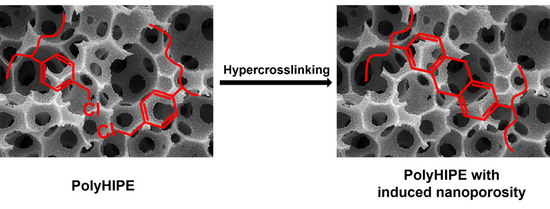
Influence of Functional Group Concentration on Hypercrosslinking of Poly(vinylbenzyl chloride) PolyHIPEs: Upgrading Macroporosity with Nanoporosity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
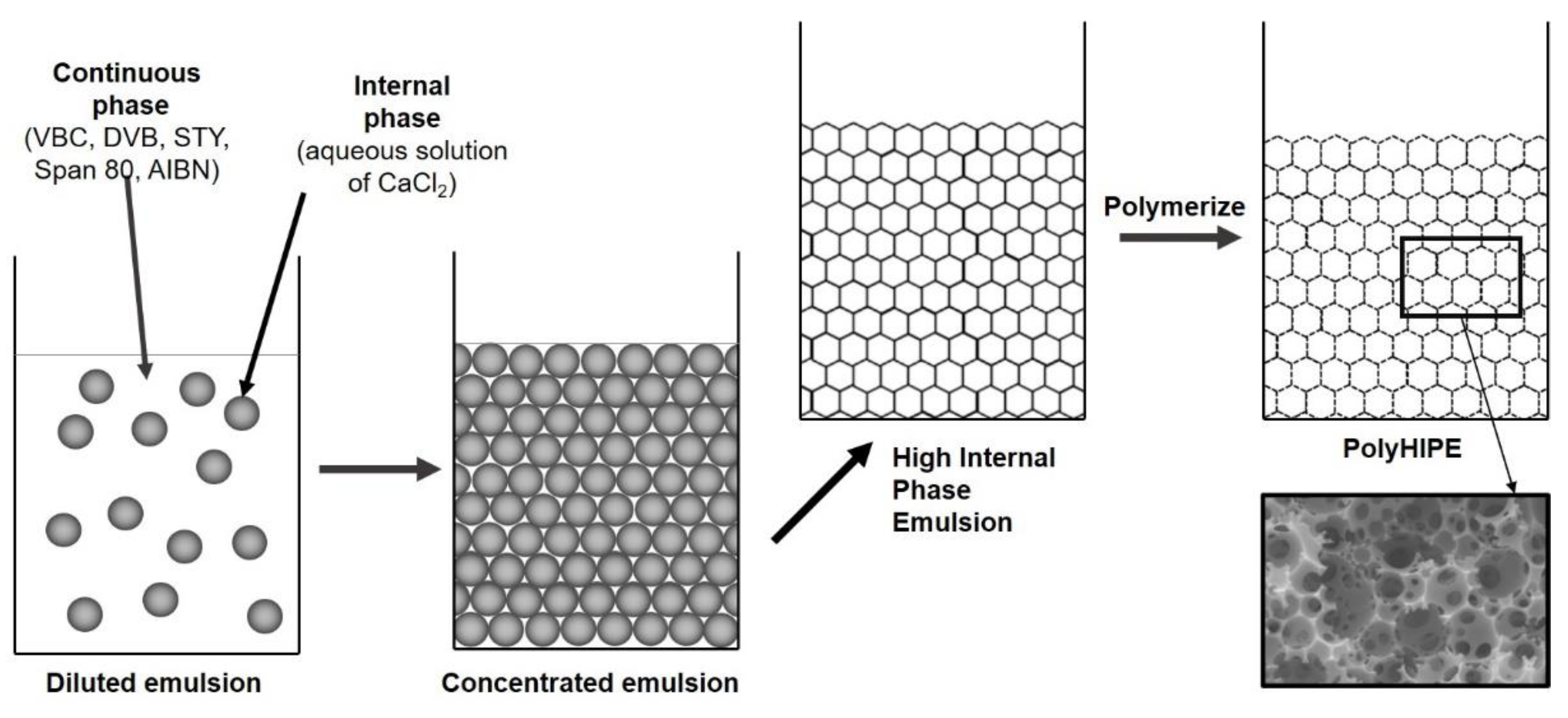
2.2. Preparation of 4-Vinylbenzyl Chloride/Divinylbenzene/Styrene Poly(High Internal Phase Emulsions) (PolyHIPEs)
2.3. Hypercrosslinking of PolyHIPEs
2.4. Characterization
3. Results
3.1. Preparation of Poly(VBC-co-DVB-co-STY)HIPEs
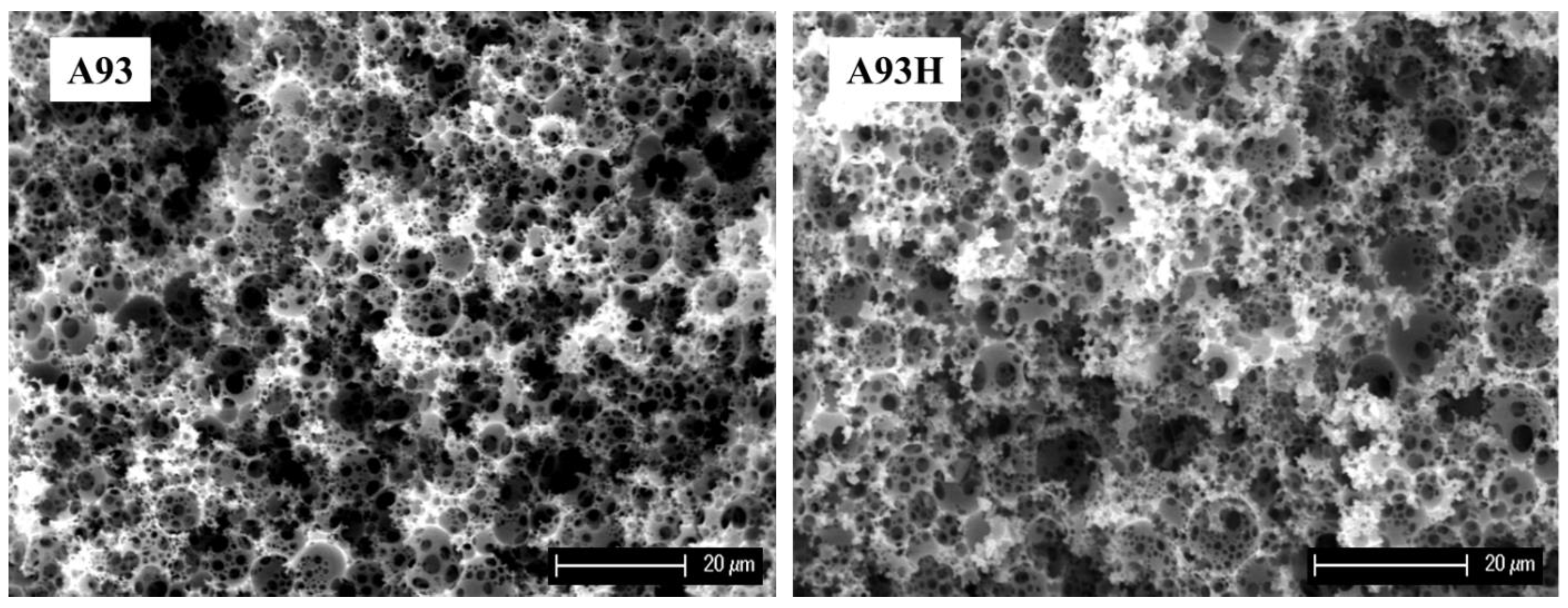
3.2. Hypercrosslinking of PolyHIPEs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davankov, V.A.; Rogozhin, V.; Tsyurupa, M.P. Macronet Polystyrene Structures for Ionites and Method of Producing Same. U.S. Patent 3,729,457, 24 April 1973. [Google Scholar]
- Tsyurupa, M.P.; Davankov, V.A. Hypercrosslinked polymers: Basic principle of preparing the new class of polymeric materials. React. Funct. Polym. 2002, 53, 193–203. [Google Scholar] [CrossRef]
- Veverka, P.; Jeřábek, K. Influence of hypercrosslinking on adsorption and absorption on or in styrenic polymers. React. Funct. Polym. 2004, 59, 71–79. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jang, J.E.; Oh, C.G.; Ihm, S.K.; Cortez, J.; Sherrington, D.C. Rapid generation and control of microporosity, bimodal pore size distribution, and surface area in Davankov-type hyper-cross-linked resins. Macromolecules 2006, 39, 627–632. [Google Scholar] [CrossRef]
- Veverka, P.; Jeřábek, K. Mechanism of hypercrosslinking of chloromethylated styrene– divinylbenzene copolymers. React. Funct. Polym. 1999, 41, 21–25. [Google Scholar] [CrossRef]
- Pulko, I.; Wall, J.; Krajnc, P.; Cameron, N.R. Ultra-high surface area functional porous polymers by emulsion templating and hypercrosslinking: Efficient nucleophilic catalyst supports. Chem. A Eur. J. 2010, 16, 2350–2354. [Google Scholar] [CrossRef]
- Dušek, K. Phase separation during the formation of three-dimensional polymers. J. Polym. Sci. Part B Polym. Lett. 1965, 3, 209–212. [Google Scholar] [CrossRef]
- Okay, O. Macroporous copolymer networks. Prog. Polym. Sci. 2000, 25, 711–779. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Davankov, V.A. Porous structure of hypercrosslinked polystyrene: State-of-the-art mini-review. React. Funct. Polym. 2006, 66, 768–779. [Google Scholar] [CrossRef]
- Sherrington, D.C. Preparation, structure and morphology of polymer supports. Chem. Commun. 1998, 2275–2286. [Google Scholar] [CrossRef]
- Sherrington, D.C.; Hodge, P. Synthesis Separations Using Functional Polymers; Wiley-VCH Verlag GmbH&Co. KGaA: Chichester, UK, 1988. [Google Scholar]
- Pastukhov, A.V.; Tsyurupa, M.P.; Davankov, V.A. Hypercrosslinked polystyrene: A polymer in a non-classical physical state. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2324–2333. [Google Scholar] [CrossRef]
- Davankov, V.A.; Tsyurupa, M.P.; Ilyin, M.; Pavlova, L. Hypercross-linked polystyrene and its potentials for liquid chromatography: A mini-review. J. Chromatogr. A 2002, 965, 65–73. [Google Scholar] [CrossRef]
- Davankov, V.A.; Sychov, C.S.; Ilyin, M.M.; Sochilina, K.O. Hypercrosslinked polystyrene as a novel type of high-performance liquid chromatography column packing material: Mechanisms of retention. J. Chromatogr. A 2003, 987, 67–75. [Google Scholar] [CrossRef]
- Penner, N.A.; Nesterenko, P.N.; Hyin, M.M.; Tsyurupa, M.P.; Davankov, V.A. Investigation of the properties of hypercrosslinked polystyrene as a stationary phase for high-performance liquid chromatography. Chromatographia 1999, 50, 611–620. [Google Scholar] [CrossRef]
- Urban, J.; Svec, F.; Fréchet, J.M.J. Hypercrosslinking: New approach to porous polymer monolithic capillary columns with large surface area for the highly efficient separation of small molecules. J. Chromatogr. A 2010, 1217, 8212–8221. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Tan, B.; Wood, C.D. Solution-processable hypercrosslinked polymers by low cost strategies: A promising platform for gas storage and separation. J. Mater. Chem. A 2016, 4, 15072–15080. [Google Scholar] [CrossRef]
- Liu, Q.; Xia, B.; Huang, J.; Liao, B.; Liu, H.; Ou, B.; Chen, L.; Zhou, Z. Hypercrosslinked polystyrene microspheres with ultrahigh surface area and their application in gas storage. Mater. Chem. Phys. 2017, 199, 616–622. [Google Scholar] [CrossRef]
- Belyakova, L.D.; Schevchenko, T.I.; Davankov, V.A.; Tsyurupa, M.P. Sorption of vapors of various substances by hypercrosslinked “styrosorb” polystyrenes. Adv. Colloid Interface Sci. 1986, 25, 249–266. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Maslova, L.A.; Andreeva, A.I.; Mrachkovskaya, T.A.; Davankov, V.A. Sorption of organic compounds from aqueous media by hypercrosslinked polystyrene sorbents ‘Styrosorbrs’. React. Polym. 1995, 25, 69–78. [Google Scholar] [CrossRef]
- Davankov, V.A.; Pavlova, L.; Tsyurupa, M.P.; Brady, J.; Balsamo, M.; Yousha, E. Polymeric adsorbent for removing toxic proteins from blood of patients with kidney failure. J. Chromatogr. B Biomed. Sci. Appl. 2000, 739, 73–80. [Google Scholar] [CrossRef]
- Wang, K.; Jia, Z.; Yang, X.; Wang, L.; Gu, Y.; Tan, B. Acid and base coexisted heterogeneous catalysts supported on hypercrosslinked polymers for one-pot cascade reactions. J. Catal. 2017, 348, 168–176. [Google Scholar] [CrossRef]
- Gu, Y.; Son, S.U.; Li, T.; Tan, B. Low-Cost Hypercrosslinked Polymers by Direct Knitting Strategy for Catalytic Applications. Adv. Funct. Mater. 2021, 31, 1–24. [Google Scholar] [CrossRef]
- Liu, X.; Xu, W.; Xiang, D.; Zhang, Z.; Chen, D.; Hu, Y.; Li, Y.; Ouyang, Y.; Lin, H. Palladium immobilized on functionalized hypercrosslinked polymers: A highly active and recyclable catalyst for Suzuki-Miyaura coupling reactions in water. New J. Chem. 2019, 43, 12206–12210. [Google Scholar] [CrossRef]
- Barby, D.; Haq, Z. Low Density Porous Cross-Linked Polymeric Material Sand Their Preparation. EP Patent 60138, 6 November 1985. [Google Scholar]
- Pulko, I.; Krajnc, P. Porous Polymer Monoliths by Emulsion Templating. Encycl. Polym. Sci. Technol. 2017, 1–28. [Google Scholar] [CrossRef]
- Cameron, N.R. High internal phase emulsion templating as a route to well-defined porous polymers. Polymer 2005, 46, 1439–1449. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Sanguramath, R.A.; Israel, S.; Silverstein, M.S. Emulsion Templating: Porous Polymers and Beyond. Macromolecules 2019, 52, 5445–5479. [Google Scholar] [CrossRef] [Green Version]
- Foudazi, R.; Qavi, S.; Masalova, I.; Malkin, A.Y. Physical chemistry of highly concentrated emulsions. Adv. Colloid Interface Sci. 2015, 220, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Naranda, J.; Sušec, M.; Maver, U.; Gradišnik, L.; Gorenjak, M.; Vukasović, A.; Ivković, A.; Rupnik, M.S.; Vogrin, M.; Krajnc, P. Polyester type polyHIPE scaffolds with an interconnected porous structure for cartilage regeneration. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Paljevac, M.; Gradišnik, L.; Lipovšek, S.; Maver, U.; Kotek, J.; Krajnc, P. Multiple-Level Porous Polymer Monoliths with Interconnected Cellular Topology Prepared by Combining Hard Sphere and Emulsion Templating for Use in Bone Tissue Engineering. Macromol. Biosci. 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Busby, W.; Cameron, N.R.; Jahoda, C.A.B. Emulsion-derived foams (PolyHIPEs) containing poly(Sε-caprolactone) as matrixes for tissue engineering. Biomacromolecules 2001, 2, 154–164. [Google Scholar] [CrossRef]
- Owen, R.; Sherborne, C.T.; Green, N.H.; Reilly, G.C.; Claeyssens, F. Emulsion templated scaffolds with tunable mechanical properties for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2016, 54, 159–172. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.; Fitzhenry, L.; White, B.; Connolly, D. Polystyrene-co-divinylbenzene polyHIPE monoliths in 1.0 mm column formats for liquid chromatography. Materials 2016, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Kovačič, S.; Krajnc, P. Macroporous monolithic poly(4-vinylbenzyl chloride) columns for organic synthesis facilitation by in situ polymerization of high internal phase emulsions. J. Polym. Sci. A Polym. Chem. 2009, 47, 6726–6734. [Google Scholar] [CrossRef]
- Huš, S.; Kolar, M.; Krajnc, P. Separation of heavy metals from water by functionalized glycidyl methacrylate poly (high internal phase emulsions). J. Chromatogr. A 2016, 1437, 168–175. [Google Scholar] [CrossRef]
- Taylor-Pashow, K.M.L.; Pribyl, J.G. PolyHIPEs for Separations and Chemical Transformations: A Review. Solvent Extr. Ion Exch. 2019, 37, 1–26. [Google Scholar] [CrossRef]
- Krajnc, P.; Leber, N.; Štefanec, D.; Kontrec, S.; Podgornik, A. Preparation and characterisation of poly(high internal phase emulsion) methacrylate monoliths and their application as separation media. J. Chromatogr. A 2005, 1065, 69–73. [Google Scholar] [CrossRef]
- Koler, A.; Paljevac, M.; Cmager, N.; Iskra, J.; Kolar, M.; Krajnc, P. Poly(4-vinylpyridine) polyHIPEs as catalysts for cycloaddition click reaction. Polymer 2017, 126, 402–407. [Google Scholar] [CrossRef]
- Kawada, K.; Okano, K.; Iskra, J.; Krajnc, P.; Cahard, D. SelectfluorTMon a PolyHIPE Material as Regenerative and Reusable Polymer-Supported Electrophilic Fluorinating Agent. Adv. Synth. Catal. 2017, 359, 584–589. [Google Scholar] [CrossRef]
- Sevšek, U.; Brus, J.; Jeřabek, K.; Krajnc, P. Post polymerisation hypercrosslinking of styrene/divinylbenzene poly(HIPE)s: Creating micropores within macroporous polymer. Polymer 2014, 55, 410–415. [Google Scholar] [CrossRef]
- Schwab, M.G.; Senkovska, I.; Rose, M.; Klein, N.; Koch, M.; Pahnke, J.; Jonschker, G.; Schmitz, B.; Hirscher, M.; Kaskel, S. High surface area polyHIPEs with hierarchical pore system. Soft Matter. 2009, 5, 1055–1059. [Google Scholar] [CrossRef]
- Israel, S.; Gurevitch, I.; Silverstein, M.S. Carbons with a hierarchical porous structure through the pyrolysis of hypercrosslinked emulsion-templated polymers. Polymer 2015, 72, 453–463. [Google Scholar] [CrossRef]
- Koler, A.; Pulko, I.; Krajnc, P. Post polymerisation hypercrosslinking with emulsion templating for hierarchical and multi-level porous polymers. Acta Chim. Slov. 2020, 67, 349–360. [Google Scholar] [CrossRef]
- Lee, J.Y.; Wood, C.D.; Bradshaw, D.; Rosseinsky, M.J.; Cooper, A.I. Hydrogen adsorption in microporous hypercrosslinked polymers. Chem. Commun. 2006, 2670–2672. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, F.S.; Sherrington, D.C.; Tetley, L. Synthesis of ultrahigh surface area monodisperse porous polymer nanospheres. Macromolecules 2006, 39, 5381–5384. [Google Scholar] [CrossRef] [Green Version]
- Rouquerol, J.; Avnir, D.; Fairbridge, D.H.; Everett, C.W.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Harkins, W.D.; Jura, G. Surfaces of Solids. XI. Determination of Decrease (pi) of Free Surface Energy of a Solid by Adsorbed Film. J. Am. Chem. Soc. 1944, 66, 1356–1362. [Google Scholar]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: New York, NY, USA, 1982. [Google Scholar] [CrossRef]

| Sample | Continuous Phase | VAP * [mL] | m (Span 80) [g] | ||
|---|---|---|---|---|---|
| m (STY) [g] | m (DVB) [g] | m (VBC) [g] | |||
| A93 | 0.181 | 0.095 | 4.747 | 18.6 | 1.264 |
| A73 | 0.899 | 0.086 | 3.724 | 17.9 | 1.185 |
| A53 | 1.563 | 0.107 | 2.696 | 17.2 | 1.162 |
| A43 | 1.909 | 0.106 | 2.225 | 16.9 | 1.035 |
| A41 | 1.98 | 0.091 | 2.104 | 16.8 | 0.83 |
| A38 | 2.091 | 0.111 | 1.936 | 16.8 | 0.834 |
| A36 | 2.111 | 0.450 | 1.539 | 16.7 | 0.887 |
| A33 | 2.253 | 0.087 | 1.679 | 16.5 | 1.005 |
| B90 | 0.173 | 0.257 | 4.579 | 18.6 | 1.339 |
| B70 | 0.877 | 0.218 | 3.567 | 17.9 | 1.199 |
| B50 | 1.583 | 0.234 | 2.573 | 17.2 | 1.089 |
| B40 | 1.926 | 0.258 | 2.035 | 16.9 | 1.076 |
| B38 | 1.98 | 0.217 | 1.933 | 16.8 | 0.916 |
| B35 | 2.096 | 0.246 | 1.794 | 16.7 | 0.817 |
| B33 | 2.187 | 0.226 | 1.630 | 16.6 | 0.815 |
| B30 | 2.279 | 0.221 | 1.532 | 16.5 | 1.035 |
| C85 | 0.188 | 0.440 | 4.372 | 18.6 | 1.227 |
| C65 | 0.566 | 0.498 | 3.858 | 18.3 | 1.194 |
| C45 | 1.223 | 0.457 | 2.814 | 17.6 | 1.122 |
| C35 | 1.922 | 0.443 | 1.788 | 16.9 | 1.087 |
| C33 | 1.978 | 0.438 | 1.678 | 16.8 | 0.835 |
| C28 | 2.196 | 0.458 | 1.386 | 16.6 | 0.814 |
| C25 | 2.256 | 0.460 | 1.290 | 16.4 | 0.993 |
| Sample | Before Hypercrosslinking | After Hypercrosslinking | |||
|---|---|---|---|---|---|
| Cl Content, wt.% | BET Surf. Area, m2/g | Cl Content, wt.% | Residual Cl, % of Orig. | BET Surf. Area, m2/g | |
| A93 | 21.90 ± 0.20 | 2.40 | 3.70 ± 0.03 | 16.9 | 709 |
| A73 | 18.00 ± 0.40 | 3.20 | 1.70 ± 0.03 | 9.4 | 636 |
| A53 | 13.90 ± 0.20 | 4.60 | 1.80 ± 0.10 | 12.9 | 410 |
| A43 | 11.70 ± 0.10 | 1.80 | 1.00 ± 0.02 | 8.5 | 279 |
| A41 | 11.20 ± 0.30 | 2.30 | 2.00 ± 0.10 | 17.9 | 304 |
| A38 | 10.30 ± 0.30 | 3.00 | 1.40 ± 0.20 | 13.6 | 122 |
| A36 | 8.20 ± 0.10 | 3.10 | 1.30 ± 0.10 | 15.9 | 5.0 |
| A33 | 9.20 ± 0.20 | 3.90 | 0.60 ± 0.03 | 6.5 | 3.9 |
| B90 | 21.30 ± 0.60 | 4.40 | 7.50 ± 0.40 | 35.2 | 585 |
| B70 | 17.80 ± 0.40 | 6.00 | 2.70 ± 0.10 | 15.2 | 491 |
| B50 | 13.70 ± 0.20 | 4.80 | 1.70 ± 0.04 | 12.4 | 412 |
| B40 | 11.00 ± 0.30 | 2.30 | 1.10 ± 0.10 | 10.0 | 196 |
| B38 | 10.40 ± 0.10 | 3.10 | 1.20 ± 0.10 | 11.5 | 217 |
| B35 | 9.60 ± 0.10 | 2.30 | 1.40 ± 0.10 | 14.6 | 70 |
| B33 | 8.90 ± 0.50 | 3.40 | 1.10 ± 0.10 | 12.4 | 8.0 |
| B30 | 8.30 ± 0.20 | 3.00 | 0.80 ± 0.10 | 9.6 | 4.2 |
| C85 | 20.40 ± 0.50 | 2.20 | 4.10 ± 0.03 | 20.1 | 536 |
| C65 | 18.20 ± 0.30 | 3.80 | 2.30 ± 0.10 | 12.6 | 547 |
| C45 | 14.30 ± 0.20 | 3.60 | 1.50 ± 0.07 | 10.5 | 350 |
| C35 | 9.60 ± | 2.10 | 1.20 ± 0.03 | 12.5 | 62 |
| C33 | 9.20 ± 0.10 | 2.60 | 1.40 ± 0.10 | 15.2 | 14 |
| C28 | 7.60 ± 0.50 | 3.10 | 0.80 ± 0.10 | 10.5 | 14 |
| C25 | 7.10 ± 0.40 | 1.80 | 1.00 ± 0.10 | 14.1 | 13 |
| Sample | Vp [cm3/g] | Vmicro [cm3/g] | Vmeso [cm3/g] | Vmicro/Vp |
|---|---|---|---|---|
| A93 (2 mol.% DVB) | 0.43 | 0.12 | 0.31 | 0.28 |
| B90 (5 mol.% DVB) | 0.40 | 0.18 | 0.22 | 0.45 |
| C85 (10 mol.% DVB) | 0.30 | 0.15 | 0.15 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koler, A.; Kolar, M.; Jeřábek, K.; Krajnc, P. Influence of Functional Group Concentration on Hypercrosslinking of Poly(vinylbenzyl chloride) PolyHIPEs: Upgrading Macroporosity with Nanoporosity. Polymers 2021, 13, 2721. https://doi.org/10.3390/polym13162721
Koler A, Kolar M, Jeřábek K, Krajnc P. Influence of Functional Group Concentration on Hypercrosslinking of Poly(vinylbenzyl chloride) PolyHIPEs: Upgrading Macroporosity with Nanoporosity. Polymers. 2021; 13(16):2721. https://doi.org/10.3390/polym13162721
Chicago/Turabian StyleKoler, Amadeja, Mitja Kolar, Karel Jeřábek, and Peter Krajnc. 2021. "Influence of Functional Group Concentration on Hypercrosslinking of Poly(vinylbenzyl chloride) PolyHIPEs: Upgrading Macroporosity with Nanoporosity" Polymers 13, no. 16: 2721. https://doi.org/10.3390/polym13162721
APA StyleKoler, A., Kolar, M., Jeřábek, K., & Krajnc, P. (2021). Influence of Functional Group Concentration on Hypercrosslinking of Poly(vinylbenzyl chloride) PolyHIPEs: Upgrading Macroporosity with Nanoporosity. Polymers, 13(16), 2721. https://doi.org/10.3390/polym13162721