Nanoclay Effect into the Biodegradation and Processability of Poly(lactic acid) Nanocomposites for Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Films Production
2.2.2. Water Vapor Transmission Rate (WVTR) and Global Migration Test
2.2.3. Biodegradation Test
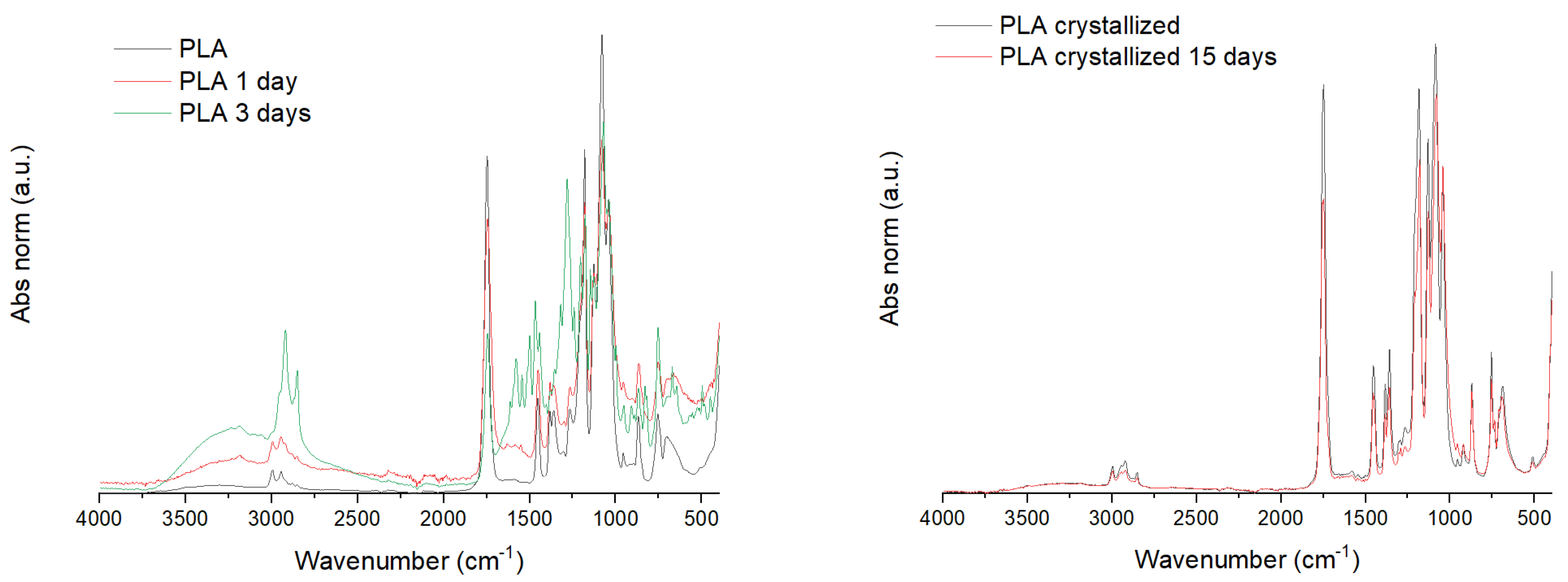
2.2.4. Structural and Thermal Characterization of the Films
2.2.5. Processability
3. Results and Discussion
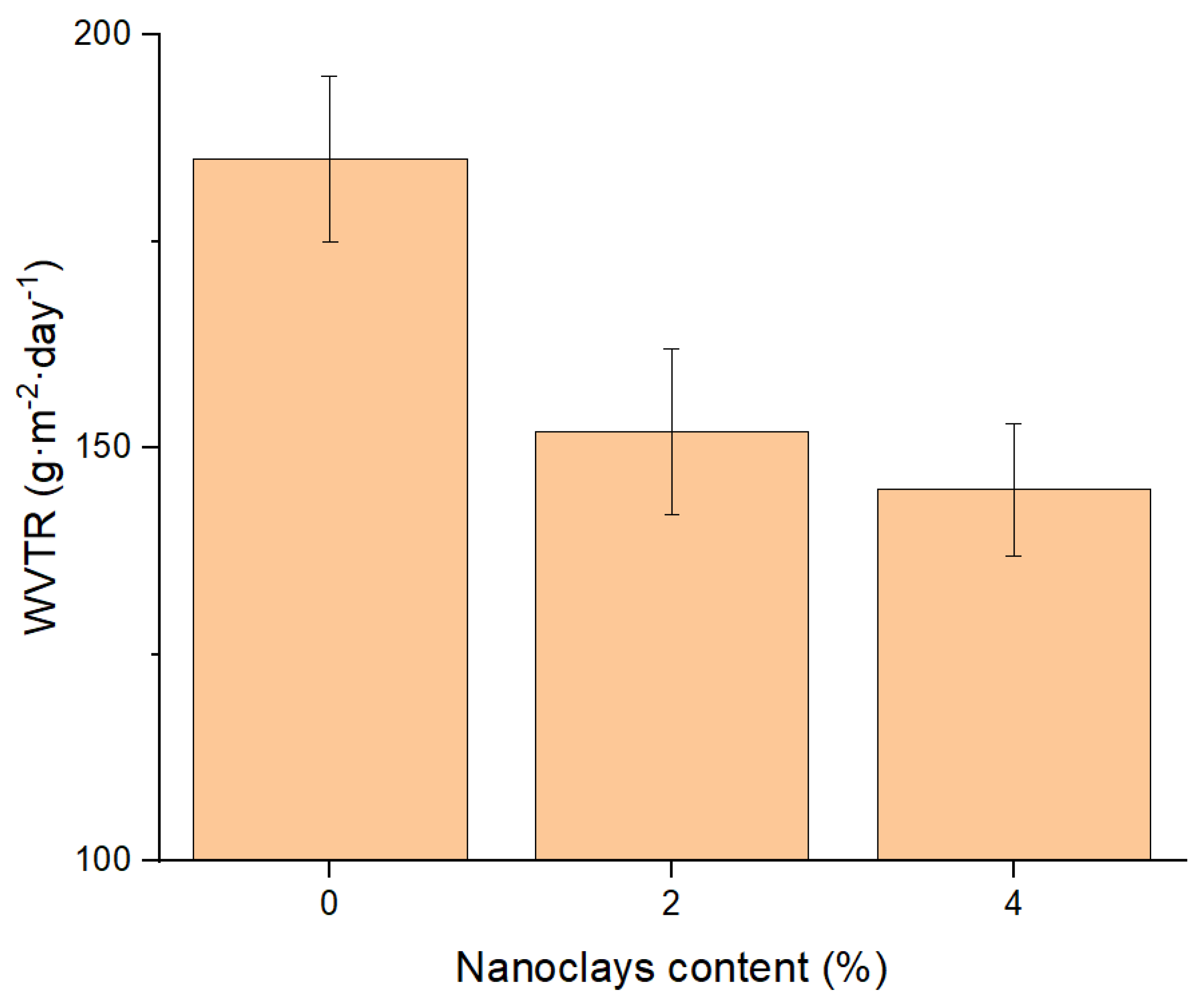
3.1. Water Vapour Transmission Rate (WVTR) and Global Migration
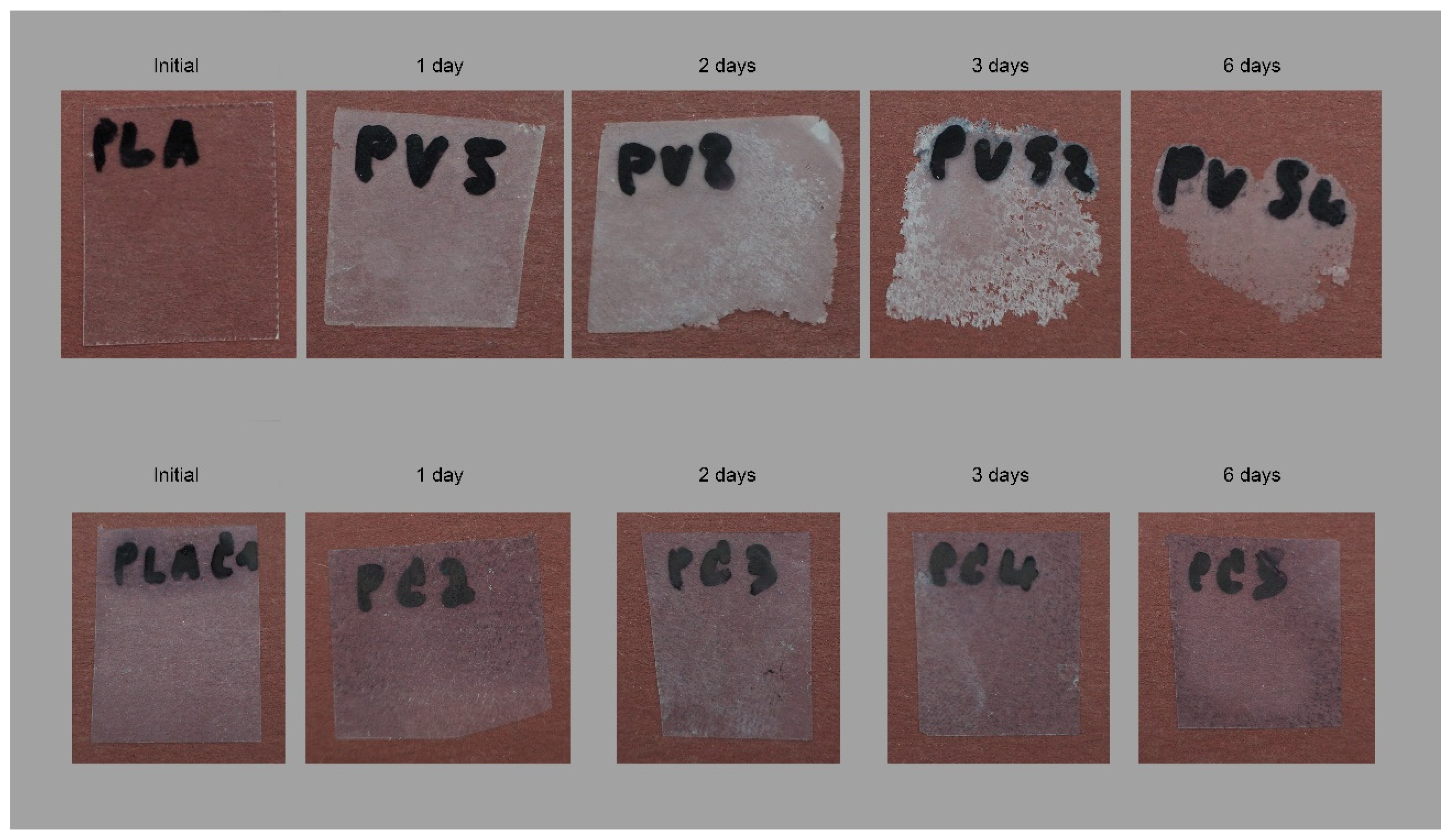
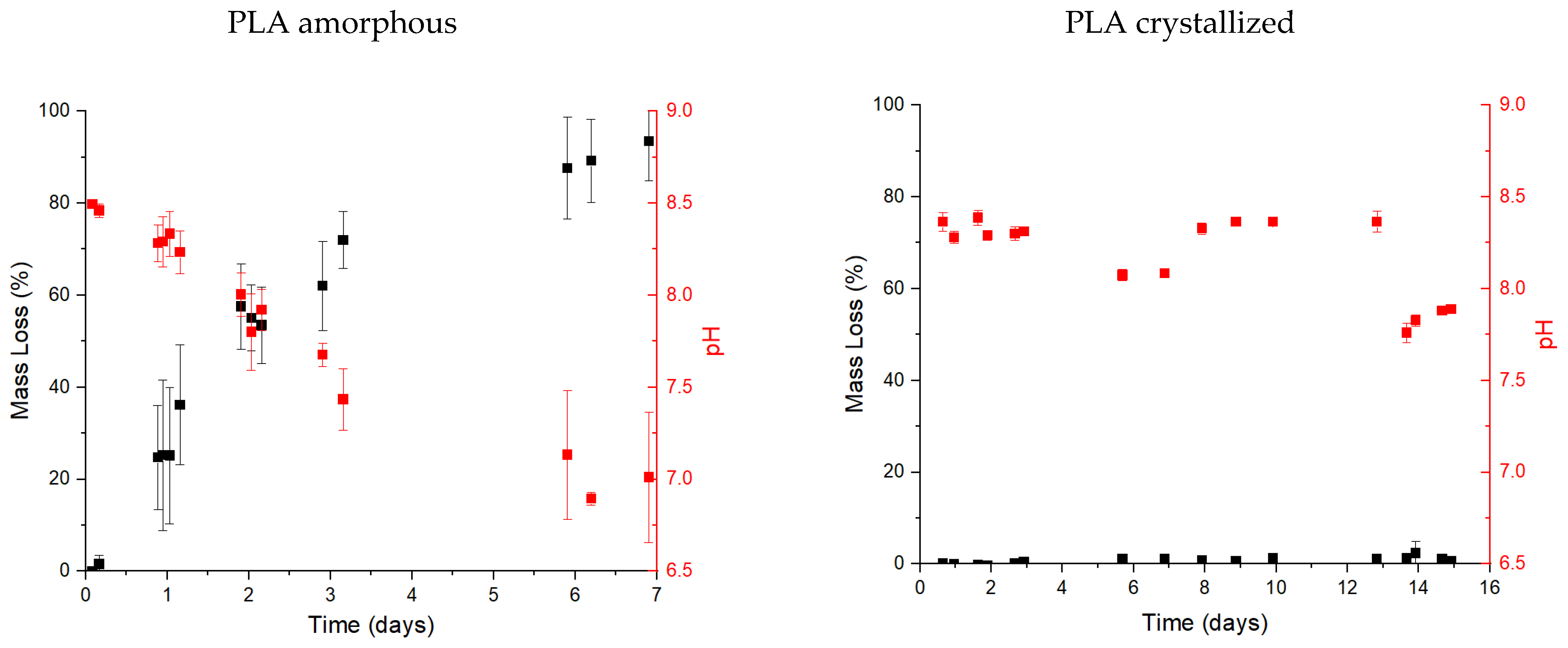
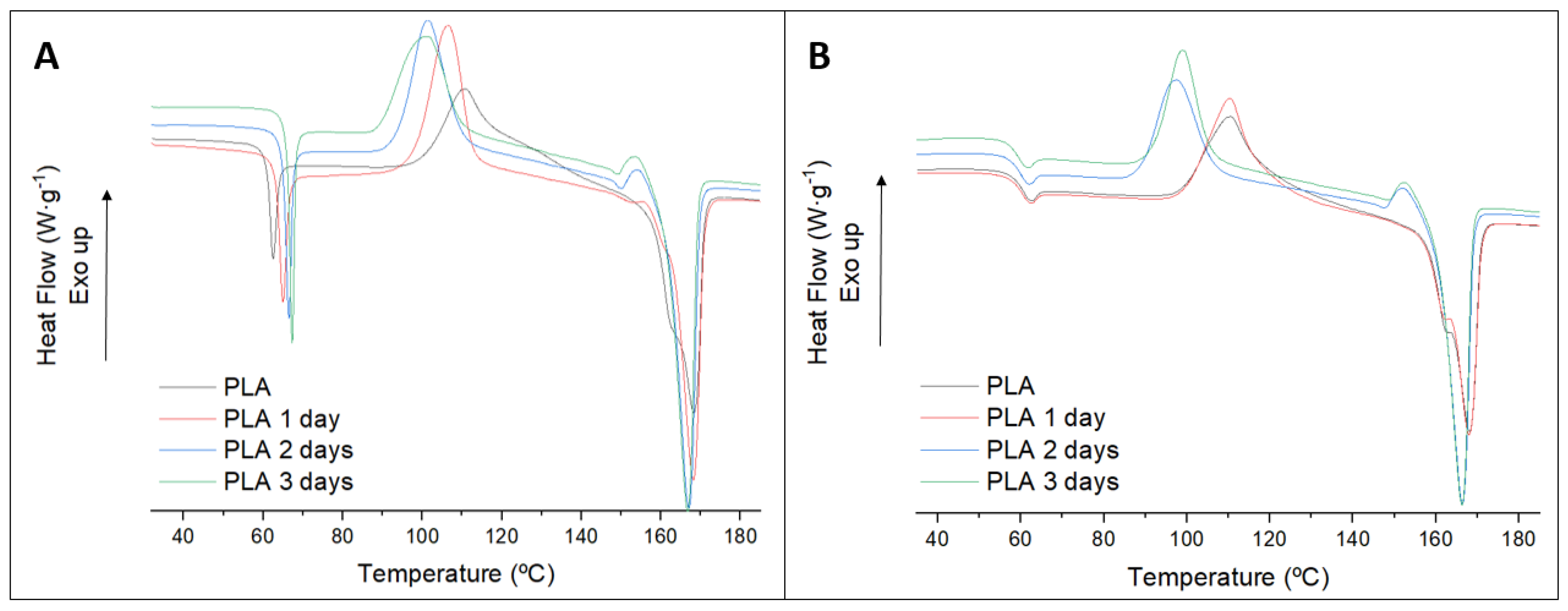
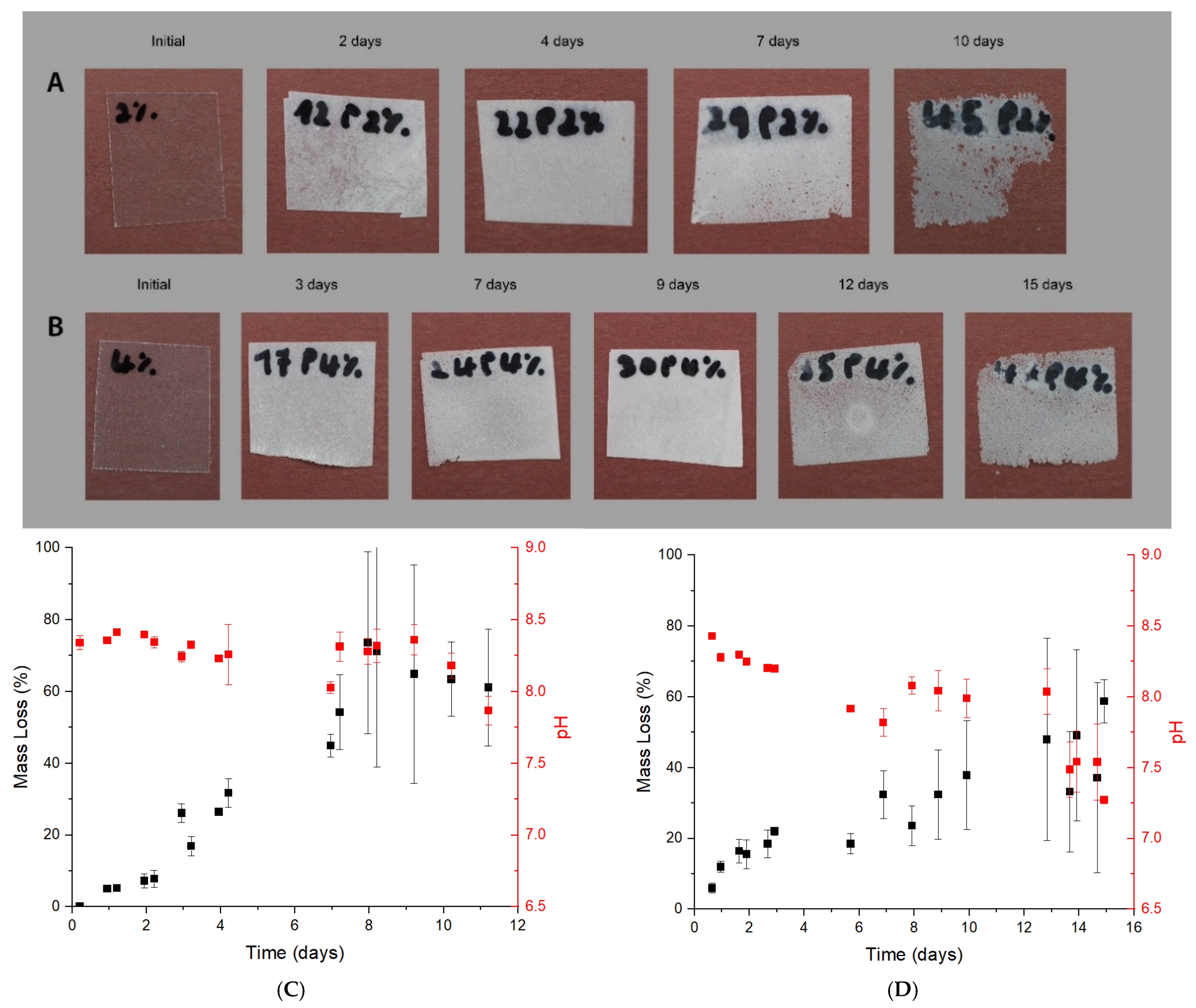
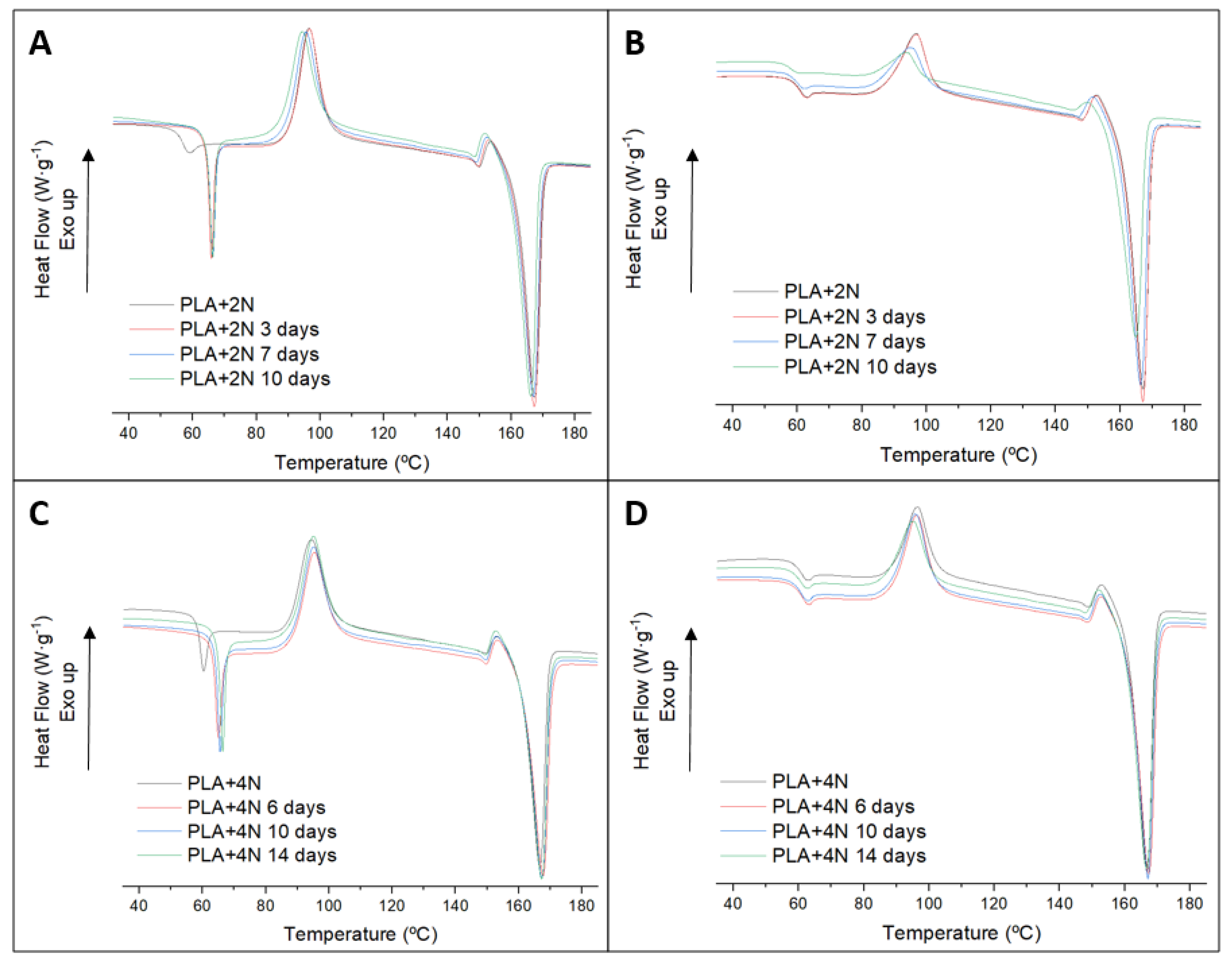
3.2. Biodegradation Test
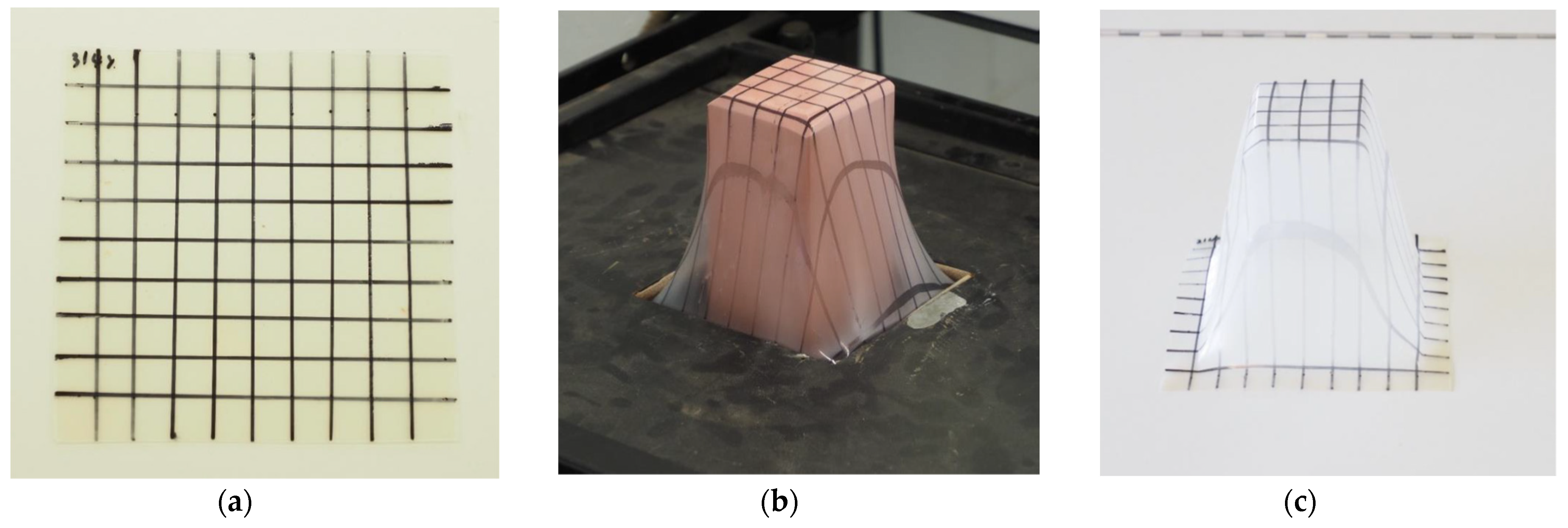
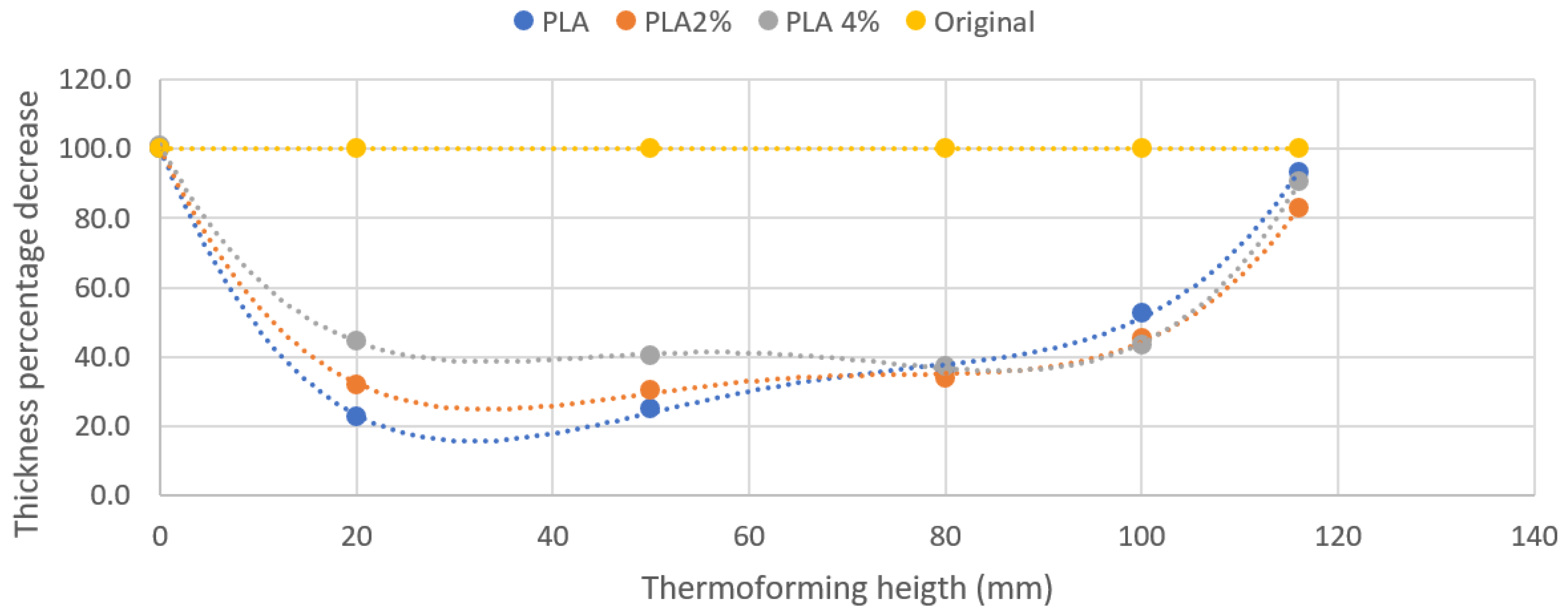
3.3. Processability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Siracusa, V.; Rosa, M.D.; Romani, S.; Rocculi, P.; Tylewicz, U. Life Cycle Assessment of multilayer polymer film used on food packaging field. Procedia Food Sci. 2011, 1, 235–239. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Su, T.; Li, P.; Wang, Z.; Anukiruthika, T.; Sethupathy, P.; Wilson, A.; Kashampur, K.; Moses, J.A.; Anandharamakrishnan, C.; et al. Multilayer packaging: Advances in preparation techniques and emerging food applications. Polym. Bull. 2018, 19, 1156–1186. [Google Scholar] [CrossRef] [Green Version]
- European Council. Directive (Eu) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the reduction of the impact of certain plastic products on the environment. Off. J. Eur. Union 2019, 155, 1–19. [Google Scholar]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Mendes, A.C.; Pedersen, G.A. Perspectives on sustainable food packaging: Is bio-based plastics a solution? Trends Food Sci. Technol. 2021, 112, 839–846. [Google Scholar] [CrossRef]
- Grizzi, I.; Garreau, H.; Li, S.; Vert, M. Hydrolytic degradation of devices based on poly(dl-lactic acid) size-dependence. Biomaterials 1995, 16, 305–311. [Google Scholar] [CrossRef]
- Wan, L.; Li, C.; Sun, C.; Zhou, S.; Zhang, Y. Conceiving a feasible degradation model of polylactic acid-based composites through hydrolysis study to polylactic acid/wood flour/polymethyl methacrylate. Compos. Sci. Technol. 2019, 181, 107675. [Google Scholar] [CrossRef]
- Baidurah, S.; Takada, S.; Shimizu, K.; Ishida, Y.; Yamane, T.; Ohtani, H. Evaluation of biodegradation behavior of poly(butylene succinate-co- butylene adipate) with lowered crystallinity by thermally assisted hydrolysis and methylation-gas chromatography. J. Anal. Appl. Pyrolysis 2012, 103, 73–77. [Google Scholar] [CrossRef]
- Im, D.; Gavande, V.; Lee, H.; Iwata, T.; Lee, W.-K. Compatibility and hydrolytic behaviors of polylactide isomer/poly(butylene succinate) mixtures by the Langmuir technique. Polym. Degrad. Stab. 2021, 186, 109517. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Eldin, R.E.S.; Serea, E.S.A.; Gomaa, N.M.; AboElmagd, G.M.; Salem, S.A.; Elsayed, Z.A.; Edrees, A.; Shams-Eldin, E.; Shalan, A.E. Advances in nanotechnology and antibacterial properties of biodegradable food packaging materials. RSC Adv. 2020, 10, 20467–20484. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, L.E. Models for the Permeability of Filled Polymer Systems. J. Macromol. Sci. Part A Chem. 1967, 1, 929–942. [Google Scholar] [CrossRef]
- Molinaro, S.; Romero, M.C.; Boaro, M.; Sensidoni, A.; Lagazio, C.; Morris, M.; Kerry, J. Effect of nanoclay-type and PLA optical purity on the characteristics of PLA-based nanocomposite films. J. Food Eng. 2013, 117, 113–123. [Google Scholar] [CrossRef]
- Das, K.; Ray, D.; Banerjee, I.; Bandyopadhyay, N.R.; Sengupta, S.; Mohanty, A.K.; Misra, M. Crystalline morphology of PLA/clay nanocomposite films and its correlation with other properties. J. Appl. Polym. Sci. 2010, 118, 143–151. [Google Scholar] [CrossRef]
- Vasile, C. Polymeric nanocomposites and nanocoatings for food packaging: A review. Materials 2018, 11, 1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.U.; Venkatanarayana, B.; Suman, K.N.S. Enhancement of mechanical properties of PLA/PCL (80/20) blend by reinforcing with MMT nanoclay. Mater. Today Proc. 2019, 18, 85–97. [Google Scholar] [CrossRef]
- Darie, R.N.; Pâslaru, E.; Sdrobis, A.; Pricope, G.M.; Hitruc, G.E.; Poiatǎ, A.; Baklavaridis, A.; Vasile, C. Effect of nanoclay hydrophilicity on the poly(lactic acid)/clay nanocomposites properties. Ind. Eng. Chem. Res. 2014, 53, 7877–7890. [Google Scholar] [CrossRef]
- Oliver-ortega, H.; Tresserras, J.; Julian, F.; Alcal, M.; Bala, A.; Espinach, F.X.; Alberto, M. Nanocomposites Materials of PLA Reinforced with Nanoclays Using a Masterbatch Technology: A Study of the Mechanical Performance and Its Sustainability. Polymers 2021, 13, 2133. [Google Scholar] [CrossRef] [PubMed]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Biodegradable polylactide and its nanocomposites: Opening a new dimension for plastics and composites. Macromol. Rapid Commun. 2003, 24, 815–840. [Google Scholar] [CrossRef]
- Malwela, T.; Ray, S.S. Enzymatic degradation behavior of nanoclay reinforced biodegradable PLA/PBSA blend composites. Int. J. Biol. Macromol. 2015, 77, 131–142. [Google Scholar] [CrossRef]
- Nofar, M.; Heuzey, M.C.; Carreau, P.J.; Kamal, M.R. Effects of nanoclay and its localization on the morphology stabilization of PLA/PBAT blends under shear flow. Polymer 2016, 98, 353–364. [Google Scholar] [CrossRef]
- Chiou, B.-S.; Wood, D.; Yee, E.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Extruded starch–nanoclay nanocomposites: Effects of glycerol and nanoclay concentration. Polym. Eng. Sci. 2007, 47, 1898–1904. [Google Scholar] [CrossRef]
- Lajarrige, A.; Gontard, N.; Gaucel, S.; Samson, M.F.; Peyron, S. The mixed impact of nanoclays on the apparent diffusion coefficient of additives in biodegradable polymers in contact with food. Appl. Clay Sci. 2019, 180, 105170. [Google Scholar] [CrossRef]
- Lai, S.M.; Wu, S.H.; Lin, G.G.; Don, T.M. Unusual mechanical properties of melt-blended poly(lactic acid) (PLA)/clay nanocomposites. Eur. Polym. J. 2014, 52, 193–206. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Tsuji, H.; Okino, R.; Daimon, H.; Fujie, K. Water vapor permeability of poly(lactide)s: Effects of molecular characteristics and crystallinity. J. Appl. Polym. Sci. 2006, 99, 2245–2252. [Google Scholar] [CrossRef]
- Naderi-Samani, H.; Loghman-Estarki, M.R.; Razavi, R.S.; Ramazani, M. The Effects of organoclay on the morphology, thermal stability, transparence and hydrophobicity properties of polyamide—Imide/nanoclay nanocomposite coatings. Prog. Org. Coatings 2017, 112, 162–168. [Google Scholar] [CrossRef]
- Lendvai, L.; Sajó, I.; Karger-Kocsis, J. Effect of Storage Time on the Structure and Mechanical Properties of Starch/Bentonite Nanocomposites. Starch Stärke 2019, 71, 1800123. [Google Scholar] [CrossRef] [Green Version]
- Trifol, J.; Plackett, D.; Sillard, C.; Hassager, O.; Daugaard, A.E.; Bras, J.; Szabo, P. A comparison of partially acetylated nanocellulose, nanocrystalline cellulose, and nanoclay as fillers for high-performance polylactide nanocomposites. J. Appl. Polym. Sci. 2016, 133, 43257. [Google Scholar] [CrossRef]
- Rhim, J.W.; Hong, S.I.; Ha, C.S. Tensile, water vapor barrier and antimicrobial properties of PLA/nanoclay composite films. LWT Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Sarfraz, J.; Gulin-Sarfraz, T.; Nilsen-Nygaard, J.; Pettersen, M.K. Nanocomposites for Food Packaging Applications: An Overview. Nanomaterials 2020, 11, 10. [Google Scholar] [CrossRef]
- Müller, C.M.O.; Laurindo, J.B.; Yamashita, F. Effect of nanoclay incorporation method on mechanical and water vapor barrier properties of starch-based films. Ind. Crop. Prod. 2011, 33, 605–610. [Google Scholar] [CrossRef]
- Granda, L.A.; Oliver-Ortega, H.; Fabra, M.J.; Tarrés, Q.; Pèlach, M.À.; Lagarón, J.M.; Méndez, J.A. Improved process to obtain nanofibrillated cellulose (CNF) reinforced starch films with upgraded mechanical properties and barrier character. Polymers 2020, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- DelRe, C.; Jiang, Y.; Kang, P.; Kwon, J.; Hall, A.; Jayapurna, I.; Ruan, Z.; Ma, L.; Zolkin, K.; Li, T.; et al. Near-complete depolymerization of polyesters with nano-dispersed enzymes. Nature 2021, 592, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Tomita, T.; Abe, N.; Kamio, Y. Purification and characterization of an extracellular poly(L-lactic acid) depolymerase from a soil isolate, amycolatopsis sp. strain K104-1. Appl. Environ. Microbiol. 2001, 67, 345–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, F. Polylactic acid (PLA)-degrading microorganisms and PLA depolymerases. ACS Symp. Ser. 2010, 1043, 405–414. [Google Scholar] [CrossRef]
- Zenkiewicz, M.; Richert, A.; Malinowski, R.; Moraczewski, K. A comparative analysis of mass losses of some aliphatic polyesters upon enzymatic degradation. Polym. Test. 2013, 32, 209–214. [Google Scholar] [CrossRef]
- Hegyesi, N.; Zhang, Y.; Kohári, A.; Polyák, P.; Sui, X.; Pukánszky, B. Enzymatic degradation of PLA/cellulose nanocrystal composites. Ind. Crop. Prod. 2019, 141, 111799. [Google Scholar] [CrossRef] [Green Version]
- Stepczyńska, M.; Rytlewski, P. Enzymatic degradation of flax-fibers reinforced polylactide. Int. Biodeterior. Biodegrad. 2018, 126, 160–166. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Puglia, D.; Petrucci, R.; Kenny, J.M.; Torre, L. Study of disintegrability in compost and enzymatic degradation of PLA and PLA nanocomposites reinforced with cellulose nanocrystals extracted from Posidonia Oceanica. Polym. Degrad. Stab. 2015, 121, 105–115. [Google Scholar] [CrossRef]
- Fundador, N.G.V.; Takemura, A.; Iwata, T. Structural Properties and Enzymatic Degradation Behavior of PLLA and Stereocomplexed PLA Nanofibers. Macromol. Mater. Eng. 2010, 295, 865–871. [Google Scholar] [CrossRef]
- Wei, H. Optimisation on thermoforming of biodegradable poly (Lactic acid) (pla) by numerical modelling. Polymers 2021, 13, 654. [Google Scholar] [CrossRef]
- Stoclet, G.; Seguela, R.; Lefebvre, J.M.; Elkoun, S.; Vanmansart, C. Strain-induced molecular ordering in polylactide upon uniaxial stretching. Macromolecules 2010, 43, 1488–1498. [Google Scholar] [CrossRef]
- Michael, N. Biopolymers: Processing and Products, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780323266987. [Google Scholar]
- Diani, J.; Liu, Y.; Gall, K. Finite Strain 3D Thermoviscoelastic Constitutive Model for Shape Memory Polymers. Polym. Eng. Sci. 2006, 46, 486–492. [Google Scholar] [CrossRef]
- Mallegni, N.; Phuong, T.V.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A. Poly(lactic acid) (PLA) based tear resistant and biodegradable flexible films by blown film extrusion. Materials 2018, 11, 148. [Google Scholar] [CrossRef] [Green Version]
- Solarski, S.; Ferreira, M.; Devaux, E. Characterization of the thermal properties of PLA fibers by modulated differential scanning calorimetry. Polymer 2005, 46, 11187–11192. [Google Scholar] [CrossRef]
- Reesha, K.V.; Panda, S.K.; Bindu, J.; Varghese, T.O. Development and characterization of an LDPE/chitosan composite antimicrobial film for chilled fish storage. Int. J. Biol. Macromol. 2015, 79, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jung, J.; Park, S.; Seo, J. Preparation and characterization of LDPE/PVA blend films filled with glycerin-plasticized polyvinyl alcohol. J. Appl. Polym. Sci. 2015, 132, 41985. [Google Scholar] [CrossRef]
- Lei, W.; Fang, C.; Zhou, X.; Yin, Q.; Pan, S.; Yang, R.; Liu, D.; Ouyang, Y. Cellulose nanocrystals obtained from office waste paper and their potential application in PET packing materials. Carbohydr. Polym. 2018, 181, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, D.; Giannoulis, A. Evaluation of the functionality of bio-based food packaging films. Polym. Test. 2018, 69, 39–51. [Google Scholar] [CrossRef]
- Pan, P.; Yang, J.; Shan, G.; Bao, Y.; Weng, Z.; Cao, A.; Yazawa, K.; Inoue, Y. Temperature-variable FTIR and solid-state 13C NMR investigations on crystalline structure and molecular dynamics of polymorphic poly(l-lactide) and poly(l-lactide)/poly(d-lactide) stereocomplex. Macromolecules 2012, 45, 189–197. [Google Scholar] [CrossRef]
- Riba, J.R.; Cailloux, J.; Cantero, R.; Canals, T.; Maspoch, M.L. Multivariable methods applied to FTIR: A powerful technique to highlight architectural changes in poly(lactic acid). Polym. Test. 2018, 65, 264–269. [Google Scholar] [CrossRef]
- Throne, J. Applied Plastics Engineering Handbook: Processing, Materials, and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780323390408. [Google Scholar]


| Sample | Water | Simulant B | ||
|---|---|---|---|---|
| Migration (mg/kg) | Migration (mg/dm2) | Migration (mg/kg) | Migration (mg/dm2) | |
| PLA | <1 | <1 | <1 | <1 |
| PLA + 4%NC | <1 | <1 | <1 | <1 |
| Material | PLA | PLA | PLA 2% | PLA 2% | PLA 4% | PLA 4% |
|---|---|---|---|---|---|---|
| HNAF (mm) | 116 ± 2 a | 152 ± 1 b | 116 ± 2 a | 135 ± 8 c | 116 ± 4 a | 137 ± 4 c |
| DFT (mm) | 17.6 ± 5 | 10.7 ± 6 | 0 | 35.1 ± 6 | 0 | 45.1 ± 7 |
| Material | PLA | PLA | PLA 2% | PLA 2% | PLA 4% | PLA 4% |
|---|---|---|---|---|---|---|
| HNAF (mm) | 116 | 152 | 116 | 135 | 116 | 137 |
| A (mm2) | 52,121 | 66,028 | 52,121 | 59,441 | 52,121 | 60,214 |
| ADR | 3.18 | 4.03 | 6.18 | 3.63 | 3.18 | 3.68 |
| PTR (%) | 68.6% | 75.2% | 68.6% | 72.4% | 68.9% | 72.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliver-Ortega, H.; Vandemoortele, V.; Bala, A.; Julian, F.; Méndez, J.A.; Espinach, F.X. Nanoclay Effect into the Biodegradation and Processability of Poly(lactic acid) Nanocomposites for Food Packaging. Polymers 2021, 13, 2741. https://doi.org/10.3390/polym13162741
Oliver-Ortega H, Vandemoortele V, Bala A, Julian F, Méndez JA, Espinach FX. Nanoclay Effect into the Biodegradation and Processability of Poly(lactic acid) Nanocomposites for Food Packaging. Polymers. 2021; 13(16):2741. https://doi.org/10.3390/polym13162741
Chicago/Turabian StyleOliver-Ortega, Helena, Victor Vandemoortele, Alba Bala, Fernando Julian, José Alberto Méndez, and Francesc Xavier Espinach. 2021. "Nanoclay Effect into the Biodegradation and Processability of Poly(lactic acid) Nanocomposites for Food Packaging" Polymers 13, no. 16: 2741. https://doi.org/10.3390/polym13162741
APA StyleOliver-Ortega, H., Vandemoortele, V., Bala, A., Julian, F., Méndez, J. A., & Espinach, F. X. (2021). Nanoclay Effect into the Biodegradation and Processability of Poly(lactic acid) Nanocomposites for Food Packaging. Polymers, 13(16), 2741. https://doi.org/10.3390/polym13162741









