Tunable Quantum Photoinitiators for Radical Photopolymerization
Abstract
:1. Introduction
2. Categories of Quantum Photoinitiators
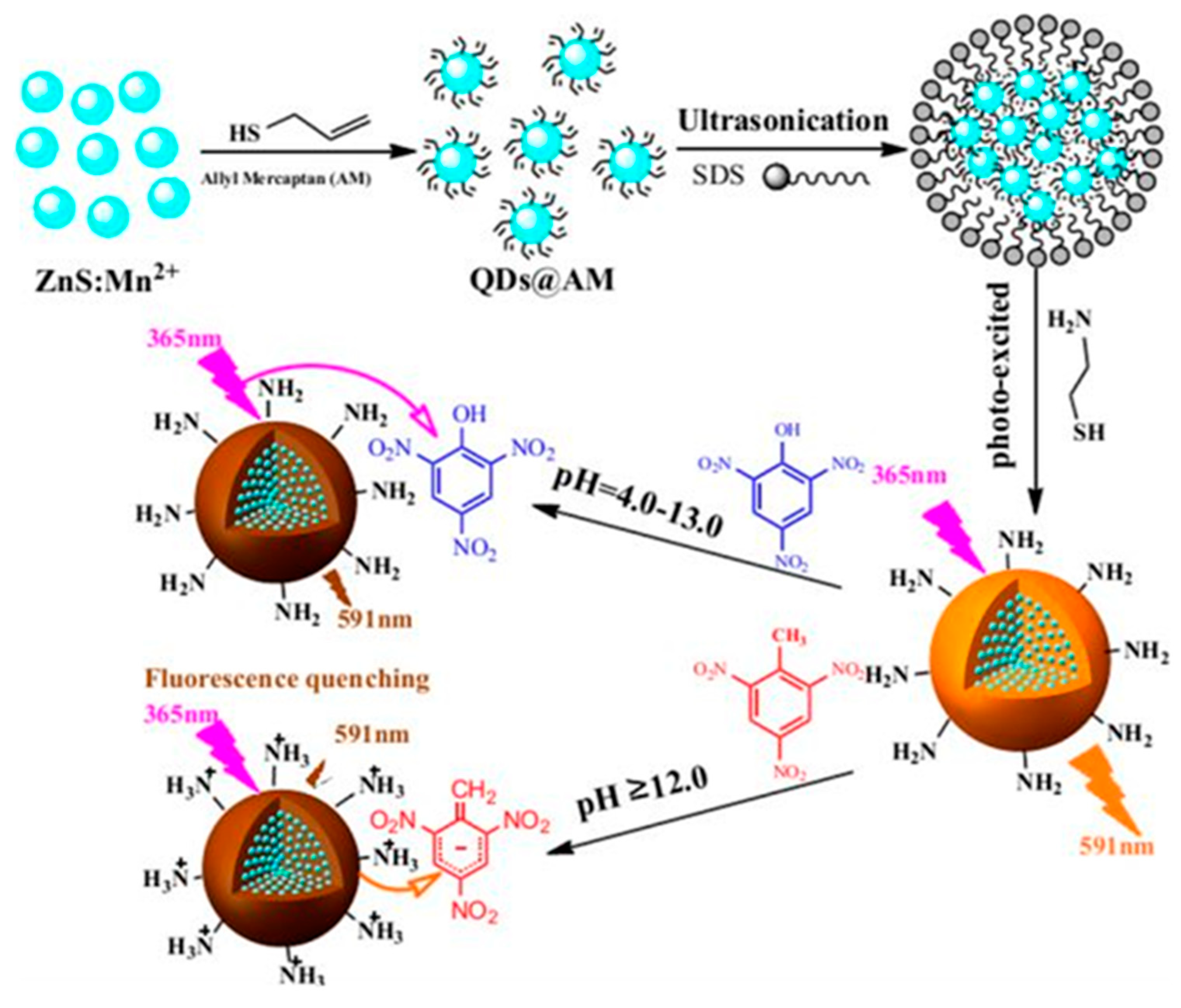
2.1. Semiconductor QPIs
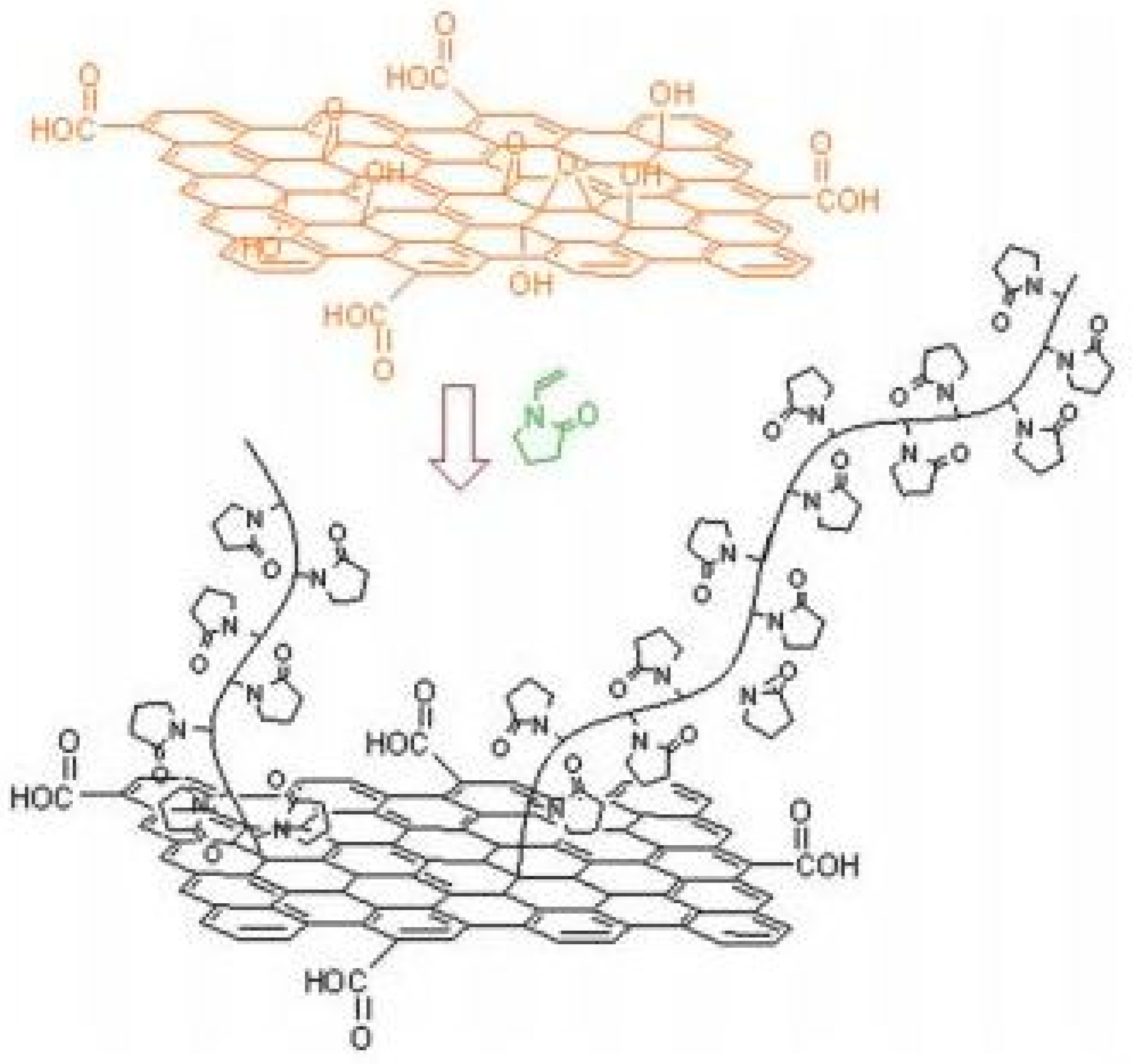
2.2. Carbon-Based QPIs
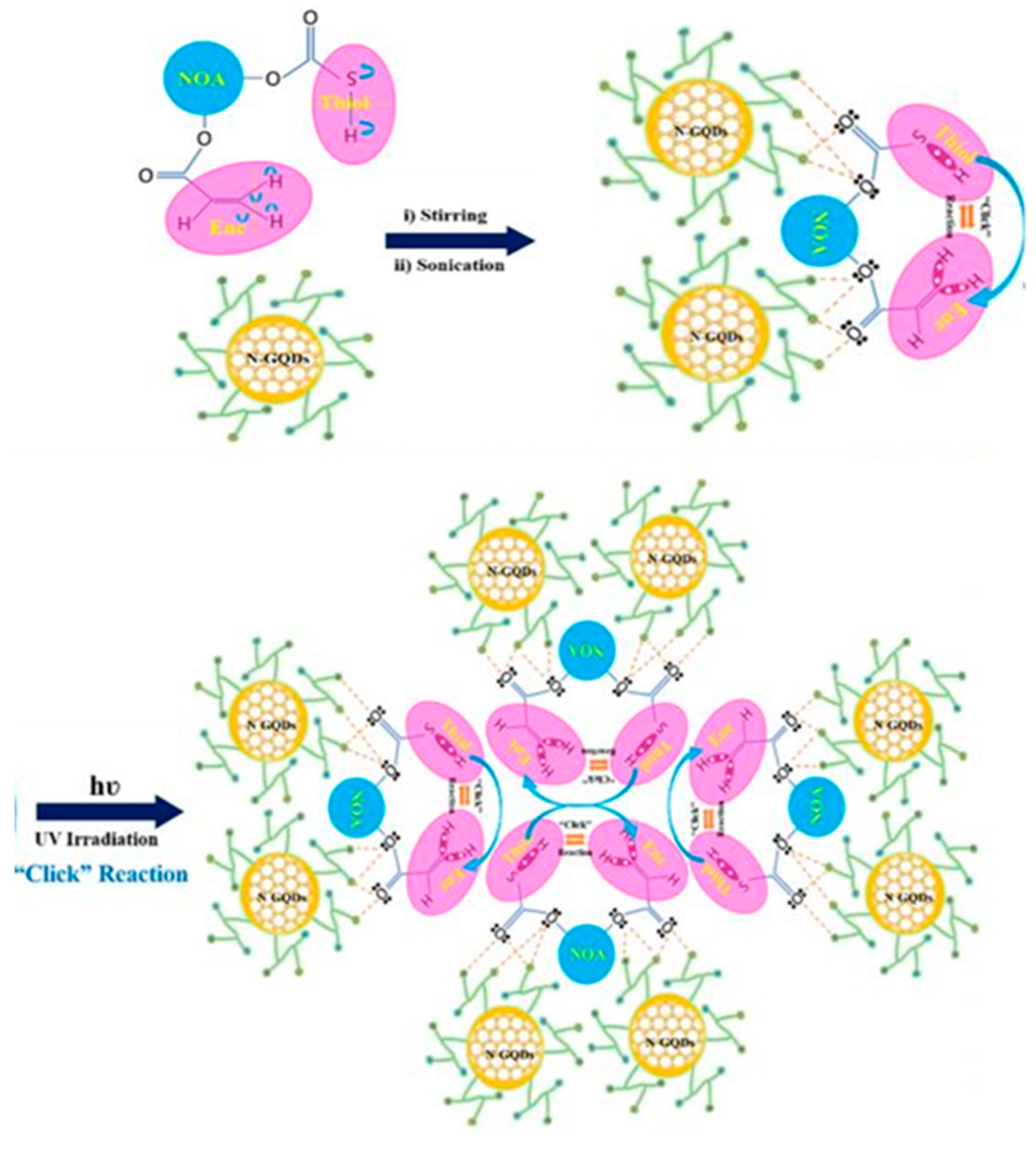
2.3. Graphene-Based QPIs
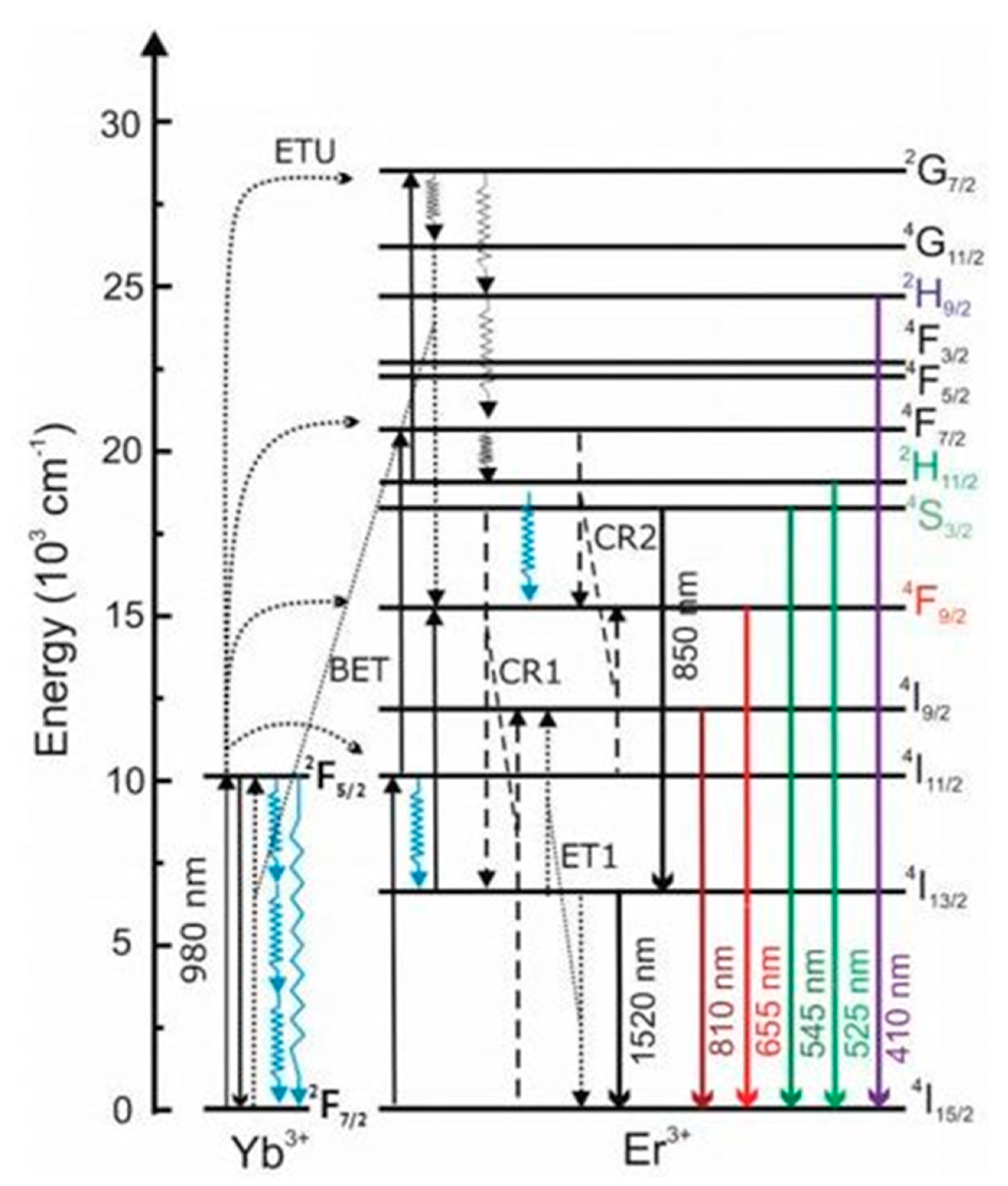
2.4. UCNPs and Hybrid QPIs
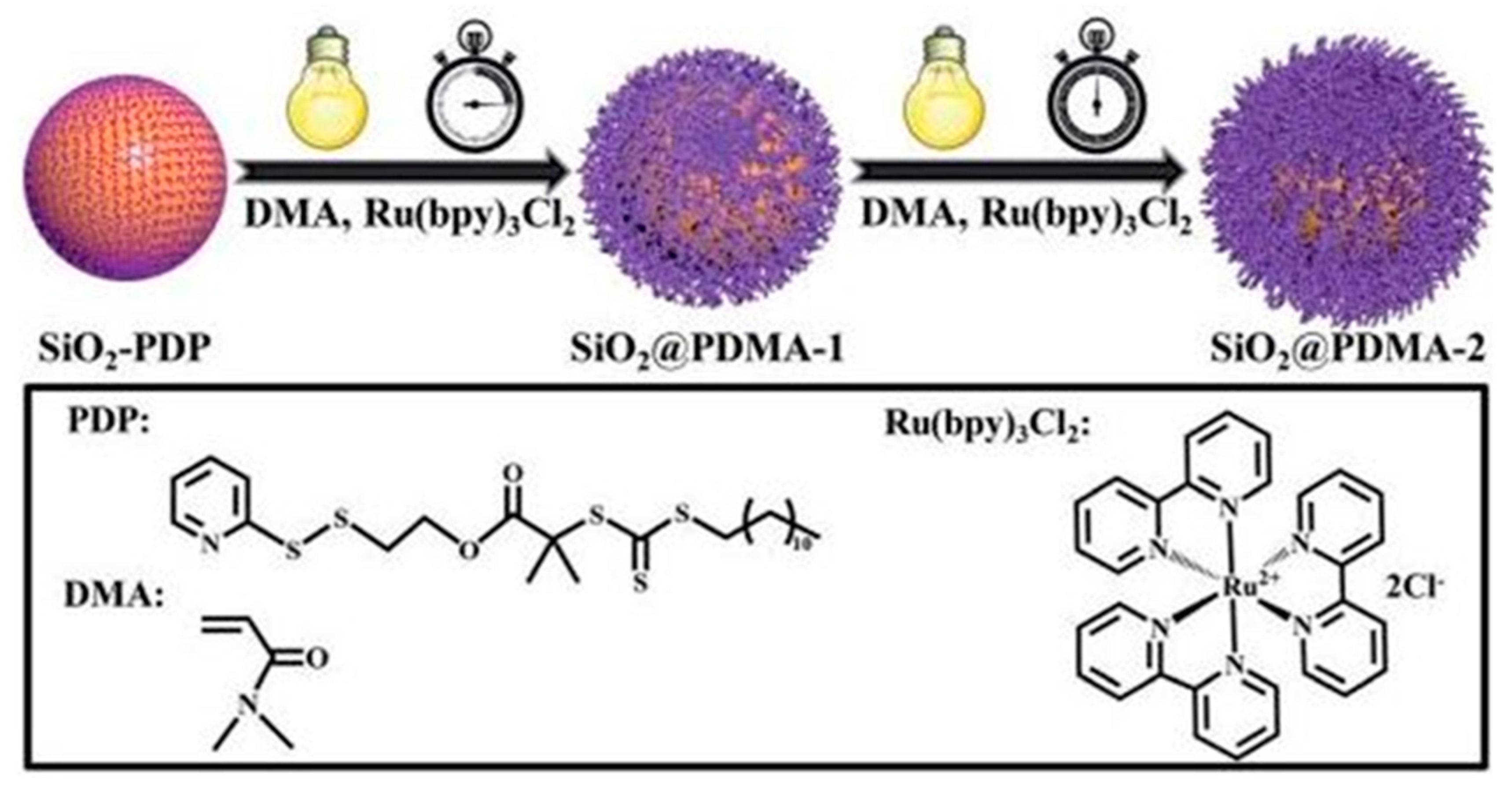
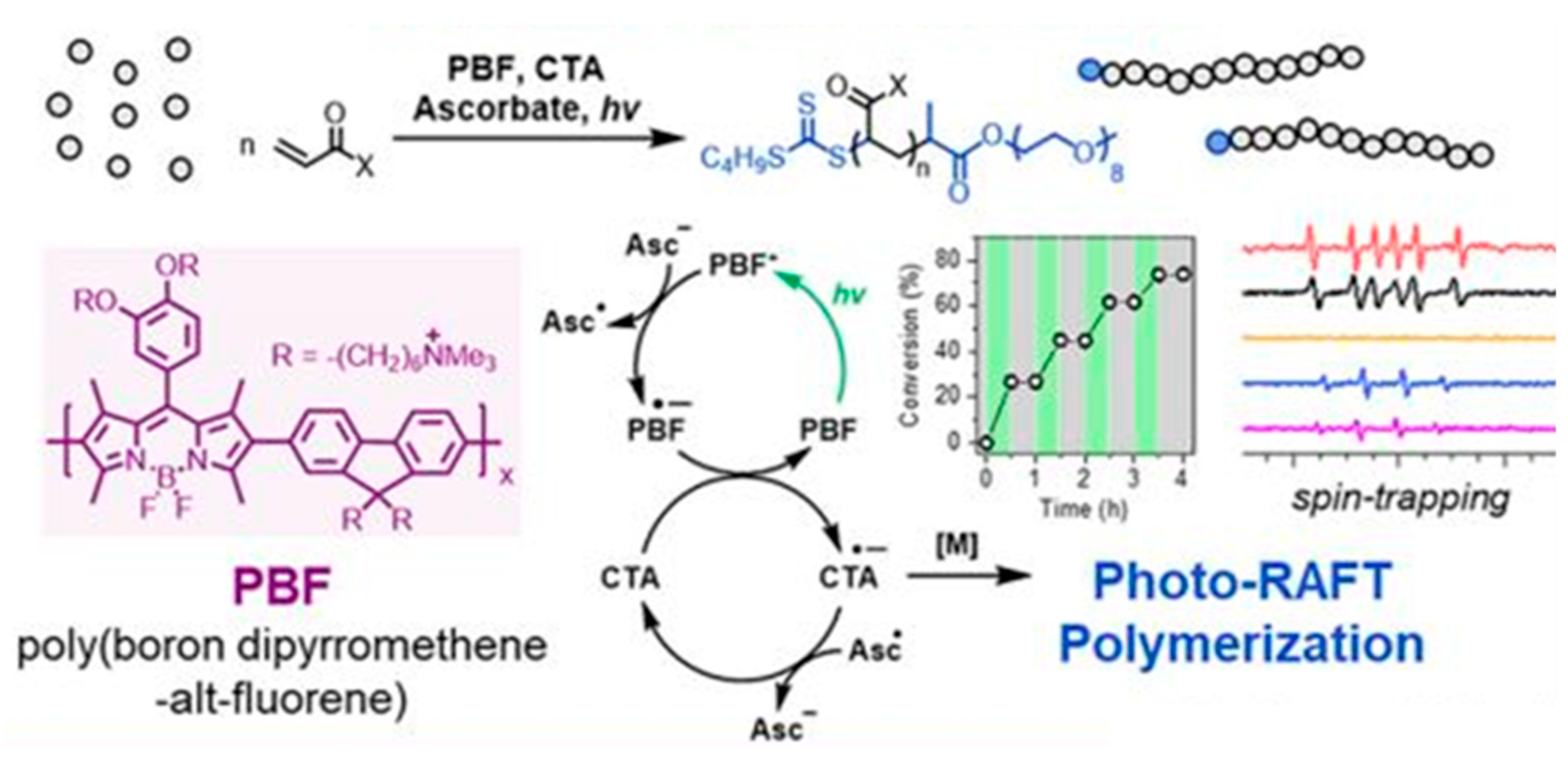
2.5. Polymer–Hybrid QPIs
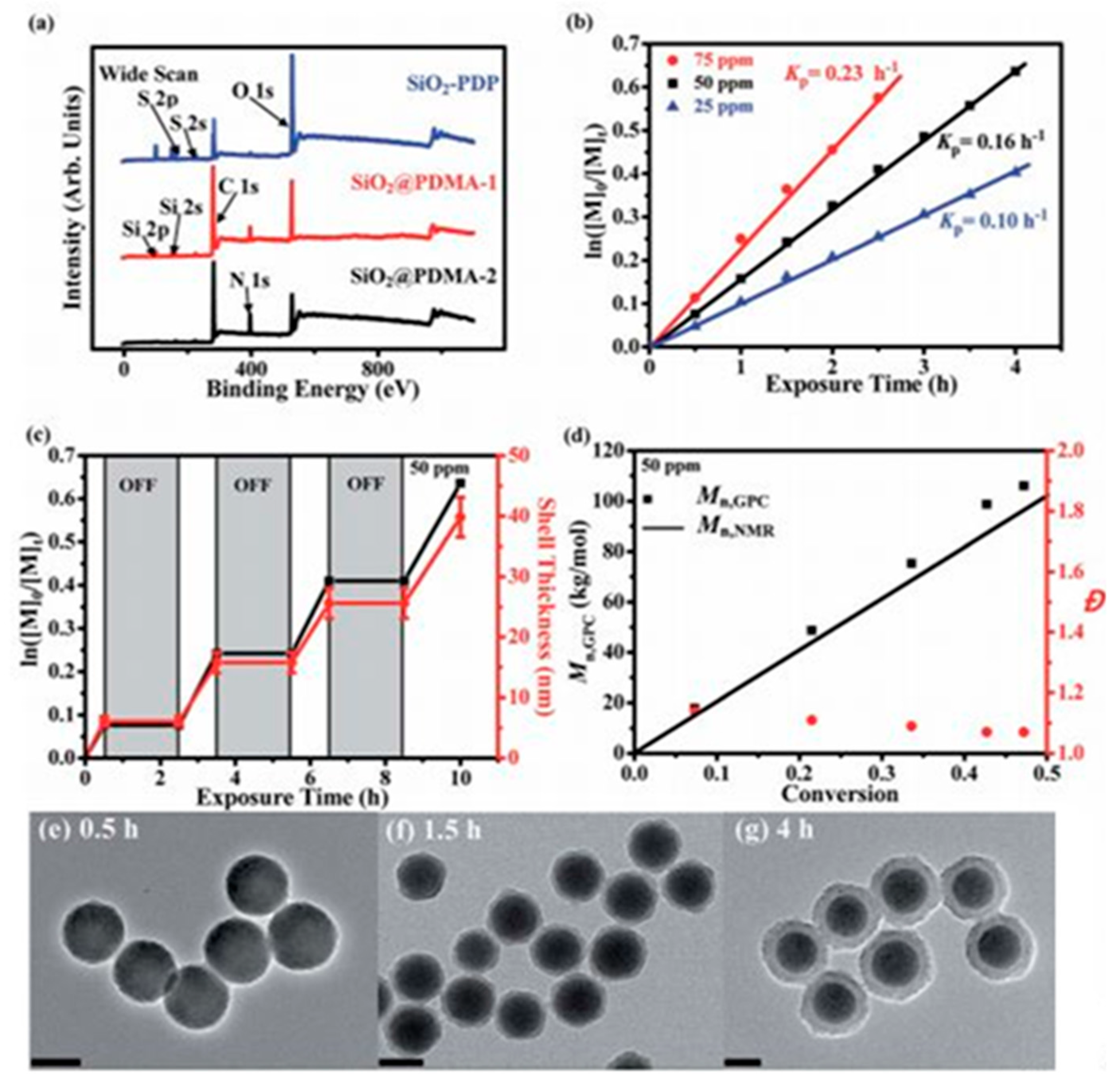
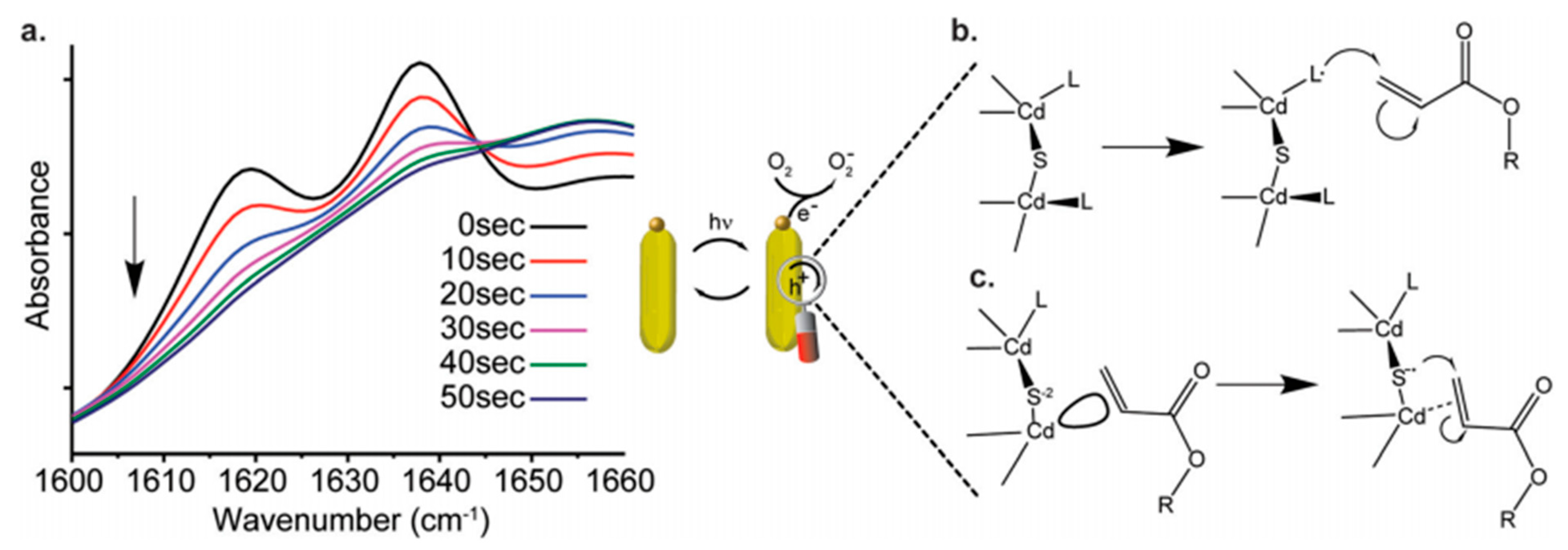
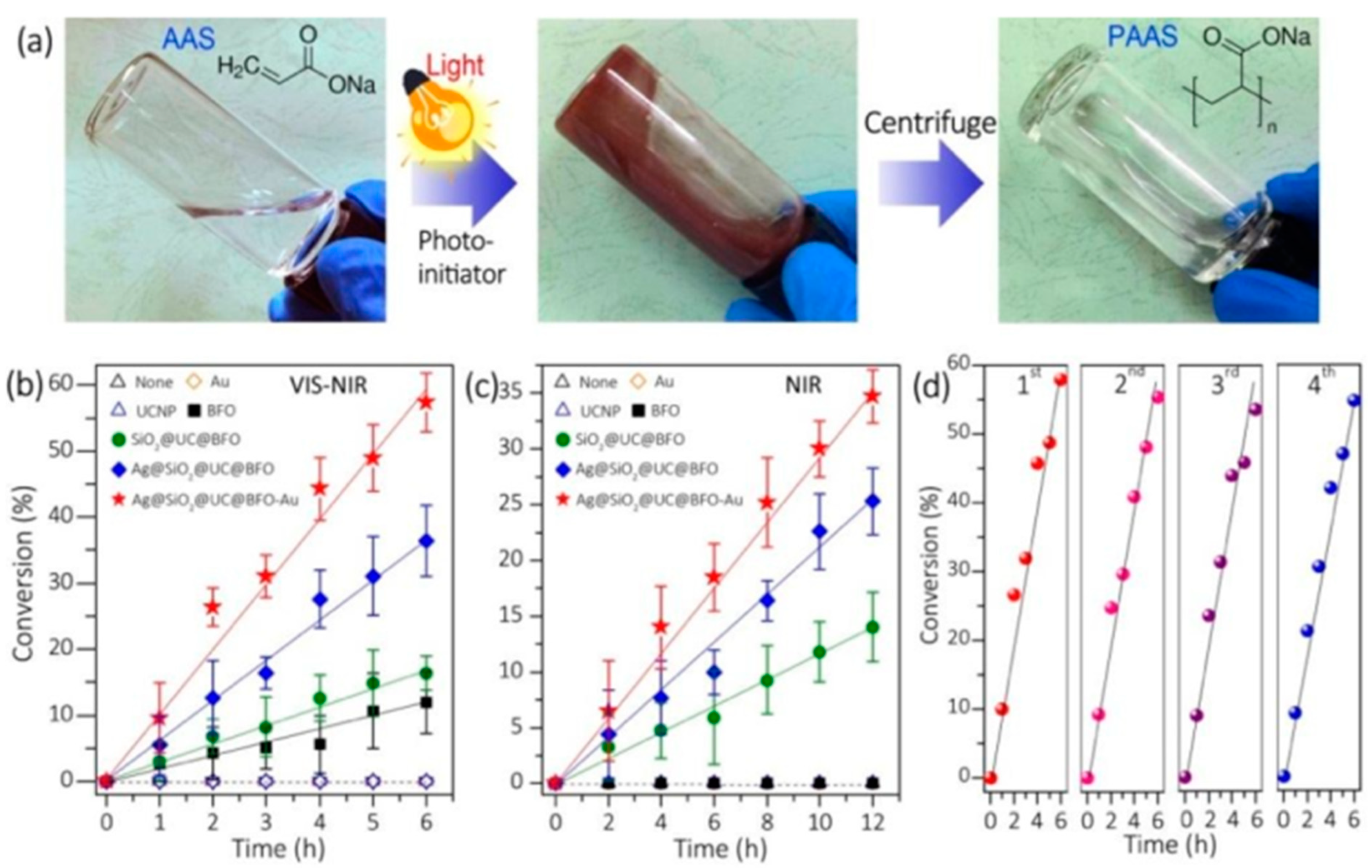
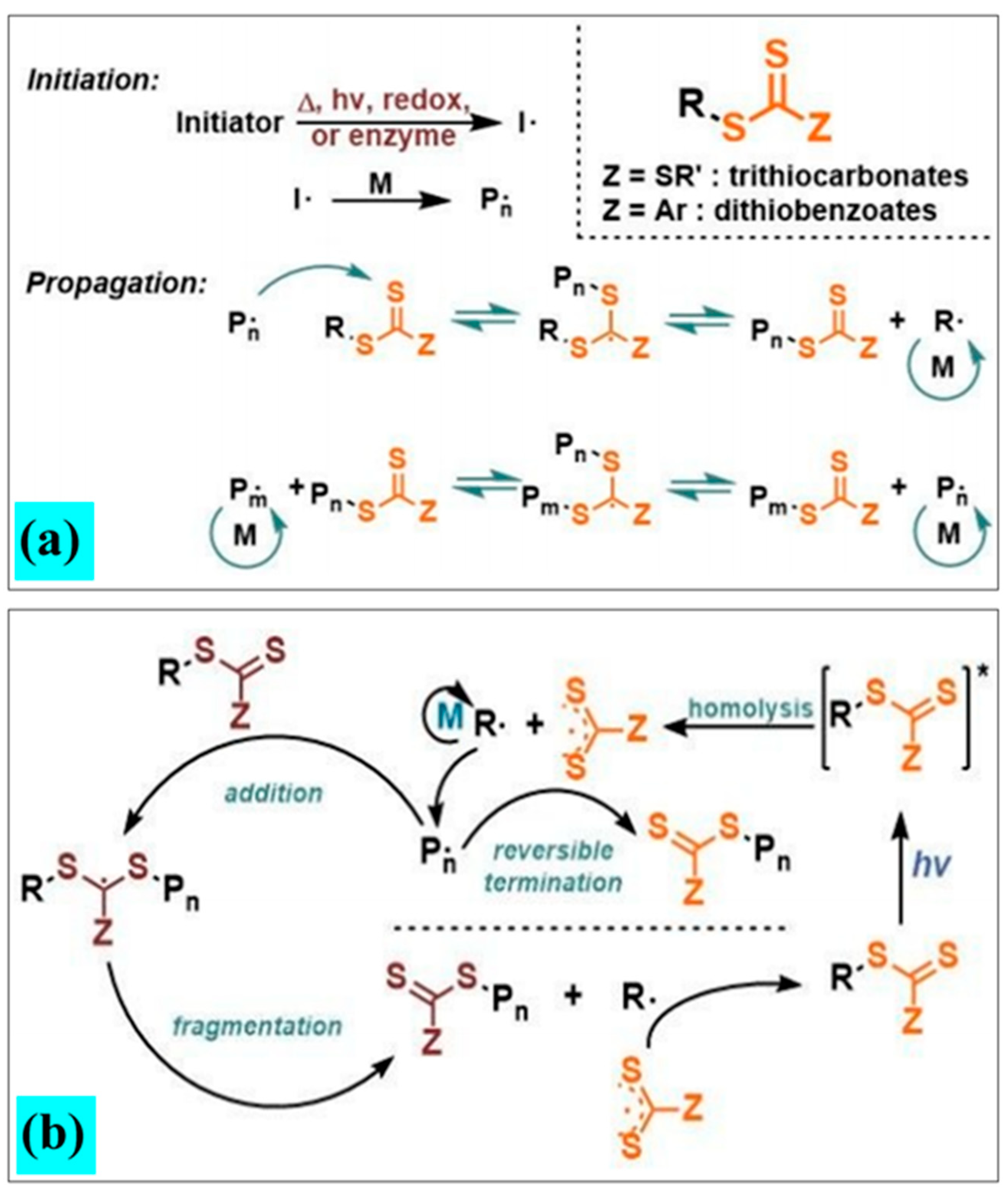
3. Applications of Photopolymers Based on QPIs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kuriacose, J.C.; Markham, M.C. Mechanism of the Photo-Initiated Polymerization of Methyl Methacrylate at Zinc Oxide Surfaces. J. Phys. Chem. 1961, 65, 2232–2236. [Google Scholar] [CrossRef]
- Waiskopf, N.; Magdassi, S.; Banin, U. Quantum Photoinitiators: Toward Emerging Photocuring Applications. J. Am. Chem. Soc. 2021, 143, 577–587. [Google Scholar] [CrossRef]
- Pinkas, A.; Waiskopf, N.; Gigi, S.; Naor, T.; Layani, A.; Banin, U. Morphology Effect on Zinc Oxide Quantum Photoinitiators for Radical Polymerization. Nanoscale 2021, 3, 7152–7160. [Google Scholar] [CrossRef]
- Waiskopf, N.; Ben-Shahar, Y.; Banin, U. Photocatalytic hybrid semiconductor–metal nanoparticles; from synergistic properties to emerging applications. Adv. Mater. 2018, 30, 1706697. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M. ZnO Nanoparticle Induced Photo-Kolbe Reaction, Fragment Stabilization and Effect on Photopolymerization Monitored by Raman–UV-Vis Measurements. Macromol. Chem. Phys. 2012, 213, 1953–1962. [Google Scholar] [CrossRef]
- Schmitt, M.; Lalevée, J. ZnO nanoparticles as polymerisation Photo-Initiator: Levulinic acid/NaOH content variation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 189–194. [Google Scholar] [CrossRef]
- Ojah, R.; Dolui, S.K. Solar radiation-induced polymerization of methyl methacrylate in the presence of semiconductor-based photocatalyst. Sol. Energy. Mater. Sol. Cells 2006, 90, 1615–1620. [Google Scholar] [CrossRef]
- Strandwitz, N.C.; Khan, A.; Boettcher, S.W.; Mikhailovsky, A.A.; Hawker, C.J.; Nguyen, T.Q.; Stucky, G.D. One-and two-photon induced polymerization of methylmethacrylate using colloidal CdS semiconductor quantum dots. J. Am. Chem. Soc. 2008, 130, 8280–8288. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, J.; Bao, S.; Wu, Q.; Wang, Q. Semiconductor nanoparticle-based hydrogels prepared via self-initiated polymerization under sunlight, even visible light. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Verbitsky, L.; Waiskopf, N.; Magdassi, S.; Banin, U. A clear solution: Semiconductor nanocrystals as photoinitiators in solvent free polymerization. Nanoscale 2019, 11, 11209–11216. [Google Scholar] [CrossRef]
- Zhou, J.; Allonas, X.; Ibrahim, A.; Liu, X. Progress in the development of polymeric and multifunctional photoinitiators. Prog. Polym. Sci. 2019, 99, 101165. [Google Scholar] [CrossRef]
- Feng, J.; Ye, D. Polymerizable ZnO photoinitiators of surface modification with hydroxyl acrylates and photopolymerization with UV-curable waterborne polyurethane acrylates. Eur. Polym. J. 2019, 120, 109252. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Atilla Tasdelen, M.; Mohamed Asiri, A.; Bahadar Khan, S.; Yagci, Y. Photoinduced atom transfer radical polymerization using semiconductor nanoparticles. Macromol. Rapid Comm. 2014, 35, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, Y.; Egap, E. Semiconductor quantum dots as photocatalysts for controlled light-mediated radical polymerization. ACS Macro Lett. 2018, 7, 184–189. [Google Scholar] [CrossRef]
- Liang, E.; Liu, M.S.; He, B.; Wang, G.X. ZnO as photocatalyst for photoinduced electron transfer–reversible addition–fragmentation chain transfer of methyl methacrylate. Adv. Polym. Technol. 2018, 37, 2879–2884. [Google Scholar] [CrossRef]
- Liao, J.; Ye, D. Improving ZnO photoinitiation efficiency by surface reaction with 2-hydroxy-2-methylpropiophenone. Eur. Polym. J. 2021, 147, 110293. [Google Scholar] [CrossRef]
- Han, Y.; Wang, F.; Lim, C.Y.; Chi, H.; Chen, D.; Wang, F.; Jiao, X. High-performance nano-photoinitiators with improved safety for 3D printing. ACS Appl. Mater. Interfaces. 2017, 9, 32418–32423. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zeng, M.; Ren, J.; Wang, J.; Fan, L.; Xu, Q. Preparation and swelling properties of graphene oxide/poly (acrylic acid-co-acrylamide) super-absorbent hydrogel nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2012, 401, 97–106. [Google Scholar] [CrossRef]
- Feng, R.; Zhou, W.; Guan, G.; Li, C.; Zhang, D.; Xiao, Y.; Zheng, L.; Zhu, W. Surface decoration of graphene by grafting polymerization using graphene oxide as the initiator. J. Mater. Chem. 2012, 22, 3982–3989. [Google Scholar] [CrossRef]
- Andryushina, N.S.; Stroyuk, O.L.; Dudarenko, G.V.; Kuchmiy, S.Y.; Pokhodenko, V.D. Photopolymerization of acrylamide induced by colloidal graphene oxide. J. Photochem. Photobiol. A 2013, 256, 1–6. [Google Scholar] [CrossRef]
- Feng, Y.; Peng, C.; Li, Y.; Hu, J.; Deng, Q.; Wu, Q.; Xu, Z. Superhydrophobic nanocomposite coatings with photoinitiated three-dimensional networks based on reactive graphene nanosheet-induced self-wrinkling patterned surfaces. J. Colloid. Interface. Sci. 2019, 536, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Sangermano, M.; Marchi, S.; Valentini, L.; Bon, S.B.; Fabbri, P. Transparent and conductive graphene oxide/poly (ethylene glycol) diacrylate coatings obtained by photopolymerization. Macromol. Mater. Eng. 2011, 296, 401–407. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Song, L.; Yu, B.; Hu, Y.; Yeoh, G.H. Preparation of UV-curable functionalized graphene/polyurethane acrylate nanocomposite with enhanced thermal and mechanical behaviors. React Funct. Polym. 2013, 73, 854–858. [Google Scholar] [CrossRef]
- Chen, Z.; Oprych, D.; Xie, C.; Kutahya, C.; Wu, S.; Strehmel, B. Upconversion-nanoparticle-assisted radical polymerization at λ= 974 nm and the generation of acidic cations. ChemPhotoChem 2017, 1, 499–503. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Chen, H.; Wang, C.; Chen, L.; Liu, J.; Liu, R. Efficient photopolymerization of thick pigmented systems using upconversion nanoparticles-assisted photochemistry. J. Polym. Sci. A Polym. Chem. 2018, 56, 994–1002. [Google Scholar] [CrossRef]
- Rocheva, V.V.; Koroleva, A.V.; Savelyev, A.G.; Khaydukov, K.V.; Generalova, A.N.; Nechaev, A.V.; Guller, A.E.; Semchishen, V.A.; Chichkov, B.N.; Khaydukov, E.V. High-resolution 3D photopolymerization assisted by upconversion nanoparticles for rapid prototyping applications. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Carneiro Neto, A.N.; Longo, R.L.; Wu, Y.; Malta, O.L.; Liu, X. Surface Plasmon–Photon Coupling in Lanthanide-Doped Nanoparticles. J. Phys. Chem. Lett. 2021, 12, 1520–1541. [Google Scholar] [CrossRef]
- Wang, K.; Peña, J.; Xing, J. Upconversion Nanoparticle-Assisted Photopolymerization. Photochem. Photobiol. 2020, 96, 741–749. [Google Scholar] [CrossRef]
- Bagheri, A.; Arandiyan, H.; Adnan, N.N.M.; Boyer, C.; Lim, M. Controlled direct growth of polymer shell on upconversion nanoparticle surface via visible light regulated polymerization. Macromolecules 2017, 50, 7137–7147. [Google Scholar] [CrossRef]
- Oprych, D.; Schmitz, C.; Ley, C.; Allonas, X.; Ermilov, E.; Erdmann, R.; Strehmel, B. Photophysics of Up-Conversion Nanoparticles: Radical Photopolymerization of Multifunctional Methacrylates Comprising Blue-and UV-Sensitive Photoinitiators. ChemPhotoChem 2019, 3, 1119–1126. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Jin, X.; Nazartchouk, A.; Liu, M.; Tong, X.; Jiang, Y.; Ni, L.; Sun, S.; Sang, Y.; et al. Plasmon enhanced upconverting core@ triple-shell nanoparticles as recyclable panchromatic initiators (blue to infrared) for radical polymerization. Nanoscale Horiz. 2019, 4, 907–917. [Google Scholar] [CrossRef]
- Jee, H.; Chen, G.; Prasad, P.N.; Ohulchanskyy, T.Y.; Lee, J. In Situ Ultraviolet Polymerization Using Upconversion Nanoparticles: Nanocomposite Structures Patterned by Near Infrared Light. Nanomaterials 2020, 10, 2054. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Wang, J.; Zhang, W.; Pan, X.; Zhang, Z.; Zhang, W.; Zhu, J.; Zhu, X. Platform of near-infrared light-induced reversible deactivation radical polymerization: Upconversion nanoparticles as internal light sources. Polym. Chem. 2016, 7, 7370–7374. [Google Scholar] [CrossRef]
- Hu, L.; Hao, Q.; Wang, L.; Cui, Z.; Fu, P.; Liu, M.; Qiao, X.; Pang, X. The in situ “grafting from” approach for the synthesis of polymer brushes on upconversion nanoparticles via NIR-mediated RAFT polymerization. Polym. Chem. 2021, 12, 545–553. [Google Scholar] [CrossRef]
- Nehlig, E.; Schneider, R.; Vidal, L.; Clavier, G.; Balan, L. Silver nanoparticles coated with thioxanthone derivative as hybrid photoinitiating systems for free radical polymerization. Langmuir 2012, 28, 17795–17802. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Yan, Y.; Wang, Y.; Guo, C.; Zhang, J. Preparation of AgCl nanocubes and their application as efficient photoinitiators in the polymerization of N-isopropylacrylamide. J. Phys. Chem. B 2015, 119, 14807–14813. [Google Scholar] [CrossRef]
- Landry, M.J.; Gellé, A.; Meng, B.Y.; Barrett, C.J.; Moores, A. Surface-plasmon-mediated hydrogenation of carbonyls catalyzed by silver nanocubes under visible light. ACS Catal. 2017, 7, 6128–6133. [Google Scholar] [CrossRef]
- McClelland, K.P.; Clemons, T.D.; Stupp, S.I.; Weiss, E.A. Semiconductor quantum dots are efficient and recyclable photocatalysts for aqueous PET-RAFT polymerization. ACS Macro Lett. 2019, 9, 7–13. [Google Scholar] [CrossRef]
- Li, S.; Han, G.; Zhang, W. Photoregulated reversible addition–fragmentation chain transfer (RAFT) polymerization. Polym. Chem. 2020, 11, 1830–1844. [Google Scholar] [CrossRef]
- Bellotti, V.; Simonutti, R. New Light in Polymer Science: Photoinduced Reversible Addition-Fragmentation Chain Transfer Polymerization (PET-RAFT) as Innovative Strategy for the Synthesis of Advanced Materials. Polymers 2021, 13, 1119. [Google Scholar] [CrossRef] [PubMed]
- He, J.Y.; Lu, M. Photoinduced electron transfer-reversible addition-fragmentation chain transfer (PET-RAFT) polymerization of acrylonitrile in miniemulsion. J. Macromol. Sci. A 2019, 56, 443–449. [Google Scholar] [CrossRef]
- Corrigan, N.; Zhernakov, L.; Hashim, M.H.; Xu, J.; Boyer, C. Flow mediated metal-free PET-RAFT polymerisation for upscaled and consistent polymer production. React. Chem. Eng. 2019, 4, 1216–1228. [Google Scholar] [CrossRef]
- Zhang, J.; Launay, K.; Hill, N.S.; Zhu, D.; Cox, N.; Langley, J.; Lalevée, J.; Stenzel, M.H.; Coote, M.L.; Xiao, P. Disubstituted aminoanthraquinone-based photoinitiators for free radical polymerization and fast 3D printing under visible light. Macromolecules 2018, 51, 10104–10112. [Google Scholar] [CrossRef]
- Pawar, A.A.; Halivni, S.; Waiskopf, N.; Ben-Shahar, Y.; Soreni-Harari, M.; Bergbreiter, S.; Banin, U.; Magdassi, S. Rapid three-dimensional printing in water using semiconductor–metal hybrid nanoparticles as photoinitiators. Nano Lett. 2017, 17, 4497–4501. [Google Scholar] [CrossRef] [Green Version]
- Scott, R.; Achtstein, A.W.; Prudnikau, A.; Antanovich, A.; Christodoulou, S.; Moreels, I.; Artemyev, M.; Woggon, U. Two photon absorption in II–VI semiconductors: The influence of dimensionality and size. Nano Lett. 2015, 15, 4985–4992. [Google Scholar] [CrossRef]
- Schafer, K.J.; Hales, J.M.; Balu, M.; Belfield, K.D.; Van Stryland, E.W.; Hagan, D.J. Two-photon absorption cross-sections of common photoinitiators. J. Photochem. Photobiol. A Chem. 2004, 162, 497–502. [Google Scholar] [CrossRef]
- Liu, W.; Xing, J.; Zhao, J.; Wen, X.; Wang, K.; Lu, P.; Xiong, Q. Giant Two-Photon Absorption and Its Saturation in 2D Organic–Inorganic Perovskite. Adv. Opt. Mater. 2017, 5, 1601045. [Google Scholar] [CrossRef]
- Krivenkov, V.; Samokhvalov, P.; Dyagileva, D.; Karaulov, A.; Nabiev, I. Determination of the single-exciton two-photon absorption cross sections of semiconductor nanocrystals through the measurement of saturation of their two-photon-excited photoluminescence. ACS Photonics 2020, 7, 831–836. [Google Scholar] [CrossRef]
- Lu, P.; Li, R.; Yao, N.; Dai, X.; Ye, Z.; Zheng, K.; Kong, W.; Fang, W.; Li, S.; Xu, Q.; et al. Enhancement of two-photon fluorescence and low threshold amplification of spontaneous emission of Zn-processed CuInS2 quantum dots. ACS Photonics. 2018, 5, 1310–1317. [Google Scholar] [CrossRef]
- Quick, M.T.; Owschimikow, N.; Khan, A.H.; Polovitsyn, A.; Moreels, I.; Woggon, U.; Achtstein, A.W. Two-photon based pulse autocorrelation with CdSe nanoplatelets. Nanoscale 2019, 11, 17293–17300. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.H.; Bertrand, G.H.; Teitelboim, A.; Sekhar, M.C.; Polovitsyn, A.; Brescia, R.; Planelles, J.; Climente, J.I.; Oron, D.; Moreels, I. CdSe/CdS/CdTe Core/Barrier/Crown Nanoplatelets: Synthesis, Optoelectronic Properties, and Multiphoton Fluorescence Upconversion. ACS Nano. 2020, 14, 4206–4215. [Google Scholar] [CrossRef]
- Alo, A.; Barros, L.W.; Nagamine, G.; Vieira, L.B.; Chang, J.H.; Jeong, B.G.; Bae, W.K.; Padilha, L.A. Simple Yet Effective Method to Determine Multiphoton Absorption Cross Section of Colloidal Semiconductor Nanocrystals. ACS Photonics 2020, 7, 1806–1812. [Google Scholar] [CrossRef]
- Xing, X.; Wang, K.; Han, X.; Qian, S.; Wang, K.; Long, H.; Wang, B.; Lu, P. Giant Quantum Yield Enhancement in CdS/MgF2/Ag Hybrid Nanobelt under Two-Photon Excitation. ACS Photonics 2020, 7, 2987–2994. [Google Scholar] [CrossRef]
- Hakobyan, K.; Gegenhuber, T.; McErlean, C.S.; Müllner, M. Visible-Light-Driven MADIX Polymerisation via a Reusable, Low-Cost, and Non-Toxic Bismuth Oxide Photocatalyst. Angew. Chem. Int. Ed. 2019, 58, 1828–1832. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Miller, K.A.; Zhu, H.; Egap, E. Lead Halide Perovskite Nanocrystals as Photocatalysts for PET-RAFT Polymerization under Visible and Near-Infrared Irradiation. ACS Macro Lett. 2020, 9, 725–730. [Google Scholar] [CrossRef]
- Cheng, B.F.; Wang, L.H.; You, Y.Z. Photoinduced electron transfer-reversible addition-fragmentation chain transfer (PET-RAFT) polymerization using titanium dioxide. Macromol. Res. 2016, 24, 811–815. [Google Scholar] [CrossRef]
- Riad, K.B.; Arnold, A.A.; Claverie, J.P.; Hoa, S.V.; Wood-Adams, P.M. Photopolymerization Using Metal Oxide Semiconducting Nanoparticles for Epoxy-Based Coatings and Patterned Films. ACS Appl. Nano. Mater. 2020, 3, 2875–2880. [Google Scholar] [CrossRef]
- Kiskan, B.; Zhang, J.; Wang, X.; Antonietti, M.; Yagci, Y. Mesoporous graphitic carbon nitride as a heterogeneous visible light photoinitiator for radical polymerization. ACS Macro Lett. 2012, 1, 546–549. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Tasdelen, M.A.; Kiskan, B.; Wang, X.; Antonietti, M.; Yagci, Y. Photochemically mediated atom transfer radical polymerization using polymeric semiconductor mesoporous graphitic carbon nitride. Macromol. Chem. Phys. 2014, 215, 675–681. [Google Scholar] [CrossRef]
- Fu, Q.; Ruan, Q.; McKenzie, T.G.; Reyhani, A.; Tang, J.; Qiao, G.G. Development of a robust PET-RAFT polymerization using graphitic carbon nitride (g-C3N4). Macromolecules 2017, 50, 7509–7516. [Google Scholar] [CrossRef]
- Jiang, J.; Ye, G.; Wang, Z.; Lu, Y.; Chen, J.; Matyjaszewski, K. Heteroatom-Doped Carbon Dots (CDs) as a Class of Metal-Free Photocatalysts for PET-RAFT Polymerization under Visible Light and Sunlight. Angew. Chemie. Int. Ed. 2018, 57, 12037–12042. [Google Scholar] [CrossRef] [PubMed]
- Hang, Z.; Yu, H.; Luo, L.; Huai, X. Nanoporous gC 3 N 4/MOF: High-performance photoinitiator for UV-curable coating. J. Mater. Sci. 2019, 54, 13959–13972. [Google Scholar] [CrossRef]
- Würth, C.; Kaiser, M.; Wilhelm, S.; Grauel, B.; Hirsch, T.; Resch-Genger, U. Excitation power dependent population pathways and absolute quantum yields of upconversion nanoparticles in different solvents. Nanoscale 2017, 9, 4283–4294. [Google Scholar] [CrossRef]
- Du, Y.; Jia, S.; Chen, Y.; Zhang, L.; Tan, J. Type I Photoinitiator-Functionalized Block Copolymer Nanoparticles Prepared by RAFT-Mediated Polymerization-Induced Self-Assembly. ACS Macro Lett. 2021, 10, 297–306. [Google Scholar] [CrossRef]
- Lu, M. Photoinduced atom transfer radical polymerization of methyl methacrylate with conducting polymer nanostructures as photocatalyst. Iran. Polym. J. 2019, 28, 167–172. [Google Scholar] [CrossRef]
- Li, X.; Ye, S.; Huang, Y.; Le Li, J.; Cai, T. Precise growth of polymer brushes on silica-based nanocomposites via visible-light-regulated controlled radical polymerization. J. Mater. Chem. A 2019, 7, 6173–6179. [Google Scholar] [CrossRef]
- Nazim, M.; Kim, B.; Lee, S.; Min, B.K.; Kim, J.H. UV-Curable Polymer–QD Flexible Films as the Downconversion Layer for Improved Performance of Cu (In, Ga) Se2 Solar Cells. Energy Fuels 2020, 34, 14581–14590. [Google Scholar] [CrossRef]
- Bai, M.; Huang, S.; Xu, S.; Hu, G.; Wang, L. Fluorescent nanosensors via photoinduced polymerization of hydrophobic inorganic quantum dots for the sensitive and selective detection of nitroaromatics. Anal. Chem. 2015, 87, 2383–2388. [Google Scholar] [CrossRef]
- Nazim, M.; Kim, J.H. Fluorescent N-Doped Graphene Quantum Dots Embedded in Transparent Polymer Films for Photon-Downconversion Applications. ACS Appl. Nano. Mater. 2020, 3, 2322–2335. [Google Scholar] [CrossRef]
- Hampton, M.J.; Templeton, J.L.; DeSimone, J.M. Direct patterning of CdSe quantum dots into sub-100 nm structures. Langmuir 2010, 26, 3012–3015. [Google Scholar] [CrossRef]
- Allan, J.M.; Mumin, M.A.; Xu, W.Z.; Al Sharari, Q.; Charpentier, P.A. Surface functionalized bare and core–shell quantum dots in poly (ethylene-co-vinyl acetate) for light selective nanocomposite films. Sol. Energy Mater. Sol. Cells 2014, 123, 30–40. [Google Scholar] [CrossRef]
- Mumin, M.A.; Xu, W.Z.; Charpentier, P.A. Quantum dots/silica/polymer nanocomposite films with high visible light transmission and UV shielding properties. Nanotechnology 2015, 26, 315702. [Google Scholar] [CrossRef] [PubMed]
- Shikinaka, K.; Nakamura, M.; Otsuka, Y. Strong UV absorption by nanoparticulated lignin in polymer films with reinforcement of mechanical properties. Polymer 2020, 190, 122254. [Google Scholar] [CrossRef]
- Xiong, F.; Wu, Y.; Li, G.; Han, Y.; Chu, F. Transparent nanocomposite films of lignin nanospheres and poly (vinyl alcohol) for UV-absorbing. Ind. Eng. Chem. Res. 2018, 57, 1207–1212. [Google Scholar] [CrossRef]
- Mumin, M.A.; Akhter, K.F.; Oyeneye, O.O.; Xu, W.Z.; Charpentier, P.A. Supercritical fluid assisted dispersion of nano-silica encapsulated CdS/ZnS quantum dots in poly (ethylene-co-vinyl acetate) for solar harvesting films. ACS Appl. Nano. Mater. 2018, 1, 3186–3195. [Google Scholar] [CrossRef]
- Bugakov, M.; Boiko, N.; Linkov, P.; Samokhvalov, P.; Efimov, A.; Abramchuk, S.; Shibaev, V. Fluorescent thermostable crosslinked poly (dodecylmethacrylate) composites based on porous polyethylene and CdSe/ZnS quantum dots. Polym. Int. 2018, 67, 1275–1281. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, X.; Zhang, Z.; Kuchel, R.P.; Namivandi-Zangeneh, R.; Corrigan, N.; Jung, K.; Liang, K.; Boyer, C. Porphyrinic Zirconium Metal–Organic Frameworks (MOFs) as Heterogeneous Photocatalysts for PET-RAFT Polymerization and Stereolithography. Angew. Chemie. 2021, 133, 5549–5556. [Google Scholar] [CrossRef]
- Allegrezza, M.L.; Konkolewicz, D. PET-RAFT Polymerization: Mechanistic Perspectives for Future Materials. ACS Macro Lett. 2021, 10, 433–446. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Lorandi, F.; DiTucci, M.J.; Sun, M.; Szczepaniak, G.; Liu, T.; Matyjaszewski, K. Conjugated Cross-linked Phenothiazines as Green or Red Light Heterogeneous Photocatalysts for Copper-Catalyzed Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 2021, 143, 9630−9638. [Google Scholar] [CrossRef]
- Lu, H.; Huang, Y.; Zhang, E.; Liu, Y.; Lv, F.; Liu, L.; Ma, Y.; Wang, S. Photocontrolled RAFT Polymerization Catalyzed by Conjugated Polymers under Aerobic Aqueous Conditions. ACS Macro Lett. 2021, 10, 996–1001. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, L.; Cui, Z.; Fu, P.; Liu, M.; Qiao, X.; Pang, X. Dual Roles of Amino-Functionalized Silicon Quantum Dots (SiQDs) for Visible-Light-Induced Surface-Initiated PET-RAFT Polymerization on Substrates. ACS Appl. Mater. Interfaces 2020, 12, 42161–42168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Egap, E. PET-RAFT polymerization catalyzed by cadmium selenide quantum dots (QDs): Grafting-from QDs photocatalysts to make polymer nanocomposites. Polym. Chem. 2020, 11, 1018–1024. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shukla, S.; Pandey, P.C.; Narayan, R.J. Tunable Quantum Photoinitiators for Radical Photopolymerization. Polymers 2021, 13, 2694. https://doi.org/10.3390/polym13162694
Shukla S, Pandey PC, Narayan RJ. Tunable Quantum Photoinitiators for Radical Photopolymerization. Polymers. 2021; 13(16):2694. https://doi.org/10.3390/polym13162694
Chicago/Turabian StyleShukla, Shubhangi, Prem C. Pandey, and Roger J. Narayan. 2021. "Tunable Quantum Photoinitiators for Radical Photopolymerization" Polymers 13, no. 16: 2694. https://doi.org/10.3390/polym13162694
APA StyleShukla, S., Pandey, P. C., & Narayan, R. J. (2021). Tunable Quantum Photoinitiators for Radical Photopolymerization. Polymers, 13(16), 2694. https://doi.org/10.3390/polym13162694







