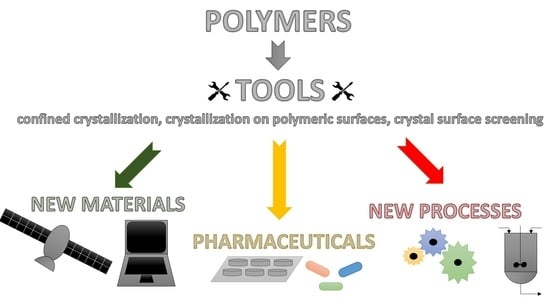
Application of Polymers as a Tool in Crystallization—A Review
Abstract
1. Introduction
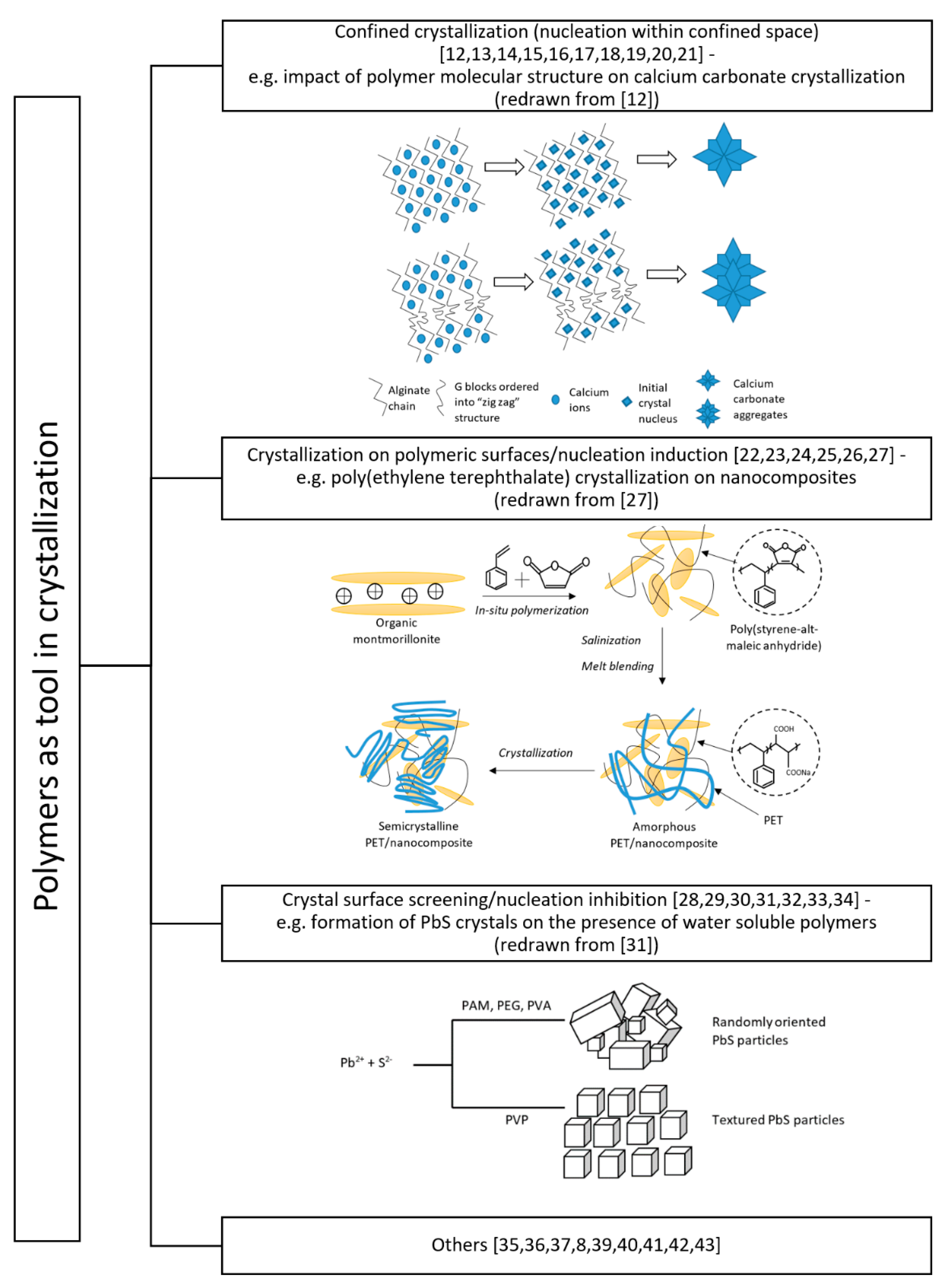
2. Basic Research
2.1. Confined Crystallization
2.2. Crystallization on Polymeric Surfaces/Nucleation Induction
2.3. Crystal Surface Screening/Nucleation Inhibition
2.4. Other Aspects of Basic Research
2.5. Discussion and Conclusions
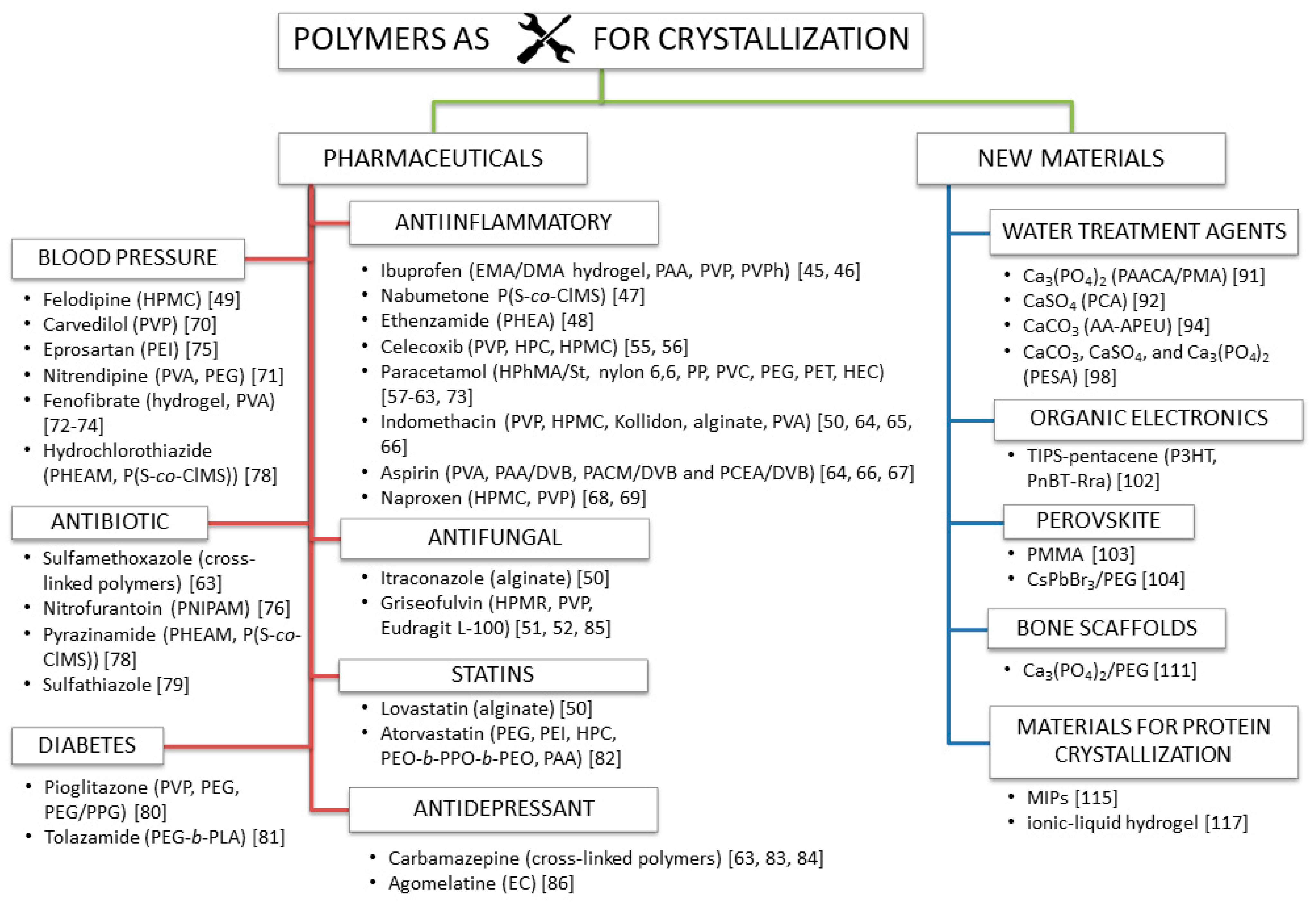
3. Present and Future Perspectives of Applications of Crystallization Phenomena in the Presence of Polymers
3.1. Polymers Used in Pharmaceuticals to Prevent the Nucleation of Crystalline Drugs or Precipitation of Amorphous Drugs
3.1.1. Antiinflammatory Drugs
3.1.2. Other Drugs
3.2. Polymers Used in Pharmaceuticals to Control the Nucleation of Crystalline Drugs or Precipitation of Amorphous Drugs
3.2.1. Antiinflammatory Drugs
3.2.2. Drugs Affecting Blood Pressure
3.2.3. Antibiotics
3.2.4. Diabetes
3.2.5. Other Drugs
3.2.6. Discussion and Conclusions
3.3. New Materials
3.3.1. Water Treatment Agents
3.3.2. Organic(-Inorganic) Electronics
3.3.3. Miscellaneous Applications
3.4. Discussion and Conclusions
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bałdyga, J.; Tyl, G.; Bouaifi, M. Application of Gaussian cubature to model two-dimensional population balances. Chem. Process Eng. 2017, 38, 393–409. [Google Scholar] [CrossRef][Green Version]
- Silva, J.E.; Paiva, A.P.; Soares, D.; Labrincha, A.; Castro, F. Plating Sludge Value-Adding by Application of Hydrometallurgical Processes. In Trends in Hazardous Materials Research; Edward, C., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2007; pp. 1–58. ISBN 978-1600213359. [Google Scholar]
- Geddert, T.; Augustin, W.; Scholl, S. Induction Time in Crystallization Fouling on Heat Transfer Surfaces. Chem. Eng. Technol. 2011, 34, 1303–1310. [Google Scholar] [CrossRef]
- Herz, A.; Malayeri, M.R.; Müller-Steinhagen, H. Fouling of roughened stainless steel surfaces during convective heat transfer to aqueous solutions. Energy Convers. Manag. 2008, 49, 3381–3386. [Google Scholar] [CrossRef]
- Bogacz, W.; Lemanowicz, M.; Al-Rashed, M.H.; Nakonieczny, D.; Piotrowski, T.; Wójcik, J. Impact of roughness, wettability and hydrodynamic conditions on the incrustation on stainless steel surfaces. Appl. Therm. Eng. 2017, 112, 352–361. [Google Scholar] [CrossRef]
- Gao, Z.; Rohani, S.; Gong, J.; Wang, J. Recent Developments in the Crystallization Process: Toward the Pharmaceutical Industry. Engineering 2017, 3, 343–353. [Google Scholar] [CrossRef]
- Bauer, J.F.; Saleki-Gerhardt, A.; Narayanan, B.A.; Chemburkar, S.R.; Patel, K.M.; Spiwek, H.O.; Bauer, P.E.; Allen, K.A. Polymorph of a Pharmaceutical. US Patent US 8674112 B2, 18 March 2014. [Google Scholar]
- Bauer, J.; Spanton, S.; Henry, R.; Quick, J.; Dziki, W.; Porter, W.; Morris, J. Ritonavir: An Etraordinary Example of Conformational Polymorphism. Pharm. Res. 2001, 18, 859–866. [Google Scholar] [CrossRef]
- Mullin, J.W. Crystallization, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2001; ISBN 0-7506-4833-3. [Google Scholar]
- Chernov, A.A. Formation of crystals in solutions. Contemp. Phys. 1989, 30, 251–276. [Google Scholar] [CrossRef]
- Lovette, M.A.; Browning, A.R.; Griffin, D.W.; Sizemore, J.P.; Snyder, R.C.; Doherty, M.F. Crystal Shape Engineering. Ind. Eng. Chem. Res. 2008, 47, 9812–9833. [Google Scholar] [CrossRef]
- Ma, Y.; Feng, Q. Alginate hydrogel-mediated crystallization of calcium carbonate. J. Solid State Chem. 2011, 184, 1008–1015. [Google Scholar] [CrossRef]
- Ma, Y.; Feng, Q.; Bourrat, X. A novel growth process of calcium carbonate crystals in silk fibroin hydrogel system. Mater. Sci. Eng. C 2013, 33, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Nindiyasari, F.; Fernandez-Diaz, L.; Griesshaber, E.; Manuel, A.J.; Sanchez-Pastor, N.; Schmahl, W.W. Influence of Gelatin Hydrogel Porosity on the Crystallization of CaCO3. Cryst. Growth Des. 2014, 14, 1531–1542. [Google Scholar] [CrossRef]
- Kosanovic, C.; Fermani, S.; Falini, G.; Kralj, D. Crystallization of Calcium Carbonate in Alginate and Xanthan Hydrogels. Crystals 2017, 7, 355. [Google Scholar] [CrossRef]
- Yokoi, T.; Kawashita, M.; Kikuta, K.; Ohtsuki, C. Crystallization of calcium phosphate in polyacrylamide hydrogels containing phosphate ions. J. Cryst. Growth 2010, 312, 2376–2382. [Google Scholar] [CrossRef]
- Velasquez-Gonzalez, O.; Campos-Escamilla, C.; Flores-Ibarra, A.; Esturau-Escofet, N.; Arreguin-Espinosa, R.; Stojanoff, V.; Cuellar-Cruz, M.; Moreno, A. Crystal Growth in Gels froms the Mechanisms of Crystal Growth to Controlof Polymorphism: New Trends on Theoretical and Experimental Aspects. Crystals 2019, 9, 443. [Google Scholar] [CrossRef]
- Choi, D.; Sonkaria, S.; Fox, S.J.; Poudel, S.; Kim, S.Y.; Kang, S.; Kim, S.; Verma, C.; Ahn, S.H.; Lee, C.S.; et al. Quantum scale biomimicry of low dimensional growth: An unusual complex amorphous precursor route to TiO2 band confinement by shape adaptive biopolymer-like flexibility for energy applications. Sci. Rep. 2019, 9, 18721. [Google Scholar] [CrossRef]
- Diao, Y.; Helgeson, M.E.; Myerson, A.S.; Hatton, T.A.; Doyle, P.S.; Trout, B.L. Controlled Nucleation from Solution Using Polymer Microgels. J. Am. Chem. Soc. 2011, 133, 3756–3759. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Helgeson, M.E.; Siam, Z.A.; Doyle, P.S.; Myerson, A.S.; Hatton, T.A.; Trout, B.L. Nucleation under Soft Confinement: Role of Polymer-Solute Interactions. Cryst. Growth Des. 2012, 12, 508–517. [Google Scholar] [CrossRef]
- Li, D.; Huang, Y.; Ratinac, K.R.; Ringer, S.P.; Wang, H. Zeolite crystallization in crosslinked chitosan hydrogels: Crystal size control and chitosan removal. Microporous Mesoporous Mater. 2008, 116, 416–423. [Google Scholar] [CrossRef]
- Curcio, E.; Fontananova, E.; Di Profio, G.; Drioli, E. Influence of the Structural Properties of Poly(vinylidene fluoride) Membranes on the Heterogeneous Nucleation Rate of Protein Crystals. J. Phys. Chem. B 2006, 110, 12438–12445. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.H.; Shih, W.-Y.; Lyster, E.; Cohen, Y. Crystallization of calcium sulfate on polymeric surfaces. J. Colloid Interface Sci. 2011, 356, 790–797. [Google Scholar] [CrossRef]
- Patel, M.A.; Nguyen, B.; Chadwick, K. Predicting the Nucleation Induction Time Based on Preferred Intermolecular Interactions. Cryst. Growth Des. 2017, 17, 4613–4621. [Google Scholar] [CrossRef]
- Solomos, M.A.; Capacci-Daniel, C.; Rubinson, J.F.; Swift, J.A. Polymorph Selection via Sublimation onto Siloxane Templates. Cryst. Growth Des. 2018, 18, 6965–6972. [Google Scholar] [CrossRef]
- Hutfles, J.; Ravichandran, S.A.; Pellegrino, J. Screening polymer surfaces in crystallization. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123869. [Google Scholar] [CrossRef]
- Xing, S.; Li, R.; Si, J.; Tang, P. In situ polymerization of poly(styrene-alt-maleic anhydride)/organic montmorillonite nanocomposites and their ionomers as crystallization nucleating agents for poly(ethylene terephthalate). J. Ind. Eng. Chem. 2016, 38, 167–174. [Google Scholar] [CrossRef]
- Yue, S.; Zhang, L.; Lu, J.; Zhang, J. Polymer-controlled crystallization of dumbbell-like ZnO hollow architectures. Mater. Lett. 2009, 63, 1217–1220. [Google Scholar] [CrossRef]
- Miculescu, F.; Rusen, E.; Mocanu, A.; Diacon, A.; Birjega, R. Hierarchical nanostructures of ZnO obtained in the presence of water soluble polymers. Powder Technol. 2013, 239, 56–58. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Zhang, Q.; Cui, Y.; Wu, Z.; Zheng, R.; Liu, L. Molecularly imprinted polymers prepared by precipitation polymerizationand used for inducing crystallization of oleanolic acid in supercritical CO2. Sep. Purif. Technol. 2011, 81, 411–417. [Google Scholar] [CrossRef]
- Preda, N.; Rusen, E.; Enculescu, M.; Matei, E.; Marculescu, B.; Enculescu, I. Polymer-assisted crystallization of low-dimensional lead sulfide particles. Phys. E Low Dimens. Syst. Nanostruct. 2011, 43, 1826–1832. [Google Scholar] [CrossRef]
- Pelin, I.M.; Popescu, I.; Suflet, D.M.; Aflori, M.; Bulacovschi, V. Influence of maleic acid copolymers on calcium orthophosphates crystallization at low temperature. J. Cryst. Growth 2013, 377, 127–135. [Google Scholar] [CrossRef]
- Lu, Y.; Tang, Y.; Xia, X. Non-isothermal crystallization of copper-containing composite based on polymer alloy of poly(ethylene oxide) and polyethylene. Thermochim. Acta 2018, 670, 61–70. [Google Scholar] [CrossRef]
- Arioglu-Tuncil, S.; Bhardwaj, V.; Taylor, L.S.; Mauer, L.J. Amorphization of thiamine chloride hydrochloride: A study of the crystallization inhibitor properties of different polymers in thiamine chloride hydrochloride amorphous solid dispersions. Food Res. Int. 2017, 99, 363–374. [Google Scholar] [CrossRef]
- Sinek, A.; Kupczak, M.; Mielańczyk, A.; Lemanowicz, M.; Yusa, S.; Neugebauer, D.; Gierczycki, A. Temperature and pH-Dependent Response of Poly(Acrylic Acid) and Poly(Acrylic Acid-co-Methyl Acrylate) in Highly Concentrated Potassium Chloride Aqueous Solutions. Polymers 2020, 12, 486. [Google Scholar] [CrossRef]
- Beyer, R.; Iacopini, S.; Palberg, T.; Schoepe, H.J. Polymer induced changes of the crystallization scenario in suspensionsof hard sphere like microgel particles. J. Chem. Phys. 2012, 136, 234906. [Google Scholar] [CrossRef] [PubMed]
- Marinović-Cincović, M.; Janković, B.; Milićević, B.; Antić, Ž.; Whiffen, R.K.; Dramićanin, M.D. The comparative kinetic analysis of the non-isothermal crystallization process of Eu3 + doped Zn2SiO4 powders prepared via polymer induced sol–gel method. Powder Technol. 2013, 249, 497–512. [Google Scholar] [CrossRef]
- Sun, H.; Luo, Y.; Yang, B.; Zhang, H.; Huang, J. Non-isothermal crystallization of biopolyesters of poly(butylenesuccinate) formed via in-situ polymerization in presence of poly(vinylbutyral). Polymer 2018, 153, 378–390. [Google Scholar] [CrossRef]
- Liu, K.; Yuan, Y.; Zhang, J. Isothermal crystallization behavior of water in poly(vinyl methyl ether) aqueous solution investigated by infrared and two-dimensional infrared correlation spectroscopy. Vib. Spectrosc. 2011, 57, 81–86. [Google Scholar] [CrossRef]
- Mandal, T.; Huang, W.; Mecca, J.M.; Getchell, A.; Porter, W.W., III; Larson, R.G. A framework for multi-scale simulation of crystal growth in the presenceof polymers. Soft Matter 2017, 13, 1904–1913. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, R.; Liang, Q.; Liu, J.; Han, Y. Control the interplay of crystallization and phase separation of conjugated polymer blends by the relative rate of nucleation and growth. Polymer 2019, 182, 121827. [Google Scholar] [CrossRef]
- Olmstead, M.M.; Ghiassi, K.B. Crystal Growth and Crystal Transformation. In Comprehensive Coordination Chemistry, 3rd ed.; Constable, E.C., Parkin, G., Que, L., Jr., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 77–86. [Google Scholar] [CrossRef]
- Bakshi, M.S.; Thakur, P.; Sachar, S.; Kaur, G.; Banipal, T.S.; Possmayer, F.; Petersen, N.O. Aqueous phase surfactant selective shape controlled synthesis of lead sulfide nanocrystals. J. Phys. Chem. C 2007, 111, 18087–18098. [Google Scholar] [CrossRef]
- The Cambridge Crystallographic Data Centre the Cambridge Structural Database (CSD). Available online: https://www.ccdc.cam.ac.uk/solutions/csd-core/components/csd/ (accessed on 10 August 2021).
- Velasco, D.; Danoux, C.B.; Redondo, J.A.; Elvira, C.; SanRoman, J.; Wray, P.S.; Kazarian, S.G. pH-sensitive polymer hydrogels derived from morpholine to prevent thecrystallization of ibuprofen. J. Control. RELEASE 2011, 149, 140–145. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yu, G.; Kim, I.W. Effects of polymeric additives on the crystallization and release behavior of amorphous ibuprofen. J. Nanomater. 2013, 2013, 503069. [Google Scholar] [CrossRef]
- Frank, D.S.; Matzger, A.J. Probing the Interplay between Amorphous Solid Dispersion Stability and Polymer Functionality. Mol. Pharm. 2018, 15, 2714–2720. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.S.; Matzger, A.J. Effect of Polymer Hydrophobicity on the Stability of Amorphous Solid Dispersions and Supersaturated Solutions of a Hydrophobic Pharmaceutical. Mol. Pharm. 2019, 16, 682–688. [Google Scholar] [CrossRef]
- Alonzo, D.E.; Raina, S.; Zhou, D.; Gao, Y.; Zhang, G.G.Z.; Taylor, L.S. Characterizing the impact of hydroxypropylmethyl cellulose on the growth and nucleation kinetics of felodipine from supersaturated solutions. Cryst. Growth Des. 2012, 12, 1538–1547. [Google Scholar] [CrossRef]
- Guan, J.; Liu, Q.; Liu, J.; Cui, Z.; Zhang, X.; Mao, S. Elucidation of alginate-drug miscibility on its crystal growth inhibition effect in supersaturated drug delivery system. Carbohydr. Polym. 2020, 230, 115601. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Nowak, S.A.; Wah, C.L. Impact of Physicochemical Properties of Cellulosic Polymers on Supersaturation Maintenance in Aqueous Drug Solutions. AAPS PharmSciTech 2018, 19, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, S.; Kushida, I.; Yamashita, T.; Hasebe, T.; Shirai, O.; Kano, K. Inhibition of crystal nucleation and growth by water-soluble polymers and its impact on the supersaturation profiles of amorphous drugs. J. Pharm. Sci. 2013, 102, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Fornells, E.; Fuguet, E.; Mañé, M.; Ruiz, R.; Box, K.; Bosch, E.; Ràfols, C. Effect of vinylpyrrolidone polymers on the solubility and supersaturation of drugs; a study using the Cheqsol method. Eur. J. Pharm. Sci. 2018, 117, 227–235. [Google Scholar] [CrossRef]
- Chavan, R.B.; Rathi, S.; Jyothi, V.G.S.S.; Shastri, N.R. Cellulose based polymers in development of amorphous solid dispersions. Asian J. Pharm. Sci. 2019, 14, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, J. Dissolution enhancement of celecoxib via polymer-induced crystallization. J. Cryst. Growth 2013, 374, 37–42. [Google Scholar] [CrossRef]
- Sodhi, I.; Mallepogu, P.; Thorat, V.P.; Kashyap, M.C.; Sangamwar, A.T. Insights on role of polymers in precipitation of celecoxib from supersaturated solutions as assessed by focused beam reflectance measurement (FBRM). Eur. J. Pharm. Sci. 2019, 137, 104983. [Google Scholar] [CrossRef]
- Sharma, M.; Trout, B.L. Effect of Pore Size and Interactions on Paracetamol Aggregation in Porous Polyethylene Glycol Diacrylate Polymers. J. Phys. Chem. B 2015, 119, 8135–8145. [Google Scholar] [CrossRef]
- Pfund, L.; Price, C.; Frick, J.; Matzger, A. Controlling Pharmaceutical Crystallization with Designed Polymeric Heteronuclei. J. Am. Chem. Soc. 2014, 137, 871–875. [Google Scholar] [CrossRef]
- Sudha, C.; Nandhini, R.; Srinivasan, K. Polymer-Induced Selective Nucleation of Mono or Ortho Polymorphs of Paracetamol through Swift Cooling of Boiled Aqueous Solution. Cryst. Growth Des. 2014, 14, 705–715. [Google Scholar] [CrossRef]
- Song, Y.; Cai, Z.; Li, Z.; Guan, G.; Jiang, Y. Preferential Orientation Effect of Polymers on Paracetamol Crystallization: Experiments and Modeling. Cryst. Growth Des. 2018, 18, 4987–4997. [Google Scholar] [CrossRef]
- Stojaković, J.; Baftizadeh, F.; Bellucci, M.A.; Myerson, A.S.; Trout, B.L. Angle-Directed Nucleation of Paracetamol on Biocompatible Nanoimprinted Polymers. Cryst. Growth Des. 2017, 17, 2955–2963. [Google Scholar] [CrossRef]
- Frank, D.S.; Matzger, A.J. Influence of Chemical Functionality on the Rate of Polymer-InducedHeteronucleation. Cryst. Growth Des. 2017, 17, 4056–4059. [Google Scholar] [CrossRef]
- Price, C.P.; Grzesiak, A.L.; Matzger, A.J. Crystalline Polymorph Selection and Discovery with Polymer Heteronuclei. J. Am. Chem. Soc. 2005, 127, 5512–5517. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Davis, R.M.; Myerson, A.S.; Trout, B.L. Control of Heterogeneous Nucleation via Rationally Designed Biocompatible Polymer Surfaces with Nanoscale Features. Cryst. Growth Des. 2015, 15, 2176–2186. [Google Scholar] [CrossRef]
- Cheng, H.; Mao, L.; Zhang, S.; Lv, H. Impacts of Polymeric Additives on Nucleation and Crystal Growth of Indomethacin from Supersaturated Solutions. AAPS PharmSciTech 2019, 20, 193. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Harada, T.; Myerson, A.; Hatton, T.; Trout, B. The role of nanopore shape in surface-induced crystallization. Nat. Mater. 2011, 10, 867–871. [Google Scholar] [CrossRef]
- Diao, Y.; Myerson, A.S.; Hatton, T.A.; Trout, B.L. Surface Design for Controlled Crystallization: The Role of Surface Chemistry and Nanoscale Pores in Heterogeneous Nucleation. Langmuir 2011, 27, 5324–5334. [Google Scholar] [CrossRef] [PubMed]
- Poornachary, S.K.; Chia, V.D.; Yani, Y.; Han, G.; Chow, P.S.; Tan, R.B.H. Anisotropic Crystal Growth Inhibition by Polymeric Additives: Impact on Modulation of Naproxen Crystal Shape and Size. Cryst. Growth Des. 2017, 17, 4844–4854. [Google Scholar] [CrossRef]
- Gupta, K.M.; Yani, Y.; Poornachary, S.K.; Chow, P.S. Atomistic Simulation To Understand Anisotropic Growth Behavior ofNaproxen Crystal in the Presence of Polymeric Additives. Cryst. Growth Des. 2019, 19, 3768–3776. [Google Scholar] [CrossRef]
- Pataki, H.; Sóti, P.; Vigh, T.; Nagy, Z.; Vajna, B.; Csontos, I.; Marosi, G. Controlled Formation of Free-Flowing Carvedilol Particles in the Presence of Polyvinylpyrrolidone. Chem. Eng. Technol. 2014, 37, 249–256. [Google Scholar] [CrossRef]
- Xia, D.; Wu, J.X.; Cui, F.; Qu, H.; Rades, T.; Rantanen, J.; Yang, M. Solvent-mediated amorphous-to-crystalline transformation of nitrendipine in amorphous particle suspensions containing polymers. Eur. J. Pharm. Sci. 2012, 46, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Eral, H.B.; O’Mahony, M.; Shaw, R.; Trout, B.L.; Myerson, A.S.; Doyle, P.S. Composite Hydrogels Laden with Crystalline Active Pharmaceutical Ingredients of Controlled Size and Loading. Chem. Mater. 2014, 26, 6213–6220. [Google Scholar] [CrossRef]
- Eral, H.B.; López-Mejías, V.; O’Mahony, M.; Trout, B.L.; Myerson, A.S.; Doyle, P.S. Biocompatible Alginate Microgel Particles as Heteronucleants and Encapsulating Vehicles for Hydrophilic and Hydrophobic Drugs. Cryst. Growth Des. 2014, 14, 2073–2082. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.; Godfrin, P.; Myerson, A.; Trout, B.; Doyle, P. Core–Shell Composite Hydrogels for Controlled Nanocrystal Formation and Release of Hydrophobic Active Pharmaceutical Ingredients. Adv. Healthc. Mater. 2016, 5, 1960–1968. [Google Scholar] [CrossRef]
- Bae, H.; Lee, J. Morphology control of eprosartan crystals via polymer-directed crystallization. J. Pharm. Investig. 2015, 45, 367–374. [Google Scholar] [CrossRef]
- Munk, T.; Baldursdottir, S.; Hietala, S.; Rades, T.; Kapp, S.; Nuopponen, M.; Kalliomäki, K.; Tenhu, H.; Rantanen, J. Crystal Morphology Modification by the Addition of Tailor-Made Stereocontrolled Poly(N-isopropyl acrylamide). Mol. Pharm. 2012, 9, 1932–1941. [Google Scholar] [CrossRef]
- Czyzewski, A.M.; Chen, S.; Bhamidi, V.; Yu, S.; Marsden, I.; Ding, C.; Becker, C.; Napier, J.J. Use of a Polymer Additive to Enhance Impurity Rejection in the Crystallization of a Pharmaceutical Compound. Org. Process Res. Dev. 2017, 21, 1493–1500. [Google Scholar] [CrossRef]
- Frank, D.S.; Zhu, Q.; Matzger, A.J. Inhibiting or Accelerating Crystallization of Pharmaceuticals by Manipulating Polymer Solubility. Mol. Pharm. 2019, 16, 3720–3725. [Google Scholar] [CrossRef]
- Torres-Moya, I.; Saikia, B.; Prieto, P.; Carrillo, J.R.; Steed, J.W. High thermal stability, pH responsive organogels of 2H-benzo[d]1,2,3-triazole derivatives as pharmaceutical crystallization media. CrystEngComm 2019, 21, 2135–2143. [Google Scholar] [CrossRef]
- Shi, N.-Q.; Lei, Y.-S.; Song, L.-M.; Yao, J.; Zhang, X.-B.; Wang, X.-L. Impact of amorphous and semicrystalline polymers on the dissolution andcrystallization inhibition of pioglitazone solid dispersions. POWDER Technol. 2013, 247, 211–221. [Google Scholar] [CrossRef]
- Gao, Y.; Olsen, K.W. Drug-Polymer Interactions at Water-Crystal Interfaces and Implicationsfor Crystallization Inhibition: Molecular Dynamics Simulations ofAmphiphilic Block Copolymer Interactions with Tolazamide Crystals. J. Pharm. Sci. 2015, 104, 2132–2141. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, H.; Lee, M.K.; Lee, J. Polymer-Directed Crystallization of Atorvastatin. J. Pharm. Sci. 2012, 101, 2941–2951. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Hata, Y.; Bjornmalm, M.; Ju, Y.; Caruso, F. Supramolecular Metal-Phenolic Gels for the Crystallization of ActivePharmaceutical Ingredients. SMALL 2018, 14, 1801202. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Whaley, K.E.; Helgeson, M.E.; Woldeyes, M.A.; Doyle, P.S.; Myerson, A.S.; Hatton, T.A.; Trout, B.L. Gel-Induced Selective Crystallization of Polymorphs. J. Am. Chem. Soc. 2012, 134, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Singh, D.; Sirkar, K.K.; Pfeffer, R. Continuous preparation of polymer coated drug crystals by solid hollow fiber membrane-based cooling crystallization. Int. J. Pharm. 2016, 499, 395–402. [Google Scholar] [CrossRef]
- Shi, X.; Hu, S.; Song, S.; Ding, Z.; Sheng, X. Selective crystallization of agomelatine from molten state induced bypolymer. J. Drug Deliv. Sci. Technol. 2019, 49, 448–454. [Google Scholar] [CrossRef]
- Parambil, J.V.; Poornachary, S.K.; Heng, J.Y.Y.; Tan, R.B.H. Template-induced nucleation for controlling crystal polymorphism: From molecular mechanisms to applications in pharmaceutical processing. CrystEngComm 2019, 21, 4122–4135. [Google Scholar] [CrossRef]
- Thakore, S.D.; Sood, A.; Bansal, A.K. Emerging role of primary heterogeneous nucleation in pharmaceutical crystallization. Drug Dev. Res. 2020, 81, 3–22. [Google Scholar] [CrossRef]
- Sood, J.; Sapra, B.; Bhandari, S.; Tiwary, A. Understanding pharmaceutical polymorphic transformations II: Crystallization variables and influence on dosage forms. Ther. Deliv. 2015, 6, 721–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shen, T.; Li, S.; Wang, X. Investigation of influence of low phosphorous co-polymer antiscalant on calcium sulfate dihydrate crystal morphologies. Desalination 2014, 348, 89–93. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhao, C.X.; Liu, X.D.; Li, W.; Wang, J.L.; Hu, Z.G. Application of poly(aspartic acid-citric acid) copolymer compound inhibitor as an effective and environmental agent against calcium phosphate in cooling water systems. J. Appl. Res. Technol. 2016, 14, 425–433. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, L.; Liu, K.; Gao, P.; Ge, H.; Fu, L. Inhibition of calcium sulfate scale by poly (citric acid). Desalination 2016, 392, 1–7. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, X.; Wang, Q.; Shan, H.; Xu, Y. Synthesis and evaluation of polyaspartic acid/furfurylamine graft copolymer as scale and corrosion inhibitor. RSC Adv. 2016, 6, 102406–102412. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, Y.; Yao, Q.; Wang, H.; Liu, Y.; Tao, W.; Chu, X.; Sun, W.; Wu, W. Synthesis of glutamic-modified polyether copolymer as a novel non-phosphorous inhibitor for calcium carbonate scales in cooling water systems. Desalin. Water Treat. 2016, 57, 19206–19215. [Google Scholar] [CrossRef]
- Du, Q.; Wang, Y.; Li, A.; Yang, H. Scale-inhibition and flocculation dual-functionality of poly(acrylic acid) grafted starch. J. Environ. Manag. 2018, 210, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Sugimura, K.; Nishio, Y. CaCO3 mineralization in polymer composites with cellulose nanocrystals providing a chiral nematic mesomorphic structure. Int. J. Biol. Macromol. 2019, 141, 783–791. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Yao, Q.; Nan, Q.; Zhang, M.; Sun, W. Inhibition and biodegradability performance of modified polyepoxysuccinic acid as a scale inhibitor against calcium carbonate. Desalin. Water Treat. 2019, 147, 211–221. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Yao, Q.; Nan, Q.; Zhang, M.; Sun, W. Synthesis of modified polyepoxysuccinic acid and evaluation of its scale inhibition on CaCO3, CaSO4, and Ca3(PO4)2 precipitation for industrial recycling water. Desalin. Water Treat. 2019, 152, 16–25. [Google Scholar] [CrossRef]
- Zahlan, H.; Saeed, W.S.; Alrasheed, R.; Alandes, N.M.; Aouak, T. Synthesis of poly (citric acid-co-glycerol) and its application as an inhibitor of CaCO3 deposition. Materials 2019, 12, 3800. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, S. Synthesis, scale inhibition and dispersion performance evaluation of the environmentally benign additive IA-AMPS-APEG copolymer. Environ. Sci. Water Res. Technol. 2019, 5, 1736–1747. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.; Yang, Z.; Xu, Q.; Yang, P.; Wang, D. Enhancement of anaerobic digestion sludge dewatering performance using in-situ crystallization in combination with cationic organic polymers flocculation. Water Res. 2018, 146, 19–29. [Google Scholar] [CrossRef]
- Chen, J.; Shao, M.; Xiao, K.; He, Z.; Li, D.; Lokitz, B.S.; Hensley, D.K.; Kilbey, S.M.; Anthony, J.E.; Keum, J.K.; et al. Conjugated Polymer-Mediated Polymorphism of a High Performance, Small-Molecule Organic Semiconductor with Tuned Intermolecular Interactions, Enhanced Long-Range Order, and Charge Transport. Chem. Mater. 2013, 25, 4378–4386. [Google Scholar] [CrossRef]
- Bi, D.; Yi, C.; Luo, J.; Decoppet Jean-Davidand Zhang, F.; Zakeeruddin, S.M.; Li, X.; Hagfeldt, A.; Gratzel, M. Polymer-templated nucleation and crystal growth of perovskite films forsolar cells with efficiency greater than 21%. Nat. Energy 2016, 1, 16142. [Google Scholar] [CrossRef]
- Ren, Y.; Hao, Y.; Zhang, N.; Arain, Z.; Mateen, M.; Sun, Y.; Shi, P.; Cai, M.; Dai, S. Exploration of Polymer-Assisted Crystallization Kinetics in CsPbBr 3 All-Inorganic solar cell. Chem. Eng. J. 2019, 392, 123805. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, B.; Li, J.; Fang, B.; Lin, Y. CdS nanodots preparation and crystallization in a polymeric colloidal nanoreactor and their characterizations. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 203–211. [Google Scholar] [CrossRef]
- Ghosh, D.; Ferfolja, K.; Drabavicius, Z.; Steed, J.W.; Damodaran, K.K. Crystal habit modification of Cu(II) isonicotinate-N-oxide complexesusing gel phase crystallisation. NEW J. Chem. 2018, 42, 19963–19970. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Zhang, C.; Cai, Y. Effect of highly carboxylated colloidal polymers on cement hydration and interactions with calcium ions. Cem. Concr. Res. 2018, 113, 140–153. [Google Scholar] [CrossRef]
- Pan, J.-H.; Wu, C.-F.; Wang, C.-A.; Lin, K.-T.; Ruan, J. Influence of horizontal distribution of polymer phases on the dispersion and crystallization of organic semiconductor triisopropylsilyl pentacene. Mater. Chem. Phys. 2018, 216, 112–119. [Google Scholar] [CrossRef]
- Purohit, B.K.; Sistla, V.S. Crystallization of inorganic salt hydrates in polymeric foam for thermalenergy storage application. J. Energy Storage 2017, 12, 196–201. [Google Scholar] [CrossRef]
- Cividanes, L.S.; Campos, T.M.B.; Bertran, C.A.; Brunelli, D.D.; Thim, G.P. Effect of urea on the mullite crystallization. J. Non. Cryst. Solids 2010, 356, 3013–3018. [Google Scholar] [CrossRef]
- Schweikle, M.; Bjørnøy, S.H.; van Helvoort, A.T.J.; Haugen, H.J.; Sikorski, P.; Tiainen, H. Stabilisation of amorphous calcium phosphate in polyethylene glycol hydrogels. Acta Biomater. 2019, 90, 132–145. [Google Scholar] [CrossRef]
- Adawy, A.; Amghouz, Z.; van Hest, J.C.M.; Wilson, D.A. Sub-Micron Polymeric Stomatocytes as Promising Templates for ConfinedCrystallization and Diffraction Experiments. SMALL 2017, 13, 1700642. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, C.; Congdon, T.R.; Gibson, M.I. Photo-polymerisation and study of the ice recrystallisation inhibition of hydrophobically modified poly(vinyl pyrrolidone) co-polymers. Eur. Polym. J. 2019, 110, 330–336. [Google Scholar] [CrossRef]
- Akshaykranth, A.; Rao, T.V.; Kumar, R.R. Growth of ZnO nanorods on biodegradable poly (lactic acid) (PLA) substrates by low temperature solution method. Mater. Lett. 2020, 259, 126807. [Google Scholar] [CrossRef]
- Saridakis, E.; Chayen, N.E. Imprinted polymers assisting protein crystallization. Trends Biotechnol. 2013, 31, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Di Profio, G.; Polino, M.; Nicoletta, F.P.; Belviso, B.D.; Caliandro, R.; Fontananova, E.; De Filpo, G.; Curcio, E.; Drioli, E. Tailored Hydrogel Membranes for Efficient Protein Crystallization. Adv. Funct. Mater. 2014, 24, 1582–1590. [Google Scholar] [CrossRef]
- Belviso, B.D.; Caliandro, R.; Salehi Shabnam Majidiand Di Profio, G.; Caliandro, R. Protein Crystallization in Ionic-Liquid Hydrogel Composite Membranes. Crystals 2019, 9, 253. [Google Scholar] [CrossRef]
- Bakshi, M.S. How Surfactants Control Crystal Growth of Nanomaterials. Cryst. Growth Des. 2016, 16, 1104–1133. [Google Scholar] [CrossRef]
| Type of Process | Polymer Used as Tool | Crystallized Substance | Reference |
|---|---|---|---|
| Confined crystallization | Alginate | CaCO3 | [12] |
| Silk fibroin | CaCO3 | [13] | |
| Gelatin | CaCO3 | [14] | |
| Alginate, xanthan | CaCO3 | [15] | |
| Polyacrylamide | Ca3(PO4)2 | [16] | |
| Biopolymer | TiO2 | [18] | |
| Poly(ethylene glycol) diacrylate | Aspirin, paracetamol | [19] | |
| Poly(ethylene glycol) diacrylate | Aspirin, paracetamol | [20] | |
| Glutaraldehyde crosslinked chitosan | Zeolite | [21] | |
| homopolymers and copolymers of nethylmorpholine methacrylamide and N,N-dimethylacrylamide | Ibuprofen | [45] | |
| Poly(ethylene glycol) diacrylate | Paracetamol | [57] | |
| Alginate | Fenofibrate | [72] | |
| Alginate | Paracetamol, fenofibrate | [73] | |
| Alginate | Fenofibrate | [74] | |
| 2H-benzo[d]1,2,3-triazole derivatives | Theophylline, sulfathiazole, sulfamerazine and niflumic acid | [79] | |
| Tannic acid-TiIV metallogel | Caffeine, carbamazepine, piroxicam | [83] | |
| Poly(ethylene glycol) diacrylate | Carbamazepine, 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile | [84] | |
| Agarose, tris-amide of trimesic acid with L-valine methyl ester, 3,3′,3″-((Benzene-1,3,5-tricarbonyl)tris(azanediyl))tris-(pyridine 1-oxide) | Copper(II) isonicotinate–N-oxide complex, Cu(NO3)2·3H2O | [106] | |
| Open cell polyurethane foam composites | Na2SO4, MgSO4·7H2O, and their mixture | [109] | |
| Polymer gels from urea, aluminum nitrate nonahydrate, tetraethylorthosilicate | Mullite | [110] | |
| Poly(ethylene glycol) | Ca3(PO4)2 | [111] | |
| Stomatocytes | KH2PO4, NaCl, NaClO3, (NH4)2SO4 | [112] |
| Type of Process | Polymer Used as Tool | Crystallized Substance | Reference |
|---|---|---|---|
| Crystallization on polymeric surfaces/ nucleation induction | Microporous poly(vinylidene fluoride) membranes | hen egg white lysozyme | [22] |
| Poly(acrylic acid), poly(sodium 4-styrenesulfonate), poly(allylamine hydrochloride), poly(ethyleneimine), polyamide pellets | CaSO4·2H2O | [23] | |
| Polyethylene, polypropylene, polyvinyl chloride, poly(vinyl alcohol), polystyrene, poly(4-aminostyre) | Benzocaine, 1,1′-bi-2-naphthol | [24] | |
| Siloxane templates | Diphenylurea | [25] | |
| Polyethylene, polyvinyl chloride, polycarbonate, PET, nylon 66, ethylene vinyl alcohol, cellulose acetate, poly(methyl methacrylate) | CaCO3 | [26] | |
| Nanocomposites | PET | [27] | |
| 2-((4-vinylphenyl)amino)-benzoic acid/divinylbenzene copolymer, 2-((4-vinylphenyl)amino)-benzoic acid/divinylbenzene copolymer, hydroxyethyl methacrylate/divinylbenzene copolymer, hydroxyethyl methacrylate/divinylbenzene copolymer | paracetamol, mefenamic acid | [58] | |
| Nylon 66, polyester, polypropylene | paracetamol | [60] | |
| 2-hydroxyethyl cellulose | paracetamol | [61] | |
| Functionalized Merrifield Resin | paracetamol | [62] | |
| Cross-linked terpolymers in which one component is the cross-linker | paracetamol, sulfamethoxazole, carbamazepine, 5-methyl2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile | [63] | |
| poly(vinyl alcohol) | Aspirin, indomethacin | [64] | |
| Poly(acrylic acid) films crosslinked with divinylbenzene | Aspirin | [66] | |
| Poly(4-acryloylmorpholine) and poly(2-carboxyethyl acrylate), each cross-linked with divinylbenzene | Aspirin | [67] | |
| poly(styrene-co-chloromethylstyrene) | Pyrazinamide, hydrochlorothiazide | [78] | |
| Ethyl cellulose | Agomelatine Form I | [86] | |
| cellulose nanocrystal/poly(2-hydroxyethyl methacrylate), cellulose nanocrystal/poly(2-hydroxyethyl methacrylate-co-acrylic acid) | CaCO3 | [96] | |
| Poly(methyl methacrylate) | Pervoskite | [103] | |
| Polyacrylamide | CdS | [105] | |
| Highly carboxylated polystyrene latex | Calcium silicate hydrate | [107] | |
| Poly(lactic acid) | ZnO | [114] | |
| Acrylamide, poly(ethylene glycol)dimethacrylate | Lysozyme, concanavalin A | [116] |
| Crystal surface screening/nucleation inhibition | Poly(sodium 4-styrenesulfonate) | ZnO | [28] |
| Poly(vinyl alcohol), polyvinylpyrolidone | ZnO | [29] | |
| Molecularly imprinted polymers (oleanolic acid as template, acrylamide as functional monomer, ethylene glycol dimethacrylate as cross-linker and azobisisobutyronitrile as initiator) | Oleanolic acid | [30] | |
| Polyacrylamide, poly(vinyl alcohol), poly(ethylene glycol), poly-N-vinyl pyrrolidone | PbS | [31] | |
| Maleic acid copolymers | Calcium orthophosphates | [32] | |
| Poly(ethylene oxide) | Copper/poly(ethylene oxide)/low density polyethylene composite | [33] | |
| Guar gum, pectin, κ-carrageenan, gelatin, polyvinylpyrrolidone | Thiamine chloride hydrochloride | [34] | |
| Poly(acrylic acid), poly(N-vinyl pyrrolidone), poly(4-vinylphenol) | Ibuprofen | [46] | |
| Polymers functionalized from a parent batch of poly(chloromethylstyrene-co-styrene) | Nabumetone | [47] | |
| Functionalized poly(N-hydroxyethyl acrylamide) | Ethenzamide | [48] | |
| Hydroxypropylmethyl cellulose | Felodipine | [49] | |
| Alginate | Lovastatin, indomethacin, itraconazole | [50] | |
| Hydroxypropyl methylcellulose and methylcellulose polymers | Griseofulvin | [51] | |
| Hydroxypropyl methylcellulose, polyvinylpyrrolidone), Eudragit L-100 | Griseofulvin, danazol | [52] | |
| Different polymers of vinylpyrrolidone and a copolymer of vinylpyrrolidone and vinylacetate | Sixteen drugs of different chemical nature | [53] | |
| Polyvinylpyrrolidone | Celecoxib | [55,56] | |
| Nylon 6/6, polypropylene, polyvinylchloride | paracetamol | [59] | |
| Polyvinylpyrrolidone, hydroxypropyl methyl cellulose, Kollidone VA64 | Indomethacin | [65] | |
| Hydroxypropyl methylcellulose, polyvinylpyrrolidone | Naproxen | [68] | |
| Polyvinylpyrrolidone | Naproxen | [69] | |
| Polyvinylpyrrolidone | Carvedilol | [70] | |
| Poly(vinyl alcohol), polyethylene glycol | Nitrendipine | [71] | |
| Poly(ethylene imine) | Eprosartan | [75] | |
| Poly(N-isopropyl acrylamide) | Nitrofurantoin | [76] | |
| Hydroxypropylmethyl cellulose, copovidone | Complex impurity | [77] | |
| Polyvinylpolypyrrolidone K30 and K90, polyethyleneglycol 6000, polyethylene-polypropylene glycol 188 | Pioglitazone | [80] | |
| Poly(ethylene glycol)-block-poly(lactic acid) | Tolazamide | [81] | |
| Poly(ethylene glycol), poly(ethylene oxide)-poly(propylene oxide) triblock, hydroxypropyl cellulose, poly(acrylic acid), poly(ethylene imine), elastin-like peptide, chitosan | Atorvastatin calcium | [82] | |
| Eudragit RL100 | Griseofulvin | [85] | |
| Poly(aspartic acid-citric acid) copolymer, polymaleic acid | Ca3(PO4)2 | [91] | |
| Poly(citric acid) | CaSO4 | [92] | |
| Polyaspartic acid/furfurylamine graft copolymer | CaCO3, Ca3(PO4)2 | [93] | |
| Acrylic acid-allyloxy polyethoxy glutamate copolymer | CaCO3 | [94] | |
| Starch-graft-poly(acrylic acid) | CaCO3 | [95] | |
| Epoxysuccinic acid derivative | CaCO3 | [97] | |
| Epoxysuccinic acid derivative | CaCO3, CaSO4, Ca3(PO4)2 | [98] | |
| Poly(Citric Acid-co-Glycerol) | CaCO3 | [99] | |
| Itaconic acid-2-acrylamido-2-methylpropanesulfonic acid allyl polyoxyethylene ether copolymer | CaCO3 | [100] | |
| Organic polymer | Struvit | [101] | |
| Regiorandom pentacene-bithiophene, poly(3-hexylthiophene) | 6,13-bis(triisopropylsilylethynyl)-pentacene | [102] | |
| Poly(ethylene glycol) | Perovskite | [104] | |
| Poly(3-hexylthiophene), poly(methyl methacrylate) | Bis(triisopropylsilylethynyl) pentacene | [108] | |
| Maleic acid and sodium ρ-styrenesulfonate copolymer | CaSO4·2H2O | [109] | |
| Poly(N-vinyl pyrrolidone) copolymers | H2O | [113] | |
| Composite membranes | Glucose Isomerase | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemanowicz, M.; Mielańczyk, A.; Walica, T.; Kotek, M.; Gierczycki, A. Application of Polymers as a Tool in Crystallization—A Review. Polymers 2021, 13, 2695. https://doi.org/10.3390/polym13162695
Lemanowicz M, Mielańczyk A, Walica T, Kotek M, Gierczycki A. Application of Polymers as a Tool in Crystallization—A Review. Polymers. 2021; 13(16):2695. https://doi.org/10.3390/polym13162695
Chicago/Turabian StyleLemanowicz, Marcin, Anna Mielańczyk, Tomasz Walica, Milena Kotek, and Andrzej Gierczycki. 2021. "Application of Polymers as a Tool in Crystallization—A Review" Polymers 13, no. 16: 2695. https://doi.org/10.3390/polym13162695
APA StyleLemanowicz, M., Mielańczyk, A., Walica, T., Kotek, M., & Gierczycki, A. (2021). Application of Polymers as a Tool in Crystallization—A Review. Polymers, 13(16), 2695. https://doi.org/10.3390/polym13162695