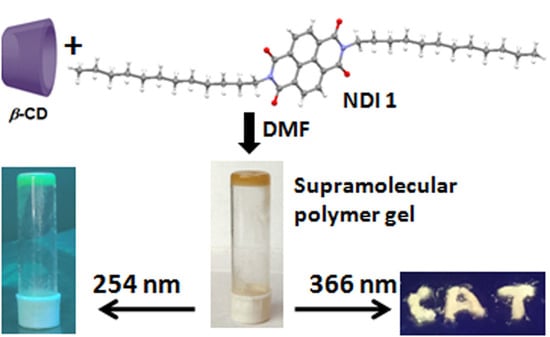
White-Light-Emitting Supramolecular Polymer Gel Based on β-CD and NDI Host-Guest Inclusion Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of NDI 1 and NDI 2
2.2. NMR Experiments
2.3. FT-IR Spectroscopy
2.4. Mass Spectrometry
2.5. UV/Vis Spectroscopy
2.6. Fluorescence Spectroscopy
2.7. Polarised Optical Microscope
2.8. Field Emission Scanning Electron Microscopy
2.9. Rheology Experiments
2.10. Single Crystal X-ray Diffraction Study
2.11. Fluorescence Lifetime Imaging Microscopy (FLIM)
3. Results and Discussions
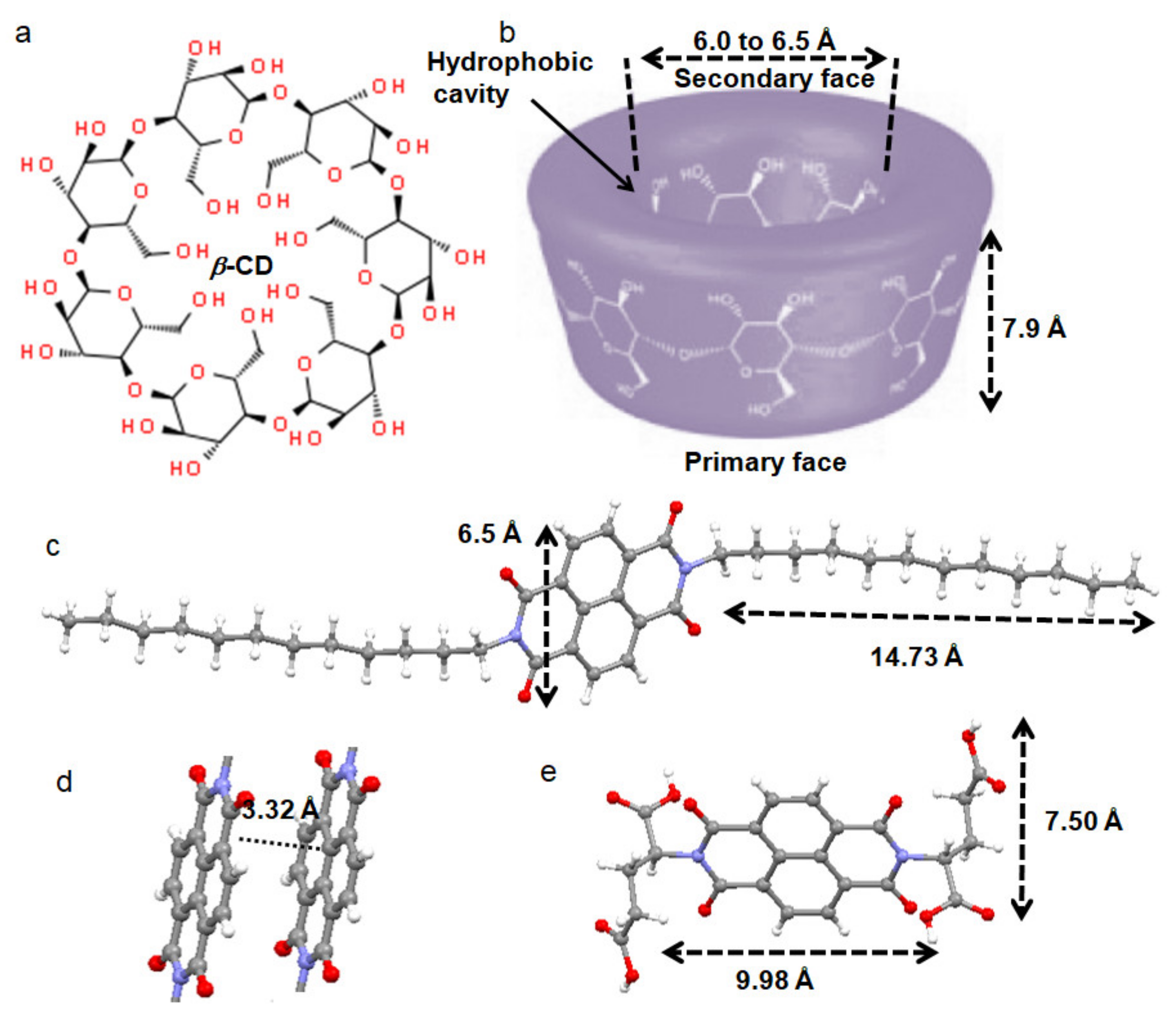
3.1. Design and Synthesis
3.2. X-ray Structures of the Building Blocks
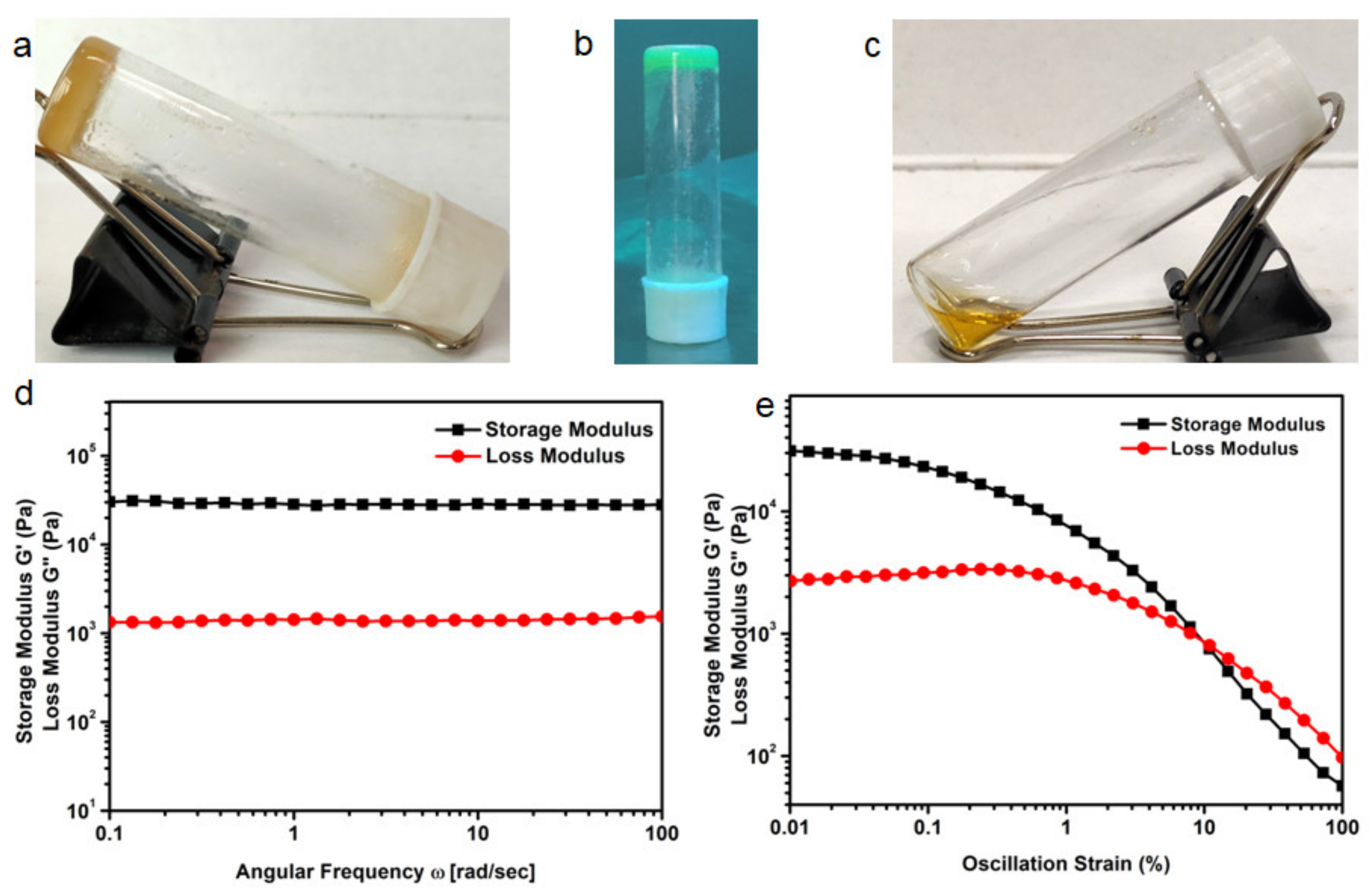
3.3. Gelation Study
3.4. Rheology Study
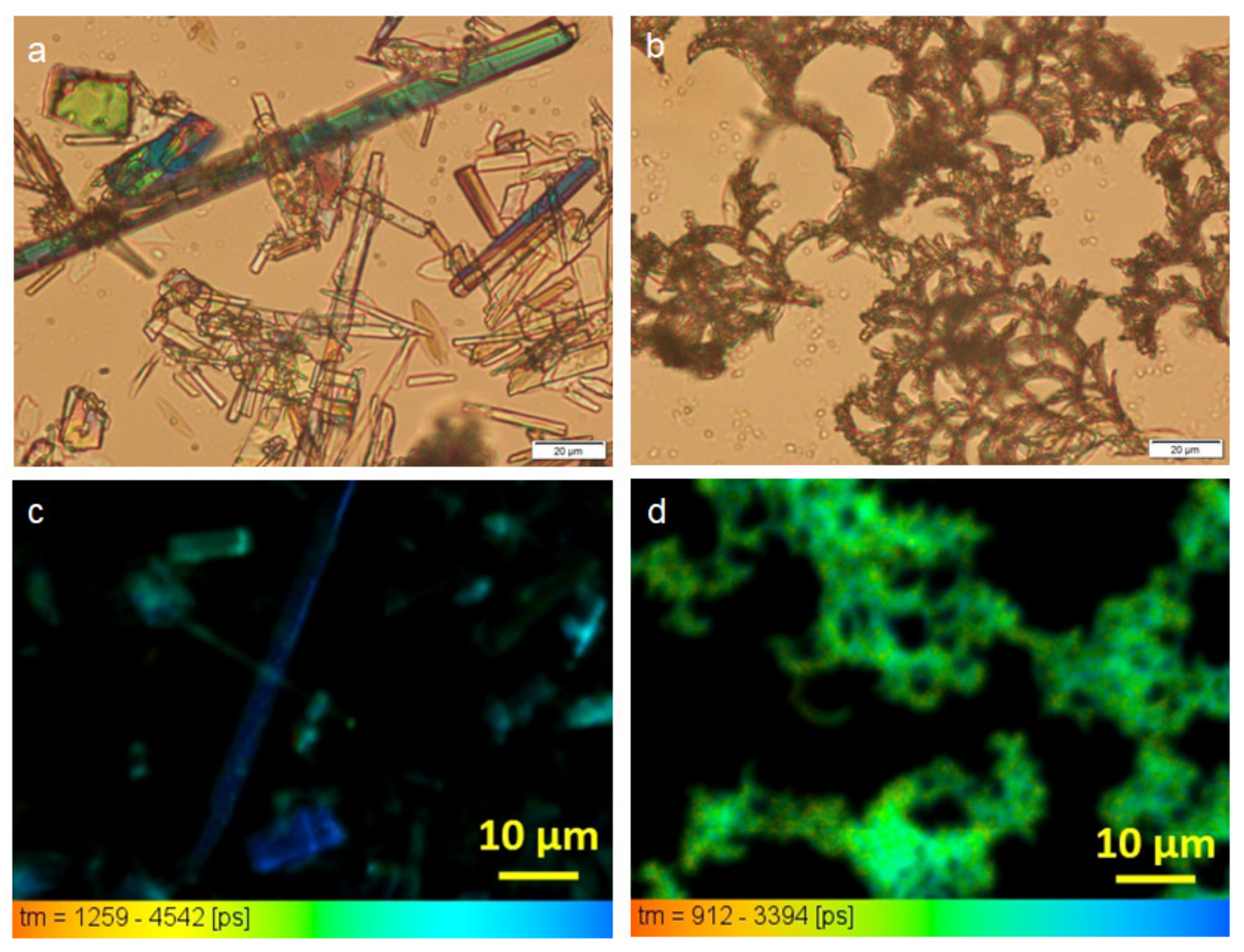
3.5. Morphology
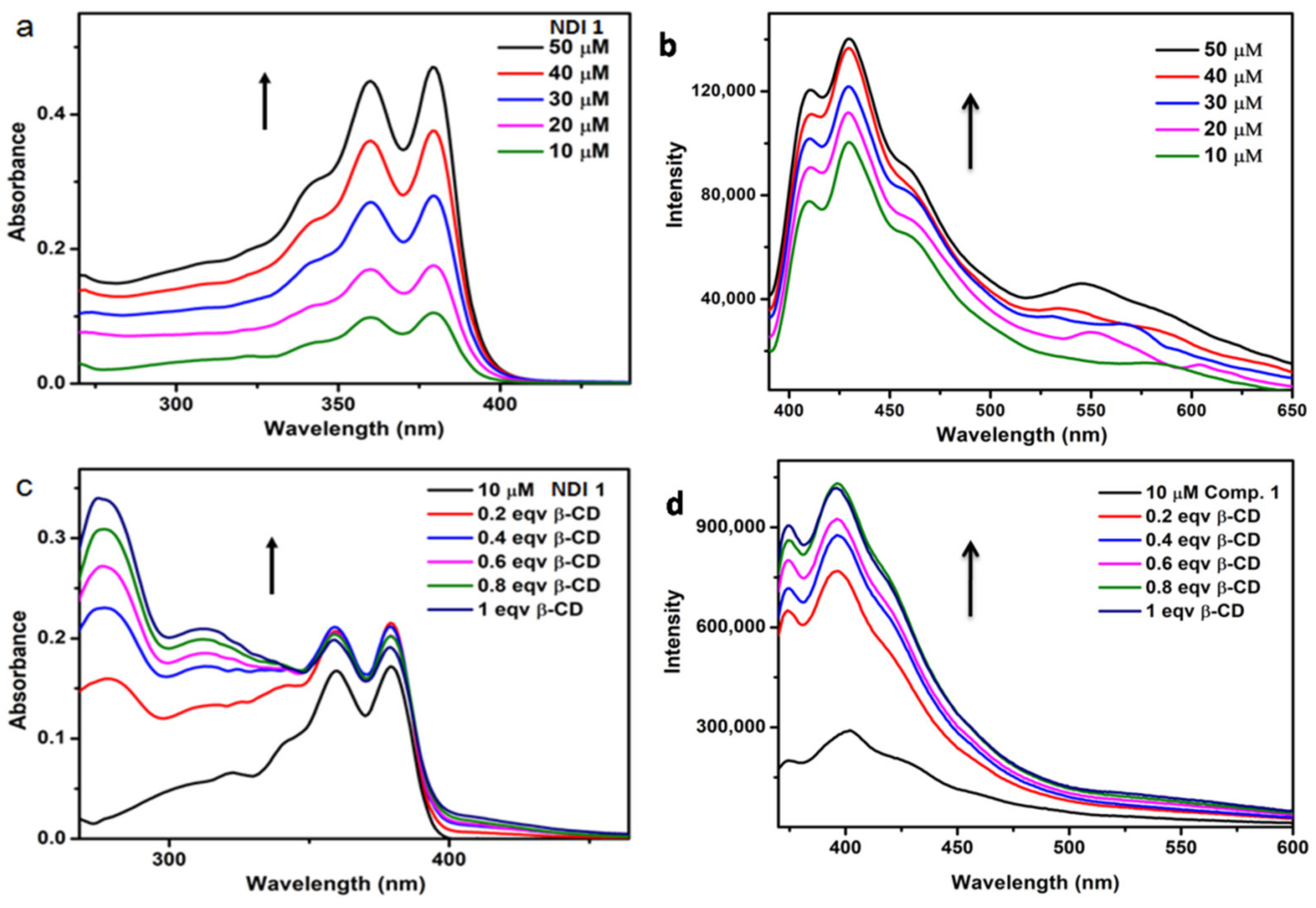
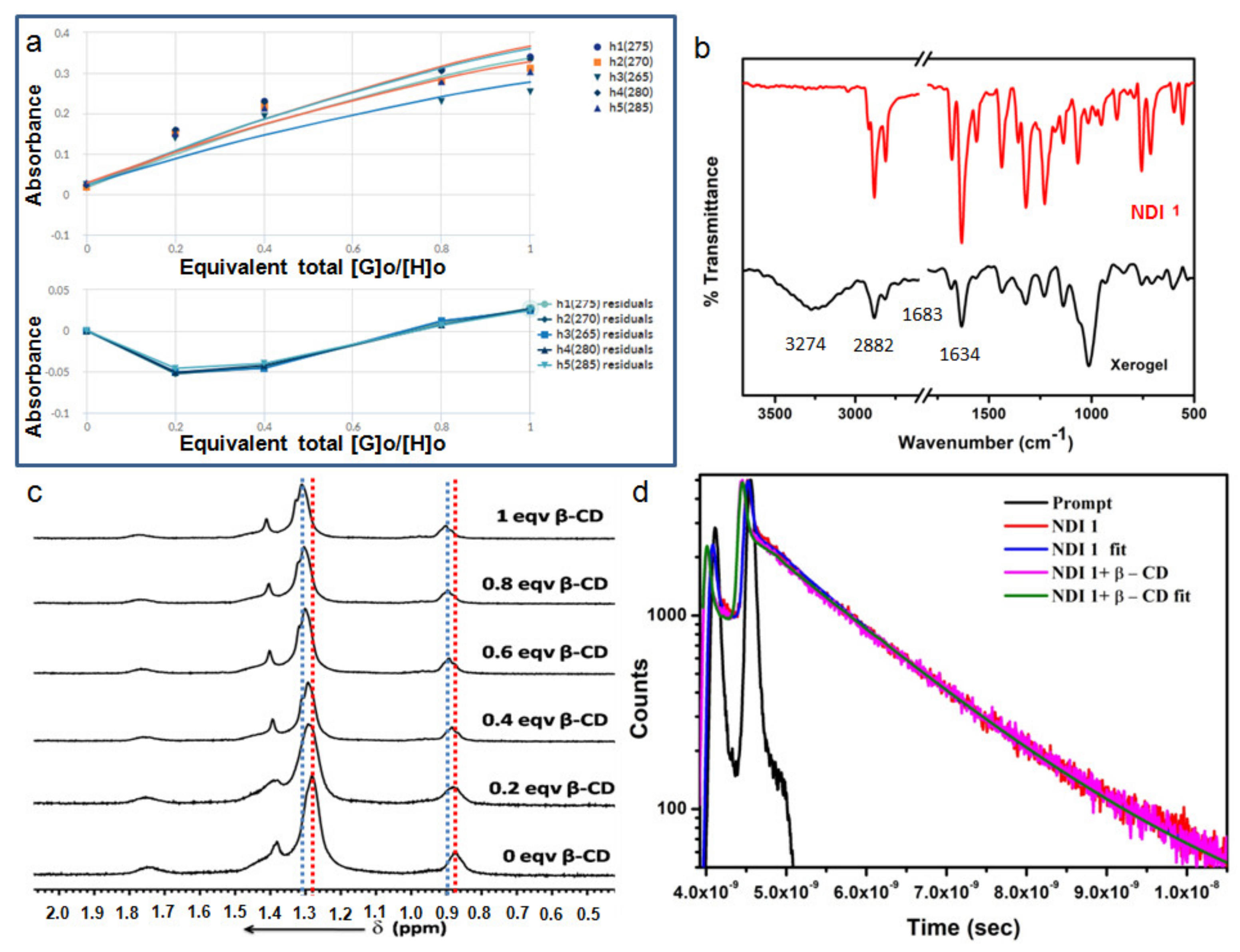
3.6. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular polymers. Chem. Rev. 2001, 101, 4071–4097. [Google Scholar] [CrossRef]
- Yang, L.; Tan, X.; Wang, Z.; Zhang, X. Supramolecular polymers: Historical development, preparation, characterization and function. Chem. Rev. 2015, 115, 7196–7239. [Google Scholar] [CrossRef]
- Krieg, E.; Bastings, M.M.C.; Besenius, P.; Rybtchinski, B. Supramolecular polymers in aqueous media. Chem. Rev. 2016, 116, 2414–2477. [Google Scholar] [CrossRef]
- Xu, F.; Pfeifer, L.; Crespi, S.; Leung, F.K.; Stuart, M.C.A.; Wezenberg, S.J.; Feringa, B.L. From photoinduced supramolecular polymerization to responsive organogels. J. Am. Chem. Soc. 2021, 143, 5990–5997. [Google Scholar] [CrossRef]
- Li, J.; Geng, L.; Wang, G.; Chu, H.; Wei, H. Self-Healable Gels for Use in Wearable Devices. Chem. Mater. 2017, 29, 8932–8952. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Vintiloiu, A.; Leroux, J.-C. Organogels and their use in drug delivery—A review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef]
- Hirst, A.R.; Escuder, B.; Miravet, J.F.; Smith, D.K. High-Tech Applications of Self-Assembling Supramolecular Nanostructured Gel-Phase Materials: From Regenerative Medicine to Electronic Devices. Angew. Chem. Int. Ed. 2008, 47, 8002–8018. [Google Scholar] [CrossRef] [PubMed]
- Kurisawa, M.; Chung, J.E.; Yang, Y.Y.; Gaoa, S.J.; Uyama, H. Injectable biodegradable hydrogels composed of hyaluronic acid–tyramine conjugates for drug delivery and tissue engineering. Chem. Commun. 2005, 34, 4312–4314. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar]
- Jayawarna, V.; Ali, M.; Jowitt, T.A.; Miller, A.F.; Saiani, A.; Gough, J.E.; Ulijn, R.V. Nanostructured Hydrogels for Three-Dimensional Cell Culture through Self-Assembly of Fluorenylmethoxycarbonyl–Dipeptides. Adv. Mater. 2006, 18, 611–614. [Google Scholar] [CrossRef]
- Pang, X.; Yu, X.; Lan, H.; Ge, X.; Li, Y.; Zhen, X.; Yi, T. Visual Recognition of Aliphatic and Aromatic Amines Using a Fluorescent Gel: Application of a Sonication-Triggered Organogel. ACS Appl. Mater. Interfaces 2015, 7, 13569–13577. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Feng, Y.; Deng, G.-J.; Liu, Z.-X.; He, Y.-M.; Fan, Q.-H. Fluorescent Dendritic Organogels Based on 2-(2′-Hydroxyphenyl)benzoxazole: Emission Enhancement and Multiple Stimuli-Responsive Properties. Chem. Eur. J. 2015, 21, 11018–11028. [Google Scholar] [CrossRef]
- Paikar, A.; Pramanik, A.; Das, T.; Haldar, D. A self-assembled peptide mimetic of a tubular host and a supramolecular polymer. Polym. Chem. 2017, 8, 396–403. [Google Scholar] [CrossRef] [Green Version]
- Harada, A.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H. Macroscopic self-assembly through molecular recognition. Nat. Chem. 2011, 3, 34–37. [Google Scholar] [CrossRef]
- Park, T.; Zimmerman, S.C. Formation of a Miscible Supramolecular Polymer Blend through Self-Assembly Mediated by a Quadruply Hydrogen-Bonded Heterocomplex. J. Am. Chem. Soc. 2006, 128, 11582–11590. [Google Scholar] [CrossRef] [PubMed]
- Ajayaghosh, A.; Praveen, V.K. π-Organogels of Self-Assembled p-Phenylenevinylenes: Soft Materials with Distinct Size, Shape, and Functions. Acc. Chem. Res. 2007, 40, 644–656. [Google Scholar] [CrossRef]
- Tu, T.; Fang, W.; Bao, X.; Li, X.; Dötz, K.H. Visual Chiral Recognition through Enantioselective Metallogel Collapsing: Synthesis, Characterization, and Application of Platinum–Steroid Low-Molecular-Mass Gelators. Angew. Chem. Int. Ed. 2011, 50, 6601–6605. [Google Scholar] [CrossRef] [PubMed]
- Rossow, T.; Seiffert, S. Supramolecular polymer gels with potential model-network structure. Polym. Chem. 2014, 5, 3018–3029. [Google Scholar] [CrossRef]
- Pal, A.; Dey, J. l-Cysteine-Derived Ambidextrous Gelators of Aromatic Solvents and Ethanol/Water Mixtures. Langmuir 2013, 29, 2120–2127. [Google Scholar] [CrossRef]
- Debnath, S.; Shome, A.; Dutta, S.; Das, P.K. Dipeptide-Based Low-Molecular-Weight Efficient Organogelators and Their Application in Water Purification. Chem. Eur. J. 2008, 14, 6870–6881. [Google Scholar] [CrossRef] [PubMed]
- Kar, T.; Mandal, S.K.; Das, P.K. Organogel–Hydrogel Transformation by Simple Removal or Inclusion of N-Boc-Protection. Chem. Eur. J. 2011, 17, 14952–14961. [Google Scholar] [CrossRef] [PubMed]
- Farinola, G.M.; Ragni, R. Electroluminescent materials for white organic light emitting diodes. Chem. Soc. Rev. 2011, 40, 3467–3482. [Google Scholar] [CrossRef] [PubMed]
- Muherjee, S.; Thilagar, P. Organic white-light emitting materials. Dye. Pigment. 2014, 110, 2–27. [Google Scholar] [CrossRef]
- Sakai, A.; Tanaka, M.; Ohta, E.; Yoshimoto, Y.; Mizuno, K.; Ikeda, H. White light emission from a single component system: Remarkable concentration effects on the fluorescence of 1,3-diaroylmethanatoboron difluoride. Tetrahedron Lett. 2012, 53, 4138–4141. [Google Scholar] [CrossRef]
- Abbel, R.; Weegen, R.V.D.; Pisula, W.; Surin, M.; Leclre, P.; Lazzaroni, R.; Meijer, E.W.; Schenning, A.P.H.J. Multicolour Self-Assembled Fluorene Co-Oligomers: From Molecules to the Solid State via White-Light-Emitting Organogels. Chem. Eur. J. 2009, 15, 9737–9746. [Google Scholar] [CrossRef]
- Lei, Y.; Liao, Q.; Fu, H.; Yao, J. Orange-Blue-Orange Triblock One-Dimensional Heterostructures of Organic Microrods for White-Light Emission. J. Am. Chem. Soc. 2010, 132, 1742–1743. [Google Scholar] [CrossRef]
- Giansante, C.; Raffy, G.; Schäfer, C.; Rahma, H.; Kao, M.-T.; Olive, A.G.L.; Guerzo, A.D. White-Light-Emitting Self-Assembled NanoFibers and Their Evidence by Microspectroscopy of Individual Objects. J. Am. Chem. Soc. 2011, 133, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Sharma, V.; Koner, A.L. Single-component white-light emission via intramolecular electronic conjugation-truncation with perylenemonoimide. Chem. Commun. 2017, 53, 7909–7912. [Google Scholar] [CrossRef] [PubMed]
- Sutar, P.; Suresh, V.M.; Maji, T.K. Tunable emission in lanthanide coordination polymer gels based on a rationally designed blue emissive gelator. Chem. Commun. 2015, 51, 9876–9879. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wu, Y.; Liu, K.; Yu, X.; Wu, B.; Wu, H.; Gong, Z.; Yi, T. Iridium complex triggered white-light-emitting gel and its response to cysteine. J. Mater. Chem. 2012, 22, 2650–2657. [Google Scholar] [CrossRef]
- Carlos, L.D.; Ferreira, R.A.S.; Bermudez, V.d.Z.; Ribeiro, S.J.L. Lanthanide-Containing Light-Emitting Organic–Inorganic Hybrids: A Bet on the Future. Adv. Mater. 2009, 21, 509–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ji, X.; Li, Z.; Zhu, C.N.; Yang, X.; Li, T.; Wu, Z.L.; Huang, F. Preparation of a white-light-emitting fluorescent supramolecular polymer gel with a single chromophore and use of the gel to fabricate a protected quick response code. Mater. Chem. Front. 2017, 1, 167–171. [Google Scholar] [CrossRef]
- Maiti, D.K.; Banerjee, A. A peptide based two component white light emitting system. Chem. Commun. 2013, 49, 6909–6911. [Google Scholar] [CrossRef]
- Praveen, V.K.; Ranjith, C.; Armaroli, N. White-light-emitting supramolecular gel. Angew. Chem. Int. Ed. 2014, 53, 365–368. [Google Scholar] [CrossRef]
- Podder, D.; Nandi, S.K.; Sasmal, S.; Haldar, D. Synergistic Tricolor Emission-Based White Light from Supramolecular Organic-Inorganic Hybrid Gel. Langmuir 2019, 35, 6453–6459. [Google Scholar] [CrossRef]
- Kozycz, L.M.; Guo, C.; Manion, J.G.; Tilley, A.J.; Lough, A.J.; Li, Y.; Seferos, D.S. Enhanced electron mobility in crystalline thionated naphthalene diimides. J. Mater. Chem. C 2015, 3, 11505–11515. [Google Scholar] [CrossRef] [Green Version]
- Molla, M.R.; Gehrig, D.; Roy, L.; Kamm, V.; Paul, A.; Laquai, F.; Ghosh, S. Self-Assembly of carboxylic acid appended naphthalene diimide derivatives with tunable luminescent color and electrical conductivity. Chem. Eur. J. 2014, 20, 760–771. [Google Scholar] [CrossRef]
- Hibbert, D.B.; Thordarson, P. The death of the Job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Ke, C.; Zhou, Y.; Xie, Z.; Alngadh, A.; Keane, D.T.; Nassar, M.S.; Botros, Y.Y.; Mirkin, C.A.; Stoddart, J.F. Concurrent covalent and supramolecular polymerization. Chem. Eur. J. 2016, 22, 12301–12306. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, M.; Tang, D.; Yan, X.; Zhang, Z.Y.; Zhou, Z.; Song, B.; Wang, H.; Li, X.; Yin, S.; et al. Fluorescent Metallacage-Core Supramolecular Polymer Gel Formed by Orthogonal Metal Coordination and Host–Guest Interactions. J. Am. Chem. Soc. 2018, 140, 7674–7680. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy Chowdhury, S.; Nandi, S.K.; Mondal, S.; Kumar, S.; Haldar, D. White-Light-Emitting Supramolecular Polymer Gel Based on β-CD and NDI Host-Guest Inclusion Complex. Polymers 2021, 13, 2762. https://doi.org/10.3390/polym13162762
Roy Chowdhury S, Nandi SK, Mondal S, Kumar S, Haldar D. White-Light-Emitting Supramolecular Polymer Gel Based on β-CD and NDI Host-Guest Inclusion Complex. Polymers. 2021; 13(16):2762. https://doi.org/10.3390/polym13162762
Chicago/Turabian StyleRoy Chowdhury, Srayoshi, Sujay Kumar Nandi, Sahabaj Mondal, Santosh Kumar, and Debasish Haldar. 2021. "White-Light-Emitting Supramolecular Polymer Gel Based on β-CD and NDI Host-Guest Inclusion Complex" Polymers 13, no. 16: 2762. https://doi.org/10.3390/polym13162762
APA StyleRoy Chowdhury, S., Nandi, S. K., Mondal, S., Kumar, S., & Haldar, D. (2021). White-Light-Emitting Supramolecular Polymer Gel Based on β-CD and NDI Host-Guest Inclusion Complex. Polymers, 13(16), 2762. https://doi.org/10.3390/polym13162762