer/graphite nanoplatelet-based dual networks for efficient thermal management. J. Mater. Chem. A 2020, 8, 20011–20020. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic phase change materials and their textile applications: An overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.C.; Sandaran, S.C. Developments in organic solid–liquid phase change materials and their applications in thermal energy storage. Energy Convers. Manag. 2015, 95, 193–228. [Google Scholar] [CrossRef] [Green Version]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid–liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Tao, W.; Cao, X.; He, Y. Fatty acids as phase change materials: A review. Renew. Sustain. Energy Rev. 2014, 29, 482–498. [Google Scholar] [CrossRef]
- Rathore, P.K.S.; Shukla, S.K. Enhanced thermophysical properties of organic PCM through shape stabilization for thermal energy storage in buildings: A state of the art review. Energy Build. 2021, 236, 110799. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, L.; Wan, H.; Liu, H.; Wu, D.; Wang, X. Construction of polyaniline/carbon nanotubes-functionalized phase-change microcapsules for thermal management application of supercapacitors. Chem. Eng. J. 2020, 396, 125317. [Google Scholar] [CrossRef]
- Yang, J.; Qi, G.-Q.; Bao, R.-Y.; Yi, K.; Li, M.; Peng, L.; Cai, Z.; Yang, M.-B.; Wei, D.; Yang, W. Hybridizing graphene aerogel into three-dimensional graphene foam for high-performance composite phase change materials. Energy Storage Mater. 2018, 13, 88–95. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Sheng, D.; Lin, C.; Ji, F.; Dong, L.; Xu, S.; Wu, H.; Yang, Y. Polyurethane-based solid-solid phase change materials with in situ reduced graphene oxide for light-thermal energy conversion and storage. Chem. Eng. J. 2018, 338, 117–125. [Google Scholar] [CrossRef]
- Jacob, R.; Bruno, F. Review on shell materials used in the encapsulation of phase change materials for high temperature thermal energy storage. Renew. Sustain. Energy Rev. 2015, 48, 79–87. [Google Scholar] [CrossRef]
- Singh, J.; Vennapusa, J.R.; Chattopadhyay, S. Protein-polysaccharide based microencapsulated phase change material composites for thermal energy storage. Carbohydr. Polym. 2020, 229, 115531. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yang, R. Efficient preparation and characterization of paraffin-based microcapsules by emulsion polymerization. J. Appl. Polym. Sci. 2019, 136, 47552. [Google Scholar] [CrossRef]
- Kahraman Döğüşcü, D.; Kızıl, Ç.; Biçer, A.; Sarı, A.; Alkan, C. Microencapsulated n -alkane eutectics in polystyrene for solar thermal applications. Sol. Energy 2018, 160, 32–42. [Google Scholar] [CrossRef]
- Srinivasaraonaik, B.; Singh, L.P.; Tyagi, I.; Rawat, A.; Sinha, S. Microencapsulation of a eutectic PCM using in situ polymerization technique for thermal energy storage. Int. J. Energy Res. 2020, 44, 3854–3864. [Google Scholar]
- Methaapanon, R.; Kornbongkotmas, S.; Ataboonwongse, C.; Soottitantawat, A. Microencapsulation of n-octadecane and methyl palmitate phase change materials in silica by spray drying process. Powder Technol. 2020, 361, 910–916. [Google Scholar] [CrossRef]
- Paneliya, S.; Khanna, S.; Utsav; Singh, A.P.; Patel, Y.K.; Vanpariya, A.; Makani, N.H.; Banerjee, R.; Mukhopadhyay, I. Core shell paraffin/silica nanocomposite: A promising phase change material for thermal energy storage. Renew. Energy 2021, 167, 591–599. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Luo, J.; Liu, Y.; Song, G.; Tang, G. Fabrication and properties of microencapsulated n-octadecane with TiO 2 shell as thermal energy storage materials. Sol. Energy 2016, 127, 28–35. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Luo, R.; Zhu, C.; Akiyama, T.; Zhang, Z. Microencapsulation of phase change materials with binary cores and calcium carbonate shell for thermal energy storage. Appl. Energy. 2016, 171, 113–119. [Google Scholar] [CrossRef]
- Weinstock, L.; Sanguramath, R.A.; Silverstein, M.S. Encapsulating an organic phase change material within emulsion-templated poly(urethane urea)s. Polym. Chem. 2019, 10, 1498–1507. [Google Scholar] [CrossRef]
- Zhu, Y.; Qin, Y.; Wei, C.; Liang, S.; Luo, X.; Wang, J.; Zhang, L. Nanoencapsulated phase change materials with polymer-SiO2 hybrid shell materials: Compositions, morphologies, and properties. Energy Convers. Manag. 2018, 164, 83–92. [Google Scholar] [CrossRef]
- Geng, X.; Gao, Y.; Wang, N.; Han, N.; Zhang, X.; Li, W. Intelligent adjustment of light-to-thermal energy conversion efficiency of thermo-regulated fabric containing reversible thermochromic MicroPCMs. Chem. Eng. J. 2021, 408, 127276. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Li, X.; Wang, B.; Feng, X.; Xu, H.; Mao, Z.; Sui, X. Cellulose nanocrystals-composited poly (methyl methacrylate) encapsulated n-eicosane via a Pickering emulsion-templating approach for energy storage. Carbohydr. Polym. 2020, 234, 115934. [Google Scholar] [CrossRef] [PubMed]
- Naikwadi, A.T.; Samui, A.B.; Mahanwar, P.A. Melamine-formaldehyde microencapsulated n-Tetracosane phase change material for solar thermal energy storage in coating. Sol. Energy Mater. Sol. Cells 2020, 215, 110676. [Google Scholar] [CrossRef]
- Cárdenas-Ramírez, C.; Jaramillo, F.; Gómez, M. Systematic review of encapsulation and shape-stabilization of phase change materials. J. Energy Storage 2020, 30, 101495. [Google Scholar] [CrossRef]
- Fang, G.; Chen, Z.; Li, H. Synthesis and properties of microencapsulated paraffin composites with SiO2 shell as thermal energy storage materials. Chem. Eng. J. 2010, 163, 154–159. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D.; Ji, S. Morphology-controlled synthesis of microencapsulated phase change materials with TiO2 shell for thermal energy harvesting and temperature regulation. Energy 2019, 172, 599–617. [Google Scholar] [CrossRef]
- Zhai, D.; He, Y.; Zhang, X.; Li, W. Preparation, Morphology, and Thermal Performance of Microencapsulated Phase Change Materials with a MF/SiO2 Composite Shell. Energy Fuels 2020, 34, 16819–16830. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, T.; Luo, G.; Zheng, B.; Huang, H.; Ma, R.; Han, X.; Chai, Y. Enhanced thermal and mechanical properties of PW-based HTPB binder using polystyrene (PS) and PS-SiO2 microencapsulated paraffin wax (MePW). J. Appl. Polym. Sci. 2018, 135, 46222. [Google Scholar] [CrossRef]
- Song, S.; Li, J.; Yang, Z.; Wang, C. Enhancement of Thermo-Physical Properties of Expanded Vermiculite-Based Organic Composite Phase Change Materials for Improving the Thermal Energy Storage Efficiency. ACS Omega 2021, 6, 3891–3899. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Li, J.; Min, X.; Guan, W.; Deng, Y.; Ning, L. Enhanced thermal conductivity of PEG/diatomite shape-stabilized phase change materials with Ag nanoparticles for thermal energy storage. J. Mater. Chem. A 2015, 3, 8526–8536. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K.; Kahwash, F.; Makhkamova, I. Enhancing thermal conductivity of paraffin wax 53–57 °C using expanded graphite. Sol. Energy Mater. Sol. Cells 2019, 200, 110026. [Google Scholar] [CrossRef]
- Feng, L.; Zheng, J.; Yang, H.; Guo, Y.; Li, W.; Li, X. Preparation and characterization of polyethylene glycol/active carbon composites as shape-stabilized phase change materials. Sol. Energy Mater. Sol. Cells 2011, 95, 644–650. [Google Scholar] [CrossRef]
- Qiu, J.; Fan, X.; Shi, Y.; Zhang, S.; Jin, X.; Wang, W.; Tang, B. PEG/3D graphene oxide network form-stable phase change materials with ultrahigh filler content. J. Mater. Chem. A 2019, 7, 21371–21377. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, K.; Wei, Q.; Ma, L.; Ye, W.; Li, H.; Zhou, B.; Yu, Z.; Lin, C.-T.; Luo, J.; et al. Thermal conductivity enhancement of phase change materials with 3D porous diamond foam for thermal energy storage. Appl. Energy. 2019, 233–234, 208–219. [Google Scholar] [CrossRef]
- Yazdani, M.R.; Ajdary, R.; Kankkunen, A.; Rojas, O.J.; Seppälä, A. Cellulose Nanofibrils Endow Phase-Change Polyethylene Glycol with Form Control and Solid-to-gel Transition for Thermal Energy Storage. ACS Appl. Mater. Interfaces 2021, 13, 6188–6200. [Google Scholar] [CrossRef]
- Jin, J.; Liu, L.; Liu, R.; Wei, H.; Qian, G.; Zheng, J.; Xie, W.; Lin, F.; Xie, J. Preparation and thermal performance of binary fatty acid with diatomite as form-stable composite phase change material for cooling asphalt pavements. Constr. Build. Mater. 2019, 226, 616–624. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, N.; Jing, Y.; Cao, X.; Yuan, Y.; Haghighat, F. Experimental and numerical investigation on dodecane/expanded graphite shape-stabilized phase change material for cold energy storage. Energy 2019, 189, 116175. [Google Scholar] [CrossRef]
- Ding, J.; Wu, X.; Shen, X.; Cui, S.; Chen, X. Form-stable phase change material embedded in three-dimensional reduced graphene aerogel with large latent heat for thermal energy management. Appl. Surf. Sci. 2020, 534, 147612. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, H.; Yang, Q.; Xiong, C.; Zhao, M.; Kobayashi, K.; Saito, T.; Isogai, A. Carboxylated nanocellulose/poly(ethylene oxide) composite films as solid-solid phase-change materials for thermal energy storage. Carbohydr. Polym. 2019, 225, 115215. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Han, N.; Gao, X.; Gao, X.; Li, W.; Zhang, X. Cellulose-based phase change fibres for thermal energy storage and management applications. Chem. Eng. J. 2021, 412, 128596. [Google Scholar] [CrossRef]
- Wang, C.; Wang, W.; Xin, G.; Li, G.; Zheng, J.; Tian, W.; Li, X. Phase change behaviors of PEG on modified graphene oxide mediated by surface functional groups. Eur. Polym. J. 2016, 74, 43–50. [Google Scholar] [CrossRef]
- Qi, G.-Q.; Liang, C.-L.; Bao, R.-Y.; Liu, Z.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Polyethylene glycol based shape-stabilized phase change material for thermal energy storage with ultra-low content of graphene oxide. Sol. Energy Mater. Sol. Cells 2014, 123, 171–177. [Google Scholar] [CrossRef]
- Zhou, L.; Tang, L.-S.; Tao, X.-F.; Yang, J.; Yang, M.-B.; Yang, W. Facile fabrication of shape-stabilized polyethylene glycol/cellulose nanocrystal phase change materials based on thiol-ene click chemistry and solvent exchange. Chem. Eng. J. 2020, 396, 125206. [Google Scholar] [CrossRef]
- Wang, H.; Deng, Y.; Wu, F.; Dai, X.; Wang, W.; Mai, Y.; Gu, Y.; Liu, Y. Effect of dopamine-modified expanded vermiculite on phase change behavior and heat storage characteristic of polyethylene glycol. Chem. Eng. J. 2021, 415, 128992. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Jia, Y.; Topham, P.D.; Wang, L. Binary shape-stabilized phase change materials based on poly(ethylene glycol)/polyurethane composite with dual-phase transition. J. Mater. Sci. 2018, 53, 16539–16556. [Google Scholar] [CrossRef] [Green Version]
- Harlé, T.; Nguyen, G.T.M.; Ledesert, B.; Mélinge, Y.; Hebert, R.L. Cross-linked polyurethane as solid-solid phase change material for low temperature thermal energy storage. Thermochim. Acta 2020, 685, 178191. [Google Scholar] [CrossRef]
- Kong, W.; Fu, X.; Yuan, Y.; Liu, Z.; Lei, J. Preparation and thermal properties of crosslinked polyurethane/lauric acid composites as novel form stable phase change materials with a low degree of supercooling. RSC Adv. 2017, 7, 29554–29562. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Liu, H.; Chen, S.; Pei, D.; Miao, J.; Zhang, X. Fabrication and properties of graphene oxide-grafted-poly(hexadecyl acrylate) as a solid-solid phase change material. Compos. Sci. Technol. 2017, 149, 262–268. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Xi, S.; Xie, H.; Yu, W. 3D porous copper foam-based shape-stabilized composite phase change materials for high photothermal conversion, thermal conductivity and storage. Renew. Energy 2021, 175, 307–317. [Google Scholar] [CrossRef]
- Jiang, F.; Cui, S.; Rungnim, C.; Song, N.; Shi, L.; Ding, P. Control of a Dual-Cross-Linked Boron Nitride Framework and the Optimized Design of the Thermal Conductive Network for Its Thermoresponsive Polymeric Composites. Chem. Mater. 2019, 31, 7686–7695. [Google Scholar] [CrossRef]
- Akhmetov, B.; Navarro, M.E.; Seitov, A.; Kaltayev, A.; Bakenov, Z.; Ding, Y. Numerical study of integrated latent heat thermal energy storage devices using nanoparticle-enhanced phase change materials. Sol. Energy 2019, 194, 724–741. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Q.; Kang, S.; Li, J.; Jia, X. Coal-based ultrathin-wall graphitic porous carbon for high-performance form-stable phase change materials with enhanced thermal conductivity. Chem. Eng. J. 2020, 395, 125112. [Google Scholar] [CrossRef]
- Kim, I.-H.; Sim, H.-W.; Hong, H.-H.; Kim, D.-W.; Lee, W.; Lee, D.-K. Effect of filler size on thermal properties of paraffin/silver nanoparticle composites. Korean J. Chem. Eng. 2019, 36, 1004–1012. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, N.; Wang, H.; Sun, R.; Wong, C.P. A newly designed paraffin@VO2 phase change material with the combination of high latent heat and large thermal conductivity. J. Colloid Interface Sci. 2020, 559, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xie, B.; Chen, D.; Chen, J.; Li, W.; Chen, Z.; Gibb, S.W.; Long, Y. Ultrathin graphite sheets stabilized stearic acid as a composite phase change material for thermal energy storage. Energy 2019, 166, 246–255. [Google Scholar] [CrossRef]
- Harish, S.; Orejon, D.; Takata, Y.; Kohno, M. Thermal conductivity enhancement of lauric acid phase change nanocomposite with graphene nanoplatelets. Appl. Therm. Eng. 2015, 80, 205–211. [Google Scholar] [CrossRef]
- Min, P.; Liu, J.; Li, X.; An, F.; Liu, P.; Shen, Y.; Koratkar, N.; Yu, Z.-Z. Thermally Conductive Phase Change Composites Featuring Anisotropic Graphene Aerogels for Real-Time and Fast-Charging Solar-Thermal Energy Conversion. Adv. Funct. Mater. 2018, 28, 1805365. [Google Scholar] [CrossRef]
- Ali, H.M.; Arshad, A.; Jabbal, M.; Verdin, P.G. Thermal management of electronics devices with PCMs filled pin-fin heat sinks: A comparison. Int. J. Heat Mass Transf. 2018, 117, 1199–1204. [Google Scholar] [CrossRef] [Green Version]
- Baby, R.; Balaji, C. Experimental investigations on phase change material based finned heat sinks for electronic equipment cooling. Int. J. Heat Mass Transf. 2012, 55, 1642–1649. [Google Scholar] [CrossRef]
- Farzanehnia, A.; Khatibi, M.; Sardarabadi, M.; Passandideh-Fard, M. Experimental investigation of multiwall carbon nanotube/paraffin based heat sink for electronic device thermal management. Energy Convers. Manag. 2019, 179, 314–325. [Google Scholar] [CrossRef]
- Jiang, G.; Huang, J.; Fu, Y.; Cao, M.; Liu, M. Thermal optimization of composite phase change material/expanded graphite for Li-ion battery thermal management. Appl. Therm. Eng. 2016, 108, 1119–1125. [Google Scholar] [CrossRef]
- Feng, C.P.; Yang, L.Y.; Yang, J.; Bai, L.; Bao, R.Y.; Liu, Z.Y.; Yang, M.B.; Lan, H.B.; Yang, W. Recent advances in polymer-based thermal interface materials for thermal management: A mini-review. Compos. Commun. 2020, 22, 100528. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Z.; Liu, Z.; Guo, Y.; Li, P.; Gao, C. Ultrahigh Thermal Conductive yet Superflexible Graphene Films. Adv. Mater. 2017, 29, 1700589. [Google Scholar] [CrossRef]
- Shen, B.; Zhai, W.; Zheng, W. Ultrathin Flexible Graphene Film: An Excellent Thermal Conducting Material with Efficient EMI Shielding. Adv. Funct. Mater. 2014, 24, 4542–4548. [Google Scholar] [CrossRef]
- Kumar, P.; Yu, S.; Shahzad, F.; Hong, S.M.; Kim, Y.-H.; Koo, C.M. Ultrahigh electrically and thermally conductive self-aligned graphene/polymer composites using large-area reduced graphene oxides. Carbon 2016, 101, 120–128. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, G.; Li, Z.; Yang, X. Custom design of solid–solid phase change material with ultra-high thermal stability for battery thermal management. J. Mater. Chem. A 2020, 8, 14624–14633. [Google Scholar] [CrossRef]
- Moore, A.L.; Shi, L. Emerging challenges and materials for thermal management of electronics. Mater. Today 2014, 17, 163–174. [Google Scholar] [CrossRef]
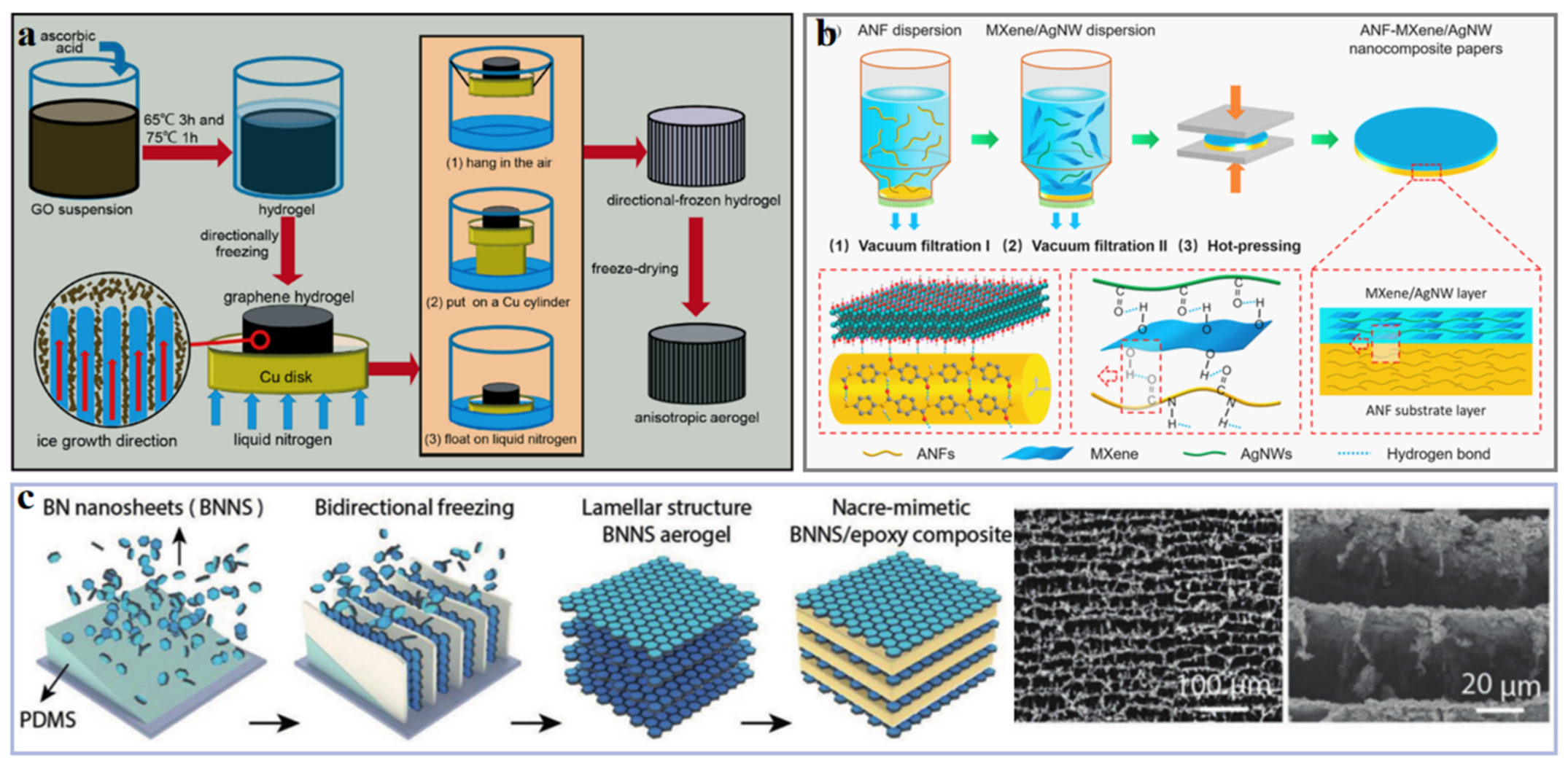
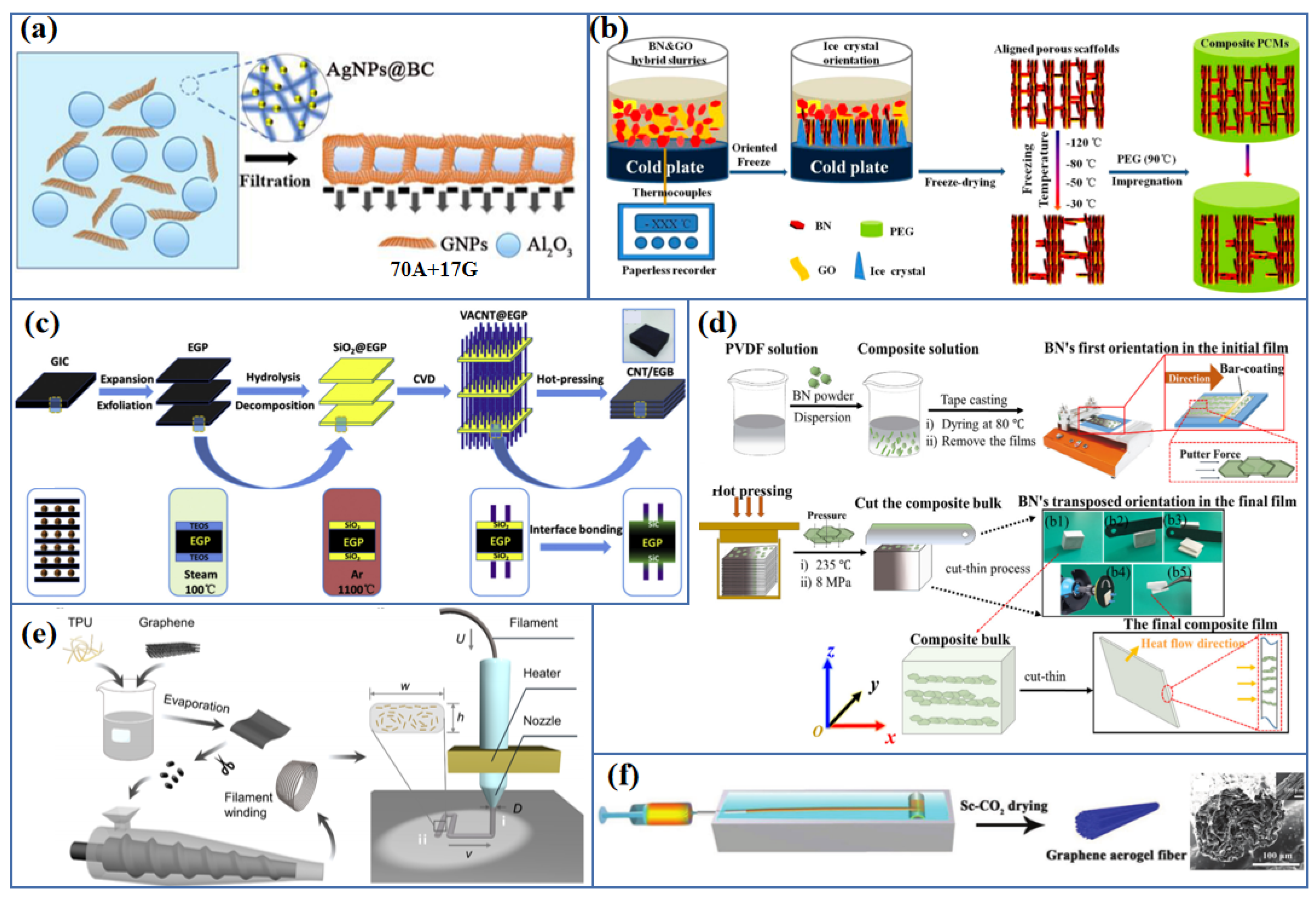
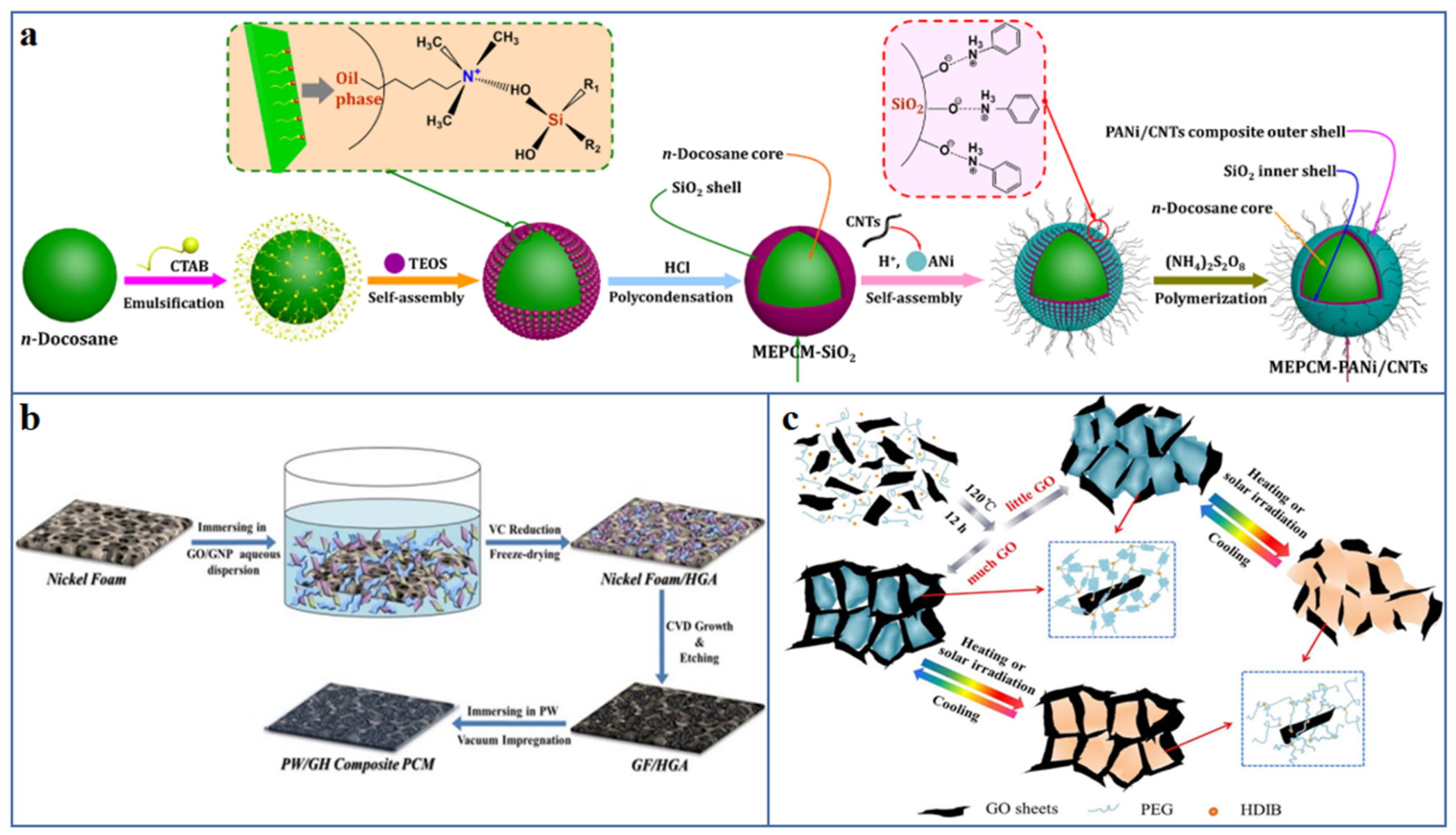



| Fillers | Thermal Conductivity (Wm−1K−1) | |
|---|---|---|
| Metals | Silver (Ag) | ~420 |
| Cupper (Cu) | 401 | |
| Aluminum (Al) | 237 | |
| Nickel (Ni) | 158 | |
| Zinc (Zn) | 121 | |
| Carbon material | Carbon fibers (CFs) | 300–1000 |
| Carbon nanotubes (CNTs) | 2000–6000 | |
| Graphite | 100–400 | |
| Graphene | 5300 | |
| Ceramics | BN | 250–300 |
| Aluminum nitride (AlN) | 300 | |
| Silicon carbide (SiC) | 120 | |
| Aluminum oxide (Al2O3) | 30–40 | |
| Solid–Liquid PCM | Advantages | Disadvantages |
|---|---|---|
| Inorganic solid–liquid PCM |
|
|
| Organic solid–liquid PCM |
|
|
| PW | Molecular Formula | Tm (°C) | Tc (°C) | ∆H (J/g) |
|---|---|---|---|---|
| n-Hexadecane | CH3(CH2)14CH3 | 18–19 | 17 | 237 |
| n-Octadecane | CH3(CH2)16CH3 | 28 | 25 | 242 |
| n-Eicosane | CH3(CH2)18CH3 | 36–37 | 31 | 247 |
| n-Docosane | CH3(CH2)20CH3 | 42–45 | 43 | 157 |
| n-Tetracosane | CH3(CH2)22CH3 | 50–51 | 48–49 | 160 |
| n-Hexacosane | CH3(CH2)24CH3 | 56 | 53–54 | 255 |
| PEG | Molecular weight (g/mol) | Tm (°C) | Tc (°C) | ∆H (J/g) |
| PEG400 | 400 | 3.2 | −24 | 91.4 |
| PEG1000 | 1000 | 32.0 | 28 | 149.5 |
| PEG2000 | 2000 | 51.0 | 35 | 181.4 |
| PEG4000 | 4000 | 59.7 | 22 | 189.7 |
| PEG10000 | 10,000 | 66.0 | 38 | 189.6 |
| PEG20000 | 20,000 | 68.7 | 38 | 187.8 |
| FA | Molecular formula | Tm (°C) | Tc (°C) | ∆H (J/g) |
| Caprylic acid | CH3(CH2)6COOH | 16–17 | - | 148–149 |
| Capric acid | CH3(CH2)8COOH | 30–32 | - | 152.7–155.46 |
| Lauric acid | CH3(CH2)10COOH | 42–44 | 39–42 | 175–190 |
| Myristic acid | CH3(CH2)12COOH | 51.5–58 | 51–52 | 178.14–210.7 |
| Palmitic acid | CH3(CH2)14COOH | 61–64 | 58–60.38 | 185.4–212.1 |
| Stearic acid | CH3(CH2)16COOH | 65–70 | 66–67 | 198.8–258.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Shi, T.; Ma, A. Recent Advances in Design and Preparation of Polymer-Based Thermal Management Material. Polymers 2021, 13, 2797. https://doi.org/10.3390/polym13162797
Zhang H, Shi T, Ma A. Recent Advances in Design and Preparation of Polymer-Based Thermal Management Material. Polymers. 2021; 13(16):2797. https://doi.org/10.3390/polym13162797
Chicago/Turabian StyleZhang, Hongli, Tiezhu Shi, and Aijie Ma. 2021. "Recent Advances in Design and Preparation of Polymer-Based Thermal Management Material" Polymers 13, no. 16: 2797. https://doi.org/10.3390/polym13162797
APA StyleZhang, H., Shi, T., & Ma, A. (2021). Recent Advances in Design and Preparation of Polymer-Based Thermal Management Material. Polymers, 13(16), 2797. https://doi.org/10.3390/polym13162797







